Abstract
Mice with homozygous null mutations in the high-density lipoprotein receptor SR-BI (scavenger receptor class B, type I) and apolipoprotein E genes fed a low-fat diet exhibit a constellation of pathologies shared with human atherosclerotic coronary heart disease (CHD): hypercholesterolemia, occlusive coronary atherosclerosis, myocardial infarctions, cardiac dysfunction (heart enlargement, reduced systolic function and ejection fraction, and ECG abnormalities), and premature death (mean age 6 weeks). They also exhibit a block in RBC maturation and abnormally high plasma unesterified-to-total cholesterol ratio (0.8) with associated abnormal lipoprotein morphology (lamellar/vesicular and stacked discoidal particles reminiscent of those in lecithin/cholesterol acyltransferase deficiency and cholestasis). Treatment with the lipid-lowering, antiatherosclerosis, and antioxidation drug probucol extended life to as long as 60 weeks (mean 36 weeks), and at 5–6 weeks of age, virtually completely reversed the cardiac and most RBC pathologies and corrected the unesterified to total cholesterol ratio (0.3) and associated distinctive abnormal lipoprotein morphologies. Manipulation of the timing of administration and withdrawal of probucol could control the onset of death and suggested that critical pathological changes usually occurred in untreated double knockout mice between ≈3 (weaning) and 5 weeks of age and that probucol delayed heart failure even after development of substantial CHD. The ability of probucol treatment to modulate pathophysiology in the double knockout mice enhances the potential of this murine system for analysis of the pathophysiology of CHD and preclinical testing of new approaches for the prevention and treatment of cardiovascular disease.
Keywords: atherosclerosis, heart failure, myocardial infarction, unesterified cholesterol
Coronary artery atherosclerosis is a major cause of myocardial infarction (MI) and death. We have described a murine model of human coronary heart disease (CHD) (1) that is based on targeted homozygous null mutations in two genes whose products play important roles in normal lipoprotein metabolism and protect mice from atherosclerosis: the high-density lipoprotein (HDL) receptor SR-BI (scavenger receptor class B, type I) (2, 3) and the lipoprotein component apolipoprotein E (apoE) (4–7).
Low-fat chow-fed, homozygous null, apoE knockout (KO) mice are hypercholesterolemic (4, 5), develop atherosclerosis between 3 and 4 months of age, and usually live a long life (>1 year), although older animals can sometimes develop coronary artery occlusions and apparently associated pathology (8, 9).
SR-BI controls HDL structure and metabolism and appears to be important for ”reverse cholesterol transport,” HDL-mediated transport of cholesterol from peripheral tissues (including atherosclerotic plaques) to the liver and then to the bile for excretion (2, 3, 10–16). SR-BI delivers cholesteryl esters and other lipids from HDL to cells via selective lipid uptake (2, 3, 10, 17, 18) and can also mediate unesterified cholesterol (UC) efflux from cells to HDL (19–21). Homozygous null SR-BI KO mice fed a low-fat diet are hypercholesterolemic (15) and exhibit abnormally low biliary cholesterol excretion (≈50%) (13, 16), but apparently no spontaneous atherosclerosis. Combined deficiencies of SR-BI and apoE (dKO) result in increased cholesterol in very low-density lipoprotein (VLDL)-sized and in abnormally large HDL-like particles and dramatically accelerated atherosclerosis (≈4 weeks of age; ref. 16).
By 5–8 weeks of age dKO mice on a low-fat diet develop many features of human CHD (occlusive atherosclerosis, MI, cardiac dysfunction, death) (1, 16) and a cholesterol-dependent block in RBC maturation (100% reticulocytosis) (22). The strikingly rapid onset of a constellation of human-like cardiovascular pathologies in dKO mice is not seen in most rodent models of hyperlipidemia and atherosclerosis (1, 23, 24). Here we have treated dKO mice with the lipid-lowering, antiatherosclerosis, and antioxidation drug probucol (25–31) and shown that it dramatically prolonged their lives, virtually completely reversed early-onset cardiovascular and most RBC pathologies, corrected their abnormally high UC to total cholesterol (TC) ratio (0.8 to 0.3) and prevented formation of abnormal lipoproteins (lamellar/vesicular and stacked discoidal particles, rouleaux) reminiscent of those in lecithin–cholesterol acyltransferase (LCAT) deficiency, cholestasis, and other cases of high UC/TC ratio (32–34). It appears that much of the probucol-sensitive pathology was a consequence of postnatal (3–5 weeks of age) processes. Manipulation of the time of probucol administration can be used as a switch or rheostat to start, arrest, or alter the rate of progression of CHD in this model, which may be useful for preclinical testing of new approaches for preventing and treating cardiovascular disease.
Methods
Animals. Mice (mixed C57BL/6 × 129 background) were maintained and fed a normal chow diet either with or without 0.5% (wt/wt) probucol supplementation [4,4,-(isopropylidenedithio)-bis-(2,6-di-tert-butylphenol; Sigma] as described (15, 16, 35). Animal procedures were in accordance with Massachusetts Institute of Technology institutional guidelines.
Morphologic, Biochemical, and Cardiac Functional Analyses. Histology using frozen sections was performed as described (1, 16) or with a minor modification of the method of Antos et al. (36). Briefly, excised hearts were rinsed in PBS, incubated in Krebs–Hanseleit solution without Ca2+ to relax the cardiac muscle, fixed in 10% formalin for 24 h at 4°C, and then infiltrated with 30% sucrose/PBS for 24 h at 4°C. Samples were frozen in Tissue Tek OCT (Sakura, Torrance, CA) and sectioned (10 μm) before staining with Masson's trichrome (Sigma; ref. 1). Cholesterol, phospholipid (PL), and triglyceride determinations were made as described (16, 35) or with kits (Wako Chemical, Richmond, VA). Two-dimensional and M-mode ECGs were performed by using very light sedation (ketamine) (37). ECGs were recorded noninvasively in conscious mice as described (1, 38) and analyzed via E-MOUSE (Mouse Specifics, Boston). All other methods were as described, including RBC characterization (22), FPLC plasma fractionation (15, 16), gravimetry and MRI analyses (1), and electron microscopy of phosphotungstic acid negatively stained lipoproteins from plasma or FPLC fractions (34). No differences were observed between males and females. A value of P < 0.05 for differences was considered significant (two-tailed, unpaired Student's t test or ANOVA test for groups calculated with Microsoft EXCEL or STATVIEW).
Results
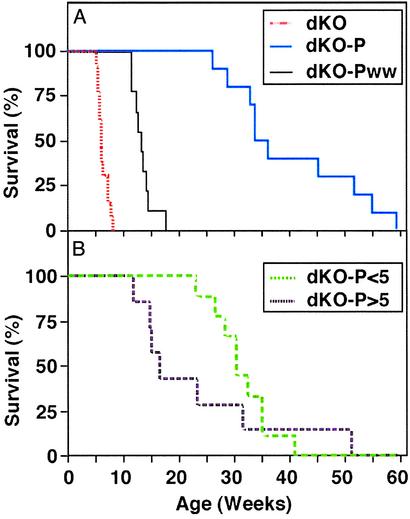
Extension of dKO Life Span by Treatment with Probucol. To use dKO mice as a model to test the influence of drugs on CHD and premature death, we fed animals a low-fat diet supplemented with probucol (0.5%). For two groups, probucol was administered to mating pairs either before or at the time of mating and continuously thereafter through weaning of offspring at 3–4 weeks of age (average age at weaning: 24.5 days). For one of these groups, designated dKO-P, probucol feeding continued throughout the animals' lives. For the other group, designated dKO-Pww (probucol withdrawn at weaning), only probucol-free chow was provided after weaning.
Fig. 1A shows that probucol treatment substantially increased the animals' life spans: the median age of death for dKO-P mice was 36 weeks (range 187–419 days), much more than 6 weeks (35–56 days) for untreated animals. For dKO-Pww mice, the median age of death was 13 weeks (80–123 days). Probucol is lipophilic and may have been available from adipose stores (39) in dKO-Pww mice for a short time after weaning.
Fig. 1.
Effects of probucol on survival. (A) dKO mice (red line, n = 13, chow diet, ref. 1), dKO-P mice (blue line, n = 10, 0.5% probucol diet from conception), and dKO-Pww mice (black line, n = 9, probucol diet from conception until weaning). (B) dKO-P<5 mice (probucol diet administered only after weaning but before 5 weeks of age, green line; n = 9) and dKO-P>5 (probucol diet administered immediately after 5 weeks of age, purple line; n = 7).
We also administered probucol only after weaning, either before 5 weeks (dKO-P<5; mean age, 4.1 weeks, range 3.9–4.6 weeks) or later (dKO-P>5; mean age, 5.2 weeks, range 5.1–5.3 weeks). Fig. 1B shows that the dKO-P<5 mice lived far longer (30.3 weeks, range 162–332 days) than untreated controls, but not as long as the dKO-P group. The average life span of the dKO-P>5 group was 16 weeks (82–359 days), about half that of the dKO-P<5 mice, but more than double that of the untreated controls. Thus, probucol treatment dramatically lengthened the lives of dKO mice, the extent of which depended on the timing and duration of administration. The findings suggest that substantial pathology that could be prevented or reversed by probucol occurred between weaning and 5 weeks of age and that the drug could extend life even when treatment began after development of substantial CHD (dKO-P>5 group, ref. 1).
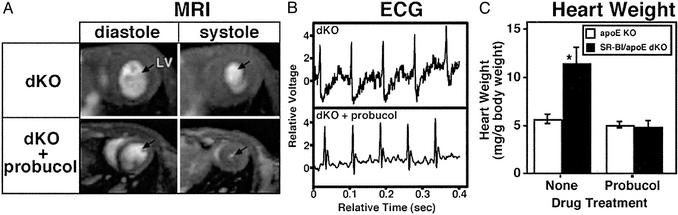
Effects of Probucol Treatment on Heart Function and Structure. We next determined whether probucol-induced extended life span was associated with correction of functional and structural cardiac defects at ≈5–6 weeks of age. Fig. 2A shows MRI short axis views of the hearts at end diastole (Left) and end systole (Right). As reported (1), the left ventricular end systolic volumes (LVESVs) were abnormally high in untreated dKO hearts (Fig. 2A Upper Right), resulting in a low ejection fraction (43 ± 13% vs. 87 ± 5% for apoE KO; not shown, ref. 1). In contrast, LVESVs (Fig. 2A Lower Right) and ejection fraction were essentially normal in dKO-P (87.7 ± 3.2%, n = 5), compared with untreated dKO mice (P = 0.005; with no probucol effect in apoE KO mice, 85.6 ± 2.0%, n = 3, P = 0.57; ref. 1). In addition, the ST depression observed in the ECGs of 50% of the untreated, unanesthetized dKO mice (1) (e.g., Fig. 2B Upper) was eliminated by probucol treatment in 10 of 11 animals examined (e.g., Fig. 2B Lower, one of the dKO-P mice exhibited a mild ST depression; data not shown).
Fig. 2.
Effects of probucol on heart function and size. Parental and offspring mice were fed either a low-fat chow or probucol (0.5% wt-wt)-supplemented diet. (A) Representative short-axis MRI images of hearts from mice (Upper, untreated dKO, n = 4; Lower, probucol-treated dKO-P, n = 5) at end diastole (Left) and end systole (Right). Blood-filled heart chambers are white, and arrows indicate left ventricles (LV). (B) Representative ECGs from 6-week-old unanesthetized, untreated (Upper, dKO; ref. 1) or probucol-treated (Lower, dKO-P, n = 11) mice. (C) Mean values (±standard deviations) of heart weights normalized to body weights for 6-week-old untreated (Left) or probucol-treated (Right) apoE KO (open bars) and dKO (filled bars) mice. Untreated dKO hearts were statistically different from the others (*, P < 0.0001, ANOVA test). Probucol treatment significantly altered the weights of dKO (P < 0.0001, t test) but not apoE KO (P = 0.09) hearts. Numbers of mice studied were: dKO, 18; dKO-P, 11; apoE KO, 8; and apoE KO-P, 4.
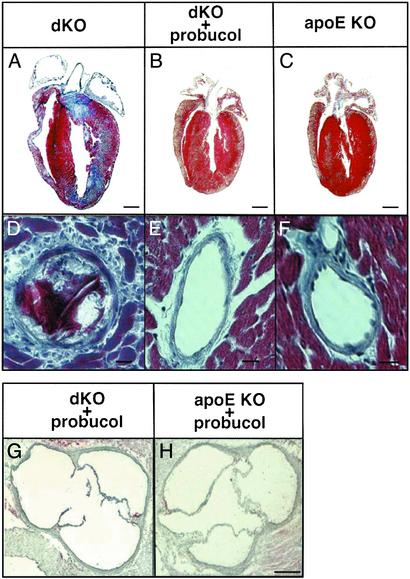
Fig. 2C shows that probucol treatment prevented the abnormal heart enlargement observed in untreated dKO, but not apoE KO, mice (1). The significantly low body weight of dKOs at ≈6 weeks of age, 16.4 ± 0.4 g [±SEM, n = 18 (1), P < 0.0002, ANOVA], was increased to 21.7 ± 1.4 g (n = 8), a value not significantly different from those of apoE KO (21.8 ± 1.6, n = 8) and probucol-treated apoE KO (22.4 ± 1.3, n = 4) controls. Probucol's effects on heart size were also readily apparent in longitudinal histologic sections stained with Masson's trichrome (Fig. 3 A–C, n = 3 for each group) and in preliminary echocardiographic analysis of wall thickness and left ventricle mass (data not shown). Unlike the untreated dKO hearts (Fig. 3A), the sizes and shapes of the dKO-P hearts (Fig. 3B) were essentially indistinguishable from those of the untreated apoE KO controls (Fig. 3C). Furthermore, the extensive MI (blue fibrotic tissue) seen throughout the untreated dKO hearts was prevented by probucol treatment. We did not observe in the dKO-P (and either treated or untreated apoE KO) hearts the occlusive, lipid-rich atherosclerotic plaques in coronary arteries (e.g., Fig. 3 E and F) or classic atherosclerosis in the root of the aortic sinus (Fig. 3 G and H) seen in all untreated dKO mice (Fig. 3D and refs. 1 and 16). Probucol's effect on the aortic root in dKO mice is noteworthy because, although it reduces aortic arch atherosclerosis (30) in older apoE single KO mice, it paradoxically enhances aortic root atherosclerosis in apoE KO mice when treatment is started at 2–4 months of age (28, 30). Probucol treatment also does not reduce aortic root atherosclerosis in fat-fed LDL receptor KO mice (40, 41). In summary, most of the functional and morphologic indicators of occlusive atherosclerotic coronary artery disease, MI, and cardiac dysfunction were eliminated in 5- to 6-week-old dKO mice by probucol treatment.
Fig. 3.
Histologic analysis of hearts. (A–F) Representative longitudinal sections of hearts from 6-week-old untreated dKO (A and D), probucol-treated dKO-P (B and E), and untreated apoE single KO (C and F) mice stained with Masson's trichrome (healthy myocardium, red; fibrotic infarcted tissue, blue). (G and H) Oil red O-stained sections through the aortic sinuses of probucol-treated dKO (G) and apoE KO (H) mice. (Scale bars: A–C, 1 mm; D–F,10 μm; G and H, 100 μm.)
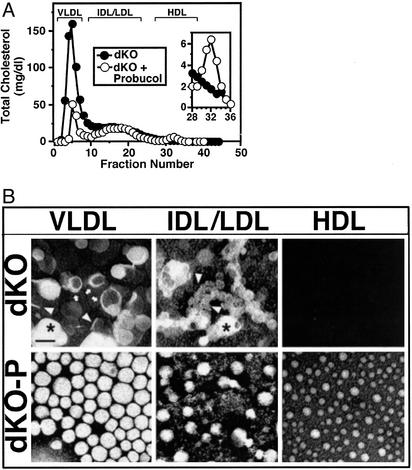
Effects of Probucol Treatment on Lipoproteins and RBC Maturation. Probucol treatment lowered by ≈2-fold the levels of plasma TC (Table 1) and PLs (Table 2) in dKO mice compared to those in untreated apoE KO mice (also see refs. 28 and 35). Treatment did not lower the plasma triglyceride level of dKO mice that was somewhat lower than that of apoE KO mice (Table 2). The lipoprotein TC profile (FPLC size fractionation, Fig. 4A) of dKO mice (Fig. 4A, filled circles) has been described (16). The abnormally large HDL particles in dKO mice are found in the VLDL/intermediate-density lipoproteins/LDL, but not the HDL, size ranges, because of the loss of SR-BI-mediated selective lipid uptake (15, 16). Unexpectedly, probucol treatment (Fig. 4A, open circles) induced the appearance of a peak of cholesterol at the position of normal-sized HDL (fractions 29–34) that contained apoA-I (preliminary analysis, data not shown) that is also seen in untreated apoE KO mice (data not shown; refs. 16 and 42). In contrast, probucol decreases the normal-size HDL peak in apoE KO mice (28). Determination of the mechanism for probucol-induced reappearance of the normal-sized HDL-like particles seen in all eight dKO-P animals examined [e.g., induced SR-BI independent selective lipid uptake (ref. 43, reviewed in ref. 2)] awaits further studies. The shapes of the lipoprotein UC and PL FPLC profiles (data not shown) were similar to that of TC. The ratios of the relative amounts of UC to TC and PL to TC in the major FPLC lipoprotein fractions are shown in Table 2.
Table 1. Effects of probucol on plasma UC and TC.
| Genotype/drug treatment | UC, mg/dl | TC, mg/dl | Ratio (UC/TC) |
|---|---|---|---|
| DKO | |||
| None (n = 11) | 781 ± 65*† | 970 ± 83*† | 0.806 ± 0.007*† |
| Probucol (n = 10) | 151 ± 21 | 456 ± 45 | 0.318 ± 0.013 |
| SR-BI+/- apoE KO | |||
| None (n = 5) | 124 ± 11§ | 421 ± 31¶ | 0.296 ± 0.012‡ |
| Probucol (n = 5) | 57 ± 3 | 244 ± 20 | 0.238 ± 0.015 |
| apoE KO | |||
| None (n = 5) | 126 ± 14§ | 433 ± 66¶ | 0.291 ± 0.02∥ |
| Probucol (n = 3) | 69 ± 6 | 249 ± 8 | 0.277 ± 0.02 |
| SR-BI KO** | |||
| None (n = 13) | 108 ± 5 | 211 ± 6 | 0.515 ± .027 |
| Probucol (n = 13) | 24 ± 2 | 110 ± 6 | 0.218 ± .012 |
| WT control** | |||
| None (n = 7) | 33 ± 2 | 103 ± 4 | 0.315 ± .011 |
| Probucol (n = 7) | 8 ± 2 | 34 ± 4 | 0.222 ± .033 |
Data are represented as mean ± standard error
P < 0.0001, compared with all the other groups with ANOVA test. Pvalues for ttest comparison with or without probucol treatment
, <0.0001
, <0.020
, <0.0003
, <0.0031
, <0.35
Blood samples from 4- to 5-week-old animals fed a low-fat chow diet were drawn, the same animals were fed a probucol supplemented diet for an additional 7-25 days, and a second set of samples was drawn (TC data are from ref. 35)
Table 2. Effects of probucol on plasma PL, triglyceride, and lipid ratios.
| dKO | dKO + probucol | apoE KO | |
|---|---|---|---|
| Plasma PL, mg/dl | 678 ± 96 (n = 8) | 246 ± 95 (n = 3) | 307 ± 51 (n = 9) |
| Plasma triglyceride, mg/dl | 53 ± 16 (n = 4) | 60 ± 44 (n = 3) | 89 ± 28 (n = 6) |
| UC × 100/TC to | |||
| PL × 100/TC ratio | |||
| Plasma* | 86:60 | 43:61 | 30:68 |
| VLDL* | 83:55 | 41:52 | 35:56 |
| IDL/LDL* | 79:60 | 37:51 | 36:62 |
| HDL* | — | 49:70 | nd |
| Plasma surface/core lipid ratio† | 6.0 | 1.1 | 1.1 |
nd, not determined.
Average of three (dKO and dKO-P) or two (apoE KO) independent determinations from individual mice. The amounts of the indicated lipids in each of the major lipoproteins [VLDL, fractions 3-9; intermediate-density lipoprotein (IDL)/LDL, fractions 10-26; HDL fractions 30-37] from the FPLC profiles were added together, and the averages were used to calculate the relative amounts of UC to TC (UC × 100/TC) and PL to TC (PL × 100/TC)
(PL + UC)/(esterified cholesterol + triglycerides)
Fig. 4.
Lipoprotein cholesterol profiles and morphology. Parental and offspring mice were fed a low-fat chow diet without or with probucol [0.5% (wt/wt) supplementation]. (A) Representative chromatograms of the TC (mg/dl of plasma) from size-fractionated (Superose 6-FPLC) plasma lipoproteins from 6-week-old untreated dKO (•) or probucol-treated dKO (○) mice are shown with the approximate elution positions of VLDL, intermediate-density lipoproteins (IDL)/LDL, and HDL. (Inset) Expanded HDL region. (B) Electron micrographs of negatively stained lipoproteins from pooled FPLC fractions. Abnormal morphologies are indicated by arrows (lamellar/vesicular), arrowheads (stacked discoidal), and asterisks (aggregated). (Scale bar = 60 nm.)
There was a surprisingly high ratio of UC to TC in both dKO and SR-BI KO mice compared with SR-BI-positive controls (Table 1). As a consequence in dKO mice the ratio of surface/polar (UC and PL) to core/nonpolar (esterified cholesterol and triglycerides) lipids was ≈6-fold larger than in apoE KO controls (Table 2). This high ratio suggested that the structures of the lipoproteins in dKO mice would be abnormal. Electron micrographs of negatively stained total plasma (not shown) and major lipoprotein fractions from dKO mice (Fig. 4B Upper) showed the presence of numerous lamellar/vesicular (Fig. 4B Upper, arrows), and stacked discoidal (Fig. 4B Upper, arrowheads) particles with abnormal morphologies, reminiscent of those (e.g., lipoprotein-X) seen in LCAT deficiency, cholestasis, and other cases of abnormally high UC (32–34). The large probucol-induced decrease (≈4.8-fold) in plasma UC lowered the abnormally high ratios of UC/TC in both dKO and SR-BI KO mice to the normal values in SR-BI-positive controls (Table 1) and lowered the surface-to-core lipid ratio in dKO mice to that in apoE KO controls (Table 2). As a consequence, the morphologies of the major lipoprotein fractions in dKO-P mice (Fig. 4B Lower) were restored to those in apoE KO controls (data not shown; ref. 34). The mechanisms underlying the abnormally high UC in untreated SR-BI-negative mice, its reversal by probucol, and its relevance to pathology in dKO mice are uncertain.
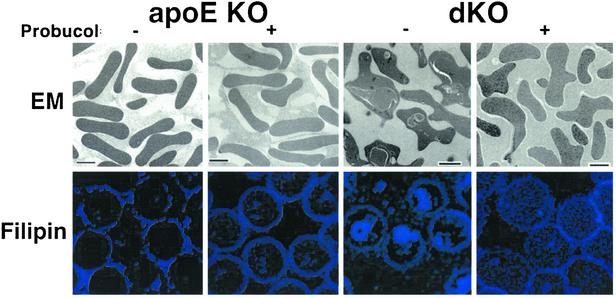
There is a profound, reversible defect in RBC maturation in dKO mice caused by excessive accumulation in precursor reticulocytes of UC (visualized by staining with the cholesterol-binding, fluorescent dye filipin, see Fig. 5 and Table 3, ref. 22). Compared with WT (data not shown, ref. 22) and apoE KO mice, the untreated dKO mice were anemic and exhibited profound reticulocytosis (irregularly shaped, precursor reticulocytes, no biconcave mature erythrocytes). Their large, macrocytic red cells (elevated mean copuscular volume) differed morphologically from classic abnormal ”target” cells seen in LCAT-deficient patients (unpublished data; refs. 44 and 45) and contained big, cholesterol-rich autophagolysosomal inclusion bodies (22). Almost all of these abnormalities, except the somewhat irregular shape (Fig. 5 Upper Right), were corrected by probucol treatment. Correction of the reticulocytosis in dKO mice by probucol may contribute to its suppression of their CHD, e.g., by improving tissue oxygenation.
Fig. 5.
Effects of probucol on RBCs. RBCs from 5-week-old untreated (-) or probucol-treated (+) apoE KO and dKO mice were examined by either transmission electron microscopy (Upper) or confocal fluorescence microscopy after staining with the cholesterol-binding, blue fluorescing dye filipin (Lower). [Scale bar (Upper) = 2 μm; magnification (Lower) = ×100.)
Table 3. Effects of probucol on RBC parameters in apoE KO and dKO mice.
| Genotype/drug treatment | Hematocrit, % | Mean corpuscular volume, fL | Reticulocytes, % of total RBCs |
|---|---|---|---|
| apoE KO | |||
| None (n = 8) | 52.5±2.1 | 57.6±0.5 | 2.8±1.2 |
| Probucol (n = 3) | 49.8±1.5 | 54.2±2.1 | 4.1±0.8 |
| dKO | |||
| None (n = 6) | 34.2±1.7 | 84.1±4.1 | 100±0 |
| Probucol (n = 3) | 50.8±2.4 | 54.2±2.3 | 4.1±0.5 |
All animals were 4-6 weeks old, except one each of probucol-treated dKO and apoE KO that were 6 months old. All values are means ± standard deviations. All of the values from the untreated dKO mice were significantly different from those from the other three groups (P < 0.0001 for mean corpuscular volume and percentage reticulocytes; P < 0.001 for hematocrit). Normal mouse reticulocyte counts range from 2% to 6%. Data for untreated animals are from ref. 22
Discussion
Mice with homozygous null mutations in the HDL receptor SR-BI and apoE genes (dKO mice) on a low-fat diet rapidly develop many cardinal features of human CHD, including hypercholesterolemia, atherosclerosis in the aortic sinus, occlusive coronary plaques, patchy MIs, cardiac enlargement and dysfunction (reduced systolic function and ejection fraction, ECG abnormalities), and premature death (50% mortality at ≈6 weeks) (1, 16). They also exhibit a block in RBC maturation (22). Their abnormally high ratio of lipoprotein surface (UC, PLs) to core (esterified cholesterol, triglycerides) lipids led to abnormal lipoprotein morphology (lamellar/vesicular and stacked discoidal particles reminiscent of those in other cases of high UC, e.g., LCAT deficiency, cholestasis, and apoE/hepatic lipase deficiency; refs. 32–34). The mechanism causing excess UC in SR-BI null (dKO, SR-BI KO) mice is unknown, but may involve the activities of LCAT, hepatic lipase, or other lipoprotein-metabolizing proteins (32–34).
Treatment of dKO mice with the lipid-lowering and antioxidation drug probucol (25–27) extended their lives (from 6 to 36 weeks) and reversed essentially all early-onset CHD-associated anatomical, functional, cellular, and biochemical pathology. A substantial portion of the probucol-sensitive pathology apparently occurred between 3 and 5 weeks of age. Potential mechanisms by which probucol treatment may have reduced atherosclerosis and/or CHD (16, 25–27, 30) in dKO mice include its ability to: (i) alter lipoprotein metabolism (25–28, 30), especially lowering TC and normalizing the UC/TC ratio (latter effect not observed in humans, ref. 46), and lipoprotein morphology, (ii) reduce the relative amounts of atherogenic versus antiatherogenic lipoproteins, possibly by inducing alternative cellular pathways of lipoprotein metabolism and restoring production of normal-size HDL, (iii) alter lipid metabolism/transport in the artery wall, (iv) prevent RBC maturation defects and thus associated hypoxia (probably caused by altered lipoprotein composition/abundance), (ν) counter effects of SR-BI deficiency on cellular metabolism, e.g., HDL/SR-BI-mediated regulation of endothelial NO synthase in endothelial cells (47, 48), and (vi) exhibit antioxidant, antiinflammatory, antihypoxia, or other lipid-lowering independent activities (25–27, 31, 49–57). Indeed, while this manuscript was in preparation, several groups reported beneficial influences of probucol in atherosclerosis-independent models of heart failure involving tachycardia (58) or coronary ligation (59). Probucol also protects against adriamycin-induced heart failure (60). Unfortunately, the detailed molecular mechanisms underlying probucol's diverse activities are not well understood (25–27, 61). Probucol's striking effects on cholesterol metabolism (UC/TC ratio) and lipoprotein morphology were likely to have played a key role in its cardioprotective effects and may help uncover its mechanism of action.
Although the rapid onset of CHD in dKO mice has many experimental advantages (1), it may, in some cases, complicate analysis of the influence of relatively slow processes, such as therapeutic angiogenesis. Varying the time and/or dosage of probucol administration can be used as a switch or rheostat to start, stop, or alter disease progression and thus make this model more useful.
In summary, the dKO mouse model of CHD exhibits many cardinal features of the human disease (1, 16). Probucol, a drug previously used to treat human hyperlipidemia and atherosclerosis/restenosis, can effect a virtually complete short-term cure and be used to alter the time of death, thus enhancing the usefulness of this model. This system should help in further investigations of the pathophysiology of CHD and be valuable for preclinical testing of new approaches for the prevention and treatment of cardiovascular disease.
Note Added in Proof: A paper in press (62) independently reports a high UC/TC ratio (≈0.5) in SR-BI single KO mice as described here.
Acknowledgments
We thank T. Hampton, W. Valle, S. Karackattu, K. Runge, D. Burstein and colleagues, J. Wong, T. A. Miettinen, H. Gylling, P. Seifert, E. Edelman, A. Yesilaltay, C. Antos, H. Kanji, M. Penman, H. Mulhern, S. Erdman, and C. Clark for technical assistance, advice, and/or helpful discussions. This work was supported by National Institutes of Health Grants HL64737 and HL66105 (to M.K.) and HL53793 and HL63609 (to M.S.) and American Heart Association Grants 9940074 (to M.S.) and 0150530N (to M.J.P.). N.C.A. is an Associate Investigator of the Howard Hughes Medical Institute. A.B. was a Human Frontiers Science Program postdoctoral fellow. H.E.M. was a postdoctoral fellow supported by the Academy of Finland, Finnish Cultural Foundation, and Yrjo Jahnsson Foundation, and D.M.Y. had an American Society of Echocardiography fellowship.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MI, myocardial infarction; CHD, coronary heart disease; HDL, high-density lipoprotein; VLDL, very low-density lipoprotein; SR-BI, scavenger receptor class B, type I; apoE, apolipoprotein E; KO, knockout; dKO, double KO; dKO-P, dKO mice with probucol throughout life; Pww, probucol withdrawn at weaning; UC, unesterified cholesterol; TC, total cholesterol; LCAT, lecithin–cholesterol acyltransferase; PL, phospholipid.
References
- 1.Braun, A., Trigatti, B. L., Post, M. J., Sato, K., Simons, M., Edelberg, J. M., Rosenberg, R. D., Schrenzel, M. & Krieger, M. (2002) Circ. Res. 90, 270-276. [DOI] [PubMed] [Google Scholar]
- 2.Krieger, M. (1999) Annu. Rev. Biochem. 68, 523-558. [DOI] [PubMed] [Google Scholar]
- 3.Krieger, M. (2001) J. Clin. Invest. 108, 793-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashima, Y., Plump, A. S., Raines, E. W., Breslow, J. L. & Ross, R. (1994) Arterioscler. Thromb. Vasc. Biol. 14, 133-140. [DOI] [PubMed] [Google Scholar]
- 5.Reddick, R. L., Zhang, S. H. & Maeda, N. (1994) Arterioscler. Thromb. Vasc. Biol. 14, 141-147. [DOI] [PubMed] [Google Scholar]
- 6.Curtiss, L. K. & Boisvert, W. A. (2000) Curr. Opin. Lipidol. 11, 243-251. [DOI] [PubMed] [Google Scholar]
- 7.Swertfeger, D. K. & Hui, D. Y. (2001) Front. Biosci. 6, D526-D535. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld, M. E., Polinsky, P., Virmani, R., Kauser, K., Rubanyi, G. & Schwartz, S. M. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2587-2592. [DOI] [PubMed] [Google Scholar]
- 9.Calara, F., Silvestre, M., Casanada, F., Yuan, N., Napoli, C. & Palinski, W. (2001) J. Pathol. 195, 257-263. [DOI] [PubMed] [Google Scholar]
- 10.Acton, S., Rigotti, A., Landschulz, K. T., Xu, S., Hobbs, H. H. & Krieger, M. (1996) Science 271, 518-520. [DOI] [PubMed] [Google Scholar]
- 11.Kozarsky, K. F., Donahee, M. H., Rigotti, A., Iqbal, S. N., Edelman, E. R. & Krieger, M. (1997) Nature 387, 414-417. [DOI] [PubMed] [Google Scholar]
- 12.Ji, Y., Wang, N., Ramakrishnan, R., Sehayek, E., Huszar, D., Breslow, J. L. & Tall, A. R. (1999) J. Biol. Chem. 274, 33398-33402. [DOI] [PubMed] [Google Scholar]
- 13.Mardones, P., Quinones, V., Amigo, L., Moreno, M., Miquel, J. F., Schwarz, M., Miettinen, H. E., Trigatti, B., Krieger, M., VanPatten, S., et al. (2001) J. Lipid. Res. 42, 170-180. [PubMed] [Google Scholar]
- 14.Sehayek, E., Ono, J. G., Shefer, S., Nguyen, L. B., Wang, N., Batta, A. K., Salen, G., Smith, J. D., Tall, A. R. & Breslow, J. L. (1998) Proc. Natl. Acad. Sci. USA 95, 10194-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigotti, A., Trigatti, B. L., Penman, M., Rayburn, H., Herz, J. & Krieger, M. (1997) Proc. Natl. Acad. Sci. USA 94, 12610-12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trigatti, B., Rayburn, H., Vinals, M., Braun, A., Miettinen, H., Penman, M., Hertz, M., Schrenzel, M., Amigo, L., Rigotti, A. & Krieger, M. (1999) Proc. Natl. Acad. Sci. USA 96, 9322-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass, C., Pittman, R. C., Weinstein, D. B. & Steinberg, D. (1983) Proc. Natl. Acad. Sci. USA 80, 5435-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein, Y., Dabach, Y., Hollander, G., Halperin, G. & Stein, O. (1983) Biochim. Biophys. Acta 752, 98-105. [DOI] [PubMed] [Google Scholar]
- 19.Gu, X., Kozarsky, K. & Krieger, M. (2000) J. Biol. Chem. 275, 29993-30001. [DOI] [PubMed] [Google Scholar]
- 20.Ji, Y., Jian, B., Wang, N., Sun, Y., Moya, M. L., Phillips, M. C., Rothblat, G. H., Swaney, J. B. & Tall, A. R. (1997) J. Biol. Chem. 272, 20982-20985. [DOI] [PubMed] [Google Scholar]
- 21.Liu, T., Krieger, M., Kan, H. Y. & Zannis, V. I. (2002) J. Biol. Chem. 277, 21576-21584. [DOI] [PubMed] [Google Scholar]
- 22.Holm, T. M., Braun, A., Trigatti, B. L., Brugnara, C., Sakamoto, M., Krieger, M. & Andrews, N. C. (2002) Blood 99, 1817-1824. [DOI] [PubMed] [Google Scholar]
- 23.Caligiuri, G., Levy, B., Pernow, J., Thoren, P. & Hansson, G. K. (1999) Proc. Natl. Acad. Sci. USA 96, 6920-6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera, V. L., Makrides, S. C., Xie, H. X., Adari, H., Krauss, R. M., Ryan, U. S. & Ruiz-Opazo, N. (1999) Nat. Med. 5, 1383-1389. [DOI] [PubMed] [Google Scholar]
- 25.Buckley, M. M., Goa, K. L., Price, A. H. & Brogden, R. N. (1989) Drugs 37, 761-800. [DOI] [PubMed] [Google Scholar]
- 26.Mashima, R., Witting, P. K. & Stocker, R. (2001) Curr. Opin. Lipidol. 12, 411-418. [DOI] [PubMed] [Google Scholar]
- 27.Pfuetze, K. D. & Dujovne, C. A. (2000) Curr. Atheroscler. Rep. 2, 47-57. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, S. H., Reddick, R. L., Avdievich, E., Surles, L. K., Jones, R. G., Reynolds, J. B., Quarfordt, S. H. & Maeda, N. (1997) J. Clin. Invest. 99, 2858-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tardif, J. C., Cote, G., Lesperance, J., Bourassa, M., Lambert, J., Doucet, S., Bilodeau, L., Nattel, S. & de Guise, P. (1997) N. Engl. J. Med. 337, 365-372. [DOI] [PubMed] [Google Scholar]
- 30.Witting, P. K., Pettersson, K., Letters, J. & Stocker, R. (2000) Arterioscler. Thromb. Vasc. Biol. 20, E26-33. [DOI] [PubMed] [Google Scholar]
- 31.Sawayama, Y., Shimizu, C., Maeda, N., Tatsukawa, M., Kinukawa, N., Koyanagi, S., Kashiwagi, S. & Hayashi, J. (2002) J. Am. Coll. Cardiol. 39, 610-616. [DOI] [PubMed] [Google Scholar]
- 32.Miller, J. P. (1990) Baillieres Clin. Endocrinol. Metab. 4, 807-832. [DOI] [PubMed] [Google Scholar]
- 33.Ng, D. S., Francone, O. L., Forte, T. M., Zhang, J., Haghpassand, M. & Rubin, E. M. (1997) J. Biol. Chem. 272, 15777-15781. [DOI] [PubMed] [Google Scholar]
- 34.Bergeron, N., Kotite, L., Verges, M., Blanche, P., Hamilton, R. L., Krauss, R. M., Bensadoun, A. & Havel, R. J. (1998) Proc. Natl. Acad. Sci. USA 95, 15647-15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miettinen, H. E., Rayburn, H. & Krieger, M. (2001) J. Clin. Invest. 108, 1717-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antos, C. L., McKinsey, T. A., Frey, N., Kutschke, W., McAnally, J., Shelton, J. M., Richardson, J. A., Hill, J. A. & Olson, E. N. (2002) Proc. Natl. Acad. Sci. USA 99, 907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scherrer-Crosbie, M., Steudel, W., Ullrich, R., Hunziker, P. R., Liel-Cohen, N., Newell, J., Zaroff, J., Zapol, W. M. & Picard, M. H. (1999) Am. J. Physiol. 277, H986-H992. [DOI] [PubMed] [Google Scholar]
- 38.Chu, V., Otero, J. M., Lopez, O., Morgan, J. P., Amende, I. & Hampton, T. G. (2001) BMC Physiol. 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto, K., Fukuda, N., Shiroi, S., Shiotsuki, Y., Nagata, Y., Tani, T. & Sakai, T. (1994) Arzneimittelforschung 44, 1059-1062. [PubMed] [Google Scholar]
- 40.Benson, G. M., Schiffelers, R., Nicols, C., Latcham, J., Vidgeon-Hart, M., Toseland, C. D., Suckling, K. E. & Groot, P. H. (1998) Atherosclerosis 141, 237-247. [DOI] [PubMed] [Google Scholar]
- 41.Yoshikawa, T., Shimano, H., Chen, Z., Ishibashi, S. & Yamada, N. (2001) Horm. Metab. Res. 33, 472-479. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, S. H., Reddick, R. L., Piedrahita, J. A. & Maeda, N. (1992) Science 258, 468-471. [DOI] [PubMed] [Google Scholar]
- 43.Pfeuffer, M. A., Richard, B. M. & Pittman, R. C. (1992) Arterioscler. Thromb. Vasc. Biol. 12, 870-878. [DOI] [PubMed] [Google Scholar]
- 44.Miettinen, H. E., Gylling, H., Tenhunen, J., Virtamo, J., Jauhiainen, M., Huttunen, J. K., Kantola, I., Miettinen, T. A. & Kontula, K. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 591-598. [DOI] [PubMed] [Google Scholar]
- 45.Gjone, E. (1974) Scand. J. Clin. Lab. Invest. Suppl. 137, 73-82. [PubMed] [Google Scholar]
- 46.Bagdade, J. D., Kaufman, D., Ritter, M. C. & Subbaiah, P. V. (1990) Atherosclerosis 84, 145-154. [DOI] [PubMed] [Google Scholar]
- 47.Yuhanna, I. S., Zhu, Y., Cox, B. E., Hahner, L. D., Osborne-Lawrence, S., Lu, P., Marcel, Y. L., Anderson, R. G., Mendelsohn, M. E., Hobbs, H. H. & Shaul, P. W. (2001) Nat. Med. 7, 853-857. [DOI] [PubMed] [Google Scholar]
- 48.Li, X. A., Titlow, W. B., Jackson, B. A., Giltiay, N., Nikolova-Karakashian, M., Uittenbogaard, A. & Smart, E. J. (2002) J. Biol. Chem. 277, 11058-11063. [DOI] [PubMed] [Google Scholar]
- 49.Carew, T. E., Schwenke, D. C. & Steinberg, D. (1987) Proc. Natl. Acad. Sci. USA 84, 7725-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kita, T., Nagano, Y., Yokode, M., Ishii, K., Kume, N., Ooshima, A., Yoshida, H. & Kawai, C. (1987) Proc. Natl. Acad. Sci. USA 84, 5928-5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ku, G., Doherty, N. S., Wolos, J. A. & Jackson, R. L. (1988) Am. J. Cardiol. 62, 77B-81B. [DOI] [PubMed] [Google Scholar]
- 52.Fruebis, J., Silvestre, M., Shelton, D., Napoli, C. & Palinski, W. (1999) J. Lipid Res. 40, 1958-1966. [PubMed] [Google Scholar]
- 53.Sugiura, T., Wada, A., Moriyama, T., Horio, M., Ueda, N., Imai, E. & Hori, M. (1999) Kidney Int. Suppl. 71, S167-S170. [DOI] [PubMed] [Google Scholar]
- 54.Aoki, M., Nata, T., Morishita, R., Matsushita, H., Nakagami, H., Yamamoto, K., Yamazaki, K., Nakabayashi, M., Ogihara, T. & Kaneda, Y. (2001) Hypertension 38, 48-55. [DOI] [PubMed] [Google Scholar]
- 55.Takabe, W., Kodama, T., Hamakubo, T., Tanaka, K., Suzuki, T., Aburatani, H., Matsukawa, N. & Noguchi, N. (2001) J. Biol. Chem. 276, 40497-40501. [DOI] [PubMed] [Google Scholar]
- 56.Robbins, M. & Topol, E. J. (2002) Clev. Clin. J. Med. 69, Suppl. 2, SII130-SII142. [DOI] [PubMed] [Google Scholar]
- 57.Inoue, K., Cynshi, O., Kawabe, Y., Nakamura, M., Miyauchi, K., Kimura, T., Daida, H., Hamakubo, T., Yamaguchi, H. & Kodama, T. (2002) Atherosclerosis 161, 353-363. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura, R., Egashira, K., Machida, Y., Hayashidani, S., Takeya, M., Utsumi, H., Tsutsui, H. & Takeshita, A. (2002) Circulation 106, 362-367. [DOI] [PubMed] [Google Scholar]
- 59.Sia, Y. T., Lapointe, N., Parker, T. G., Tsoporis, J. N., Deschepper, C. F., Calderone, A., Pourdjabbar, A., Jasmin, J. F., Sarrazin, J. F., Liu, P., et al. (2002) Circulation 105, 2549-2555. [DOI] [PubMed] [Google Scholar]
- 60.Singal, P. K., Li, T., Kumar, D., Danelisen, I. & Iliskovic, N. (2000) Mol. Cell. Biochem. 207, 77-86. [DOI] [PubMed] [Google Scholar]
- 61.Tomimoto, S., Tsujita, M., Okazaki, M., Usui, S., Tada, T., Fukutomi, T., Ito, S., Itoh, M. & Yokoyama, S. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 394-400. [DOI] [PubMed] [Google Scholar]
- 62.Van Eck, M., Twisk, J., Hoekstra, M., Van Rij, B. T., Van Der Lans, C. A., Bos, I. S., Kruijt, J. K., Kuipers, F. & Van Berkel, T. J., (2003) J. Biol. Chem. 278, in press. [DOI] [PubMed]