Abstract
Post-translational modification by ubiquitin and ubiquitin-related proteins plays critical roles in protein degradation and in regulation of essential cellular processes. In mammals, transcription grinds to a halt during late spermiogenesis due to compaction of the spermatid genome, which creates a special need for robust post-transcriptional regulation. Here we report the finding of a novel mouse ubiquitin-like protein, UBL4B. Ubl4b is a testis-specific autosomal gene. Ubl4b lacks introns and evidently arose from an X-linked intron-bearing housekeeping gene, Ubl4a, by retroposition during mammalian evolution. While Ubl4a is expressed throughout spermatogenesis, Ubl4b is restricted to post-meiotic germ cells. Ubl4a is highly conserved, but Ubl4b has undergone rapid evolution and may have evolved new functions. Our data suggest that evolution of Ubl4b is not due to meiotic sex chromosome inactivation (MSCI). Alternatively, origination of Ubl4b was due to MSCI, but Ubl4b eventually evolved to be restricted to post-meiotic germ cells.
Keywords: ubiquitin, meiosis, retrogene, MSCI, sex chromosomes, XY body, spermiogenesis, testis, germ cells
1. Results and discussion
Protein modification by ubiquitin regulates diverse cellular processes (Schwartz and Hochstrasser, 2003). Ubiquitin is a highly conserved 76-amino acid (aa) protein tag. Covalent modification of proteins by multiple ubiquitin tags usually targets proteins for degradation. However, mono-ubiquitination often modulates protein functions in a non-proteolytic manner. In addition, a number of ubiquitin-like proteins (UBLs), such as SUMO and NEDD8, have been found to covalently modify other proteins (Schwartz and Hochstrasser, 2003). In contrast to small protein modifiers such as phosphoryl and acetyl groups, ubiquitin and UBLs are much larger and thus can dramatically alter protein conformation and protein-protein interactions. Ubiquitin is important in the regulation of spermatogenesis, including modifications of histone and mitochondria (Bebington, et al., 2001). SUMO-1, a ubiquitin-related factor, might be involved in many aspects of spermatogenesis as well (Vigodner and Morris, 2005). Both ubiquitin and SUMO-1 are widely expressed. Here we report the identification and characterization of a novel testis-specific ubiquitin-like protein, UBL4B and its ancestral gene product, UBL4A.
1.1 Ubl4b is a retroposed homologue of Ubl4a
Tex15 is a testis- and ovary-specific gene of unknown functions (Wang, et al., 2001). In a search for TEX15-interacting proteins, we identified a novel ubiquitin-like protein by yeast two-hybrid. This new protein is homologous to UBL4A (ubiquitin-like 4A) and thus is referred to as UBL4B. However, the function of UBL4A is unknown (Toniolo, et al., 1988). Interestingly, the Ubl4a gene consists of three introns, but Ubl4b lacks introns, suggesting that Ubl4b originated from Ubl4a by retro(trans)position during evolution (Fig. 1A). While Ubl4a maps to the X chromosome, Ubl4b is autosomal (on mouse Chr. 3). Both proteins harbor the 72-aa ubiquitin domain, which is conserved in ubiquitin and ubiquitin-like proteins (Schwartz and Hochstrasser, 2003). UBL4A and UBL4B consist of 157 aa and 188 aa respectively, and are 44% identical at the protein sequence level (Fig. 1A). However, Ubl4b displays no significant homology with Ubl4a at the nucleotide level, suggesting that their sequences are highly diverged.
Fig. 1.
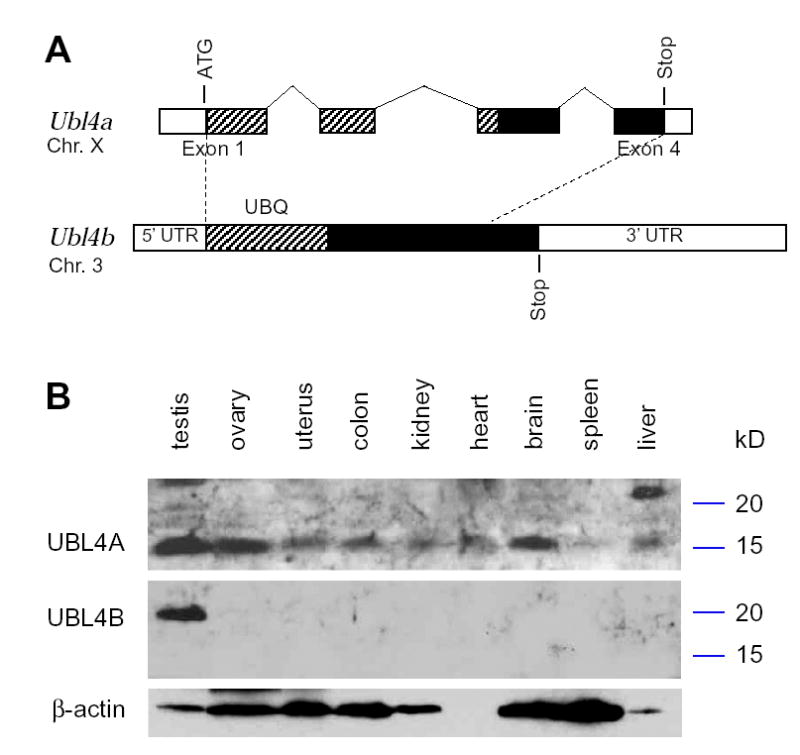
Ubl4b is a testis-specific retrogene. (A) Comparison of the exon/intron structures of mouse Ubl4a and Ubl4b. Exons 1 and 4 of Ubl4a are labeled. Introns and exons are drawn to scale. Coding region is shown in hatch pattern and black. The hatched region indicates the ubiquitin (UBQ) domain. There is little sequence identity between Ubl4a and Ubl4b at the nucleotide level. (B) Western blot analysis of UBL4A and UBL4B in mouse tissues. Bacterially expressed recombinant UBL4A and UBL4B were specifically recognized with respective antibodies (data not shown). Western blotting with pre-immune sera was negative (data not shown). Molecular weight markers are shown in kD.
1.2 Testis-specific expression of Ubl4b
In the mammalian genome, retroposed genes are very common, but most of them are transcriptionally silent (Lander, et al., 2001, Venter, et al., 2001). To determine whether Ubl4b is expressed, we performed RT-PCR on mRNAs from multiple mouse tissues including testis. Our studies show that Ubl4a is ubiquitously expressed but Ubl4b is testis-specific (data not shown). We raised antibodies against recombinant UBL4A and UBL4B respectively. Western blot analysis confirmed the ubiquitous pattern of UBL4A and testis-specificity of UBL4B (Fig. 1B). The apparent molecular weight of UBL4A is 15 kD and that of UBL4B is 20 kD. In addition, these antibodies were specific to UBL4A or UBL4B and did not show cross-reactivity (Fig. 1B).
1.3 Ubl4b is specifically expressed in post-meiotic male germ cells
To determine whether transcription of Ubl4a and Ubl4b is developmentally regulated during spermatogenesis, we examined their expression in juvenile testes by northern blot analysis. We found that Ubl4a was expressed at a relatively constant level during postnatal development of testis (Fig. 2A). In contrast, Ubl4b is not expressed in testes of days 14, 16, and 18, when the most advanced germ cells are spermatocytes, suggesting that Ubl4b is not expressed during male meiosis. Ubl4b begins to express at day 24, when round spermatids first appear. These data demonstrate that Ubl4b is transcribed post-meiotically in male germ cells in a manner similar to that of the Prm1 (protamine 1) gene (Fig. 2A).
Fig. 2.
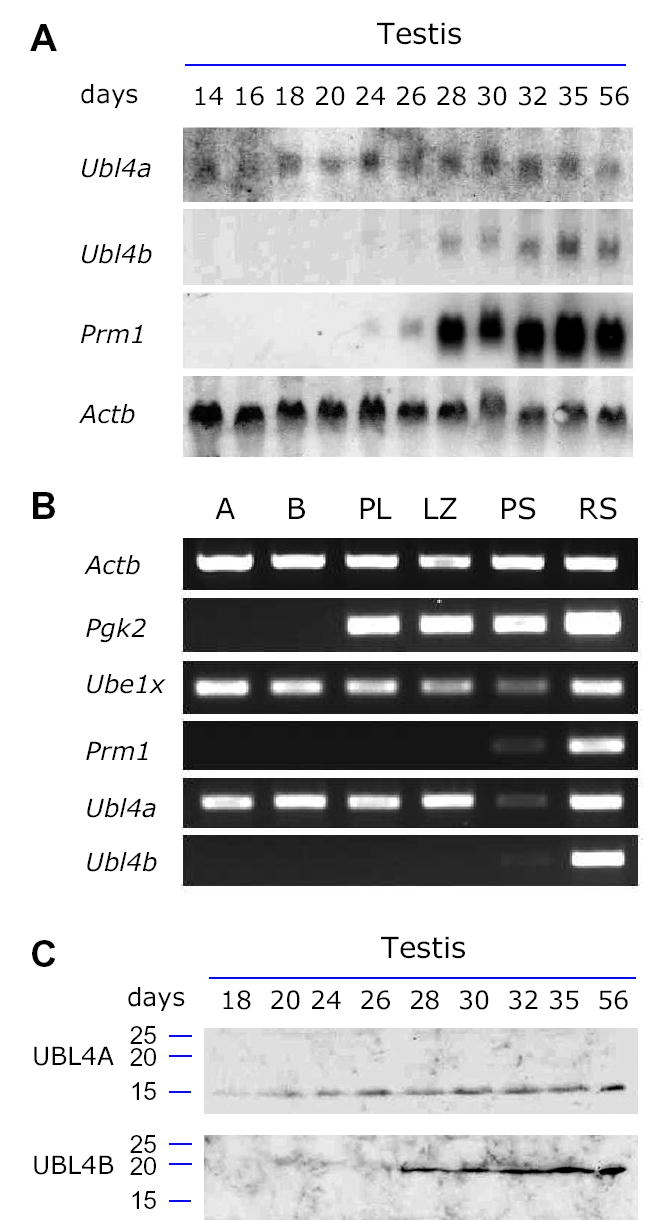
Differential expression of UBL4A and UBL4B during spermatogenesis. Postnatal juvenile testes were collected. The number of days after birth was indicated. (A) Northern blot analysis. ATTT serves as a ubiquitous gene control. Prm1 is a post-meiotically expressed germ cell-specific gene. (B) RT-PCR expression analysis using germ cell preparations. Relative levels of transcripts among different germ cell types were assayed by RT-PCR. Four control genes were used as previously described (Wang, et al., 2005). In addition to Actb and Prm1, Pgk2 initiates transcription at the onset of meiosis. Ube1x is a control for undergoing MSCI as previously documented (Odorisio, et al., 1996). A: type A spermatogonia; B: type B spermatogonia; PL: preleptotene spermatocytes; LZ: leptotene plus zygotene spermatocytes; PS: adult pachytene spermatocytes; RS: round spermatids. (C) Western blot analysis. 30 μg of testicular protein extracts for each sample was used. Molecular weight standards were marked in kD.
We then examined the relative levels of transcripts in different spermatogenic cell populations by RT-PCR (Fig.2B). The expression level of Ubl4a is comparable in spermatogonia, early spermatocytes (preleptotene through zygotene) and round spermatids. In contrast, the transcript level of Ubl4a in pachytene spermatocytes is sharply reduced, suggesting that transcription of Ubl4a is subject to MSCI. As expected, Ubl4b is only expressed in round spermatids
Furthermore, we examined the relative protein levels of UBL4A and UBL4B in juvenile testes. Western blot analysis shows that the abundance of UBL4A is relatively constant (Fig. 2C). In contrast, the UBL4B is first detected in testes of day 28, when round spermatids differentiate into elongating spermatids, suggesting that UBL4B is restricted to late spermiogenesis.
1.4 Differential localization of UBL4A and UBL4B in male germ cells
To determine the subcellular localization of UBL4A and UBL4B in male germ cells, we immunostained adult testis sections with anti-UBL4A and anti-UBL4B antibodies. Immunofluorescence analysis reveals that UBL4A is a cytoplasmic protein. UBL4A is present in both somatic cells (Leydig cells and Sertoli cells) and germ cells (spermatogonia, spermatocytes and spermatids) (Fig. 3A). In contrast to the ubiquitous distribution of UBL4A, UBL4B is a germ cell-specific cytoplasmic protein (Fig. 3C). UBL4B is not present in somatic cells (Leydig cells or Sertoli cells). Furthermore, UBL4B is present in elongated spermatids, but not in spermatocytes and round spermatids (Fig. 3C), suggesting that UBL4B function is restricted to late spermiogenesis. In addition, we performed immunostaining of epididymal sperm (online supplementary data Fig. S1). The anti-UBL4A antibodies appeared to weakly stain the sperm tail (both middle and principal pieces) and the periphery of the sperm head. By contrast, anti-UBL4B antibodies gave rise to a strong staining of the middle piece but a weak staining of the principal piece.
Fig. 3.
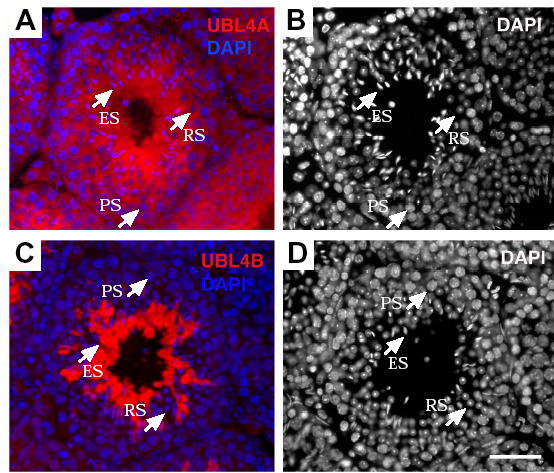
Subcellular localization of UBL4A and UBL4B in male germ cells. Adult mouse testis sections were immunostained with anti-UBL4A or anti-UBL4B antibodies (both shown in red). Nuclear DNA was stained with DAPI. (A) UBL4A is distributed in the cytoplasm of all germ cells. (C) Localization of UBL4B is restricted to elongating spermatids. Panels B and D are identical to Panels A and C, respectively, except that nuclei staining alone is shown to distinguish among different testicular cell types. PS, pachytene spermatocytes; RS, round spermatids; ES, elongating spermatids. Scale bar, 50 μm
Expression of UBL4B depends on the stages of seminiferous tubules. Spermatogenesis in mice is divided into 12 stages, each of which consists of a unique association of differentiating germ cells (Russell, et al., 1990). To precisely determine when UBL4B is translated, we immuno-stained testis sections with anti-UBL4B antibodies, anti-ACRV1 (acrosomal vesicle protein 1) antibodies, and DAPI. ACRV1 (previously known as SP-10) is an acrosomal component (Reddi, et al., 1995). According to the morphology of acrosomes and nuclei, the stage of seminiferous tubules can be determined. Our results show that UBL4B is present in Step 13 spermatids at an extremely low level (Fig. 4A). The abundance of UBL4B dramatically increases in Step 14 spermatids (Fig. 4B), and the high level of UBL4B persists in elongated spermatids of Steps 15 and 16 (Fig. 4C, D). At the conclusion of Stage VIII, Step 16 spermatids are released from the seminiferous epithelium. In the subsequent Stage IX, UBL4B is not observed in elongating spermatids of Step 9 (Fig. 4E). A low level of UBL4B is not observed until in spermatids at Step 12 (Fig. 4F). In summary, UBL4A is present in all types of germ cells (Fig. 5A). UBL4B is not observed in spermatogonia, spermatocytes, or spermatids of Steps 1–11. UBL4B is present in spermatids of Steps 12 and 13 at an extremely low level. However, UBL4B is abundant in spermatids of Steps 14–16 (Fig. 5B).
Fig. 4.
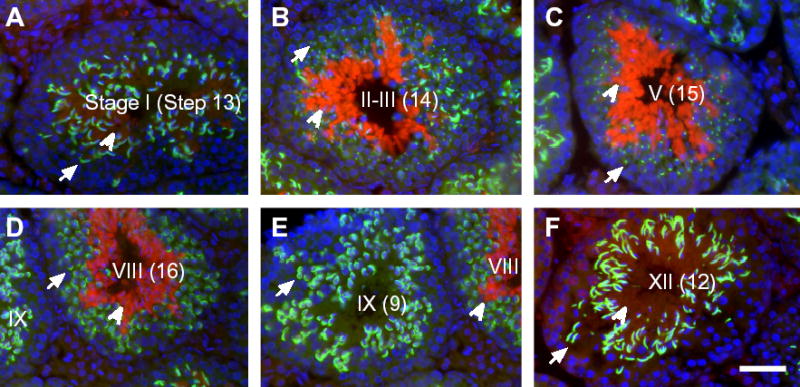
Stage-dependent distribution of UBL4B in mouse spermiogenesis. (A-F) Expression of UBL4B in representative tubules. Testis sections were immunostained with anti-UBL4B antibodies (red, indicated by arrow heads), anti-ACRV1 (green, indicated by arrows), and DAPI (blue). Anti-UBL4B antiserum did not show cross reactivity with UBL4A (Fig. 1C). The morphology of acrosomes (stained by anti-ACRV1 antibodies) and nuclei (stained by DAPI) was used to determine the stages of seminiferous tubules. The stage number is shown in roman numerals. The Arabic number in parenthesis designates the step of most advanced spermatids. Elongated spermatids are multiple layers of germ cells located in the lumen of seminiferous tubules. (D, E) Comparison of two adjacent tubules at Stages VIII and IX. As Step 16 spermatozoa are released from the seminiferous epithelium in Stage VIII, UBL4B is not observed in the subsequent Stage IX tubules. Scale bar, 50 μm.
Fig. 5.
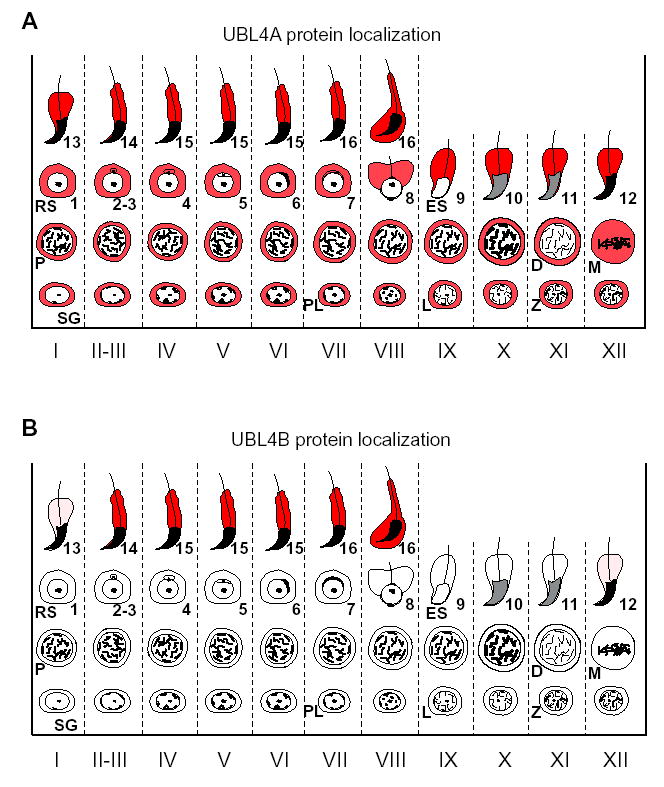
Summary of UBL4A and UBL4B protein localization during spermatogenesis. Expression of UBL4A and UBL4B protein is shown in red. Stages (I-XII) of spermatogenesis are shown at the bottom. Representative types of germ cells are indicated. SG, spermatogonia; PL, preleptotene spermatocytes; L, leptotene; Z, zygotene; P, pachytene; D, diplotene; M, metaphase spermatocyte; RS, round spermatid; ES, elongating spermatid.
1.5 Ubl4b retroposition predates the separation of human and marsupial lineages
To determine when the Ubl4b retroposition occurred during evolution, we searched for Ubl4b orthologues in other mammalian genomes. We identified the human UBL4B gene (NM_203412). The UBL4B gene maps to the same syntenic region between mouse and human and, as expected, is flanked by the same genes (ALX3 and SLC6A17) in both species. We also found UBL4B orthologues in chimpanzee, dog, cattle, and opossum. These data suggest that the Ubl4b retroposition probably occurred at least 170 million years ago, prior to the radiation of therian mammals (Fig. 6A).
Fig. 6.
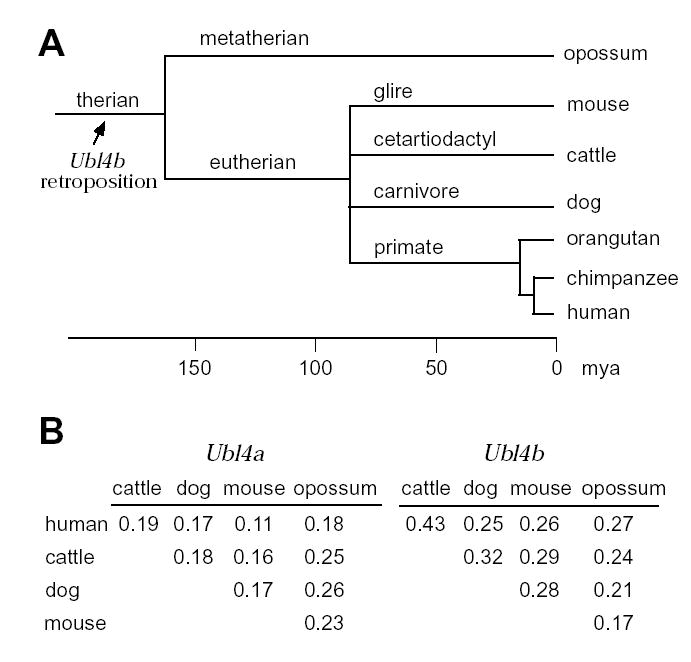
Evolution of the Ubl4b retrogene. (A) Timing of the Ubl4b retroposition. The timescale was drawn as described previously (mya, million years ago) (Kumar and Hedges, 1998). By searching the genomic sequence databases, we identified the Ubl4b orthologues in opossum, mouse, cattle, dog, orangutan, and human. (B) Ka/Ks ratios calculated from pairwise comparisons in five species. Calculation was performed for Ubl4a and Ubl4b separately. The cDNA sequences of Ubl4a and Ubl4b orthologues were retrieved from GenBank. The opossum Ubl4a cDNA sequence was electronically predicted from genomic sequences generated by Broad Institute (Cambridge, MA, USA). The coding sequences were aligned using CLUSTAL W (Jeanmougin, et al., 1998). The Ka/Ks ratios were calculated according to Li’s method as implemented in the GCG software (Li, 1993).
To determine whether Ubl4b has undergone rapid evolution, we analyzed the rates of non-synonymous substitution (Ka) and synonymous substitution (Ks) (Li, 1993). A nucleotide substitution in protein-coding regions is synonymous or silent if it causes no amino acid change. Non-synonymous substitutions refer to both missense and nonsense mutations. The Ka/Ks ratio of the Ubl4b orthologues in most cases is substantially higher than that of the Ubl4a orthologues (Fig. 6B), suggesting that the Ubl4b gene has evolved more rapidly. In addition, the Ka/Ks ratio of Ubl4b is consistently < 1, indicating that the UBL4B protein, like UBL4A, has been subject to purifying selection.
A number of X-derived mammalian retrogenes have been reported (Wang, 2004). A recent genome-wide survey has identified a large number of putative new X-derived retrogenes in humans and mice (Emerson, et al., 2004). These studies demonstrate an excess of retroposition from the X chromosome. It has been hypothesized that the driving force behind preferential X-originated retroposition is the meiotic sex chromosome inactivation (MSCI) (McCarrey and Thomas, 1987, Emerson, et al., 2004, Wang, 2004). During male meiosis of mammals, sex chromosomes are sequestered into a so-called “XY” body and become transcriptionally inactive. By contrast, autosomes are transcriptionally active. Due to MSCI, some X-linked house-keeping genes have evolved autosomal “backup” testis-specific genes by retroposition. In support of the MSCI hypothesis for the evolution of X-derived retrogenes, most known testis-specific retrogenes initiate transcription during male meiosis (McCarrey and Thomas, 1987, Dahl, et al., 1990, Sedlacek, et al., 1999, Elliott, et al., 2000, Dass, et al., 2001, Bradley, et al., 2004, Rohozinski and Bishop, 2004).
However, two lines of evidence suggest that evolution of the Ubl4b retrogene might not be due to MSCI. First, the UBL4A protein persists throughout spermatogenesis including meiosis. Despite MSCI, the UBL4A protein level in meiotic germ cells is not decreased, suggesting that the UBL4A protein has a long half-life. Second, Ubl4b is transcribed post-meiotically and the UBL4B protein is detected in elongated spermatids but not in spermatocytes. However, the Ubl4b expression level or protein level in spermatocytes might be below the detection limit of our methods (northern blotting, western blotting, and immunofluorescence). Alternatively, the original evolution of Ubl4b could be due to MSCI. Once established as a functional gene, Ubl4b subsequently diverged from Ubl4a and became restricted to post-meiotic germ cells.
2. Experimental procedures
2.1 Production of polyclonal antibodies
The entire mouse Ubl4a coding region was cloned in the pQE-30 vector (QIAGEN). The 6xHis-UBL4A (1–157aa) fusion protein was expressed in E. coli and affinity purified with Ni-NTA resin. The full-length mouse UBL4B (188 aa) was expressed as a GST fusion protein in E. coli using the pGEX4T-1 vector and affinity purified with glutathione sepharose. The UBL4B moiety was released from the fusion protein by enzymatic cleavage with thrombin and recovered for immunization. Purified 6xHis-UBL4A and UBL4B were used to immunize rabbits (Cocalico Biologicals, Inc). Antiserum was used for western blot analysis (1:500).
2.2 Northern blot and RT-PCR analysis
Total RNAs were extracted from mouse tissues by using TRIzol reagent (Invitrogen). 20 μg total RNAs were electrophoresed on a 1% agarose/formaldehyde gel, transferred to a GeneScreen Plus nylon membrane (PerkinElmer), pre-hybridized in Rapid-hyb Buffer (Amersham Biosciences) for 1 hour at 65°C, hybridized with 32P-labeled DNA probes for 1–2 hours at 65°C, and washed. The hybridized blot was exposed to Kodak BioMax MR film (Eastman Kodak). Probes were labeled with [α-32P]dCTP using Ready-To-Go DNA Labeling Beads kit (Amersham Biosciences). In Fig. 2A, the same blot was re-used for hybridization. The blot was stripped of the previous probe, and was hybridized with a different gene probe.
Populations of cells highly enriched for specific spermatogenic cell types were the same as previously used (Wang, et al., 2005). A semi-quantitative RT-PCR technique was used for expression analysis as previously described (Wang, et al., 2005). Poly(A)+ RNAs were used for reverse transcription (RT). To avoid saturation of PCR, products were removed after various cycles of PCR (25–35 cycles) and analyzed by agarose gel electrophoresis. Each RT-PCR was done twice with similar results. Controls without reverse transcriptase were done in parallel and were negative (data not shown). The following gene-specific primers were used: Actb, AGAAGAGCTATGAGCTGCCT and TCATCGTACTCCTGCTTGCT; Pgk2, AAGTTTGATGAGAATGCTAAAGT and CCTCCTCCTATAATGGTGACA; Ube1x, TGTCCACACCCACTTACT and GCACTCTGCAACTCCTGG; Prm1, ACAAAATTCCACCTGCTCACA and GTTTTTCATCGGCGGTGGC; Ubl4a, GGAATGTAGCCTACAGGT and CATAGCCTCAGTCACTTCAG; Ubl4b, GAAGATGCAGCCTGAAGGTA and CTCGAGTTCCGATGCAACA.
2.3 Immunofluorescence analysis
Mouse testes were fixed in 4% paraformaldehyde (PFA) for 3 hours at 4°C, dehydrated in 30% sucrose overnight, submerged in tissue freezing medium, and frozen in dry ice/95% ethanol bath. 8-μm sections were cut using the Reichert-Jung CryoStat at –20°C. For immunostaining of testis sections, specific anti-UBL4A or anti-UBL4B antibodies were affinity-purified using the immunoblot method (Harlow and Lane, 1998). Guinea pig anti-ACRV1 antibodies were used to stain acrosomes (1:500). Texas red or FITC-conjugated secondary antibodies were used (Vector Laboratories). The care and use of mice were within standard ethical guidelines and were approved by the Institutional Animal Care and Use Committee at University of Pennsylvania.
2.4 GenBank accession numbers
Ubl4a sequences: mouse, NM_145405; human, NM_014235; cattle, XM_580675; dog, XM_538208; opossum, DQ522240. Ubl4b sequences: mouse, NM_026261; human, NM_203412; cattle, XM_607835; dog, XM_537036; opossum, DQ522241.
Supplementary Material
Supplementary Figure S1.
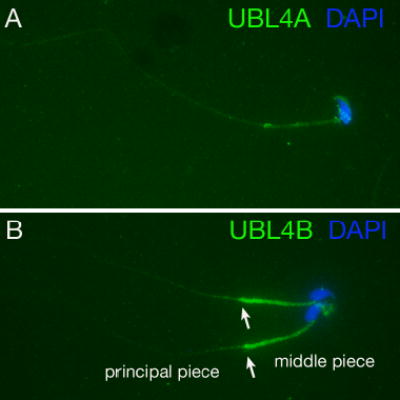
Localization of UBL4A and UBL4B in epididymal sperm. Sperm from epididymis were fixed in 4% PFA and were immunostained with affinity-purified anti-UBL4A (green) or anti-UBL4B antibodies (green) and DAPI (blue). (A) Anti-UBL4A antibodies weakly stained both sperm tail and periphery of sperm head. (B) Anti-UBL4B antibodies produced a strong staining of the middle piece and a relatively weak staining of the principal piece. Two sperm were shown in (B). The arrows demarcate the possible junction between the middle piece and principal piece.
Acknowledgments
We thank E. Gleason for technical assistance, J. Pan for mouse testis RNA samples, J.R. McCarrey for germ cell RNA samples, and P. P. Reddi for anti-ACRV1 antibodies. We thank S. Y. Fuchs and S. B. Moss for comments on the manuscript. This work was supported by the Startup fund from the University of Pennsylvania and an NIH/NICHD grant (HD 045866) to PJW.
References
- Bebington C, Doherty FJ, Fleming SD. The possible biological and reproductive functions of ubiquitin. Hum Reprod Update. 2001;7:102–111. doi: 10.1093/humupd/7.1.102. [DOI] [PubMed] [Google Scholar]
- Bradley J, Baltus A, Skaletsky H, Royce-Tolland M, Dewar K, Page DC. An X-to-autosome retrogene is required for spermatogenesis in mice. Nat Genet. 2004;36:872–876. doi: 10.1038/ng1390. [DOI] [PubMed] [Google Scholar]
- Dahl HH, Brown RM, Hutchison WM, Maragos C, Brown GK. A testis-specific form of the human pyruvate dehydrogenase E1 alpha subunit is coded for by an intronless gene on chromosome 4. Genomics. 1990;8:225–232. doi: 10.1016/0888-7543(90)90275-y. [DOI] [PubMed] [Google Scholar]
- Dass B, McMahon KW, Jenkins NA, Gilbert DJ, Copeland NG, MacDonald CC. The gene for a variant form of the polyadenylation protein CstF-64 is on chromosome 19 and is expressed in pachytene spermatocytes in mice. J Biol Chem. 2001;276:8044–8050. doi: 10.1074/jbc.M009091200. [DOI] [PubMed] [Google Scholar]
- Elliott DJ, Venables JP, Newton CS, Lawson D, Boyle S, Eperon IC, Cooke HJ. An evolutionarily conserved germ cell-specific hnRNP is encoded by a retrotransposed gene. Hum Mol Genet. 2000;9:2117–2124. doi: 10.1093/hmg/9.14.2117. [DOI] [PubMed] [Google Scholar]
- Emerson JJ, Kaessmann H, Betran E, Long M. Extensive gene traffic on the mammalian X chromosome. Science. 2004;303:537–540. doi: 10.1126/science.1090042. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1998. Using Antibodies: A Laboratory Manual. [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Li WH. Unbiased estimation of the rates of synonymous and nonsynonymous substitution. J Mol Evol. 1993;36:96–9. doi: 10.1007/BF02407308. [DOI] [PubMed] [Google Scholar]
- McCarrey JR, Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987;326:501–505. doi: 10.1038/326501a0. [DOI] [PubMed] [Google Scholar]
- Odorisio T, Mahadevaiah SK, McCarrey JR, Burgoyne PS. Transcriptional analysis of the candidate spermatogenesis gene Ube1y and of the closely related Ube1x shows that they are coexpressed in spermatogonia and spermatids but are repressed in pachytene spermatocytes. Dev Biol. 1996;180:336–343. doi: 10.1006/dbio.1996.0305. [DOI] [PubMed] [Google Scholar]
- Reddi PP, Naaby-Hansen S, Aguolnik I, Tsai JY, Silver LM, Flickinger CJ, Herr JC. Complementary deoxyribonucleic acid cloning and characterization of mSP-10: the mouse homologue of human acrosomal protein SP-10. Biol Reprod. 1995;53:873–881. doi: 10.1095/biolreprod53.4.873. [DOI] [PubMed] [Google Scholar]
- Rohozinski J, Bishop CE. The mouse juvenile spermatogonial depletion (jsd) phenotype is due to a mutation in the X-derived retrogene, mUtp14b. Proc Natl Acad Sci U S A. 2004;101:11695–11700. doi: 10.1073/pnas.0401130101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Cache River Press; 1990. Histological and Histopathological Evaluation of the Testis; p. 286. [Google Scholar]
- Schwartz DC, Hochstrasser M. A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem Sci. 2003;28:321–328. doi: 10.1016/S0968-0004(03)00113-0. [DOI] [PubMed] [Google Scholar]
- Sedlacek Z, Munstermann E, Dhorne-Pollet S, Otto C, Bock D, Schutz G, Poustka A. Human and mouse XAP-5 and XAP-5-like (X5L) genes: identification of an ancient functional retroposon differentially expressed in testis. Genomics. 1999;61:125–132. doi: 10.1006/geno.1999.5931. [DOI] [PubMed] [Google Scholar]
- Toniolo D, Persico M, Alcalay M. A "housekeeping" gene on the X chromosome encodes a protein similar to ubiquitin. Proc Natl Acad Sci U S A. 1988;85:851–855. doi: 10.1073/pnas.85.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Vigodner M, Morris PL. Testicular expression of small ubiquitin-related modifier-1 (SUMO-1) supports multiple roles in spermatogenesis: silencing of sex chromosomes in spermatocytes, spermatid microtubule nucleation, and nuclear reshaping. Dev Biol. 2005;282:480–492. doi: 10.1016/j.ydbio.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Wang PJ. X chromosomes, retrogenes and their role in male reproduction. Trends Endocrinol Metab. 2004;15:79–83. doi: 10.1016/j.tem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27:422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- Wang PJ, Page DC, McCarrey JR. Differential expression of sex-linked and autosomal germ-cell-specific genes during spermatogenesis in the mouse. Hum Mol Genet. 2005;14:2911–2918. doi: 10.1093/hmg/ddi322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


