Abstract
We have examined the expression of the HEMA1 gene, which encodes the key chlorophyll synthesis enzyme glutamyl-tRNA reductase, during the phytochrome A-mediated far-red light (FR) block of greening response in Arabidopsis. Our results demonstrate that the FR block of greening comprises two separate responses: a white light (WL) intensity-independent response that requires 3 d of FR and is associated with a loss of expression of the nuclear genes HEMA1 and Lhcb following the transfer to WL (transcriptionally coupled response) and a WL intensity-dependent response that is induced by 1 d of FR and is transcriptionally uncoupled. Both responses required phytochrome A. The transcriptionally uncoupled response correlated with a deregulation of tetrapyrrole synthesis and potential photooxidative damage and was inhibited by cytokinin. The transcriptionally coupled FR response was additive with the loss of expression following Norflurazon-induced photobleaching and was absent in the presence of sucrose or after lower fluence rate (1 μmol m−2 s−1) FR treatments. Both pathways leading to the loss of nuclear gene expression were inhibited by overexpression of NADPH:protochlorophyllide oxidoreductase, indicating a role for plastid signaling in the FR-mediated pathway. The significance of identifying a distinct phytochrome A-mediated plastid signaling pathway is discussed.
The coordinated synthesis of chlorophylls and chlorophyll-binding proteins is critically important during emergence of the etiolated seedling into light, and is essential for the normal development of functional chloroplasts. The key regulatory step in chlorophyll synthesis is the formation of 5-aminolevulinic acid (ALA), which is rate limiting for the tetrapyrrole pathway (Beale and Castelfranco, 1974). As a consequence, inhibition of ALA synthesis through inhibitors or anti-sense approaches (Höfgen et al., 1994; Kumar and Söll, 2000) has a dramatic effect on chloroplast development, resulting in plants that are highly susceptible to photobleaching. In a converse manner, excess ALA also affects chloroplast development (Kittsteiner et al., 1991) and leads to an overaccumulation of porphyrins, which can lead to severe photooxidative damage (Reinbothe et al., 1996).
Uncoupling tetrapyrrole synthesis from chloroplast development may also be achieved by irradiating etiolated seedlings with far-red (FR) light (Barnes et al., 1996; Runge et al., 1996). Growth of Arabidopsis seedlings under continuous FR (FRc) can induce partial photomorphogenesis with reduced hypocotyl elongation growth and cotyledon expansion. These responses are characteristic of the FR-high-irradiance response (FR-HIR; Mancinelli, 1994), which is a specific function of phytochrome A (Smith, 1995). FRc irradiation can also activate many of the processes required for chloroplast development. These include the induction of nuclear genes encoding chlorophyll a/b-binding proteins and other photosynthetic proteins (Kuno et al., 2000; Tepperman et al., 2001) and the transcription of chloroplast genes and replication of plastid DNA (DuBell and Mullet, 1995a, 1995b; Chun et al., 2001). However, because photoconversion of protochlorophyllide (Pchlide) to chlorophyllide (Chlide) by the enzyme NADPH:Pchlide oxidoreductase (POR) is inefficient under FR wavelengths, chlorophyll synthesis is severely limited under these conditions. Thus, phytochrome A-mediated induction of selected facets of plastid development may occur in the absence of conditions that allow the synthesis of corresponding levels of chlorophyll.
It has previously been shown that seedlings of Arabidopsis and tomato (Lycopersicon esculentum) grown under prolonged FR not only fail to accumulate chlorophyll, but also are unable to green when subsequently transferred to white light (WL; Barnes et al., 1995; van Tuinen et al., 1995). This phenomenon, known as the FR block of greening response, has been characterized at the molecular level as a depletion of PORA (and partially of PORB) and the concomitant loss of the ordered membrane system of the prolamellar body (Barnes et al., 1996; Runge et al., 1996). Each of these effects displays characteristics of an FR-HIR and is dependent on phytochrome A activity (Barnes et al., 1996). Further evidence that the loss of POR is crucial to the FR block of greening comes from a transgenic approach in which overexpression of PORA was able to maintain WL-mediated greening in FR-pretreated seedlings (Runge et al., 1996; Sperling et al., 1997). It is likely that PORA has two important roles in maintaining chlorophyll synthesis in WL. Not only is it required for light-dependent chlorophyll synthesis, but it has a role in buffering against photooxidative damage that occurs during the illumination of etiolated, Pchlide-containing tissues (Runge et al., 1996). This second role has been largely supported by subsequent studies with PORA- and PORB-overexpressing lines (Sperling et al., 1997, 1999).
However, it is interesting that POR overexpression could not fully counteract the FR block, and FR-pretreated transgenic seedlings still contained approximately two-thirds of the chlorophyll levels of dark-treated controls (Sperling et al., 1997). This suggests that although the level of POR is a critical determinant of this response, other FR-mediated processes may also be important. Prolonged FR irradiation has been shown to cause the formation of aberrant plastids in developing cotyledons, and the irreversible nature of the FR block of greening response has been attributed to the ensuing degradation of plastid components suggested by the formation of vesicles in the stroma (Barnes et al., 1996). One possibility is that this FR-mediated effect on plastid integrity leads to the loss of a plastid signal required for normal expression of photosynthetically related genes. Such a signal has been previously identified through the action of photobleaching herbicides (Taylor, 1989; Susek and Chory, 1992) and is required for the expression of numerous photosynthetically related nuclear genes (Kusnetsov et al., 1996), including Lhcb in Arabidopsis (Susek et al., 1993). This signal is also closely involved with phytochrome signaling pathways (López-Juez et al., 1998) and can be thought of as gating phytochrome responses (McCormac et al., 2001) in a similar way to that proposed for circadian control of light-induced gene expression (Millar and Kay, 1996).
We have examined the hypothesis that plastid signaling is involved in the FR block of greening response by analyzing the expression profile of HEMA1. This gene, which encodes glutamyl-tRNA reductase, the first committed enzyme of tetrapyrrole synthesis, is strongly expressed in photosynthetic tissues and is regulated by light, including an FR-HIR, and a plastid signal (Ilag et al., 1994; Kumar et al., 1999; McCormac et al., 2001). However, the light-regulated expression pattern of HEMA1 is not identical to other light-regulated genes, as HEMA1 does not exhibit a very low-fluence response (McCormac et al., 2001) and utilizes light signaling pathways that partly diverge from those for light regulation of Lhcb (A.C. McCormac and M.J. Terry, unpublished data). Given the importance of tetrapyrroles in the FR block of greening response and the increasingly significant relationship between tetrapyrroles and plastid signaling (Kropat et al., 2000; Vinti et al., 2000; La Rocca et al., 2001; Mochizuki et al., 2001; Møller et al., 2001), the expression of HEMA1 under these conditions is likely to be highly informative for our understanding of plastid development.
RESULTS
The FR Block of Greening Response Is Associated with an Inhibition of Promoter Responsiveness to WL
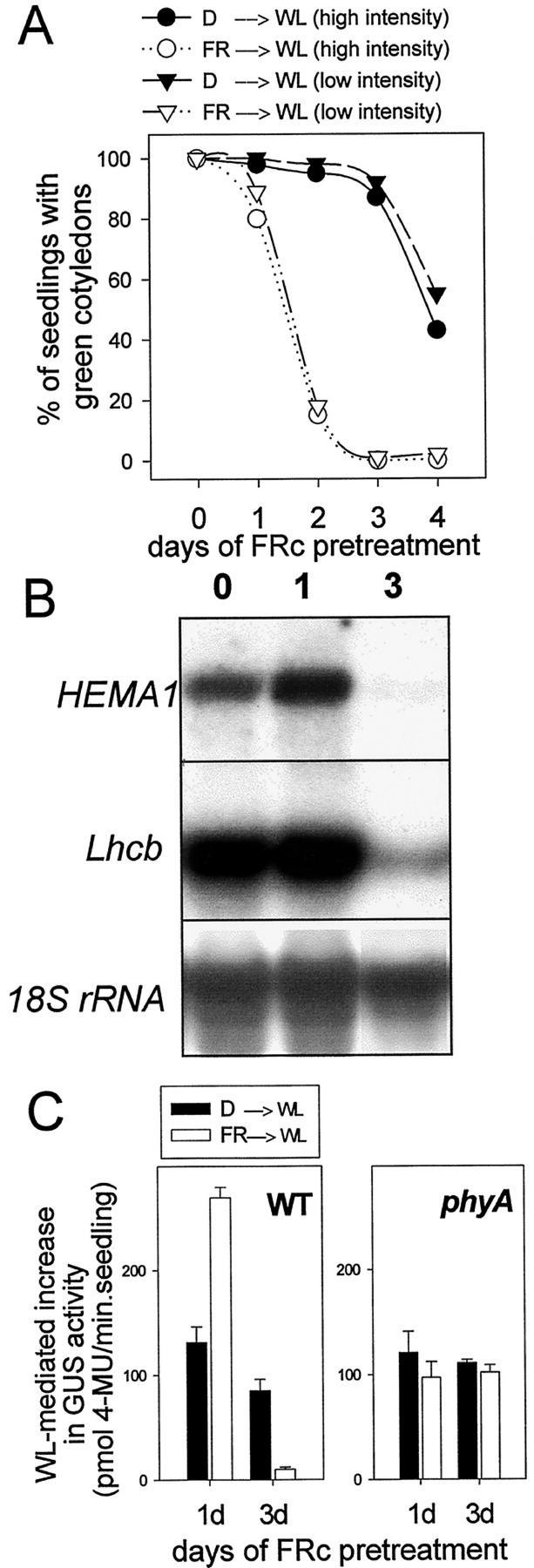
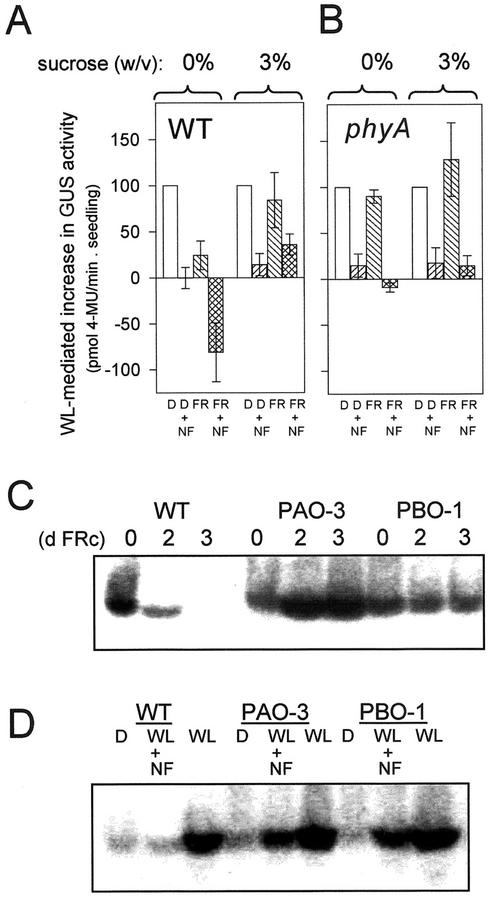
Figure 1A shows that, as seen previously (Barnes et al., 1996; Runge et al., 1996), 3 d of FRc (10 μmol m−2 s−1) irradiation of etiolated seedlings fully inhibited their ability to green under subsequent WL, whereas dark-grown seedlings at the same developmental age could green normally. This effect was independent of WL intensity (Fig. 1A; Barnes et al., 1996) and was also found to be associated with a loss of HEMA1 and Lhcb expression (3 d FRc; Fig. 1B). Seedlings that had received a pretreatment of just 1 d of FRc suffered only a small, but consistently observed, loss of greening capacity that was enhanced by high-intensity WL (Fig. 1A). These seedlings were still able to accumulate high levels of HEMA1 and Lhcb mRNA (1 d FRc; Fig. 1B). Under these conditions, FRc stimulated subsequent WL induction of HEMA1 expression by up to 2-fold compared with seedlings transferred directly from darkness to WL (Fig. 1B). This stimulation was most evident under low-intensity (8 μmol m−2 s−1) WL, but was absent under high-intensity (250 μmol m−2 s−1) WL (data not shown). These results were confirmed using transgenic Arabidopsis seedlings expressing a HEMA1 promoter:gusA construct (Fig. 1C). Furthermore, the 1-d FRc-mediated stimulation of WL-induced HEMA1 expression and the 3-d FRc-mediated inhibition of HEMA1 expression following transfer to WL were absent in a phyA genetic background, indicating that these are both phytochrome A-mediated responses (Fig. 1C). Therefore, the FR block of greening response is characterized not only as a progressive loss of greening ability, but also by a marked change in the transcriptional response to WL.
Figure 1.
FRc preirradiation leads to a reduction in greening and nuclear gene expression following transfer to WL. A, Greening of cotyledons under different WL fluence rates after growth in the dark or FRc. Seedlings were germinated without Suc in the dark for 2 d. They were subsequently grown for a further period (as indicated) under FRc (white symbols) or were maintained in the dark (black symbols) for the equivalent period. Seedlings were then transferred to a WL source of 250 μmol m−2 s−1 (circles) or 8 μmol m−2 s−1 (triangles). B, HEMA1 and Lhcb mRNA accumulation under WL (130 μmol m−2 s−1) following a 1- or 3-d FRc pretreatment. Seedlings were grown without Suc. The RNA gel blot was sequentially hybridized with probes for HEMA1, Lhcb, and 18S transcripts. C, Effect of an FR pretreatment on the subsequent response to WL of a HEMA1 promoter:β-glucuronidase A (gusA) transgene. Transgenic WT or phyA Arabidopsis seedlings were germinated for 1 d in the dark followed by a 1- or 3-d FRc treatment (or equivalent darkness). Seedlings were then transferred to WL (8 μmol m−2 s−1) for 3 d prior to measurement of GUS activity. Data shown are the mean ± se (n = 3).
The FR Block of Greening Response Develops without Inhibition of HEMA1 Transcription in the Presence of Suc
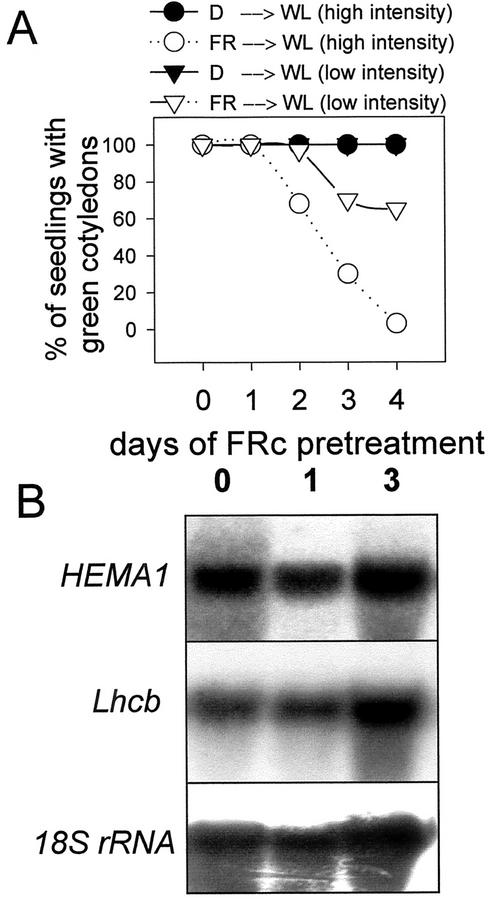
A full FR block of greening response was avoided if seedlings were grown on a medium supplemented with 3% (w/v) Suc (Fig. 2A; Barnes et al., 1996). However, even in the presence of Suc, FR pretreatments still elicited a partial impairment of the greening response as compared with that in dark-grown seedlings (Fig. 2A). This partial block of greening was not seen following FR irradiation of phyA lines (data not shown). In contrast to the FR block of greening response in the absence of Suc (Fig. 1A), the partial FR block in wild-type (WT) lines was shown to be dependent on WL intensity (Fig. 2A). In addition, for seedlings grown on 3% (w/v) Suc, an FR pretreatment produced no discernible inhibition of HEMA1 or Lhcb transcript accumulation following transfer to 130 μmol m−2 s−1 WL (Fig. 2B), even with a 75% inhibition of greening capacity. The accumulation of GUS activity driven by the HEMA1 promoter also showed a normal response to WL following a 3-d FRc preirradiation and was even elevated compared with the response of dark-grown seedlings in low-intensity WL (data not shown). Thus, under these conditions, the effect of an FR preirradiation was to separate HEMA1 expression from chlorophyll accumulation.
Figure 2.
Suc inhibits the loss of nuclear gene expression in FRc-grown seedlings transferred to WL. A, Greening of cotyledons under different WL fluence rates after growth in darkness or FRc. Seedlings were germinated on 3% (w/v) Suc in the dark for 2 d. They were subsequently grown for a further period (as indicated) under FRc (white symbols) or were maintained in darkness (black symbols) for the equivalent period. Seedlings were then transferred to a WL source of 250 μmol m−2 s−1 (circles) or 8 μmol m−2 s−1 (triangles). B, HEMA1 and Lhcb mRNA accumulation under WL (130 μmol m−2 s−1) following a 1- or 3-d FRc pretreatment. Seedlings were grown on 3% (w/v) Suc. The RNA gel blot was sequentially hybridized with probes for HEMA1, Lhcb, and 18S transcripts.
The response characteristics on Suc are similar to those seen in the absence of Suc, but following a short period (1 d) of FR irradiation (Fig. 1, A and B) and they enable us to define two separate responses leading to an FR block of greening. The first response requires only 1 d of FRc and results in a WL intensity-dependent, incomplete loss of greening ability that is not associated with a loss of HEMA1 and Lhcb expression. We subsequently refer to this as the transcriptionally uncoupled response. The second response requires a longer period of FRc (3 d), is independent of WL intensity, and leads to a complete loss of greening ability (<10%) and the inhibition of HEMA1 and Lhcb expression. This WL intensity-independent response is specifically inhibited by Suc and is now referred to as the transcriptionally coupled response.
Different Transcriptional Responses under FRc Define the Two FR Block of Greening Responses under WL
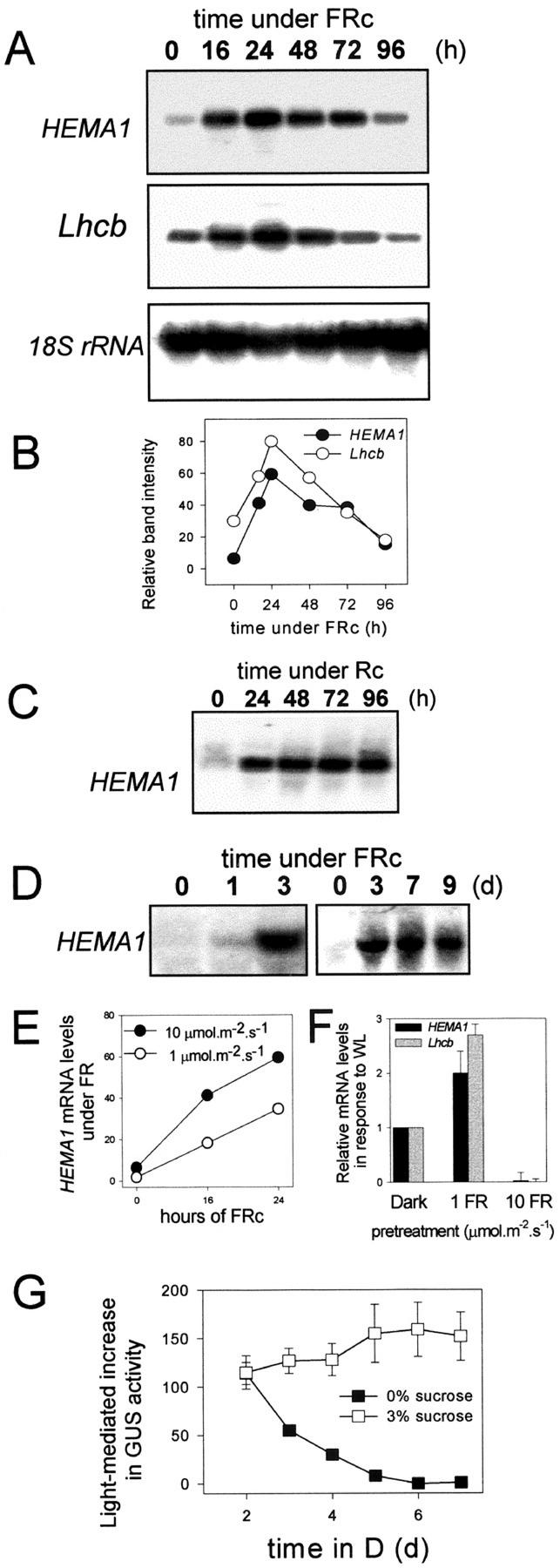
To further define the characteristics of the two response pathways leading to the FR block of greening, we examined the expression of HEMA1 during FRc. Figure 3A shows a time course of HEMA1 and Lhcb expression for a 4-d period of FR irradiation. A peak in HEMA1 and Lhcb mRNA levels occurred at around 1 d from the start of the FR treatment and declined thereafter, returning almost to dark levels by d 4 (Fig. 3, A and B). This type of short-lived expression profile is comparable with that reported previously for a wide range of plastid-associated nuclear genes under FRc (Tepperman et al., 2001), but is in contrast to the sustained transcriptional response seen under Rc (Fig. 3C). The post-peak phase of FR-mediated HEMA1 expression (i.e. ≥72 h FRc; Fig. 3A) temporally coincides with the inability to reinitiate transcription when exposed to WL (3 d FRc; Fig. 1B). Because Suc can prevent the loss of HEMA1 expression in WL after a prolonged FRc treatment (Fig. 2B), we tested whether it could also block the loss of FR-induced HEMA1 expression. Figure 3D shows that the loss of HEMA1 expression under FRc was prevented by the presence of 3% (w/v) Suc with maximal expression levels (detected after 3 d of FRc) remaining for ≥7d. However, the presence of 3% (w/v) Suc also substantially reduced the accumulation of HEMA1 (and Lhcb; data not shown) mRNA seen within the first 24 h of FRc (Fig. 3, compare A and D). This is consistent with previous reports of the effect of Suc on phytochrome A signaling (Dijkwel et al., 1997).
Figure 3.
The response of the HEMA1 promoter to light is subject to developmental aging, FR fluence rate, and Suc. A, Transcript levels of HEMA1 and Lhcb under prolonged (16–96 h) FR irradiation. Seedlings were grown without Suc and were germinated for 2 d in the dark before transfer (at time = 0) to FRc. B, Densitometry scans of band intensities as shown in (A). C, HEMA1 transcript levels under prolonged (24–96 h) R irradiation. Seedlings were grown without Suc and were germinated for 2 d in the dark before transfer (at time = 0) to Rc. D, HEMA1 mRNA levels in seedlings grown under prolonged (1–9 d) FR irradiation in the presence of 3% (w/v) Suc. Seedlings were germinated for 3 d in the dark prior to transfer (at t = 0) to FRc. E, HEMA1 mRNA levels in seedlings grown under 1 or 10 μmol m−2 s−1 FRc. F, HEMA1 mRNA levels under WL in seedlings grown previously for 3 d in the dark or under 1 or 10 μmol m−2 s−1 FRc. G, Effect of aging on the subsequent response of a HEMA1 promoter:gusA construct to WL. Seedlings were grown with or without 3% (w/v) Suc in the dark and were subsequently transferred to WL for 3 d prior to measurement of GUS activity. Data shown are the mean ± se (n = 5).
Phytochrome A-mediated FR responses are also typically affected by the rate of FR delivery (Mancinelli, 1994). Therefore, we examined this relationship for the transcriptional response to FR and the FR block of greening response by comparing the effect of irradiating seedlings (grown without Suc) with FRc at 1 μmol m−2 s−1 (Fig. 3E) instead of 10 μmol m−2 s−1 (Fig. 3, A and E). Under the lower fluence rate, the levels of HEMA1 mRNA showed the same pattern of transient up-regulation, but the rate of transcriptional increase and magnitude of the peak were reduced (Fig. 3E). A 3-d pretreatment under 1 μmol m−2 s−1 FRc failed to induce the FR-mediated block of the WL transcriptional response (Fig. 3F). Instead, the FR-irradiated seedlings displayed the characteristics of the transcriptionally uncoupled FR block of greening, showing strong nuclear transcription (Fig. 3F) and a WL intensity-dependent loss of greening (data not shown). In each case, phyA mutants demonstrated that there was an absolute requirement for phytochrome A for the response to FR (data not shown).
To test whether the decline in mRNA accumulation under FRc (Fig. 3B; 24–96 h) could be explained as a general loss of light responsiveness rather than the specific loss of phytochrome A-mediated signaling, we examined the effect of prolonging the period in darkness on the ability of seedlings to induce HEMA1 in response to WL. There was a progressive loss of HEMA1 light responsiveness to WL (Fig. 3G) and also FR (data not shown). This effect was also seen in phyA mutants, which demonstrates that this is a time-dependent, but phytochrome A-independent, response (data not shown). In addition, a time-dependent depletion of greening in dark-grown seedlings was seen in the absence of Suc (Fig. 1A). When 3% (w/v) Suc was included in the growth medium, the time-dependent loss of promoter responsiveness to WL (Fig. 3G) and FR (data not shown) was prevented, suggesting that starvation may be a key factor in this process. Thus, it appears that in darkness or FR light (in the absence of exogenous sugars), nuclear genes progress into a nonresponsive state. By contrast, under R (Fig. 4C) or WL, the transcriptional response escapes this process.
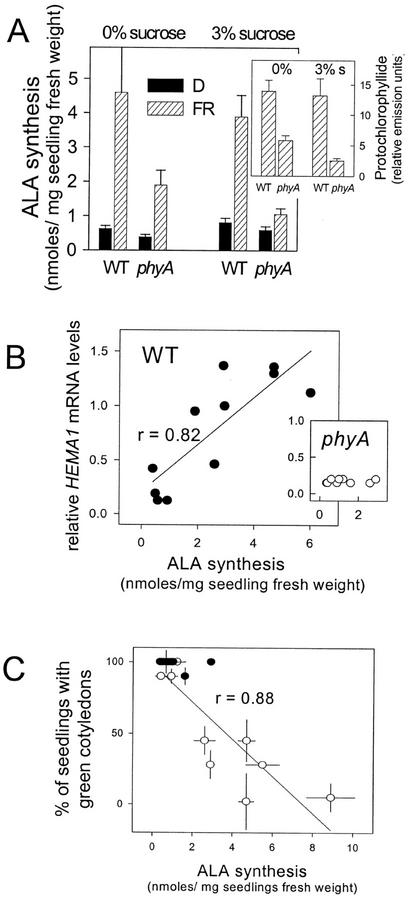
Figure 4.
Relationship between HEMA1 expression, ALA synthesis, and greening ability in FRc-grown seedlings. A, ALA synthesis rates in seedlings of WT and the phyA mutant grown in the dark (black columns) or under 1 d of FRc (hatched columns), with or without 3% (w/v) Suc (as indicated). The inset graph shows the corresponding levels of Pchlide accumulated in the FR-irradiated WT and phyA seedlings. Data shown are the mean ± se (n = 3). B, Relationship between relative HEMA1 mRNA levels (as measured by densitometry scans of RNA gel blots) and corresponding rates of ALA synthesis in WT and phyA (inset) seedlings. Seedlings were allowed to germinate in the presence of 3% (w/v) Suc for 2 d in the dark and were transferred to FRc or maintained in the dark for an additional period of 1 or 3 d. Each datum point shows the individual value under either irradiation condition for these two incubation times for the Landsberg erecta (Ler) and Colombia (Col) backgrounds. C, Relationship between greening capacity measured after transfer to WL and ALA synthesis rates prior to transfer in dark- and FRc-grown WT (○) and phyA (●) seedlings. Seedlings were grown as for B. Data shown are the mean ± se (n = 3).
These results demonstrate that a transcriptionally coupled block of greening response can proceed in the absence of light, although more slowly than under high-intensity FRc (see also Fig. 1A) and therefore appears to have no absolute requirement for phytochrome A. In contrast, the transcriptionally uncoupled effect on greening has a strict requirement for phytochrome A at the seedling stage (see also Fig. 2A). In addition, the two FR block of greening responses, as observed in WL, can be further distinguished by the different patterns of transcriptional response during the preceding period of FR irradiation. Thus, for FR irradiation to accelerate the development of the transcriptionally coupled block of greening response, a maximal FR-HIR must be achieved. By contrast, a submaximal FR-HIR can trigger the transcriptionally uncoupled block of greening.
The Transcriptionally Uncoupled Response Is Associated with an Increase in ALA Synthesis
The FR block of greening has previously been ascribed, at least in part, to the photoexcitation of excess Pchlide in the absence of adequate POR buffering (Runge et al., 1996; Sperling et al., 1997), and we hypothesized that the WL-dependent, transcriptionally uncoupled response was likely to be related to these processes. ALA synthesis is the rate-limiting step in Pchlide accumulation (Beale and Castelfranco, 1974), and GluTR activity and HEMA1 expression are believed to be key determinants of this activity (see McCormac et al., 2001). Because FRc resulted in an increase in HEMA1 expression (Fig. 3, A and B), we tested whether HEMA1 induction could also contribute to the transcriptionally uncoupled response by increasing ALA synthesis and, therefore, the pool of photosensitive Pchlide.
Figure 4 shows that irradiation with FRc significantly elevated ALA synthesis in WT seedlings (Fig. 4A) and this was correlated (r = 0.82) with HEMA1 mRNA levels under a range of different conditions (Fig. 4B). On 3% (w/v) Suc, the FRc induction of ALA synthesis was also seen, consistent with the observation previously (Fig. 2) that although Suc inhibits the transcriptionally coupled response, the transcriptionally uncoupled response is retained. In phyA seedlings, the FR-mediated increase in ALA synthesis was significantly reduced on 0% and 3% (w/v) Suc (Fig. 4A). The greater increase in ALA synthesis in WT seedlings grown under FRc also correlated with a higher level of Pchlide in WT versus phyA seedlings in FRc (Fig. 4A, inset). In contrast to the WT response, phyA seedlings showed no correlation between ALA synthesis rates and HEMA1 mRNA levels (Fig. 4B, inset), indicating that the FR-mediated increase in ALA synthesis in phyA was not attributable to transcriptional regulation of HEMA1. This strongly suggests a role for posttranscriptional effects on GluTR expression and/or activity in regulating ALA synthesis under these conditions.
We next investigated the relationship between ALA synthesis rates and subsequent greening capacity. Figure 4C shows this data for WT and phyA lines grown under conditions in which only the transcriptionally uncoupled response is occurring (i.e. 1–3 d of FRc with Suc or 1 d of FRc without Suc). There is a significant (r = 0.88) inverse relationship between ALA synthesis rates and the ability to green following transfer to WL. This suggests that increased ALA synthesis contributes to the transcriptionally uncoupled response.
Loss of Nuclear Gene Expression following FRc- and Norflurazon (NF)-Induced Photobleaching Is Additive
The loss of nuclear gene expression in the transcriptionally coupled FR block of greening response is reminiscent of the loss of HEMA1 expression following plastid photobleaching (McCormac et al., 2001). Therefore, we investigated the interaction between these two responses. Figure 5A shows that the carotenoid biosynthesis inhibitor NF inhibits HEMA1 expression under WL following growth in darkness on 0% or 3% (w/v) Suc. This response is the same in WT and phyA seedlings (Fig. 5, A and B). NF does not affect the HEMA1 response of etiolated seedlings while under FR (data not shown). However, when NF and a 3-d FR pretreatment were combined on 0% (w/v) Suc, the inhibitory effect on the subsequent WL responsiveness of the HEMA1 promoter exceeded that of either of the individual treatments (Fig. 5A). It should be noted that immediately prior to transfer to WL, the FR-irradiated seedlings in each case had higher levels of HEMA1 expression than dark controls and so had the potential to show a higher level of transcriptional inhibition under WL. However, direct observation of HEMA1 mRNA levels by northern blotting demonstrates that in the presence of NF, a substantial level of HEMA1 mRNA remains (data not shown). Therefore, the magnitude of the NF-mediated reduction of GUS activity was not limited following darkness, and only the combination of FR and NF produced the minimum promoter activity (Fig. 5A). The additive transcriptional effect of NF and an FR pretreatment was dependent on phytochrome A (Fig. 5B). In contrast, the transcriptionally uncoupled response, as seen in WT seedlings grown on 3% (w/v) Suc (Fig. 2), did not enhance the NF-mediated inhibition of the HEMA1 WL response (Fig. 5A). Therefore, these results show that the loss of nuclear gene expression during the FRc-mediated transcriptionally coupled response and following NF-induced photobleaching is additive. This may result from two inputs into the same plastid signaling pathway or through two independent pathways.
Figure 5.
POR overexpression rescues the loss of HEMA1 expression in WL following growth in FRc or NF. A, Effect of a combined FR preirradiation and NF treatment on the WL response of a HEMA1 promoter:gusA reporter gene. WT seedlings were grown with or without 3% (w/v) Suc for 3 d in FRc or darkness prior to transfer to WL (130 μmol m−2 s−1) for an additional 3 d. NF was included in the media at 5 μm and resulted in the cotyledons appearing completely white under all treatments. Values were normalized to the respective dark control levels (=100) within each experiment. Data shown are the mean ± se (n = 5). B, HEMA1 promoter:gusA expression in phyA seedlings under the same conditions as shown in A. C, The effect of POR overexpression on HEMA1 expression. Seedlings of PORA- or PORB-overexpressors (PAO-3 and PBO-1) were germinated in the dark without Suc and were irradiated for 0 to 3 d under FRc. HEMA1 mRNA accumulation was measured following transfer to WL (130 μmol m−2 s−1). D, The effect of 5 μm NF on the accumulation of HEMA1 mRNA in WL-irradiated seedlings of WT and transgenic lines overexpressing PORA (PAO-3) or PORB (PBO-1). Seedlings were grown in the absence of Suc for 3 d in the dark prior to transfer to WL for an additional 3 d.
POR Overexpression Leads to a Maintenance of Nuclear Gene Expression following FRc- and NF-Induced Photobleaching
It has previously been shown that overexpression of POR can partially rescue the FR block of greening response, and this has been attributed to an inhibition of WL intensity-dependent photooxidative damage (Runge et al., 1996; Sperling et al., 1997). We wanted to examine whether POR overexpression could also affect the transcriptionally coupled response and, therefore, we subjected transgenic Arabidopsis lines overexpressing PORA (PAO-3) or PORB (PBO-1; Sperling et al., 1997) to conditions resulting in the loss of HEMA1 in WT seedlings (i.e. 2–3 d of FRc on 0% [w/v] Suc). Upon transfer to WL, FR-treated PAO-3 and PBO-1 seedlings retained full expression of HEMA1 (Fig. 5C). Thus, POR overexpression inhibited the transcriptionally coupled FR block of greening response. The plastid localization of POR in the overexpressing lines (Sperling et al., 1997, 1999) supports the idea that loss of HEMA1 expression following FRc is the consequence of a signal emanating from the plastid.
We also tested the effect of POR overexpression on the loss of plastid signaling following NF treatment. The cotyledons of NF-treated seedlings of WT and both POR-overexpressing lines appeared totally white. However, as seen for the WL transcriptional response following FRc, the POR-overexpressing seedlings accumulated significant levels of HEMA1 mRNA (Fig. 5D), indicating that positive plastid signaling was still functional despite photobleaching of the cotyledons. Therefore, POR overexpression results in a phenotype that is apparently similar to that of the genomes uncoupled mutants in which nuclear gene expression is maintained in the absence of functional plastids (Susek et al., 1993). However, in this case, it is not yet known whether POR overexpression results in a reduced level of plastid damage or has a direct affect on plastid signaling.
The Transcriptionally Uncoupled But Not the Transcriptionally Coupled FR Block of Greening Response Is Inhibited by Cytokinin
We have described the FR block of greening as comprising two separate responses with respect to their different sensitivities to WL intensity and Suc and their effects on nuclear gene expression. Furthermore, there is a time-dependent transcription effect that is superimposed on these processes. Thus, the FR block of greening is a complex process encompassing a phytochrome A-dependent FR-HIR and phytochrome-independent effects. To try to isolate these components further, we investigated the effects of cytokinin treatment, as this hormone has previously been shown to influence greening capacity (e.g. Kusnetsov et al., 1998).
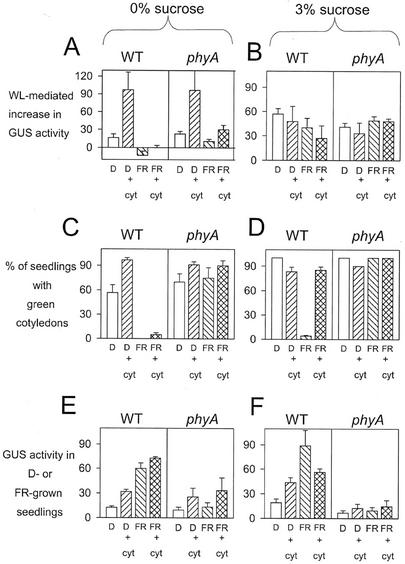
WT and phyA seedlings grown for 5 d in the dark in the absence of Suc showed a time-dependent loss of light responsiveness (see Fig. 3) and, therefore, showed a characteristically poor transcriptional response of the HEMA1 promoter to a WL treatment (Fig. 6A) and a partially reduced greening response (Fig. 6C). Application of the cytokinin 6-benzylaminopurine (BAP) inhibited both of these time-dependent processes (Fig. 6, A and C). In contrast, the inhibition of promoter responsiveness and cotyledon greening seen in WT seedlings subject to FRc (the transcriptionally coupled response) was not prevented by cytokinin (Fig. 6, A and C). Analysis of HEMA1 expression in darkness or FR light showed that cytokinin increased HEMA1 expression by an equal increment in both conditions (Fig. 6E) and, therefore, the FR response relative to darkness was unchanged.
Figure 6.
The effect of cytokinin on the FR block greening response and HEMA1 expression. A and B, The effect of cytokinin on WL-mediated induction of HEMA1 expression. WT and phyA seedlings were germinated in the dark for 2 d, transferred to FRc (or maintained in the dark) for 3 d, and then transferred to WL for an additional 3 d prior to measurement of GUS activity. Seedlings were grown in the absence (A) or presence (B) of 3% (w/v) Suc, and data shown are the mean ± se (n = 3). C and D, The effect of cytokinin on greening following transfer to WL. Seedlings were grown as shown in A and B and greening was measured in the absence (C) or presence (D) of 3% (w/v) Suc. Data shown are the mean ± se (n = 3). E and F, The effect of cytokinin on HEMA1 expression in the dark and FRc. WT and phyA seedlings were germinated in the dark for 2 d and were transferred to FRc (or maintained in the dark) for 3 d prior to measurement of GUS activity. Seedlings were grown in the absence (E) or presence (F) of 3% (w/v) Suc, and data shown are the mean ± se (n = 3).
When seedlings were grown on 3% (w/v) Suc, an FR pretreatment had little affect on the subsequent HEMA1 response to WL (Fig. 6B), as seen previously (Fig. 2), and cytokinin did not further influence this (Fig. 6B). For seedlings grown in darkness on 3% (w/v) Suc, the greening capacity was very slightly reduced by the presence of cytokinin (Fig. 6D). However, cytokinin could almost completely prevent the transcriptionally uncoupled block of greening response following FR irradiation of WT seedlings (Fig. 6D). Again, cytokinin stimulated HEMA1 expression in dark-grown seedlings (Fig. 6F), but in contrast to the effect on 0% (w/v) Suc (Fig. 6E), cytokinin inhibited the FR-mediated increase in HEMA1 expression on 3% (w/v) Suc (Fig. 6F).
These results demonstrate that exogenous cytokinin can rescue the transcriptionally uncoupled response as it develops in the presence of Suc, but that it does not block the transcriptionally coupled response. However, there is also an effect of cytokinin on the time-dependent, FR-independent inhibition of greening. Therefore, the response to cytokinin defines a developmental separation of the two FR block of greening responses and can also isolate the FR-mediated transcriptionally coupled response from FR-independent events.
DISCUSSION
Two Distinct Responses Leading to an FR Block of Greening
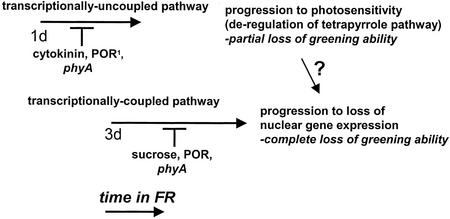
Here, we demonstrate that the FR block of greening comprises two distinct FR-dependent responses. As shown in Figure 7, the two responses can be distinguished by a number of different parameters. Following the onset of FRc irradiation, the first response detected (within 1 d of FRc) is a WL intensity-dependent incomplete loss of greening ability with a retention of WL induction of HEMA1 and Lhcb expression. We have shown that this response, which we have termed the transcriptionally uncoupled response, is prevented by the phyA mutation and by cytokinin treatment. In addition, it has previously been demonstrated that overexpression of POR can inhibit this response (Sperling et al., 1997). Following longer periods of FRc irradiation (3 d of FRc), a WL intensity-independent response is also observed (Fig. 7). This response is characterized by a complete loss of greening ability and the inhibition of HEMA1 and Lhcb expression following transfer to WL, and is inhibited by Suc and POR overexpression. We have termed this response the transcriptionally coupled response and it is also absent in the phyA mutant, consistent with the FR fluence rate dependence of this process. We have also identified a time-dependent effect on greening and the transcriptional light response that proceeds in the absence of FR.
Figure 7.
Model for the progression of the FR block of greening response. FR block of greening is comprised of two separate phytochrome A-dependent responses that can be separated based on their dependence on WL intensity and their effects on greening and nuclear gene expression. The transcriptionally uncoupled response is WL intensity dependent, results in a partial loss of greening following transfer to WL, and is inhibited by cytokinin, the phyA mutation, and POR overexpression. The transcriptionally coupled response is WL intensity independent, and results in a complete loss of greening ability and an inhibition of HEMA1 and Lhcb gene expression in WL. This response is inhibited by Suc, the phyA mutation, and POR overexpression and requires high fluence rate FRc. 1Sperling et al. (1997).
The Transcriptionally Uncoupled Response Results from a Perturbation of the PORA:Pchlide Ratio Leading to Increased Photosensitivity to WL
Previous studies on the FR block of greening response have demonstrated that there is an FR-HIR-mediated depletion of PORA in WT seedlings (Barnes et al., 1996; Runge et al., 1996) and that this leads to an overaccumulation of nonphotoactive Pchlide (Lebedev et al., 1995; Runge et al., 1996). This Pchlide species cannot be reduced to Chlide upon illumination and therefore acts as a potent sensitizer for photooxidative damage to plastids (Reinbothe et al., 1996). The combination of photooxidative damage and reduced availability of POR for chlorophyll synthesis leads to a loss of greening ability in WL. Consistent with this interpretation, overexpression of POR has been shown to protect against the FR block of greening by decreasing the amount of nonphotoactive Pchlide present in seedlings prior to the shift to WL (Sperling et al., 1997, 1999). Because the effects of photooxidative damage are dependent on WL intensity, we believe that the transcriptionally uncoupled response characterized in this study corresponds to an increase in photosensitivity through inhibition of POR synthesis as shown previously (Barnes et al., 1996; Runge et al., 1996; Sperling et al., 1997). One aspect of the block of greening response is that there is an FR-dependent increase in total Pchlide (Fig. 4; Sperling et al., 1997, 1999). This would be expected to compound any depletion in POR. Here, we show that the increase in Pchlide results from an FR-HIR-mediated up-regulation of HEMA1 expression and ALA synthesis (Fig. 4). The correlation between increased ALA synthesis and loss of greening ability under conditions resulting in the transcriptionally uncoupled FR block of greening response (Fig. 4) supports the hypothesis that the transcriptionally uncoupled response is a consequence of deregulation of the tetrapyrrole pathway (Fig. 7).
The transcriptionally uncoupled response was inhibited by the presence of cytokinin. The effect of cytokinin in inhibiting the greening response of FR-treated WT seedlings grown on 3% (w/v) Suc (Fig. 6D) corresponded to a decrease in the FR-mediated induction of HEMA1 expression (Fig. 6F). Cytokinin has also been shown to increase PORA levels (Kusnetsov et al., 1998), and these combined effects could well be sufficient to maintain adequate buffering of Pchlide when exposed to WL. It should also be noted that in addition to blocking the transcriptionally coupled response, Suc slightly delayed the transcriptionally uncoupled response (Fig. 2A). Again, this corresponded to a delay in the induction of HEMA1 (Fig. 3D) and in the down-regulation of POR expression (Barnes et al., 1996). These results are all consistent with the PORA:Pchlide ratio being the determining factor in the transcriptionally uncoupled FR block of greening response.
With regard to the physiological significance of these results, the observation here that a 1-d FR pretreatment (or longer irradiations with low-intensity FRc) could enhance the subsequent transcriptional response to low-intensity WL (Fig. 1C) has some precedence in reports that an FRc pretreatment caused an amplification of the low fluence growth response to a subsequent RL treatment (Casal, 1995). Stimulation of ALA synthesis in WL through an FR pretreatment has also been noted before (e.g. Masoner and Kasemir, 1975). Therefore, it appears that an FR-HIR serves a similar function as a R light pulse (followed by a short darkness incubation period) in priming the autotrophic transition in the emerging seedling, in this case, for example, under dense canopy shade.
The Transcriptionally Coupled Response Results in the Loss of Nuclear Gene Expression through an FR-HIR Effect on Plastid Gating of Phytochrome Signaling
The transcriptionally coupled response was characterized by the almost complete loss of HEMA1 and Lhcb expression in WL following transfer from prolonged (>2 d) FR treatments (Fig. 1). Because this effect is also seen using the HEMA1 promoter:gusA lines, it clearly results from a loss of promoter-driven transcriptional activity as opposed to transcript stability. Suc (Fig. 2), but not cytokinin (Fig. 6), inhibited the development of the transcriptionally coupled response. We propose that the loss of nuclear gene expression results from an FR-HIR-mediated inhibition of a plastid signal that gates HEMA1 and Lhcb gene expression. This proposal is based on the similarity of this response to the characteristic loss of nuclear gene expression following NF-induced photobleaching and the ability of POR overexpression to rescue HEMA1 expression (Fig. 5). Because POR is localized to plastids in these overexpressors (Sperling et al., 1997, 1999), it is reasonable that its ability to rescue HEMA1 expression results from alterations in plastid function.
It is not clear whether the deterioration of the plastid signal in the transcriptionally coupled response occurs directly during the FR irradiation period or subsequently under WL as the result of sensitization by the FR pretreatment. However, our current hypothesis is that plastid signaling is lost during the period of FRc irradiation. This hypothesis is based on the observations that the complete loss of greening ability and nuclear gene expression was independent of WL intensity (Fig. 1), but was dependent on the FR fluence rate (Fig. 3F). Furthermore, there was an additive interaction of an FR pretreatment with NF-induced photobleaching (Fig. 5), which suggests that plastid signaling is not completely abolished by either treatment alone. Although it is not possible to say whether these two treatments use the same or independent signaling pathways, these results do suggest that at least some element of the transcriptional block was irreversibly determined during the FR preirradiation period. This is in contrast to the effect of NF-induced photobleaching, which occurs exclusively during the WL irradiation period.
A number of events may be occurring during the period of FRc that could lead to the loss of plastid signaling. Previous studies on the effects of FR irradiation on plastid structure have revealed that this treatment leads to a deficiency in the ordered membrane system of the prolamellar body (Oelmüller et al., 1986; Barnes et al., 1996; Sperling et al., 1997). This can be largely attributed to the dramatic decline in PORA (Runge et al., 1996), which is the major protein component of the prolamellar body (Ryberg and Sundqvist, 1982; Ikeuchi and Murakami, 1983). In addition, the development of vesicles, which may be related to degradation of plastid components, has also been observed (Barnes et al., 1996). These ultrastructural changes all occur during the FR treatment, prior to transfer to WL, and seedlings treated with 2 d of darkness following the FR pretreatment do not recover greening ability (Barnes et al., 1996), indicating that FRc leads to an irreversible arrest of plastid development. It is interesting that Suc, which inhibits the WL-independent loss of nuclear gene expression, also inhibits vesicle formation (Barnes et al., 1996). It is plausible that this FR-mediated deterioration of plastid structure would compromise plastid/nuclear signaling and is consistent with the observation here of a specific FR-HIR-mediated impairment of nuclear gene expression. Because POR overexpression rescues the observable effects of FRc on plastid ultrastructure (Sperling et al., 1997, 1999), the demonstration that POR overexpression also rescues HEMA1 expression supports the idea that FRc-mediated changes in plastid ultrastructure lead to a loss of plastid signaling. In this context, it may be that cytokinin can mediate plastid repair (sufficient to permit greening) in the event of low-level damage such as within the transcriptionally uncoupled response, but cannot overcome this more severe damage associated with the transcriptionally coupled response.
Loss of nuclear gene expression can also be seen following transfer to WL after longer growth periods (>4 d) in darkness (Fig. 3). In this experiment, Arabidopsis seedlings showed a progressive loss of the HEMA1 promoter response to light and this was independent of FR and phytochrome. Lhcb and RBCS mRNA levels have previously been reported to be subject to light-independent developmental mechanisms (Brusslan and Tobin, 1992), but it is equally possible that this is simply the result of starvation. The inhibition of this response by 3% (w/v) Suc supports such an idea. The decline in the transcriptional response of seedlings while still under FRc displayed a similar time course to the FR-independent loss of WL induction, and this response was also prevented by the presence of 3% (w/v) Suc (Fig. 3). However, the time-dependent loss of transcriptional activity is distinct from that seen following the complete FR block of greening response. First, the use of the GUS reporter showed that an FR pretreatment resulted in a significantly greater level of HEMA1 promoter inhibition under subsequent WL than could be accounted for by an extended dark period (Fig. 1C). Second, the time-dependent loss of greening ability and promoter responsiveness, as seen in dark-grown seedlings in the absence of Suc, was alleviated by cytokinin, whereas the FR-dependent loss of greening and HEMA1 expression was not (Fig. 6, A and C). The most likely explanation is that in the absence of Suc or cytokinin, these time- or starvation-dependent effects proceed within FR-irradiated seedlings in parallel with FR-specific responses. In contrast, when seedlings are transferred to R or WL, they become dissociated from such a time-dependent loss of transcriptional activity (Fig. 3C) through the photosynthetic supply of sugars or direct chloroplast signaling.
There is clearly a close link between the transcriptionally uncoupled response proposed to act through a perturbation of the PORA:Pchlide ratio and the transcriptionally coupled response in which it is proposed that irreversible changes in plastid ultrastructure result in a loss of nuclear gene expression. The important role of POR in determining plastid structure and the ability of POR overexpression to rescue both responses, at least in part (Fig. 5; Sperling et al., 1997), supports such a hypothesis and suggests that the effects caused by the transcriptionally uncoupled response may have an input into the transcriptionally coupled response (Fig. 7). Given the temporal separation of the two FR-mediated responses, it is possible to think of them proceeding sequentially following the onset of FRc, with the transcriptionally uncoupled response leading to the transcriptionally coupled response. However, as cytokinin inhibits the transcriptionally uncoupled response while having no effect on the transcriptionally coupled response, there is clearly no absolute requirement for the transcriptionally uncoupled response for the transcriptionally coupled response to proceed.
Implications for Plastid-Nuclear Signaling
In this study, we have identified a plastid-signaling pathway that is affected by prolonged periods of FRc irradiation and is at least partially independent from the pathway inhibited by NF-induced photobleaching. Previous studies have also concluded that more than one plastid-signaling pathway must exist (Vinti et al., 2000; Mochizuki et al., 2001). There are a number of similarities between the FRc dependence of plastid signaling described here and previous observations. For example, treatment of barley (Hordeum vulgare) seedlings with the carotenoid inhibitor Amitrole (but not NF) results in the loss of Lhcb and RbcS expression in low-intensity, nonphotodamaging WL (La Rocca et al., 2001). In a similar manner, inhibition of etioplast development resulting from the reduced accumulation of Pchlide and POR in the phytochrome chromophore-deficient aurea mutant of tomato (Terry and Kendrick, 1999; Terry et al., 2001) also leads to reduced Lhcb expression in the darkness (Sharrock et al., 1988; Ken-Dror and Horwitz, 1990). This can also be considered as a WL intensity-independent loss of plastid signaling. The possibility that changes in plastid development through FRc, inhibition of chromophore biosynthesis, and Amitrole treatment all lead to inhibition of the same plastid signaling pathway is intriguing and will require further experiments. One common feature of these conditions is that they all perturb tetrapyrrole biosynthesis (Terry and Kendrick, 1999; La Rocca et al., 2001). This observation, together with our finding that POR overexpression can sustain this signaling response, supports the growing evidence that tetrapyrroles play a major role in mediating plastid signaling (Kropat et al., 2000; Vinti et al., 2000; Mochizuki et al., 2001; Møller et al., 2001). However, the exact role of tetrapyrroles in these pathways is still unknown and further work is clearly needed to elucidate this. The identification of a new pathway leading to a phytochrome A-dependent loss of plastid signaling will provide a new experimental system for addressing questions on the mechanisms involved in plastid-nuclear signaling.
MATERIALS AND METHODS
Plant Material
Transgenic lines expressing a HEMA1promoter:gusA reporter construct were the kind gift of Andrea Fischer and Prof. Dieter Söll (Yale University, New Haven, CT) and have been described previously (McCormac et al., 2001). Two homozygous lines were used in these experiments, each containing the full-length (−2,435/+252) HEMA1 promoter region fused upstream of the GUS coding sequence. The Arabidopsis WT ecotypes Col and Ler and the phyA (Col and Ler) mutant used in this study were kindly provided by Drs. Haruko Okamoto and Xing-Wang Deng (Yale University).
Surface-sterilized seeds were plated onto a 1% (w/v) agar medium containing Murashige and Skoog salts (Murashige and Skoog, 1962) and were supplemented with 0% or 3% (w/v) Suc as indicated in “Results.” Plates were placed at 4°C in darkness for 2 d prior to receiving a 30-min WL irradiation to synchronize germination. Unless indicated otherwise, seeds were allowed to germinate in darkness at 23°C for 2 d (0% [w/v] Suc) or 3 d (3% [w/v] Suc) prior to transfer to the FR light source. Where indicated, NF (kindly provided by Prof. John Gray, University of Cambridge, Cambridge, UK) was added to the medium at a level of 5 μm. For treatments with the cytokinin BAP, seeds were first germinated on sterile filters placed over Murashige and Skoog medium for 1 d in darkness and were then transferred to plates containing 10 mg L−1 (44 μm) BAP.
Light Sources
Broad-band WL was provided by white fluorescent tubes (400–700 nm = 130 μmol m−2 s−1 unless indicated otherwise). This fluence rate was equivalent to high-intensity WL (250 μmol m−2 s−1; Figs. 1 and 2) for the greening response. Narrow waveband sources were provided by light-emitting diode displays in environmental control chambers (Percival Scientific, Boone, IA). R light corresponded to a peak at 669 nm (25-nm bandwidth at 50% of peak magnitude) with a fluence rate of 80 μmol m−2 s−1. FR from the light-emitting diodes had a peak at 739 nm (25-nm bandwidth at 50% of peak magnitude) and was passed through two filters (nos. 116 and 172; Lee Filters, Andover, UK) to remove λs <700 nm to give a final fluence rate of 10 μmol m−2 s−1.
RNA Gel-Blot Analysis
RNA gel-blot analysis was performed using total RNA (30 μg lane−1) exactly as described previously (McCormac et al., 2001). The HEMA1 probe used was a 3′-cDNA fragment that is gene specific (Kumar et al., 1996; McCormac et al., 2001). The Lhcb probe (kindly provided by Dr. Joanne Chory, The Salk Institute, La Jolla, CA) contained the majority of the coding region of the Lhcb1*2 gene and is predicted to crosshybridize with other members of the Lhcb gene family (McCormac et al., 2001).
GUS Fluorometric Analysis
Quantitative assays of GUS activity in seedling cotyledons were conducted exactly as described previously (McCormac et al., 2001).
ALA Biosynthesis Assay
Seedlings were incubated, under the appropriate light conditions, in 100 mm sodium phosphate buffer (pH 7.0), with or without 40 mm levulinic acid (Sigma-Aldrich, Poole, UK), at 23°C for 7 h. Seedlings were then blotted dry, frozen in liquid nitrogen, and stored at −80°C. The frozen seedlings were ground in 20 mm sodium phosphate buffer (pH 7.0), incubated on ice for 20 min, and centrifuged at 8,500g in a benchtop microfuge for 5 min. The supernatant was incubated with ethylacetoacetate at 100°C followed by the addition of modified Ehrlich's reagent (Urata and Granick, 1963). Absorbance was read at 526, 553, and 600 nm and the concentration of ALA was calculated using a molar absorption coefficient of 7.45 × 104 m−1 cm−1.
Pigment Extraction
Pchlide was extracted based on the method of Rebeiz et al. (1975). Etiolated Arabidopsis seedlings were homogenized in acetone:0.1 m NH4OH (90:10, v/v). The extract was centrifuged at 8,500g for 2 min and the pellet was re-extracted as above. The supernatants were combined and mixed with an equal volume of hexane. The aqueous and hexane fractions were collected separately, and relative fluorescence emission spectra were recorded using a fluorescence spectrophotometer (F-2000; Hitachi, Tokyo) with an excitation wavelength of 440 nm. Greening is shown as the percentage of seedlings with visibly green cotyledons. Comparison with direct measurements of chlorophyll extractions (Moran, 1982) showed that such an assessment was linearly correlated to total chlorophyll within a given genetic background.
ACKNOWLEDGMENTS
We thank Andrea Fischer and Prof. Dieter Söll (Yale University) for the HEMA1 promoter:gusA lines, Dr. Gregory Armstrong (Ohio State University, Columbus) for the PORA and PORB overexpressing lines, Dr. Xing-Wang Deng (Yale University) for seeds of phyA and hy1, Prof. John Gray (University of Cambridge) for the generous gift of Norflurazon, and Drs. Haruko Okamoto (University of Oxford), James Weller (University of Tasmania, Hobart, Australia), and Enrique López-Juez (Royal Holloway College, University of London) for critical reading of the manuscript.
Footnotes
This work was supported by the United Kingdom Biology and Biotechnology Research Council (grant no. 51/P10162). M.J.T. is a Royal Society University Research Fellow.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.003806.
LITERATURE CITED
- Barnes SA, Nishizawa NK, Quaggio RB, Whitelam GC, Chua N-H. Far-red light blocks greening of Arabidopsis seedlings via a phytochrome A-mediated change in plastid development. Plant Cell. 1996;8:601–613. doi: 10.1105/tpc.8.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Quaggio RB, Chua N-H. Phytochrome signal transduction: characterisation of pathways and isolation of mutants. Phil Trans Roy Soc Lond B Biol Sci. 1995;350:67–74. doi: 10.1098/rstb.1995.0139. [DOI] [PubMed] [Google Scholar]
- Beale SI, Castelfranco PA. The biosynthesis of δ-aminolevulinic acid in higher plants: accumulation of δ-aminolevulinic acid in greening plant tissues. Plant Physiol. 1974;53:291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusslan JA, Tobin EM. Light-dependent developmental regulation of CAB gene expression in Arabidopsis thaliana seedlings. Proc Natl Acad Sci USA. 1992;89:7791–7795. doi: 10.1073/pnas.89.16.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. Coupling of phytochrome B to the control of hypocotyl growth in Arabidopsis. Planta. 1995;196:23–29. doi: 10.1007/BF00193213. [DOI] [PubMed] [Google Scholar]
- Chun L, Kawakami A, Christopher DA. Phytochrome A mediates blue light and UV-A-dependent chloroplast gene transcription in green leaves. Plant Physiol. 2001;125:1957–1966. doi: 10.1104/pp.125.4.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel PP, Huijser C, Weisbeek PJ, Chua N-H, Smeekens SC. Sucrose control of phytochrome A signaling in Arabidopsis. Plant Cell. 1997;9:583–595. doi: 10.1105/tpc.9.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBell AN, Mullet JE. Continuous far-red light activates plastid DNA synthesis in pea leaves but not full cell enlargement or an increase in plastid number per cell. Plant Physiol. 1995a;109:95–103. doi: 10.1104/pp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBell AN, Mullet JE. Differential transcription of pea chloroplast genes during light-induced leaf development: Continuous far-red light activates chloroplast transcription. Plant Physiol. 1995b;109:105–112. doi: 10.1104/pp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfgen R, Axelsen KB, Kannangara CG, Schüttke I, Pohlenz H-D, Willmitzer L, Grimm B, von Wettstein D. A visible marker for antisense mRNA expression in plants: inhibition of chlorophyll synthesis with a glutamate-1-semialdehyde aminotransferase antisense gene. Proc Natl Acad Sci USA. 1994;91:1726–1730. doi: 10.1073/pnas.91.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Murakami S. Separation and characterization of prolamellar bodies and prothylakoids from squash etioplasts. Plant Cell Physiol. 1983;24:71–80. [Google Scholar]
- Ilag LL, Kumar AM, Söll D. Light regulation of chlorophyll biosynthesis at the level of 5-aminolevulinate formation in Arabidopsis. Plant Cell. 1994;6:265–275. doi: 10.1105/tpc.6.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ken-Dror S, Horwitz BA. Altered phytochrome regulation of greening in an aurea mutant of tomato. Plant Physiol. 1990;92:1004–1008. doi: 10.1104/pp.92.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittsteiner U, Mostowska A, Rüdiger W. The greening process in cress seedlings: pigment accumulation and ultrastructure after application of 5-aminolevulinic acid and complexing agents. Physiol Plant. 1991;81:139–147. [Google Scholar]
- Kropat J, Oster U, Rüdiger W, Beck CF. Chloroplast signalling in the light induction of nuclear HSP70 genes requires the accumulation of chlorophyll precursors and their accessibility to cytoplasm/nucleus. Plant J. 2000;24:523–531. doi: 10.1046/j.1365-313x.2000.00898.x. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Chaturvedi S, Söll D. Selective inhibition of HEMA gene expression by photooxidation in Arabidopsis thaliana. Phytochemistry. 1999;51:847–850. doi: 10.1016/s0031-9422(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Csankovszki G, Söll D. A second and differentially expressed glutamyl-tRNA reductase gene from Arabidopsis thaliana. Plant Mol Biol. 1996;30:419–426. doi: 10.1007/BF00049321. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Söll D. Antisense HEMA1 RNA expression inhibits heme and chlorophyll biosynthesis in Arabidopsis. Plant Physiol. 2000;122:49–55. doi: 10.1104/pp.122.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno N, Muramatsu T, Hamazato F, Furuya M. Identification by large-scale screening of phytochrome-regulated genes in etiolated seedlings of Arabidopsis using a fluorescent differential display technique. Plant Physiol. 2000;122:15–24. doi: 10.1104/pp.122.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnetsov V, Bolle C, Lübberstedt T, Sopory S, Herrmann RG, Oelmüller R. Evidence that the plastid signal and light operate via the same cis-acting elements in the promoters of nuclear genes for plastid proteins. Mol Gen Genet. 1996;252:631–639. doi: 10.1007/BF02173968. [DOI] [PubMed] [Google Scholar]
- Kusnetsov V, Herrmann RG, Kulaeva ON, Oelmüller R. Cytokinin stimulates and abscisic acid inhibits greening in etiolated Lupinus luteus cotyledons by affecting the expression of the light-sensitive protochlorophyllide oxidoreductase. Mol Gen Genet. 1998;259:21–28. doi: 10.1007/pl00008626. [DOI] [PubMed] [Google Scholar]
- La Rocca NL, Rascio N, Oster U, Rüdiger W. Amitrole treatment of etiolated barley seedlings leads to deregulation of tetrapyrrole synthesis and to reduced expression of Lhc and RbcS genes. Planta. 2001;213:101–108. doi: 10.1007/s004250000477. [DOI] [PubMed] [Google Scholar]
- Lebedev N, van Cleve B, Armstrong G, Apel K. Chlorophyll synthesis in a deetiolated (det340) mutant of Arabidopsis without NADPH-protochlorophyllide (Pchlide) oxidoreductase (POR) A and photoactive Pchlide-F655. Plant Cell. 1995;7:2081–2090. doi: 10.1105/tpc.7.12.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Juez E, Jarvis RP, Takeuchi A, Page AM, Chory J. New Arabidopsis cue mutant suggest a close connection between plastid- and phytochrome-regulation of nuclear gene expression. Plant Physiol. 1998;118:803–815. doi: 10.1104/pp.118.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli AL. The physiology of phytochrome action. In: Kendrick RE, Kronenburg GHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 211–270. [Google Scholar]
- Masoner M, Kasemir H. Control of chlorophyll synthesis by phytochrome: the effect of phytochrome on the formation of 5-aminolevulinate in mustard seedlings. Planta. 1975;126:111–117. doi: 10.1007/BF00380614. [DOI] [PubMed] [Google Scholar]
- McCormac AC, Fischer A, Kumar AM, Söll D, Terry MJ. Regulation of HEMA1 expression by phytochrome and a plastid signal during de-etiolation in Arabidopsis thaliana. Plant J. 2001;25:549–561. doi: 10.1046/j.1365-313x.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- Millar AJ, Kay SA. Integration of circadian and phototransduction pathways in the network controlling CAB gene transcription in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:15491–15496. doi: 10.1073/pnas.93.26.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA. 2001;98:2053–2058. doi: 10.1073/pnas.98.4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller SG, Kunkel T, Chua N-H. A plastidic ABC protein involved in intercompartmental communication of light signaling. Genes Dev. 2001;15:90–103. doi: 10.1101/gad.850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982;69:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Oelmüller R, Levitan I, Bergfeld R, Rajasekhar VK, Mohr H. Expression of nuclear genes as affected by treatments acting on the plastids. Planta. 1986;168:482–492. doi: 10.1007/BF00392267. [DOI] [PubMed] [Google Scholar]
- Rebeiz CA, Mattheis JR, Smith BB, Rebeiz CC, Dayton DF. Chloroplast biogenesis: biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys. 1975;171:549–567. doi: 10.1016/0003-9861(75)90065-x. [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Apel K, Lebedev N. Evolution of chlorophyll biosynthesis: the challenge to survive photooxidation. Cell. 1996;86:703–705. doi: 10.1016/s0092-8674(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Runge S, Sperling U, Frick G, Apel K, Armstrong GA. Distinct roles for light-dependent NADPH:protochlorophyllide oxidoreductases (POR) A and B during greening in higher plants. Plant J. 1996;9:513–523. doi: 10.1046/j.1365-313x.1996.09040513.x. [DOI] [PubMed] [Google Scholar]
- Ryberg M, Sundqvist C. Characterization of prolamellar bodies and prothylakoids fractionated from wheat etioplasts. Physiol Plant. 1982;56:125–132. [Google Scholar]
- Sharrock RA, Parks BM, Koornneef M, Quail PH. Molecular analysis of the phytochrome deficiency in an aurea mutant of tomato. Mol Gen Genet. 1988;213:9–14. [Google Scholar]
- Smith H. Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- Sperling U, Frick G, van Cleve B, Apel K, Armstrong GA. Pigment-protein complexes, plastid development and photooxidative protection. In: Argyroudi-Akoyunoglou JH, Senger H, editors. The Chloroplast: From Molecular Biology to Biotechnology. Dordrecht, The Netherlands: Kluwer Academic Press; 1999. pp. 97–102. [Google Scholar]
- Sperling U, van Cleve B, Frick G, Apel K, Armstrong GA. Overexpression of light-dependent PORA or PORB in plants depleted of endogenous POR by far-red light enhances seedling survival in white light and protects against photooxidative damage. Plant J. 1997;12:649–658. doi: 10.1046/j.1365-313x.1997.00649.x. [DOI] [PubMed] [Google Scholar]
- Susek RE, Ausubel FM, Chory J. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell. 1993;74:787–799. doi: 10.1016/0092-8674(93)90459-4. [DOI] [PubMed] [Google Scholar]
- Susek RE, Chory J. A tale of two genomes: role of a chloroplast signal in coordinating nuclear and plastid genome expression. Aust J Plant Physiol. 1992;19:387–399. [Google Scholar]
- Taylor WC. Regulatory interactions between nuclear and plastid genomes. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:211–233. [Google Scholar]
- Tepperman JM, Zhu T, Chang H-S, Quail PH. Multiple transcription-factor genes are early targets of phytochrome A signalling. Proc Natl Acad Sci USA. 2001;98:9437–9442. doi: 10.1073/pnas.161300998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MJ, Kendrick RE. Feedback inhibition of chlorophyll synthesis in the phytochrome chromophore-deficient aurea and yellow-green-2 mutants of tomato. Plant Physiol. 1999;119:143–152. doi: 10.1104/pp.119.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MJ, Ryberg M, Raitt CE, Page AM. Altered etioplast development in phytochrome chromophore-deficient mutants. Planta. 2001;214:314–325. doi: 10.1007/s004250100624. [DOI] [PubMed] [Google Scholar]
- Urata G, Granick S. Biosynthesis of α-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963;238:811–820. [PubMed] [Google Scholar]
- van Tuinen A, Kerchoffs LHJ, Nagatani A, Kendrick RE, Koornneef M. Far-red light-insensitive phytochrome A-deficient mutants of tomato. Mol Gen Genet. 1995;246:133–141. doi: 10.1007/BF00294675. [DOI] [PubMed] [Google Scholar]
- Vinti G, Hills A, Campbell S, Bowyer JR, Mochizuki N, Chory J, López-Juez E. Interactions between hy1 and gun mutants of Arabidopsis, and their implications for plastid/nuclear signalling. Plant J. 2000;24:883–894. doi: 10.1046/j.1365-313x.2000.00936.x. [DOI] [PubMed] [Google Scholar]