Abstract
Astrocytes can respond to neurotransmitters released at the synapse by generating elevations in intracellular Ca2+ concentration ([Ca2+]i) and releasing glutamate that signals back to neurones. This discovery opens new perspectives for the possible participation of these glial cells in actual information processing by the brain and raises the hypothesis that astrocyte activation by neuronal signals plays a key role in distinct, functional events. Depending on the level of neuronal activity, the [Ca2+]i response that is activated by neurotransmitters can either remain restricted to an astrocytic process or it can propagate as an intracellular [Ca2+]i wave to other astrocytic processes in contact with different neurones, astrocytes, microglia or endothelial cells of cerebral arterioles. Glutamate release triggered by the [Ca2+]i rise at the astrocytic process represents a feedback, short-distance signal that affects synaptic transmission locally. The release of glutamate as well as of other compounds far away from the site of initial activation represents a feedforward, long-distance signal that can be involved in the regulation of distinct processes. For instance, through the release of vasoactive molecules from the astrocytic processes in contact with cerebral arterioles, the neurone–astrocyte–endothelial cell signalling pathway plays a pivotal role in the neuronal control of vascular tone. In this article we will review recent results that should persuade us to reshape our current thinking on the roles of astroglial cells in the brain. We propose that neurones and astrocytes represent an integral unit that has a distinctive role in different fundamental events in brain function. Furthermore, while recent findings provide important evidences for the vesicular hypothesis of glutamate release, we discuss also the proposals for a possible physiological role of hemichannels and purinergic P2X7 receptors in glutamate release from astrocytes. A full clarification of the functional significance of the bidirectional communication that astrocytes establish with neurones as well as with other brain cells represents one of the most intriguing challenges in neurobiological research at the moment and should fuel stimulating debates in years to come.
Introduction
A presynaptic terminal and a postsynaptic neuronal membrane in close apposition are considered the basic functional units of the chemical synapse, i.e. the site where neurones pass on information. At most synapses, a non-neuronal cell – the astrocyte – is also intimately associated with neuronal membranes, but most studies relegate this glial cell to a mere passive neurone-supportive element of the synapse. Indeed, astrocytes efficiently perform neurone-supportive operations, such as the capture of neurotransmitters from the synaptic cleft and the release of energetic substrates which are essential to metabolically sustain high synaptic activities (Magistretti & Pellerin, 1996). However, over the last decade a number of studies have conclusively shown that astrocytes are anything but spectators in the dialogue that neurones carry out at the synapse. Indeed, they behave like additional targets of neuronal signals and respond to the synaptic release of the neurotransmitter, such as glutamate, with elevations in the intracellular Ca2+ concentration ([Ca2+]i) (Porter & McCarthy, 1996; Pasti et al. 1997). A turning point in glial cell research is represented by the finding that [Ca2+]i elevations in cultured astrocytes mediate the release of glutamate which, in turn, triggers ionotropic glutamate receptor-mediated [Ca2+]i increases in nearby neurones (Parpura et al. 1994). By demonstrating the ability of astrocytes to release glutamate through a Ca2+-dependent, most likely vesicular (Parpura et al. 1995a,b; Jeftinija et al. 1997; Pasti et al. 1997, 2001; Calegari et al. 1999; Araque et al. 2000) mechanism, the many studies originating from this pioneering observation have provided the bulk of the data which support the existence of a reciprocal communication system between neurones and astrocytes. The recent evidence for the presence of [Ca2+]i oscillations in astrocytes in vivo (Hirase et al. 2004) similar to those observed in astrocytes from acute brain slices underlines the importance of neurone–astrocyte interactions in brain function.
Numerous reviews on the role of astrocytes in the brain have been recently published (Bezzi et al. 2001; Haydon, 2001; Auld & Robitaille, 2003; Nedergaard et al. 2003; Newman, 2003). We refer the reader to these excellent works for a comprehensive discussion of the aspects in neurone–astrocyte communication which are only briefly mentioned here. We will specifically address our review to two main issues which, in our opinion, are particularly relevant for understanding the rules governing neurone–astrocyte signalling. The first concerns the activation of astrocytes by synaptically released neurotransmitters and their signalling back to neurones. We will attempt to gather the available data into a novel conceptual framework in which neurones and astrocytes represent integral units which perform distinct, multiple tasks in brain functioning. The second issue concerns the mechanism(s) that are the basis of the release of signalling molecules such as glutamate and ATP. Specifically we will critically discuss the recent, intriguing hypothesis that opening of large pores in the membrane, such as hemichannels and/or P2X7 receptors, mediates the release of glutamate and ATP from astrocytes.
Astrocyte response to neuronal activity
It is now established that astrocytes respond to the synaptic release of various neurotransmitters, such as glutamate (Porter & McCarthy, 1996; Pasti et al. 1997), GABA (Kang et al. 1998), noradrenaline (Duffy & MacVicar, 1995; Kulik et al. 1999) and acetylcholine (Shelton & McCarthy, 2000; Araque et al. 2002) with transient, often repetitive elevations in the [Ca2+]i which primarily depend on activation of metabotropic neurotransmitter receptors linked to phospholipase C, production of inositol 1,4,5-trisphosphate (InsP3), activation of InsP3 receptors, and, finally, release of Ca2+ from intracellular Ca2+ pools (Berridge, 1993; Pozzan et al. 1994). These [Ca2+]i elevations, and the following activation of Ca2+-dependent signalling pathways, regulate the release of various signalling molecules, including classical neurotransmitters, from activated astrocytes (Parpura et al. 1994; Pasti et al. 1997; Bezzi et al. 1998; Kang et al. 1998; Benz et al. 2004).
To identify and characterize the rules which govern the reciprocal communication between neurones and astrocytes, it is essential to decipher the properties of the astrocyte response to neuronal activity. The most crucial issue is to understand if and how neurones can shape the spatial–temporal aspects of the astrocyte response, i.e. the general pattern, amplitude and duration of the [Ca2+]i rise, and, in the case of an oscillatory response, the frequency of [Ca2+]i oscillations. Indeed, all these features may dictate the timing and amount of Ca2+-dependent release of signalling molecules from the astrocyte (Pasti et al. 1997; Parpura & Haydon, 2000; Zonta et al. 2003c).
The following section will focus on the diversity of astrocyte responses to synaptically released neurotransmitters.
Low and high neuronal activity evokes in astrocytes different responses
The results that have been obtained suggest that astrocytes from the hippocampus (Porter & McCarthy, 1996; Pasti et al. 1997; Kang et al. 1998), Bergmann glia from the cerebellum (Matyash et al. 2001) as well as glial cells in contact with cholinergic synapses in culture (Smit et al. 2001) are generally activated by intense stimulation of neuronal afferents (short trains of stimuli applied at 20–50 Hz). This type of stimulation mimicks episodes of high synaptic activity and is frequently used to induce the potentiation of synaptic transmission, the so-called long-term potentiation (LTP). The response of the astrocyte is most often represented by a transient, repetitive [Ca2+]i elevation that is initiated at the level of distal processes, generally at multiple sites, and can then diffuse along the process as a regenerative [Ca2+]i wave that reaches the soma as well as other processes (Pasti et al. 1997; Parri & Crunelli, 2003). In contrast, low frequency stimulation of neuronal afferents (single pulses applied at 0.1–0.3 Hz) either fails to induce appreciable astrocyte Ca2+ responses (Porter & McCarthy, 1996; Pasti et al. 1997), or results in a [Ca2+]i elevation which remains restricted to individual astrocyte processes (Pasti et al. 1997; Grosche et al. 1999; Parri & Crunelli, 2003). These observations suggest that astrocytes can respond differently to high and low synaptic activity and integrate neuronal signals deriving from multiple sites of activation into propagating [Ca2+]i waves.
Astrocytes as detectors of neuronal activity
The astrocyte response to high neuronal activity could not, however, be considered simply an all-or-none response. Support for this conclusion derives from results obtained initially in cultured astrocytes (Pasti et al. 1995) and then confirmed in astrocytes from acute brain slices (Pasti et al. 1997; Kang et al. 1998). In slice preparations, either from the hippocampus or the neocortex, when the frequency of the stimulus applied to neuronal afferents was increased (thereby increasing their firing rate), the frequency of [Ca2+]i oscillations in astrocytes, as measured at the soma, also increased. Increasing the intensity of the stimulus (thereby recruiting additional fibres that were not initially stimulated) also resulted in an increased frequency of [Ca2+]i oscillations in astrocytes. It is likely that the progressive increase in [Ca2+]i oscillation frequency which is observed at the astrocyte soma upon increasing levels of neuronal activity derives from a higher number of activated sites at the astrocyte distal processes. Indeed, it has been often observed that the frequency of [Ca2+]i oscillations at the level of the process is higher than that at the level of the soma (Pasti et al. 1997; Parri & Crunelli, 2003). Ultimately, the increased [Ca2+]i oscillation frequency at the processes, which are in the proximity of active synaptic sites, depends on the fact that the glutamate concentration in the extrasynaptic space increases as a function of synaptic activity.
These studies lead to two main conclusions. First, astrocytes are accurate detectors of neuronal activity. This conclusion is clearly supported by the qualitatively different response to low and high synaptic activity as well as by the increase in the frequency of [Ca2+]i oscillations which parallels the increase in the level of synaptic activity. Second, the frequency of neuronal activity-dependent [Ca2+]i oscillations in astrocytes represents the code of neurone-to-astrocyte communication, allowing neurones to inform astrocytes about the levels of synaptic activity.
An important issue that stems from these observations is the identification and characterization of the functional role(s) of this neurone-to-astrocyte signalling. To accomplish this task, the following questions should be addressed. Do the local [Ca2+]i elevation and the global, repetitive [Ca2+]i oscillations evoked by low and high synaptic activity, respectively, reflect distinct functional responses of activated astrocytes? Why do astrocytes have to be informed about the status of neuronal activity?
Taking into account data mainly obtained from brain slice preparations, we propose a model in which the [Ca2+]i responses of astrocytes to low and high neuronal activity are decoded into feedback and feedforward signals, respectively. It should be noted that some features of the model are only based on indirect evidence and assumptions, and thus need to be further investigated.
Neurones and astrocytes act as a multifunctional unit in the brain
A model of the astrocyte response: feedback and feedforward signals
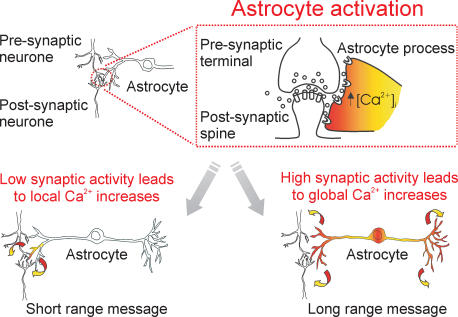
The release by astrocytes of neuroactive molecules, such as glutamate, may encode two qualitatively different messages with distinct functional consequences (Fig. 1). In the case of low synaptic activity, following [Ca2+]i elevations in the astrocytic processes glutamate may be rapidly released and its action may be spatially restricted to the perisynaptic space of active synapses. As such, glutamate acts on metabotropic glutamate receptors, which are known to be expressed in neuronal membranes at the border of the synaptic cleft, and/or on extrasynaptic ionotropic glutamate receptors located pre- and/or postsynaptically. Other factors that may potentially shape the action of astrocytic glutamate include the geometry of the contact between the neuronal synapse and the astrocytic membrane, and the number and localization of neurotransmitter transporters on astrocytic and neuronal membranes relative to neurotransmitter receptors. Finally, the whole process may also be conditioned by the distinct circuitry of inhibitory and excitatory synaptic connections in the various brain districts and by the heterogeneity among different astrocyte subtypes (Matthias et al. 2003). On the basis of these theoretical assumptions the action of glutamate is predicted to be rather complex. Experimental data from neurone–astrocyte cocultures (Araque et al. 1998) and intact tissue preparations, such as acute brain slices (Porter & McCarthy, 1996; Pasti et al. 1997; Kang et al. 1998; Fiacco & McCarthy, 2004; Liu et al. 2004) and whole-mount retinas (Newman & Zahs, 1998; Newman, 2003) confirm this complexity and indicate that glutamate of astrocytic origin can have both excitatory and inhibitory effects on synaptic transmission. The response of the astrocyte to low or moderate synaptic activity may thus represent a complex but short-distance signal which works as a feedback mechanism to locally affect synaptic transmission.
Figure 1. A hypothetical model for the diversity of the astrocyte response to neuronal activity.
Low synaptic activity triggers [Ca2+]i elevations which remain restricted to activated astrocyte processes. This short-distance message evokes in astrocytes a Ca2+-dependent release of neuroactive agents that may work as a feedback mechanism to locally affect neuronal transmission. High neuronal activity triggers a Ca2+ signal which propagates to other processes, and possibly other astrocytes, and is oscillatory in nature. This long-distance message works as a feedforward mechanism to transfer information on neuronal activity to other cells remote from the initial site of activation at the astrocyte process.
When neuronal activity is high, the response is far more complex than that triggered by low neuronal activity. First of all, the release of the neurotransmitter is elevated in a high number of synaptic loci and it thus results in concurrent and repetitive activation of multiple sites on the same astrocytic process and/or on different processes. This part of the response differs from that triggered by low neuronal activity only in quantitative terms, the end result being a local glutamate release at multiple neurone–astrocyte contacts. More importantly, the qualitative difference between the two responses relies on the propagating [Ca2+]i wave, which often follows the [Ca2+]i elevation at the process, and the presence of global, relatively periodic [Ca2+]i oscillations. The [Ca2+]i wave allows the Ca2+ signal to be transferred from the periphery to other specialized districts of the cell, such as the soma and nucleus, possibly affecting gene expression. Furthermore, once propagated to other processes, the [Ca2+]i increase can trigger the release of signalling molecules, far from the site of initial excitation, at distant synapses that are not necessarily active. In cell culture experiments, the [Ca2+]i wave can also be transferred to other astrocytes, mainly through the release of ATP (Hassinger et al. 1996; Cotrina et al. 1998; Guthrie et al. 1999) and possibly glutamate (Innocenti et al. 2000), resulting in the activation of a large number of cells (Sul et al. 2004). Indeed, since its discovery, the propagating [Ca2+]i wave has been proposed to represent in astrocytes a form of long-distance signalling (Cornell-Bell et al. 1990; Smith, 1994).
[Ca2+]i oscillations in astrocytes in situ have also been observed to occur spontaneously and form with [Ca2+]i transients in neurones a correlated network activity (Aguado et al. 2002). While in individual astrocytes [Ca2+]i oscillations can occur independently of neuronal activity (Nett et al. 2002), the pattern of synchronous oscillations in the astrocyte network is deeply affected when neuronal activity is abolished by tetrodotoxin (Aguado et al. 2002). Oscillations in the [Ca2+]i thus represent an additional feature of the astrocyte response to high neuronal activity. The observation that astrocytes can decode the information concerning the level of neuronal activity into [Ca2+]i oscillations of variable frequencies supports the original proposal that these oscillations represent a flexible coding system, a sort of digital signalling in the control of a wide array of physiological processes (Woods et al. 1986; Jacobs et al. 1988). It is noteworthy that [Ca2+]i oscillations control the release of signalling molecules, such as glutamate and prostaglandin E2, from astrocytes. This release is often pulsatile and regulated more by the frequency than by the amplitude of [Ca2+]i oscillations (Pasti et al. 1997; Zonta et al. 2003b). It follows that the strict control exerted by neurones on the frequency of [Ca2+]i oscillations implies that the [Ca2+]i-dependent release of these molecules is also under a dynamic control of neurones. Oscillations and propagating [Ca2+]i waves triggered in astrocytes by high neuronal activity can ultimately represent a feedforward, long-distance message which provides a bridge between different neuronal populations. This long-distance message may allow the spreading of the excitation (or the inhibition), according to the spatial and temporal domains specifically defined by astrocyte–neurone interactions.
Interestingly, this pathway could allow neurones to transfer signals not only to other neurones and astrocytes, but also to other cell types in the brain, such as microglia (Fig. 2). Clues for the existence of a neurone–astrocyte–microglia signalling pathway have been obtained in acute brain slice preparations in which a transient, outwardly rectifying current, probably mediated by ATP, was recorded from microglial cells coincident with the passage of astrocyte [Ca2+]i waves (Schipke et al. 2002). A neurone–astrocyte–microglial cell signalling pathway may certainly have distinct roles in the physiology and pathophysiology of the brain.
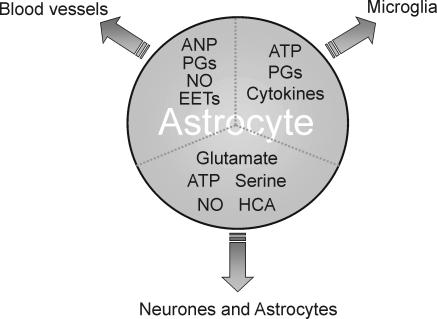
Figure 2. Different targets of neurone-to-astrocyte signalling pathway.
By releasing neuroactive molecules, including neurotransmitters, astrocytes may directly participate in information processing in the neurone–astrocyte network. By releasing vasoactive agents, such as nitric oxide (NO), prostanoids and atrial natriuretic peptide (ANP), astrocytes belonging to the neurone–astrocyte–blood vessel pathway participate in the regulation of cerebral blood flow. By releasing ATP and prostanoids astrocytes may signal to microglia thus influencing the activation of these cells and the profile of their secreted products. Following a brain trauma or a metabolic insult, reactive astrocytes also become a rich source of cytokines which can amplify the signal to microglia. EETs, epoxyeicosatrienoic acids; HCA, homocysteic acid; PGs, prostaglandins.
The [Ca2+]i signal triggered in astrocytes by the synaptic release of glutamate has also been shown to propagate to astrocyte endfeet in contact with cerebral arterioles (Zonta et al. 2003b). This finding, together with the observation that the frequency of oscillations in astrocytes is regulated by the level of synaptic activity, hints at the possibility that activated astrocytes are mediators of a neuronal activity-dependent event which involves blood vessels. This neurone–astrocyte–cerebral blood vessel pathway (Fig. 2) was indeed found to participate in the regulation of the cerebral blood flow (CBF) response to high neuronal activity, the so-called functional hyperaemia (see below). The neurone–astrocyte–blood vessel pathway might be also involved in the control of the permeability of the blood–brain barrier.
The consequences of astrocyte activation by high neuronal activity in the function of the brain can therefore be far more complex and quite distinct from those initiated by localized [Ca2+]i elevations.
An example of a feedforward message: neuronal activity-dependent [Ca2+]i oscillations in astrocytes control cerebral microcirculation
A specific physiological role of neuronal activity-dependent [Ca2+]i oscillations in astrocytes as a feedforward, long-distance message to blood vessels was recently established. It has long been known that astrocytic processes are in close structural association with both synapses and cerebral vessels (Golgi, 1885; Peters et al. 1991; Ventura & Harris, 1999). The astrocyte signal – which essentially encodes information about the level of neuronal activity in the form of propagating [Ca2+]i oscillations of specific frequencies – was observed to be transferred through astrocytic processes to cerebral microarterioles. The inhibition of astrocyte Ca2+ responses by specific antagonists of the mGluR results in the impairment of neuronal activity-dependent vasodilatation, while the selective activation by a patch pipette of single astrocytes in contact with arterioles triggers their relaxation. Results obtained in adult rats in vivo corroborated the role of astrocyte activation in the mechanism that couples synaptic activity to CBF. In adult rats, the CBF response in the somatosensory cortex upon contralateral forepaw stimulation was significantly reduced after systemic injection of the same mGluR antagonists that in brain slices blocked the activation of astrocytes by neuronal activity. At the level of these astrocyte endfeet, [Ca2+]i elevations trigger the release of a cyclooxygenase product, presumably prostaglandin E2, which mediates the dilatation of cerebral vessels (Zonta et al. 2003b,c).
The [Ca2+]i increase triggered by neuronal stimulation in a distinct astrocyte was often observed to be followed by [Ca2+]i elevations in other astrocytes surrounding the same arteriole. The timing for the [Ca2+]i response in adjacent astrocytes is compatible with that for a [Ca2+]i wave propagating between these cells. Importantly, a recent study provides convincing evidence for gap junctional communication in perivascular astrocytes (Simard et al. 2003): (i) Cx43 and purinergic receptors, i.e. the basic elements which mediated the propagation of the [Ca2+]i wave in cultured astrocytes, are highly expressed at astrocyte processes in contact with blood vessels; (ii) filling of single astrocytes in proximity of a blood vessel with Lucifer yellow results in the diffusion of the dye to other astrocyte endfeet surrounding the vessel wall; (iii) local puffing of ATP triggers a robust [Ca2+]i increase in astrocyte endfeet; (iv) a [Ca2+]i elevation in individual astrocytes triggered by direct electrical stimulation can migrate to other perivascular astrocytes. The [Ca2+]i response induced by neuronal activity may thus involve a number of perivascular astrocytes not necessarily in direct contact with active synaptic sites (Anderson & Nedergaard, 2003; Zonta et al. 2003a). The activation of these astrocytes by a propagating [Ca2+]i signal may result in an upstream and/or downstream vasodilatation and this may have a major impact on the overall conductance of the vascular network in that region.
While the astrocyte control of arteriole tone was clearly shown to depend on a COX product, presumably PGE2, this COX product is not necessarily released from astrocytes since it may be provided by other cells (i.e. endothelial cells) activated by astrocytes through either PGE2 itself or other unidentified signalling molecules. Results from recent experiments in cultured astrocytes (Zonta et al. 2003c) provide support for the hypothesis that activated astrocytes release PGE2. In that study, biosensor cells – HEK cells transfected with the PGE2 receptor EP1 – were used to investigate the possible release of the PGE2 and to obtain insights into its spatial and temporal characteristics. The results obtained indicate that the same stimulus which evokes [Ca2+]i oscillations in astrocytes in situ and, in turn, vasodilatation (i.e. the mGluR agonist (15,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD)), also triggers a pulsatile, frequency-encoded release of PGE2 from cultured astrocytes. The pulsatility of PG release from astrocytes together with the short half-life of this molecule, may account for the duration of the blood flow response: the dilating action of PGs triggered by a [Ca2+]i peak may soon vanish unless another [Ca2+]i peak can provide more PG. Taking into account that the duration and frequency of [Ca2+]i oscillations are strictly dependent on sustained neuronal activity – and vanish when synaptic activity is back to resting conditions – this observation may provide a mechanistic background for the temporal relationship between neuronal activity and blood flow response.
PGE2 can act directly on smooth muscle cells to trigger vasodilatation or indirectly through activation of endothelial cells which, in turn, mediate vasodilatation. It should also be taken into account that astrocytes may activate endothelial cells through gap junctional communication, a form of communication between these two cells which has been demonstrated to exist in culture (Braet et al. 2001) and may be present also in situ. This adds a further step of complexity to the mechanism that underlines the astrocyte control of arteriole tone. Astrocytes may transfer [Ca2+]i elevations to endothelial cells which, in turn, may release vasodilating agents, such as NO or prostaglandin I2. Endothelial cell activation might thus represent the final step in the astrocyte control of arteriole tone. Because of the presence of tight junctions in endothelial cells, the [Ca2+]i signal may ultimately involve many endothelial cells. Co-ordination of large and small arteriole behaviour by this signal may be pivotal for the adaptation of blood flow to altered energy demands of active neurones.
Beside COX products, other vasodilatory agents which are known to be released upon astrocyte activation, such as epoxyeicosatrienoic acids (EETs; cytochrome P450 epoxyegenase products), may be involved. Support for a role of these molecules derives from the evidence that glutamate can induce production of EETs in cultured astrocytes (Harder et al. 1998), and that inhibition of P450 activity results in the decrease of the blood flow response to glutamate and NMDA in vivo (Alkayed et al. 1996, 1997). ATP release from astrocytes can also be involved in the control of arteriole tone. ATP can elicit a powerful dilatation of cerebral microarterioles in the rat brain by acting on purinergic and/or adenosine receptors after its extracellular conversion to adenosine by ectoenzymes (Ibayashi et al. 1991).
In summary, many aspects in the cellular and molecular mechanism underlying the astrocyte action in the coupling between neuronal activity and blood flow remain to be investigated.
On the mechanisms of glutamate and ATP release from astrocytes
Different mechanisms can mediate the release of signalling molecules from astrocytes (Attwell, 1994; Bezzi et al. 1998; Haydon, 2001; Kržan et al. 2003) (Fig. 3). We will focus on the release mechanisms of two of these molecules: glutamate and ATP. Glutamate is widely recognized to be the main excitatory neurotransmitter in the brain. ATP is also of major physiological relevance. Once released from axon terminals through a Ca2+-dependent, vesicular-mediated mechanism, ATP is proposed to participate in excitatory synaptic transmission and Ca2+ signalling by acting on purinergic receptors, both ionotropic (P2X) and metabotropic (P2Y) (North & Barnard, 1997; Khakh, 2001). These receptors are widely expressed by neurones from the central nervous system, and they are also present in astrocytes (Kukley et al. 2001).
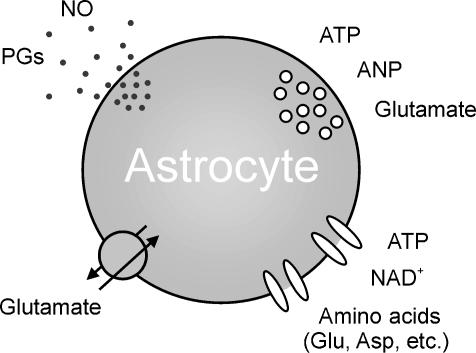
Figure 3. Multiple mechanisms mediate the release of signalling molecules from astrocytes.
Classical neurotransmitters, such as glutamate and ATP, can be released from cultured astrocytes through a Ca2+-dependent, most likely vesicular-mediated, mechanism. ATP, NAD+, glutamate and other amino acids can be also released through the opening of large conductance channels, such as volume-sensitive anion channels, connexin hemichannels and P2X7 receptors. Reverse operation of glutamate transporters represents an additional mechanism that can lead to a slow increase in the extracellular concentration of glutamate. Signalling molecules, such as PGs and NO, may diffuse freely accross the membrane. Due to their short half-life, the action of these molecules is spatially restricted to nearby target cells. It is noteworthy that other neuroactive molecules, such as the neurotrophins and the NMDA receptor co-agonist d-serine, are released from astrocytes. Given that their release mechanism has not yet been clarified, they are not included in the figure.
Regarding glutamate release, both Ca2+-independent, such as the reversal operation of glutamate transporters (Szatkowski et al. 1990; Attwell et al. 1993), and Ca2+-dependent mechanisms have been proposed. A number of studies provide indirect, although compelling, evidence that under physiological conditions glutamate can be released from astrocytes through a Ca2+-dependent mechanism that shares common properties with the vesicle-mediated mechanism of the neurotransmitter release in neurones (Parpura et al. 1995a,b; Jeftinija et al. 1997; Calegari et al. 1999; Araque et al. 2000; Pasti et al. 2001; Kreft et al. 2004; Zhang et al. 2004). Recent results provide significant support for the vesicle hypothesis of glutamate release from astrocytes (Bezzi et al. 2004). Using postembedding immunogold labelling, vesicles expressing the vesicular glutamate transporters (VGLUT1/2) and the vesicular SNARE protein cellubrevin have been detected in hippocampal astrocytes in situ. By total internal reflection fluorescence microscopy, distinct fusion events of fluorescently tagged VGLUT-positive vesicles were revealed in cultured astrocytes following stimuli that trigger [Ca2+]i elevations. Most important, in strict temporal correlation with these events, NMDA receptor-mediated [Ca2+]i elevations were detected in NMDA receptor transfected insulinoma-1 cells used as glutamate biosensors. These observations provide convincing evidence for the existence in astrocytes of a Ca2+-dependent exocytosis of glutamate-containing vesicles.
Glutamate and other amino acids have also been reported to be released from astrocytes through volume-sensitive anion channels (Kimelberg et al. 1990; Kimelberg & Mongin, 1998). Opening of these channels can allow the delivery of these molecules into the extracellular space along their concentration gradient, since their concentration in the cytosol is in the millimolar range while in the extracellular space it is in the low micromolar range (Nedergaard et al. 2002). Whether this release may occur under physiological conditions and whether it may be regulated by [Ca2+]i is, however, unclear.
As to ATP release, the ATP-binding cassette transporters (Abraham et al. 1993), the cystic fibrosis transmembrane conductance regulator or other volume-regulated anion channels (Queiroz et al. 1999; Hisadome et al. 2002; Anderson et al. 2004) are possible mechanisms for ATP release. Furthermore, evidence for a Ca2+-dependent release of ATP-containing vesicles has been recently reported (Coco et al. 2003).
Connexin hemichannels and/or P2X7 receptors have also been recently proposed as possible pathways for the delivery of both glutamate (Duan et al. 2003; Ye et al. 2003) and ATP (Cotrina et al. 1998) (Fig. 3). Here we will review recent data on this issue and focus our discussion on the possible role of these novel release pathways under physiological and non-physiological conditions.
Release of glutamate and ATP under low external Ca2+. Involvement of connexin hemichannels and/or P2X7 receptors
Connexin hemichannels, also called connexons, are hexagonal multimers made up of six connexin macromolecules. They represent a heterogeneous family of hemichannels with different permeation and regulatory properties. When opposed to another connexon in an adjacent membrane, a connexon forms a gap junction intercellular channel. However, when unopposed, a connexon can form a single-membrane-spanning channel which can allow the passage of molecules of relatively large molecular weight (< 1 kDa). Its general features are a voltage dependence, a modulation by either phosphorylation and pH and an increase of the open probability at low extracellular concentrations of divalent ions (Goodenough & Paul, 2003). Connexins, particularly connexin 43, are highly expressed in astrocytes where they mediate gap junctional intercellular communication (Dermietzel et al. 1989; Dermietzel & Spray, 1993; Simard et al. 2003).
Clues for the possibility that connexon openings in astrocytes allow the release of ATP and glutamate derive from the observations that the [Ca2+]i waves evoked by lowering the extracellular Ca2+ in cultured astrocytes (Zanotti & Charles, 1997) are accompanied by a large increase in the extracellular concentration of both ATP (Cotrina et al. 1998, 2000; Stout et al. 2002; Coco et al. 2003) and glutamate (Ye et al. 2003). This release was sensitive to anandemide (Coco et al. 2003) and to flufenamic acid, carbenoxolone, octanol and other blockers of gap junctions (Ye et al. 2003). In an elegant study which used a real-time bioluminescence imaging technique, Nedergaard and collaborators (Arcuino et al. 2002) provided evidence for the release of ATP from single astrocytes. These authors observed that the [Ca2+]i wave caused by lowering extracellular Ca2+ is initiated at distinct, individual astrocytes, which operate as triggers for the propagating [Ca2+]i wave. The [Ca2+]i signal in these cultured astrocytes is associated with a transient opening of a non-selective membrane channel, as assessed by the selective uptake of the fluorescent dye propidium iodide. Although the opening of this large membrane pore is transient, and thus does not lead to cell death, it apparently allows a highly localized release of ATP. Other studies confirm that at low divalent extracellular concentrations cultured astrocytes can take up dyes, such as Lucifer yellow, and this uptake is blocked by gap junctional blockers (Ye et al. 2003). Furthermore, patch-clamp recordings from cultured astrocytes revealed inward currents activated by low Ca2+ concentrations, and these currents are blocked by flufenamic acid and by increasing extracellular Mg2+ concentration (Stout et al. 2002). All these observations provide convincing evidence that, at least at low extracellular concentrations of divalent ions, a large pore in the plasma membrane could open in the astrocytic membrane and allow intracellular molecules, such as ATP and glutamate, to exit following their concentration gradient. Hemichannels are good candidates as mediators of this type of release.
However, it is worthwhile noting that attributing a specific function to connexin hemichannels is hampered by our insufficent knowledge of their properties and by the lack of satisfactory pharmacological tools for their specific inhibition. Compounds such as octanol, flufenamic acid, AGA and carbenoxolone are commonly used to block gap junctions and hemichannels, but their mechanisms of action are not known. Indeed, none of these compounds have been demonstrated to specifically interact with connexins. Rather, they can have other effects, including the induction of [Ca2+]i elevations and [Ca2+]i waves, and it is likely that, at least for some of them, the mechanism of action is indirect (Rouach et al. 2003). A complementary approach therefore appears necessary for unambiguously identifying a hemichannel role in the release of glutamate and ATP from astrocytes.
Activation of the P2X7 purinergic receptor has been recently proposed to represent an additional, novel pathway for glutamate release from astrocytes (Duan et al. 2003). Like connexons, the opening of this receptor forms a pore that is permeable to molecules of relatively large molecular mass and it is greatly enhanced by low extracellular concentrations of divalent ions (Khakh, 2001). The release of amino acids from cultured astrocytes is observed to be (i) higher upon stimulation with benzoyl-ATP than with ATP (the latter being a weak P2X agonist), (ii) greatly potentiated in divalent ion-free medium, and (iii) blocked by P2X antagonists. Amino acid release induced by ATP in divalent ion-free medium is unaffected after cell incubation with either BAPTA-AM or EGTA plus thapsigargin indicating the Ca2+ independence of this release (Duan et al. 2003).
While these results from cultured astrocytes clearly indicate that under selected experimental conditions the P2X7 receptor allows the delivery of glutamate into the extracellular space, no compelling evidence has been reported that under physiological conditions activation of astrocyte P2X7 receptors results in formation of large pores. On the other hand, under physiological extracellular concentrations of Ca2+, ATP is known to trigger a significant release of glutamate (Jeremic et al. 2001; Coco et al. 2003). However this release can be due to a Ca2+-dependent pathway. Indeed, by activating both ionotropic, Ca2+-permeable P2X receptors and/or metabotropic P2Y receptors, ATP can evoke an intracellular Ca2+ increase that, in turn, mediates glutamate release. Furthermore, it is worthwhile underlining that, while an immunocytochemical study provides an indication of the presence in hippocampal astrocytes of all P2X receptors (with the exception of the P2X5 type) (Kukley et al. 2001), no evidence yet exists for a functional expression in these cells of the P2X7 receptor.
It thus appears well established that, under particular experimental conditions, such as the virtual absence of divalent cations in the extracellular space, connexin hemichannels and P2X7 receptors can open and allow the passage of small molecules, including glutamate and ATP. It is noteworthy that they can also open under pathological conditions, such as brain ischaemia and spreading depression. Their possible involvement in the mechanism(s) leading to cell death, for example contributing to produce large excytotoxic concentrations of glutamate in the extracellular space, is a matter of debate and will not be discussed further. However, it is worthwhile underlining that the opening of these channels can, indeed, cause the loss of ionic gradients and of cytoplasmic components, change in cell volume, Ca2+ influx and, ultimately, cell death. The relevant point here is whether or not a large and non-selective channel, such as the connexin hemichannel, opens under physiological conditions and thus contributes to the normal functioning of the cell.
Can hemichannels mediate ATP release fromastrocytes under physiological conditions?
Under normal extracellular concentrations of Ca2+ and Mg2+, hemichannels should remain in a permanent, closed state (Goodenough & Paul, 2003). In contrast to this view, results obtained in cultured 3T3 fibroblasts, which constitutively expressed Cx43, demonstrate that these connexin hemichannels can open and mediate the release of the nucleotide NAD+ into the extracellular space (Bruzzone et al. 2001). This release could be blocked by 18β-glycyrrhetinic acid, a gap junctional blocker, and by an antisense-Cx43 oligonucleotide. Evidence has also been provided that cultured astrocytes release NAD+ through the same pathway (Verderio et al. 2001). In addition, a recent study in cultured endothelial cells from rat brain cell lines provides further indication for ATP release through hemichannels without deleterious consequences for the cell (Braet et al. 2003). A peptide which binds to an extracellular domain of connexins, namely Gap 26, was found to block the propagation of the [Ca2+]i waves in endothelial cells due to ATP release induced by photo-liberation of InsP3 in a single cell (Braet et al. 2003). To be compatible with cell survival, openings of these hemichannels should, obviously, be transient and regulated. A recent study in HeLa cell transfected with the cDNA for the rat Cx43 (Contreras et al. 2003) confirms this assumption and provides the following electrophysiological characterization of hemichannel gating properties: (i) transient single channel openings can occur at potentials more positive than +60 mV; (ii) openings are sensitive to gap junctional blockers and La3+, and (iii) openings increase, although modestly, in the absence of extracellular Ca2+. While hemichannel activity is not detectable at the resting potential by electrophysiological techniques, a significant dye uptake into the cells can be observed after the prolonged incubation with ethidium bromide. Therefore, infrequent or brief openings may occur, although such a low activity in heterologous systems forced to overexpress the hemichannels can hardly be considered a convincing indication of hemichannel openings under normal conditions.
Only a few studies on a possible role of hemichannel-mediated release of ATP in cultured astrocytes under physiological extracellular concentrations of divalent ions have been performed and the results obtained are conflicting. Stout et al. (2002) reported that gap junction and connexin hemichannel blockers reduce ATP release, while quinine, an agonist of connexin hemichannels, increases ATP release and [Ca2+]i wave propagation. In contrast, Coco et al. (2003) found that under normal conditions the release of ATP triggered by either phorbol 12-myristate 13-acetate (PMA) or mechanical astrocyte stimulation was insensitive to the connexin hemichannel blockers flufenamic acid and anandamide, which, conversely, drastically reduced the release of ATP triggered by low divalent ions. It is noteworthy that anandamide (Coco et al. 2003), but not flufenamic acid (C. Verderio, personal communication), inhibited the NAD+ influx down its concentration gradient, confirming its effectiveness as a hemichannel blocker. Furthermore, by means of sucrose gradient analysis, these authors provide evidence that a fraction of ATP in astrocytes, as previously shown in other cell types (Unsworth & Johnson, 1990), is stored and released in vesicles through a Ca2+-dependent mechanism. In particular, they observed that the release of ATP is: (i) Ca2+ dependent, being inhibited by astrocyte incubation with the intracellular Ca2+ chelator BAPTA-AM, (ii) partly inhibited by tetanus neurotoxin, which impairs exocytosis by cleavage of the SNARE protein synaptobrevin, and (iii) reduced by bafilomycin A1, which also impaired ATP storage in vesicles. Interestingly enough, the protein kinase C activator PMA and the metabotropic glutamate receptor agonist t-ACPD, were equally effective in inducing the release of glutamate, while PMA, but not t-ACPD, induced the release of ATP. Glutamate and ATP may thus be stored in different organelles, raising the possibility that the exocytosis of glutamate- and ATP-containing vesicles can be differentially regulated by Ca2+ signalling (Coco et al. 2003) (Fig. 3).
According to the results available from experiments in cultured astrocytes, it therefore seems likely that the two mechanisms, i.e. the Ca2+-dependent and the Ca2+-independent ATP release mediated by hemichannels, do not overlap, the first being present under normal, the second under particular, experimental conditions. Whether or not this view can hold in in vivo conditions is a question to be addressed.
In conclusion, while many studies from different laboratories demonstrate that hemichannels can mediate ATP release from cultured astrocytes under low divalent concentrations, no compelling evidence exists for their possible role in ATP release from astrocytes under physiological conditions. It is clear that a more comprehensive approach together with the development of new tools, such as specific connexin antagonists, are necessary to gain further insights into connexin function.
Conclusions
Emerging evidence has highlighted the capability of the astrocyte to work as both a target of neuronal signals, i.e. a non-neuronal postsynaptic membrane responsive to neurotransmitters, and a source of signalling molecules, i.e. a non-neuronal terminal that releases neurotransmitters as well as other neuroactive agents. The astrocyte's action remains, however, under the strict control of neurones. Indeed, by determining the astrocyte's type of response (i.e. short- or long-distance signals), neurones shape the output of activated glial cells and thus determine a qualitatively distinct functional consequence. The neurone-to-astrocyte signalling pathway thus represents an integral unit which serves multiple, diverse roles. Ultimately, this multifunctional unit contributes to achieve the rich diversity in neuronal transmission which is a fundamental aspect of brain function.
Acknowledgments
We thank Paulo Magalhães for critical reading of the manuscript. The original work described in this review was supported by grants from the Armenize–Harvard University Foundation, the Italian University and Health Ministries (FIRB, RBNE01RHZM_003; RBAU015PZE_003) and ST/Murst: ‘Neuroscienze’.
References
- Abraham EH, Prat AG, Gerweck L, Seneveratne T, Arceci RJ, Kramer R, Guidotti G, Cantiello HF. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci U S A. 1993;90:312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado F, Espinosa-Parrilla JF, Carmona MA, Soriano E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J Neurosci. 2002;22:9430–9444. doi: 10.1523/JNEUROSCI.22-21-09430.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henderson L, Harder DR. Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol. 1996;271:1541–1546. doi: 10.1152/ajpheart.1996.271.4.H1541. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Martín ED, Perea G, Arellano JI, Buño W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci. 2002;22:2443–2450. doi: 10.1523/JNEUROSCI.22-07-02443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Arcuino G, Lin JH-C, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D. Glia and neurons in dialogue. Nature. 1994;369:707–708. doi: 10.1038/369707a0. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Auld DS, Robitaille R. Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron. 2003;40:389–400. doi: 10.1016/s0896-6273(03)00607-x. [DOI] [PubMed] [Google Scholar]
- Benz B, Grima G, Do KQ. Glutamate-induced homocysteic acid release from astrocytes: possible implication in glia-neuron signaling. Neuroscience. 2004;124:377–386. doi: 10.1016/j.neuroscience.2003.08.067. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Vesce S, Volterra A. Neuron-astrocyte cross-talk during synaptic transmission: physiological and neuropathological implications. Prog Brain Res. 2001;132:255–265. doi: 10.1016/S0079-6123(01)32081-2. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Braet K, Paemeleire K, D'Herde K, Sanderson MJ, Leybaert L. Astrocyte-endothelial cell calcium signals conveyed by two signalling pathways. Eur J Neurosci. 2001;13:79–91. [PubMed] [Google Scholar]
- Braet K, Vandamme W, Martin PEM, Evans WH, Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemichannels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- Calegari F, Coco S, Taverna E, Bassetti M, Verderio C, Corradi N, Matteoli M, Rosa P. A regulated secretory pathway in cultured hippocampal astrocytes. J Biol Chem. 1999;274:22539–22547. doi: 10.1074/jbc.274.32.22539. [DOI] [PubMed] [Google Scholar]
- Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Sáez JC, Bukauskas FF, Bennett MVL. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci U S A. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH-C, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CCG, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH-C, López-García JC, Naus CCG, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Spray DC. Gap junctions in the brain: where, what type, how many and why? Trends Neurosci. 1993;16:186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Traub O, Hwang TK, Beyer E, Bennett MV, Spray DC, Willecke K. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci U S A. 1989;86:10148–10152. doi: 10.1073/pnas.86.24.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci. 1995;15:5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004;24:722–732. doi: 10.1523/JNEUROSCI.2859-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgi C. Sulla fina anatomia degli organi centrali del sistema nervoso. Riv Sper Fremiat Med Leg Alienazioni Ment. 1885;11:72–123. [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron–glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- Hassinger TD, Guthrie PB, Atkinson PB, Bennett MV, Kater SB. An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci U S A. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hirase H, Qian L, Barthó P, Buzsáki G. Calcium dynamics of cortical astrocytic networks in vivo. PLOS Biol. 2004;2:494–499. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J General Physiol. 2002;119:511–520. doi: 10.1085/jgp.20028540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibayashi S, Ngai AC, Meno JR, Winn HR. Effects of topical adenosine analogs and forskolin on rat pial arterioles in vivo. J Cereb Blood Flow Metab. 1991;11:72–76. doi: 10.1038/jcbfm.1991.8. [DOI] [PubMed] [Google Scholar]
- Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20:1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HT, Elliott DJ, Math VB, Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988;202:185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jeftinija SD, Jeftinija KV, Stefanovic G. Cultured astrocytes express proteins involved in vesicular glutamate release. Brain Res. 1997;750:41–47. doi: 10.1016/s0006-8993(96)00610-5. [DOI] [PubMed] [Google Scholar]
- Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem. 2001;77:664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Mongin AA. Swelling-activated release of excitatory amino acids in the brain: relevance for pathophysiology. Contributions Nephrol. 1998;123:240–257. doi: 10.1159/000059916. [DOI] [PubMed] [Google Scholar]
- Kreft M, Stenovec M, Rupnik M, Grilc S, Krzan M, Potokar M, Pangrsic T, Haydon PG, Zorec R. Properties of Ca2+-dependent exocytosis in cultured astrocytes. Glia. 2004;46:437–445. doi: 10.1002/glia.20018. [DOI] [PubMed] [Google Scholar]
- Kržan M, Stenovec M, Kreft M, Pangršic T, Grilc S, Haydon PG, Zorec R. Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J Neurosci. 2003;23:1580–1583. doi: 10.1523/JNEUROSCI.23-05-01580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Barden JA, Steinhäuser C, Jabs R. Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia. 2001;36:11–21. doi: 10.1002/glia.1091. [DOI] [PubMed] [Google Scholar]
- Kulik A, Haentzsch A, Luckermann M, Reichelt W, Ballanyi K. Neuron-glia signaling via α1 adrenoceptor-mediated Ca2+ release in Bergmann glial cells in situ. J Neurosci. 1999;19:8401–8408. doi: 10.1523/JNEUROSCI.19-19-08401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q-S, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci U S A. 2004;101:3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular bases of brain energy metabolism and their relevance to functional brain imaging: evidence for a prominent role of astrocytes. Cereb Cortex. 1996;6:50–61. doi: 10.1093/cercor/6.1.50. [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Hüttmann K, Matyash M, Kettenmann H, Steinhäuser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V, Filippov V, Mohrhagen K, Kettenmann H. Nitric oxide signals parallel fiber activity to bergmann glial cells in the mouse cerebellar slice. Mol Cell Neurosci. 2001;18:664–670. doi: 10.1006/mcne.2001.1047. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995a;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Liu F, Brethorst S, Jeftinija K, Jeftinija S, Haydon PG. Alpha-latrotoxin stimulates glutamate release from cortical astrocytes in cell culture. FEBS Lett. 1995b;360:266–270. doi: 10.1016/0014-5793(95)00121-o. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience. 2003;120:979–992. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- Pasti L, Pozzan T, Carmignoto G. Long-lasting changes of calcium oscillations in astrocytes. A new form of glutamate-mediated plasticity. J Biol Chem. 1995;270:15203–15210. doi: 10.1074/jbc.270.25.15203. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HdF. The Fine Structure Of The Nervous System: Neurons and Their Supporting Cells. New York: Oxford University Press; 1991. [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Meyer DK, Meyer A, Starke K, von Kügelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience. 1999;91:1171–1181. doi: 10.1016/s0306-4522(98)00644-7. [DOI] [PubMed] [Google Scholar]
- Rouach N, Segal M, Koulakoff A, Giaume C, Avignone E. Carbenoxolone blockade of neuronal network activity in culture is not mediated by an action on gap junctions. J Physiol. 2003;553:729–745. doi: 10.1113/jphysiol.2003.053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Boucsein C, Ohlemeyer C, Kirchhoff F, Kettenmann H. Astrocyte Ca2+ waves trigger responses in microglial cells in brain slices. FASEB J. 2002;16:255–257. doi: 10.1096/fj.01-0514fje. [DOI] [PubMed] [Google Scholar]
- Shelton MK, McCarthy KD. Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J Neurochem. 2000;74:555–563. doi: 10.1046/j.1471-4159.2000.740555.x. [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, Lodder H, van der Schors RC, van Elk R, Sorgedrager B, Brejc K, Sixma TK, Geraerts WP. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature. 2001;411:261–268. doi: 10.1038/35077000. [DOI] [PubMed] [Google Scholar]
- Smith SJ. Neural signalling. Neuromodulatory astrocytes. Curr Biol. 1994;4:807–810. doi: 10.1016/s0960-9822(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CCG, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Sul J-Y, Orosz G, Givens RS, Haydon PG. Astrocytic connectivity in the hippocampus. Neuron Glia Biol. 2004;1:3–11. doi: 10.1017/s1740925x04000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- Unsworth CD, Johnson RG., Jr ATP compartmentation in neuroendocrine secretory vesicles. Ann N Y Acad Sci. 1990;603:353–363. doi: 10.1111/j.1749-6632.1990.tb37685.x. [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Bruzzone S, Zocchi E, Fedele E, Schenk U, De Flora A, Matteoli M. Evidence of a role for cyclic ADP-ribose in calcium signalling and neurotransmitter release in cultured astrocytes. J Neurochem. 2001;78:646–657. doi: 10.1046/j.1471-4159.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- Woods NM, Cuthbertson KSR, Cobbold PH. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986;319:600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- Ye Z-C, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti S, Charles A. Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signaling. J Neurochem. 1997;69:594–602. doi: 10.1046/j.1471-4159.1997.69020594.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pangršic T, Kreft M, Kržan M, Li N, Sul J-Y, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. Fusion related release of glutamate from astrocytes. J Biol Chem. 2004;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Carmignoto G. Response: Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003a;26:344–345. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann K-A, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003b;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Zonta M, Sebelin A, Gobbo S, Fellin T, Pozzan T, Carmignoto G. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J Physiol. 2003c;553:407–414. doi: 10.1113/jphysiol.2003.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]