Abstract
Antitumor antibodies with the same specificity as cytotoxic T lymphocytes that recognize antigenic peptides encoded by tumor-associated genes and presented by MHC class I molecules would be valuable tools to analyze the antigenicity or target tumor cells in vivo. To obtain a human antibody directed against a peptide encoded by gene melanoma-associated antigen (MAGE)-A1 and presented by HLA-A1 molecules, we selected a large phage Fab antibody repertoire on a recombinant version of the complex HLA-A1–MAGE-A1 produced by in vitro refolding. One of the selected phage antibodies shows binding to HLA-A1 complexed with the MAGE-A1 peptide, but does not show binding to HLA-A1 complexed with a peptide encoded by gene MAGE-A3 and differing from the MAGE-A1 peptide by only three residues. Phages carrying this recombinant antibody bind to HLA-A1+ cells only after in vitro loading with MAGE-A1 peptide. These results indicate that nonimmunized phage Fab libraries are a source of antibodies with a T cell antigen receptor-like specificity. The human anti-HLA-A1–MAGE-A1 antibody described here may prove very useful for monitoring the cell surface expression of these complexes, and eventually, as a targeting reagent for the specific immunotherapy of HLA-A1 patients bearing a MAGE-A1-positive tumor.
Keywords: phage display, major histocompatibility complex, T cell antigen receptor-like antibodies, tumor targeting
The past few years have seen the resurgence of a strong interest in tumor vaccine development (1). This is in part attributable to an increased understanding of the immune response to tumors, especially in the case of melanomas. It is now well established that human melanoma cells often express antigens that are recognized by cytotoxic T lymphocytes (CTL) derived from tumor-bearing patients. These CTLs have been used as tools to identify genes that code for tumor antigens, such as those of the melanoma-associated antigen (MAGE) gene family, which includes at least 17 related genes, namely MAGE-A1 to A12, MAGE-B1 to B4, and MAGE-C1 (2). The MAGE genes are expressed by tumors of various histological types, but they are silent in normal cells, with the exception of male germ-line cells that do not carry HLA class I molecules and are therefore unable to present antigens to CTL. Hence, antigens encoded by MAGE-A, -B, or -C genes should be strictly tumor specific. Because the MAGE antigens are shared by many tumors and on account of their strict tumor specificity, they are of particular interest for cancer immunotherapy.
Gene MAGE-A1 was isolated because it encoded an antigen presented on HLA-A1 molecules to autologous CTL of a melanoma patient (3). It is frequently expressed in metastatic melanomas (48%), esophageal squamous cell carcinomas (53%), head and neck squamous cell carcinomas (28%), non-small cell lung carcinomas (49%), or the bladder carcinomas (22%). The antigenic peptide presented by HLA-A1 molecules is EADPTGHSY (4). Several other MAGE-A1 epitopes recognized by CTL have been identified. These are presented by HLA-A3, -A24, -A28, -B53, -Cw2, -Cw3, and -Cw16 (5).
Although there is ample evidence for the presence of these antigens on a variety of tumors, they are seemingly unable to elicit an adequate antitumor immune response. Many modern cancer immunotherapies are therefore designed to induce or enhance T cell reactivity against tumor antigens. Clinical trials involving therapeutic vaccination of cancer patients with antigenic peptides or proteins are in progress (4). In a recently completed trial, 25 tumor-bearing HLA-A1 melanoma patients with advanced disease received three s.c. injections of a MAGE-A3 peptide presented by HLA-A1 (6). Tumor regression was observed in seven patients; three of these were complete. No increase in anti-MAGE CTL could be detected in the blood of these patients, including those with tumor regression. These regressions occurred very slowly, suggesting that they may have been caused by a weak immune response. To explain how these tumor regressions are obtained, and why the majority of patients do not appear to respond to the vaccines, one wishes to have precise information about the presence of the target tumor antigen on the tumor cell surface, before and after the vaccination. Currently, expression of MAGE-A and HLA class I genes is assessed with reverse transcription–PCR on tumor samples, or by immunological detection of certain MAGE-A proteins in tumor cells (7), by using a mAb such as 57B that detects MAGE-A4 in tissues, and with mAb W6/32HL that detects the presence of mature HLA class I molecules. However, positive results from these assays do not imply display of the antigenic complex. Multiple protein interactions are required for efficient assembly of MHC class I heavy chain and β2 microglobulin (β2m) with endogenous peptides (8). Peptide processing and transport into the endoplasmic reticulum involves the transporters associated with antigen processing, (TAP-1 and TAP-2), and the proteasome complex, which also includes the MHC-encoded low molecular weight proteins LMP-2 and LMP-7 (8). Loss of expression or down-regulation of any of these proteins may allow tumor cells to escape recognition by CD8+ CTLs (9). Similarly, it has been shown that a mutation in the β2m gene is a frequent event leading to the loss of HLA class I surface expression in melanomas (10). Thus, a direct visualization of the HLA-A1–MAGE-A1 complex on the tumor cell surface would be the ideal way to ensure its presence. Soluble T cell receptors would be ideal for this purpose. Unfortunately, it has been proven difficult to engineer these molecules (11) and their inherent low affinity for their target may limit their use as detection reagents. Antibodies that specifically recognize a peptide–MHC complex have already been used to study MHC class I or II antigen presentation (12–18), to localize and quantify antigen-presenting cells (APC) displaying a T cell epitope (13, 19–21), specifically mask an autoimmune T cell epitope (22, 23), or as a targeting tool in a mouse model (24). However, selecting such reagents remains a difficult task and several failures have been reported (25, 26). The available antibodies have been obtained after immunization of mice with recombinant peptide–MHC complexes or peptide-loaded TAP-deficient APC, and recently by selection from phage-antibody libraries made from immunized transgenic mice (27). Immunization with such complexes is extremely time-consuming. Moreover, all these antibodies are of murine origin, and cannot be used repetitively in patients because of the likely development of a human anti-mouse antibody response.
We report here the selection of a fully human Fab fragment directed against the HLA-A1–MAGE-A1 complex by selection from a large nonimmune phage-antibody repertoire.
Materials and Methods
Cloning of HLA-A1 Heavy Chain.
The cDNA of the HLA-A*0101 allele was amplified by PCR with the primers 5′-GCGGCGGCGGCCATGGGCTCCCACTCCATGAGG-3′ and 5′-CGGCAGGAGAGCGGCCGCGAGCTCCCATCTCAGGG-3′ (Eurogentec, Seraing), containing the underlined NcoI and NotI restriction sites, respectively. The PCR products were ethanol precipitated, digested with NcoI and NotI enzymes, gel purified, and ligated into the plasmid pET21d (Novagen) digested with the same enzymes. The constructs were transformed into Escherichia coli strain DH5α and some clones containing an insert were sequenced. Clones with a correct sequence were then transformed into E. coli strain BL21DE3 for production. The plasmid pHNβ2m was used to produce the β2m (28), also in E. coli.
In Vitro Refolding of the Peptide–MHC Complexes.
The peptide–MHC complexes were in vitro refolded from inclusion bodies produced in E. coli as described (28).
Biotinylation of the Refolded Complex.
Centricon-10 units were used to exchange the buffer for 50 mM NaHCO3 and concentrate the complex to 1 mg/ml. EZ-link sulfo NHS-SS biotin (0.01 volume of 10 mM; Pierce) was added (a final concentration of 100 μM corresponds to a 5:1 ratio of biotin:complex) and the solution was incubated for 30 min at room temperature. The biotinylated complex was separated from the free biotin by gel filtration on a Superdex 200 column (Amersham Pharmacia).
Monoclonal Antibodies.
The mAb W6/32HL (anti-HLA class I heavy chain/β2m complexes), W6/32HK (inactive variant of W6/32.HL), HB28 (BBM1) (anti-human β2m), HC-A2 (anti-HLA-A heavy chains), and TÜ155 (anti-HLA-A/β2m complexes with peptide-dependent reactivity) have been described (29–31). TÜ114 (IgMκ) was produced by standard techniques and recognizes HLA-A–β2m complexes independent of the presence of peptide in the binding groove.
Selection of Phage-Antibodies on Biotinylated Complexes.
A large human Fab library containing 3.7 × 1010 antibody fragments was used for the selection (32). Phages (1013) were first preincubated 1 h at room temperature in 2% nonfat dry milk-PBS in an immunotube coated with streptavidin (10 μg/ml) to deplete for streptavidin binders. Streptavidin–coated paramagnetic beads (200 μl; Dynal, Oslo) were also incubated in 2% milk-PBS for 1 h at room temperature. Phages were subsequently incubated for 1 h with decreasing amounts of biotinylated complexes (500, 100, 20, and 4 nM for rounds 1–4, respectively). Streptavidin beads were added, and the mixture was left for 15 min on a rotating wheel. After 15 washes with 0.1% Tween-PBS, bound phages were eluted by a 10-min incubation with 60 μl of 50 mM DTT, thus breaking the disulfide bond in between the complex and the biotin. The eluted phages were diluted in PBS to 1 ml and 0.5 ml were used to infect E. coli strain TG1 cells grown to the logarithmic phase (OD600 of 0.5). The infected cells were plated for amplification as described (33). After infection of TG1 cells for 30 min at 37°C, bacteria were grown overnight at 30°C on agar plates.
The diversity of the selected antibodies was determined by means of DNA fingerprinting (34). The insert of different clones was amplified by PCR with primers pUC-reverse (5′-AGCGGATAACAATTTCACACAGG-3′) and fd-tet-seq24 (5′-TTTGTCGTCTTTCCAGACGTTAGT-3′) and digested with the enzyme BstNI before analysis on agarose gel.
Phage ELISA.
Specificity of individual Fab fragments was assessed by ELISA with indirectly coated complexes as described (35).
Flow Cytometry Analysis on Peptide-Loaded Cells.
The Epstein–Barr virus (EBV)-transformed B cell lines MZ2 (A1, A29, B37, B44, Cw6, and Cw16), LG2 (A24, A32, B35, B44, and Cw6), and AVL3 (A1, A2, B27, B44, Cw5, and Cw7) or melanoma cell lines MZ2-MEL 3.0 and MZ2-MEL 2.2 (3), were stained to demonstrate the ability of fd-Fab-G8 to bind the native HLA-A1–MAGE-A1 complex. About 106 cells were used for each experiment. B cells were washed twice in PBS, incubated for 30 min at 37°C in PBS containing 100 μM peptide, and then washed twice again with ice-cold 2% milk-PBS. Melanoma cells were directly resuspended in 2% milk-PBS. All subsequent washes and incubations were done in ice-cold 2% milk-PBS. Cells were incubated for 1 h at 4°C with phage-antibodies (1 × 1010 cfu) in 100 μl, washed three times, incubated with 100 μl of goat anti-fd polyclonal antibody (diluted 1/500), washed three times again and finally incubated with 100 μl of FITC-conjugated rabbit anti-goat antibody (Dako; diluted 1/50). After three washes, cells were resuspended in 500 μl of ice-cold PBS. Detection of fluorescent cells was performed by means of flow cytometry on a FACScalibur (Becton Dickinson) and the results were analyzed with the cellquest program (Becton Dickinson).
Measurements of Fab–Antigens Interaction by BIAcore Biosensor.
Fab-G8 was purified from E. coli periplasmic fraction as already described (32). Kinetic measurements were performed by surface plasmon resonance. PBS (pH 8)/0.05% Tween20 was chosen as running buffer. An NTA-chip (Amersham Pharmacia) was activated with 500 μM NiCl2 for 1 min at 10 μl/min. Approximately 800 response units of hexahistidine-tagged Fab (20 μg/ml) was immobilized and the peptide–MHC complexes were subsequently injected at a flow rate of 20 μl/min to minimize rebinding effects. A blank (injection of the antibody only) was subtracted to each curve to take in account the slightly decreasing baseline caused by the Fab dissociation. The channel was regenerated by injection of 250 mM EDTA during 2 min (36).
Results
Production of HLA-A1-β2m–Peptide Complex.
HLA-A1 heavy chains and β2m were produced as inclusion bodies in an E. coli expression system. The yields of purified inclusion bodies were 25 and 36 mg/l for the heavy chain and β2m, respectively. Recombinant products of the expected sizes (33 and 12 kDa for HLA-A1 and β2m, respectively) were visualized with SDS/PAGE analysis (Fig. 1A). An additional band, presumably corresponding to a degradation product, was detected in the heavy chain preparation. However, this did not seem to interfere with further experiments. The purified inclusion bodies were dissolved in urea buffer. The heavy chain and β2m solutions were then diluted in a folding buffer, in the presence of the MAGE-A1 peptide, and the complexes were allowed to fold over 36 h at 4°C. The mixture was submitted to gel filtration and three major peaks were observed (Fig. 1B). SDS/PAGE analysis indicated that the first peak, with a molecular weight of ≈48 kDa, contained HLA-A1 heavy chain and β2m (Fig. 1C). This peak was absent from a control folding experiment conducted in the absence of antigenic peptide. We concluded that it corresponded to HLA-A1–β2 m–MAGE-A1 peptide complexes. The second peak corresponded to β-lactamase and the third peak was composed of free β2m. Under these conditions, up to 1.2 mg of purified complexes could be obtained from a refolding experiment by using 6 mg of heavy chain, 5 mg of β2m, and 2 mg of peptide. Thus, the observed yield was ≈10%.
Figure 1.
(A) Analysis of purified inclusion bodies by SDS/PAGE and Coomassie staining. Two microliters of HLA-A1 (lane 1) or β2m (lane 2) inclusion bodies in freezing buffer were loaded on the gel. The expected size of the full-length heavy chain product is indicated with an arrow. (B) Gel filtration profile of the folding mixture, after an incubation of 36 h at 4°C. The mixture was concentrated by ultracentrifugation and loaded on a Superdex 200 column. The shoulder after peak 3 is a component of the folding buffer. (C) SDS/PAGE analysis and Coomassie staining of the peaks obtained by gel filtration. Lanes 1–3 correspond to 20 μl of fractions from peaks 1–3, respectively.
Assessment of Correct Folding of the Recombinant Complexes.
We verified that the recombinant complex could stimulate CTL clone 82/30, which specifically recognizes the MAGE-A1 peptide (EADPTGHSY) presented by HLA-A1 molecules (37). The CTL clone produced tumor necrosis factor when incubated in microwells coated with the HLA-A1–MAGE-A1 complex (Fig. 2A). No production of tumor necrosis factor was observed when CTL 82/30 was incubated with a recombinant HLA-A2–MAGE-A3 complex produced and purified with the same methods as for the HLA-A1–MAGE-A1 complexes. The HLA-A1–MAGE-A1 complexes did not stimulate another CTL clone that recognizes a peptide presented by HLA-A2 molecules. We concluded that at least a fraction of the HLA-A1–MAGE-A1 complexes were folded in such a way that they could bind the T cell receptors displayed by the specific CTL clone.
Figure 2.
(A) T cell activation assay with the recombinant HLA-A1–MAGE-A1 complexes. Microwells were coated with the indicated concentrations of HLA-A1–MAGE-A1 (A1MA1) or HLA-A2–MAGE-A3 (A2MA3) complexes, and washed. CTL clones 82/30 (anti-HLA-A1–MAGE-A1) or 413/13 (against a peptide presented on HLA-A2 molecules) were added at 3,000 cells per well. After 24 h, the concentration of TNF present in the culture medium was measured by testing its cytotoxicity on the TNF-sensitive WEHI-164c13 cells. (B) ELISA with anti-HLA mAbs. Equivalent amounts of proteins were coated directly on plastic (striped, aggregates; gray, β2m; and white, complex) or on streptavidin-coated plastic (black, biotinylated complex) and binding of several mAbs was tested. PBS, no antibody; W6/32, mAb W6/32HL binding to heavy chain–β2m dimers; HK, inactive mutant of W6/32HK; HB28, mAb binding to β2m; HC-A2, mAb binding to a nonconformational epitope of HLA molecules; and TÜ114 and TÜ155, two conformation-sensitive mAbs.
We decided to biotinylate the complexes to avoid a possible conformational change of the molecules caused by passive absorption onto plastic during phage library selection procedures. The coupling reagent was chosen to possess a disulfide bond between the reactive group and the biotin. This allowed for easy separation of the complex with bound phage-antibody from biotin-streptavidin particles by using reducing conditions. Biotinylated complexes were added to streptavidin-coated wells and their recognition by a panel of anti-HLA mAb was compared with aggregates and β2m (Fig. 2B). They were recognized by antibody W6/32HL which recognizes heavy chain-β2m dimers (29), the anti-β2m antibody HB28, antibody HC-A2 which recognizes a nonconformational epitope on HLA class I molecules (30), and two conformation-sensitive antibodies recognizing HLA-A–β2m complexes either in the presence (TÜ155) or also absence (TÜ114) of a peptide in the groove (31). Conversely, the aggregates were only recognized by HC-A2 and HB28. These results confirmed the correct conformation of the biotinylated complexes.
Selection of Recombinant Anti-HLA-A1–MAGE-A1 Antibodies from a Phage Display Library.
In preliminary experiments, phage displaying a large repertoire (3.7 × 1010 of human recombinant Fab fragments) (32) were incubated with the biotinylated complexes and subsequently incubated with streptavidin-coated beads. To avoid the selection of antistreptavidin antibodies, the phage population was preincubated on streptavidin-coated immunotubes before selection and the bound phages were eluted with DTT. Breaking the disulfide bond between the biotin and the HLA-A1–MAGE-A1 complexes prevented the retrieval of phage bound to streptavidin. A 117-fold enrichment was obtained after four rounds of this selection procedure, and 92 clones out of 94 were binding to the HLA-A1–MAGE-A1 complexes. Without these precautions, only streptavidin-binding phage antibodies were isolated (data not shown).
The diversity of the selected antibodies was assessed by means of DNA fingerprinting, identifying 14 different patterns. The fine specificity of clones representative of each pattern was analyzed by ELISA on wells coated with HLA-A1 complexes containing either the MAGE-A1 (EADPTGHSY) or the MAGE-A3 (EVDPIGHLY) peptides, which differ by only three residues (Fig. 3). For most antibodies (11/14), no difference was observed for the binding assay to either of the two peptides. Two antibodies, such as clone D2, appeared to bind slightly better to the complexes containing the MAGE-A1 peptide. One recombinant antibody, G8, bound to HLA-A1–MAGE-A1 but not at all to HLA-A1–MAGE-A3 complexes.
Figure 3.
Examples of specificity of recombinant antibodies selected for binding to HLA-A1–MAGE-A1 complexes. Microwells were coated with biotinylated BSA, washed, incubated with streptavidin, washed, and incubated with biotinylated HLA-A1–MAGE-A1 (black bars) or HLA-A1–MAGE-A3 (white bars) complexes. Suspensions of phages displaying the recombinant Fab fragments were then added. After washing, bound phages were detected with a mAb recognizing the p8 protein. Positive and negative controls were mAbs TÜ155 and W6/32HK (HK), respectively.
Characterization of Recombinant Fab Fragment G8.
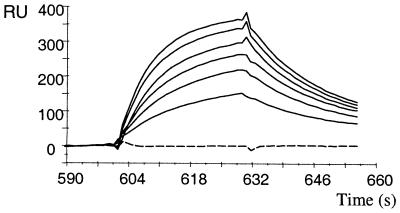
The soluble Fab fragment G8 was purified by metal affinity chromatography from the periplasm of E. coli by using the hexahistidine tag fused to its CH1 domain (32). Approximately 1 mg of pure material could be obtained from 1 liter of culture medium. The specificity of Fab-G8 was analyzed by surface plasmon resonance in a BIAcore instrument. In a classical-binding experiment, with the antigen immobilized on the chip, we could not find conditions that eluted the antibody without also dissociating the β2m from the HLA-A1 heavy chain. We resorted to immobilizing antibody Fab-G8 through its hexahistidine tag, and running the complexes in the soluble phase. These experiments confirmed that Fab-G8 bound to HLA-A1–MAGE-A1 but not to HLA-A1–MAGE-A3 complexes (Fig. 4). Binding to HLA-A1–MAGE-A1 occurred with on- and off-rates of 1.8 × 105 M−1⋅s−1 and 0.045 s−1, respectively, resulting in a KD (koff/kon) of 250 nM.
Figure 4.
BIAcore analysis of Fab fragment G8. Purified Fab-G8 was immobilized on an NTA-chip after NiCl2 activation and the HLA-A1–peptide complexes were run in solution. Full lines: HLA-A1–MAGE-A1 complexes (top to bottom: 625, 542, 458, 375, 292, and 208 nM). Broken line: HLA-A1–MAGE-A3 complexes (625 nM).
Binding of fd-Fab-G8 to Cells Carrying the HLA-A1–MAGE-1 Antigen.
Preliminary experiments indicated that a binding of the purified Fab-G8 could not be visualized on HLA-A1 cells incubated with the MAGE-A1 peptide. This was not surprising, considering the low affinity of the recombinant antibody and the low antigen density on the cell surface. To increase the avidity of the interaction, we recloned the Fab-G8 gene for display on fd phage. This expression system does not need a helper phage and the resulting avidity is therefore higher than with the phagemid system in which the antibody–gp3 fusion product competes with wild-type gp3 during assembly of the capsid.
EBV-transformed B cells that did or did not express the HLA-A1 gene were incubated first with the MAGE-A1 or MAGE-A3 peptides. These cell lines are TAP+. Consequently, only a minor fraction of HLA molecules can be loaded by this method, whereas the vast majority still displays endogenous peptides. The cells were washed, and incubated further with a suspension of fd-Fab-G8 phage. Bound phages were detected by sequential incubations with polyclonal goat anti-fd antibodies and rabbit anti-goat Ig antibodies coupled to fluorescein (Fig. 5). For the two HLA-A1 EBV-B cell lines that were tested, a shift in fluorescence intensity was observed when the cells were incubated with the MAGE-A1 but not with the MAGE-A3 peptide or when the cells were used without loading (data not shown). No shift was observed with a fd phage displaying an anti-ras Fab antibody. Furthermore, no shift in fluorescence intensity was observed when fd-Fab-G8 was incubated with an HLA-A1− EBV-B cell line, loaded with MAGE-A1 or MAGE-A3. These results demonstrate that Fab-G8 specifically recognizes HLA-A1–MAGE-A1 antigenic complexes in situ.
Figure 5.
Labeling EBV-transformed B cells with phage-antibody fd-Fab-G8. Cells were incubated with 100 μM peptide MAGE-1-A1 (thick lines) or MAGE-3-A1 (thin lines), and labeled with fd-Fab-G8 or a control fd phage. Bound phages were detected by sequential incubations with polyclonal goat anti-fd antibodies and rabbit anti-goat Ig antibodies coupled to fluorescein.
Binding of fd-Fab-G8 to (Unloaded) Melanoma Cell Lines.
To confirm that Fab-G8 was capable of binding endogenously generated peptide–MHC complexes that might be present at a much lower density on the cell surface, we repeated the flow cytometry experiments with HLA-A1+ and MAGE-A1− or MAGE-A1+ melanoma cell lines. fd-Fab-G8 gave the same fluorescence intensity compared with the control on HLA-A1+, MAGE-A1− cells but a higher intensity was obtained for fd-Fab-G8 compared with the control when a MAGE-A1+ cell line was used (Fig. 6). The fluorescence shift was moderate compared with in vitro-loaded B cells, but this has to be expected because melanoma cells are expressing a lower density of HLA molecules and natural antigen processing is thought to produce a low density of HLA-A1–MAGE-A1 complexes on the cell surface. These results show that fd-Fab-G8 is capable of binding to cells which express the MHC–peptide complex at a density most likely to be found on MAGE-A1-expressing tumor cells.
Figure 6.
Labeling melanoma cell lines with phage-antibody fd-Fab-G8. Melanoma cells MZ2-MEL 3.0 (HLA-A1+, MAGE-A1+) or MZ2-MEL 2.2 (HLA-A1+, MAGE-A1−) were incubated with phage antibodies fd-Fab-G8 (thick lines) or control phage antibodies (thin lines). Bound phages were detected by sequential incubations with polyclonal goat anti-fd antibodies and rabbit anti-goat Ig antibodies coupled to fluorescein.
Discussion
In this work, we have chosen to employ a large nonimmunized repertoire of human Fab fragments (32) to directly select human reagents with fine specificity for HLA class I–MAGE-A1, a well-characterized tumor antigen already used in clinical trials (38, 39). Until now, the few approaches that have been successful in isolating such T cell antigen receptor-like antibodies have used sophisticated immunization protocols, involving the injection of recombinant complexes or TAP-deficient in vitro loaded APC in syngeneic or even MHC-transgenic mice (40). The obvious advantage of this technique is a strong enrichment for peptide–MHC binders, and the use of transgenic mice may be helpful in reducing the frequency of pan-MHC reactive antibodies. However, these approaches are very time-consuming and the murine origin of the selected antibodies is a major drawback to possible future therapeutic applications. Indeed, antibodies specific for this tumor-associated antigen may eventually be used as a targeting reagent to deliver toxins or cytokines (24) specifically to the tumor site. In search of a more generic method to isolate human antibodies to MHC–peptide complexes, we have explored the use of very large nonimmunized phage–antibody libraries.
We used the phage display technique to select human antibody fragments from a large nonimmune library. One of the crucial factors determining the success of this approach relates to the state of the antigen used for selection. The conformation of the antigen has to be as “natural” as possible. We tested several methods to produce a recombinant version of the complex needed for the selection, including secretion of a single-chain peptide–HLA molecule in E. coli periplasm and expression in Drosophila cells (data not shown), but only in vitro refolding from inclusion bodies produced in E. coli yielded enough correctly folded protein. Numerous HLA complexes (class I and II) have been refolded in vitro, including HLA-A2, -B27, -B35, -B53 -G, -E, DR2, and DRB, demonstrating the versatility of this method for HLA molecules (41–45). However, this is the first report describing the production of a recombinant HLA-A1. This recombinant complex was biotinylated to minimize conformational changes of the antigen that may occur as a consequence of passive adsorption onto plastic (46, 47).
Using direct selection in solution with this antigen, we could isolate 14 different antibodies binding to the complex, with one clone (G8) showing the capability to bind in a peptide-specific manner. This antibody fragment binds in ELISA to HLA-A1–MAGE-A1 but not to HLA-A1–MAGE-A3. These two peptides display only three aa differences. This extreme specificity could be confirmed by BIAcore experiments. More importantly, phages displaying G8 did not bind HLA-A1+ cells unless they were in vitro loaded with MAGE-A1 peptide, demonstrating the absence of binding for HLA-A1 complexes loaded with endogenous peptides. MAGE-A1 and MAGE-A3 share the same main anchor residues for HLA-A1 (Asp in position 3 or P3, and Tyr in P9) and secondary anchor residue Pro in P4. Moreover, P1, P6, and P7 are also identical. The residue in P2 (Ala in MAGE-A1; Val in MAGE-A3) is probably not important for binding because the side chain is thought to be buried in the groove (48). Interestingly, the remaining residues in P5 and P8 have their side chain pointing out of the groove in a molecular model of HLA-A1–MAGE-A3 (48). MAGE-A1 has in P5 and P8 two residues (Thr and Ser) displaying hydroxyl groups that can be involved in hydrogen bonds, conversely to the residues displayed by MAGE-A3 (Ile and Leu). Therefore, the residues in P5 and P8 have a high probability of being involved in the differential binding to CTL82/30 TCR as well as the Fab antibody G8.
As shown by surface plasmon resonance studies, Fab-G8 has an affinity of 250 nM for the complex HLA-A1–MAGE-A1. This rather low affinity was not expected because the Fab fragment was selected from a very large repertoire of 3.7 × 1010 independent clones. A large number of binders directed to diverse antigens have already been selected from this repertoire, most of them having an affinity in the 5–30 nM range (32). The fact that the Fab-G8 antibody survived four rounds of selection in competition with all of the pan-reactive antibodies is surprising, but might be explained by a high expression level, inducing a high display level of the Fab-p3 fusion protein on phage. No other peptide-specific binders could be selected from the library, and a depletion step with HLA-A1–MAGE-A3 complexes did not favor the selection of MAGE-A1-specific binders. Hence, such peptide-specific binders seem to be rare in the library despite its size. It has to be kept in mind that the targeted epitope is a peptide deeply buried inside the MHC molecule. Only between 100–300 Å of peptide bound to a MHC class I molecule is actually available for direct recognition (49). Antibodies binding to proteins contact usually 800 Å of their ligand (50). Consequently, peptide specificity can be obtained only if the major interactions between the antibody and the complex are made with the peptide. The present affinity may be sufficient for staining purposes, but is most likely too weak for in vivo targeting purposes. The next step is thus to mature the affinity of this antibody without losing its fine specificity. This goal is difficult to reach, because the HLA-A1 chain is thought to contribute to 65–85% of the (peptide–MHC)–antibody interface. To overcome this problem, we are now performing an affinity maturation of G8 by directed randomization of complementarity determining region H3, and reselection (51). Although it is not known which residues of the antibody are involved in the peptide recognition, it is predominantly the H3 loop that dominates in the antigen interaction. Indeed, a gain of up to 18-fold in affinity without loss of peptide specificity has already been achieved (Chames et al., unpublished work). Moreover, these studies may also define which residues are directly interacting with the peptide and allow for more targeted affinity maturation. Furthermore, with such antigen–antibody interaction profiles, it may be possible to build antibody libraries with a propensity to bind HLA–peptide complexes in a peptide-specific manner.
We succeeded in selecting a human antibody binding specifically to the complex HLA-A1–MAGE-A1. This antibody may now be used to detect the presence of this specific T cell epitope by flow cytometry and possibly immunohistochemistry or immunoprecipitation and should be very useful for analysis of MAGE-1-based immunotherapies. Indeed G8, as Fab or phage-Fab, may be used to check the expression of this T cell epitope on tumor cells, before and during vaccination with MAGE-A1 peptide, or APCs loaded with MAGE-A1 (39). The display efficiency of this complex at the APC surface could also be monitored after transfection with MAGE-1 gene or after in vitro peptide loading.
G8 is the first human antibody directed against a class I peptide–MHC complex. This presents many opportunities. This human antibody is directed against a well-characterized and very specific human tumor marker and in principle should be an interesting candidate as targeting moiety in an immunocytokine (52), immunotoxin (24), or in a bispecific antibody (53), in particular after antibody affinity maturation. However, the main limitation for all of these applications may be the density of the specific epitope on the cell surface. Indeed, only a small fraction of the 104-105 HLA-A1 complexes displayed per cell are expected to contain the MAGE-A1 peptide. Possibly a more sensitive and selective antitumor reactivity in vivo could be obtained by retargeting of T cell achieved by fusion of Fab-G8 with the CD3 ζ or γ chain (54, 55). Preliminary data suggest that a fusion protein between Fab-G8 and the CD3γ chains, once transfected into human PBL, is able to redirect T cells specifically toward MAGE-A1+ melanoma cells (unpublished work). As Fab-G8 already has an affinity 5- to 500-fold higher than found for TCRs, it will be very interesting to compare the behavior of these Fab-G8-displaying T cells with CTL 82/30 that harbors a natural TCR directed against the same epitope.
To conclude, this work demonstrates that very large human nonimmunized phage libraries can be used to rapidly select antibodies of exquisite TCR-like specificity and highlights the potential of such molecules for immunodiagnostic and immunotherapeutic applications.
Acknowledgments
We thank Mrs. G. Wille for expert technical assistance and A. Ziegler for critical reading of the manuscript, and Drs. R. Bolhuis and R. Willemsen for sharing unpublished data. This work has been funded by a European Union Biotech Grant Bio4-CT97–2196.
Abbreviations
- MAGE
melanoma-associated antigen
- CTL
cytotoxic T lymphocyte
- TAP
transporter associated with antigen processing
- TCR
T cell antigen receptor
- EBV
Epstein–Barr virus
- APC
antigen-presenting cell
References
- 1.Hemmila M R, Chang A E. J Surg Oncol. 1999;70:263–274. doi: 10.1002/(sici)1096-9098(199904)70:4<263::aid-jso14>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Gillespie A M, Coleman R E. Cancer Treat Rev. 1999;25:219–227. doi: 10.1053/ctrv.1999.0126. [DOI] [PubMed] [Google Scholar]
- 3.van der Bruggen P, Traversari C, Chomez P, Lurquin C, de Pleanm E, van den Eynde B, Knuth A, Boon T. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 4.Maeurer M J, Storkus W J, Kirkwood J M, Lotze M T. Melanoma Res. 1996;6:11–24. doi: 10.1097/00008390-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Chaux P, Luiten R, Demotte N, Vantomme V, Stroobant V, Traversari C, Russo V, Schultz E, Cornelis G R, Boon T, et al. J Immunol. 1999;163:2928–2936. [PubMed] [Google Scholar]
- 6.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier M H, Rankin E, Parmiani G, Arienti F, Humblet Y, et al. Int J Cancer. 1999;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Kariyama K, Higashi T, Kobayashi Y, Nouso K, Nakatsukasa H, Yamano T, Ishizaki M, Kaneyoshi T, Toshikuni N, Ohnishi T, et al. Br J Cancer. 1999;81:1080–1087. doi: 10.1038/sj.bjc.6690810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pamer E, Cresswell P. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 9.Seliger B, Hohne A, Knuth A, Bernhard H, Ehring B, Tampe R, Huber C. Clin Cancer Res. 1996;2:1427–1433. [PubMed] [Google Scholar]
- 10.Hicklin D J, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. J Clin Invest. 1998;101:2720–2729. doi: 10.1172/JCI498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wulfing C, Pluckthun A. J Mol Biol. 1994;242:655–669. doi: 10.1006/jmbi.1994.1615. [DOI] [PubMed] [Google Scholar]
- 12.Day P M, Yewdell J W, Porgador A, Germain R N, Bennink J R. Proc Natl Acad Sci USA. 1997;94:8064–8069. doi: 10.1073/pnas.94.15.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porgador A, Yewdell J W, Deng Y, Bennink J R, Germain R N. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhong G, Reis e Sousa C, Germain R N. Proc Natl Acad Sci USA. 1997;94:13856–13861. doi: 10.1073/pnas.94.25.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong G, Sousa C R, Germain R N. J Exp Med. 1997;186:673–682. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dadaglio G, Nelson C A, Deck M B, Petzold S J, Unanue E R. Immunity. 1997;6:727–738. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 17.Kleijmeer M J, Morkowski S, Griffith J M, Rudensky A Y, Geuze H J. J Cell Biol. 1997;139:639–649. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eastman S, Deftos M, DeRoos P C, Hsu D H, Teyton L, Braunstein N S, Hackett C J, Rudensky A. Eur J Immunol. 1996;26:385–393. doi: 10.1002/eji.1830260218. [DOI] [PubMed] [Google Scholar]
- 19.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman R M. J Exp Med. 1997;186:665–672. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy D B, Lo D, Rath S, Brinster R L, Flavell R A, Slanetz A, Janeway C A., Jr Nature (London) 1989;338:765–768. doi: 10.1038/338765a0. [DOI] [PubMed] [Google Scholar]
- 21.Murphy D B, Rath S, Pizzo E, Rudensky A Y, George A, Larson J K, Janeway C A., Jr J Immunol. 1992;148:3483–3491. [PubMed] [Google Scholar]
- 22.Aharoni R, Teitelbaum D, Arnon R, Puri J. Nature (London) 1991;351:147–150. doi: 10.1038/351147a0. [DOI] [PubMed] [Google Scholar]
- 23.Puri J, Arnon R, Gurevich E, Teitelbaum D. J Immunol. 1997;158:2471–2476. [PubMed] [Google Scholar]
- 24.Reiter Y, Di Carlo A, Fugger L, Engberg J, Pastan I. Proc Natl Acad Sci USA. 1997;94:4631–4636. doi: 10.1073/pnas.94.9.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamminen W L, Wraith D, Barber B H. Eur J Immunol. 1987;17:999–1006. doi: 10.1002/eji.1830170716. [DOI] [PubMed] [Google Scholar]
- 26.Rubin B, Malissen B, Jorgensen P N, Zeuthen J. Res Immunol. 1989;140:67–74. doi: 10.1016/0923-2494(89)90007-2. [DOI] [PubMed] [Google Scholar]
- 27.Andersen P S, Stryhn A, Hansen B E, Fugger L, Engberg J, Buus S. Proc Natl Acad Sci USA. 1996;93:1820–1824. doi: 10.1073/pnas.93.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garboczi D N, Hung D T, Wiley D C. Proc Natl Acad Sci USA. 1992;89:3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnstable C J, Bodmer W F, Brown G, Galfre G, Milstein C, Williams A F, Ziegler A. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 30.Seitz C, Uchanska-Ziegler B, Zank A, Ziegler A. Mol Immunol. 1998;35:819–827. doi: 10.1016/s0161-5890(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 31.Hutter H, Hammer A, Blaschitz A, Hartmann M, Ebbesen P, Dohr G, Ziegler A, Uchanska-Ziegler B. Cell Tissue Res. 1996;286:439–447. doi: 10.1007/s004410050713. [DOI] [PubMed] [Google Scholar]
- 32.de Haard H J, van Neer N, Reurs A, Hufton S E, Roovers R C, Henderikx P, de Bruïne A P, Arends J W, Hoogenboom H R. J Biol Chem. 1999;274:18218–18230. doi: 10.1074/jbc.274.26.18218. [DOI] [PubMed] [Google Scholar]
- 33.Marks J D, Hoogenboom H R, Bonnert T P, McCafferty J, Griffiths A D, Winter G. J Mol Biol. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 34.Clackson T, Hoogenboom H R, Griffiths A D, Winter G. Nature (London) 1991;352:624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- 35.Henderikx P, Kandilogiannaki M, Petrarca C, von Mensdorff-Pouilly S, Hilgers J H, Krambovitis E, Arends J W, Hoogenboom H R. Cancer Res. 1998;58:4324–4332. [PubMed] [Google Scholar]
- 36.Nieba L, Nieba-Axmann S E, Persson A, Hamalainen M, Edebratt F, Hansson A, Lidholm J, Magnusson K, Karlsson A F, Pluckthun A. Anal Biochem. 1997;252:217–228. doi: 10.1006/abio.1997.2326. [DOI] [PubMed] [Google Scholar]
- 37.Traversari C, van der Bruggen P, Luescher I F, Lurquin C, Chomez P, Van Pel A, De Plaen E, Amar-Costesec A, Boon T. J Exp Med. 1992;176:1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherji B, Chakraborty N G, Yamasaki S, Okino T, Yamase H, Sporn J R, Kurtzman S K, Ergin M T, Ozols J, Meehan J, et al. Proc Natl Acad Sci USA. 1995;92:8078–8082. doi: 10.1073/pnas.92.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu X, Chakraborty N G, Sporn J R, Kurtzman S H, Ergin M T, Mukherji B. Cancer Res. 1996;56:2479–2483. [PubMed] [Google Scholar]
- 40.Engberg J, Krogsgaard M, Fugger L. Immunotechnology. 1999;4:273–278. doi: 10.1016/s1380-2933(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 41.Allan D S, Colonna M, Lanier L L, Churakova T D, Abrams J S, Ellis S A, McMichael A J, Braud V M. J Exp Med. 1999;189:1149–1156. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Callaghan C A, Tormo J, Willcox B E, Blundell C D, Jakobsen B K, Stuart D I, McMichael A J, Bell J I, Jones E Y. Protein Sci. 1998;7:1264–1266. doi: 10.1002/pro.5560070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arimilli S, Cardoso C, Mukku P, Baichwal V, Nag B. J Biol Chem. 1995;270:971–977. doi: 10.1074/jbc.270.2.971. [DOI] [PubMed] [Google Scholar]
- 44.Stockel J, Meinl E, Hahnel C, Malotka J, Seitz R, Drexler K, Wekerle H, Dornmair K. J Biol Chem. 1994;269:29571–29578. [PubMed] [Google Scholar]
- 45.Parker K C, Carreno B M, Sestak L, Utz U, Biddison W E, Coligan J E. J Biol Chem. 1992;267:5451–5459. [PubMed] [Google Scholar]
- 46.Butler J E, Ni L, Nessler R, Joshi K S, Suter M, Rosenberg B, Chang J, Brown W R, Cantarero L A. J Immunol Methods. 1992;150:77–90. doi: 10.1016/0022-1759(92)90066-3. [DOI] [PubMed] [Google Scholar]
- 47.Davies J, Dawkes A C, Haymes A G, Roberts C J, Sunderland R F, Wilkins M J, Davies M C, Tendler S J, Jackson D E, Edwards J C. J Immunol Methods. 1994;167:263–269. doi: 10.1016/0022-1759(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 48.Luescher I F, Romero P, Kuznetsov D, Rimoldi D, Coulie P, Cerottini J C, Jongeneel C V. J Biol Chem. 1996;271:12463–12471. doi: 10.1074/jbc.271.21.12463. [DOI] [PubMed] [Google Scholar]
- 49.Fremont D H, Matsumura M, Stura E A, Peterson P A, Wilson I A. Science. 1992;257:919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- 50.Davies D R, Padlan E A, Sheriff S. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- 51.Hoogenboom H R. Trends Biotechnol. 1997;15:62–70. doi: 10.1016/S0167-7799(97)84205-9. [DOI] [PubMed] [Google Scholar]
- 52.Reisfeld R A, Becker J C, Gillies S D. Melanoma Res. 1997;7, Suppl. 2:S99–S106. [PubMed] [Google Scholar]
- 53.Beun G D, van de Velde C J, Fleuren G J. Immunol Today. 1994;15:11–15. doi: 10.1016/0167-5699(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 54.Altenschmidt U, Kahl R, Moritz D, Schnierle B S, Gerstmayer B, Wels W, Groner B. Clin Cancer Res. 1996;2:1001–1008. [PubMed] [Google Scholar]
- 55.Weijtens M E, Willemsen R A, Valerio D, Stam K, Bolhuis R L. J Immunol. 1996;157:836–843. [PubMed] [Google Scholar]