Abstract
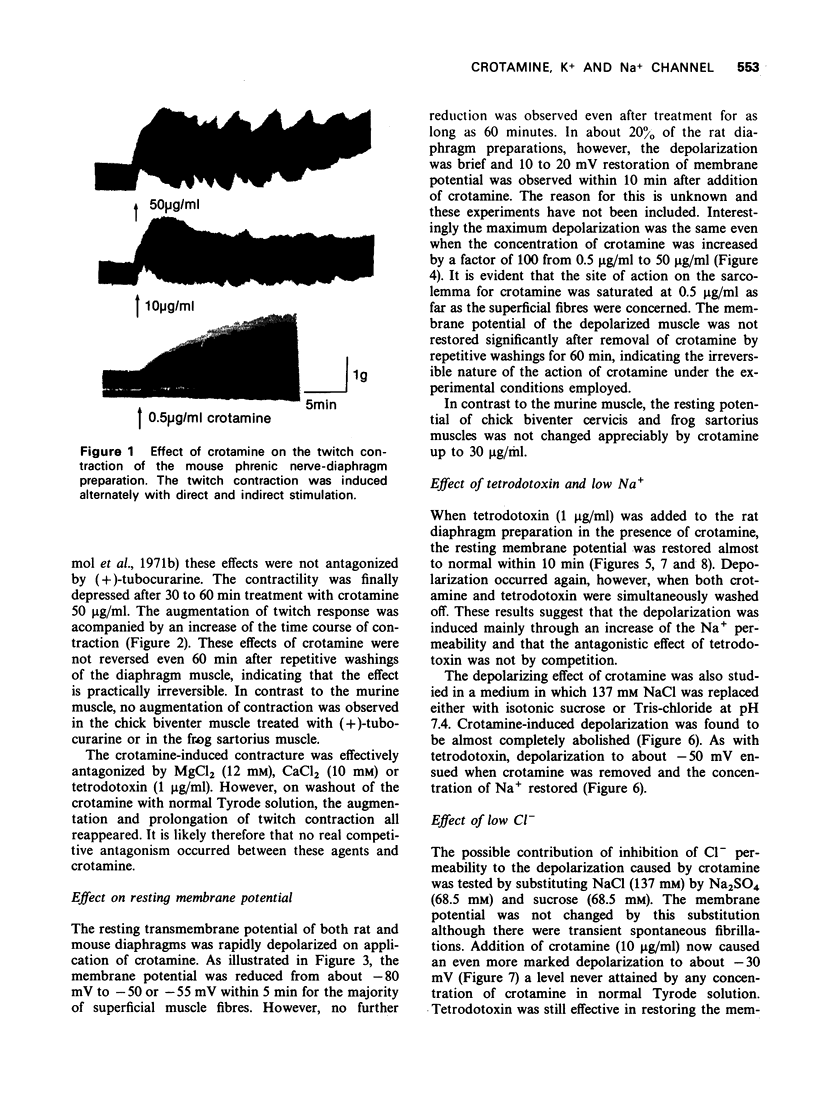
1 Crotamine (0.5 μg/ml) augmented the single twitch response of the rat and mouse isolated diaphragm to direct stimulation and prolonged the time course of contraction. At higher doses (10 to 50 μg/ml), contracture was observed with spontaneous fibrillation.
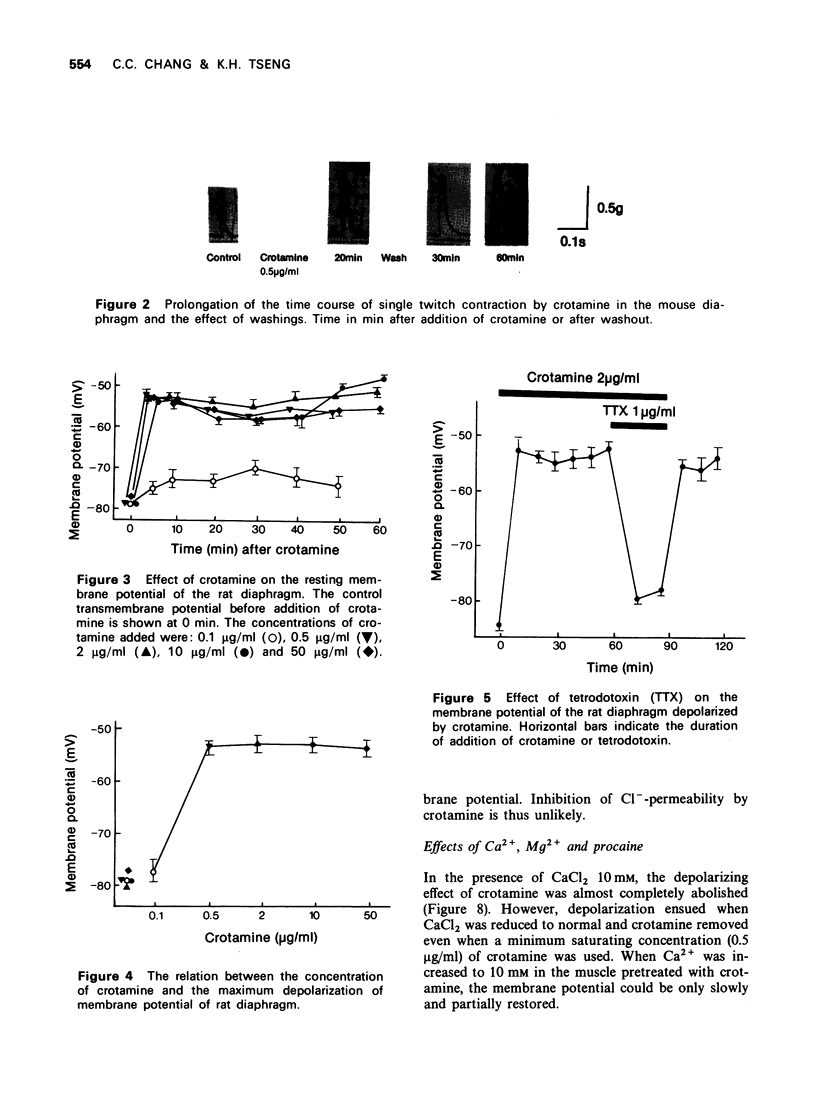
2 The resting membrane potential of diaphragm was rapidly depolarized to about -50 mV within 5 minutes. No increase of depolarization occurred on prolongation of the incubation time or increase of crotamine concentration from 0.5 μg/ml to 50 μg/ml. The effect was not reversed by washing.
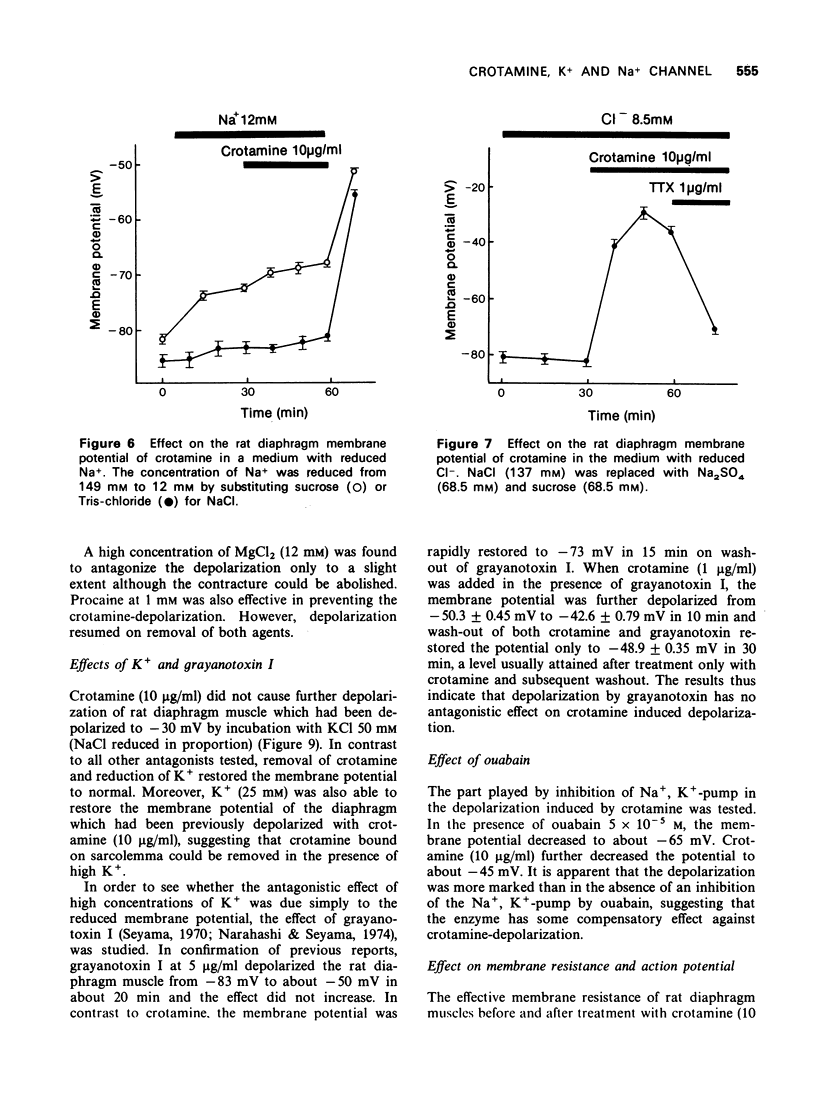
3 Tetrodotoxin, low Na+ (12 mM), Ca2+ (10 mM) and procaine (1 mM) prevented the crotamine-depolarization. However, depolarization resumed when crotamine and the antagonists were removed.
4 Low Cl- (8.5 mM) and pretreatment with ouabain enhanced depolarization by crotamine.
5 High K+ (25 to 50 mM) prevented the further depolarization by crotamine and the membrane potential was restored to normal on washout of crotamine with normal Tyrode solution.
6 Effective membrane resistance was decreased by about 50% by crotamine.
7 24Na-influx of the rat diaphragm was increased by crotamine. 42K-influx was slightly increased if tetrodotoxin was also present but was decreased in the absence of tetrodotoxin.
8 No effect on the miniature and evoked endplate potential of the rat diaphragm was observed. Skeletal muscles from frog and chick were not affected.
9 It is inferred that crotamine acts on a molecule regulating the Na+ - permeability of the Na+ channel of murine muscles. It is proposed that extracellular K+ depresses the permeability of the Na+ channel by acting on the same regulator molecule.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., OTANI T. Response of single motoneurons to direct stimulation in toad's spinal cord. J Neurophysiol. 1955 Sep;18(5):472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- BARRIO A., BRAZIL O. V. Neuromuscular action of the Crotalus terrificus terrificus (Laur.) poisons. Acta Physiol Lat Am. 1951 Dec;1(4):291–308. [PubMed] [Google Scholar]
- Bartels-Bernal E., Rosenberry T. L., Daly J. W. Effect of batrachotoxin on the electroplax of electric eel: evidence for voltage-dependent interaction with sodium channels. Proc Natl Acad Sci U S A. 1977 Mar;74(3):951–955. doi: 10.1073/pnas.74.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Ray R., Morrow C. S. Membrane potential dependent binding of scorpion toxin to action potential Na+ ionophore. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2682–2686. doi: 10.1073/pnas.73.8.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheymol J., Bourillet F., Roch-Arveiller M., Thach Toan Effets neuromusculaires des venins des deux variétés de Crotalus durissus terrificus. Arch Int Pharmacodyn Ther. 1969 May;179(1):40–55. [PubMed] [Google Scholar]
- Cheymol J., Gonçalves J. M., Bourillet F., Roch-Arveiller M. Action neuromusculaire comparée de la crotamine et du venin de Crotalus durissus terrificus var. crotaminicus. 1. Sur préparations neuromusculaires in situ. Toxicon. 1971 Jul;9(3):279–286. doi: 10.1016/0041-0101(71)90081-x. [DOI] [PubMed] [Google Scholar]
- Cheymol J., Gonçalves J. M., Bourillet F., Roch-Arveiller M. Action neuromusculaire comparée de la crotamine et du venin de Crotalus durissus terrificus var. crotaminicus. 2. Sur préparations isolées. Toxicon. 1971 Jul;9(3):287–289. doi: 10.1016/0041-0101(71)90082-1. [DOI] [PubMed] [Google Scholar]
- Cohen M., Palti Y., Adelman W. J. Ionic dependence of sodium currents in squid axons analyzed in terms of specific ion "channel" interactions. J Membr Biol. 1975 Dec 4;24(3-4):201–223. doi: 10.1007/BF01868623. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E., Deroubaix E., Tazieff-Depierre T. Effect of toxin II isolated from scorpion venom on action potential and contraction of mammalian heart. J Mol Cell Cardiol. 1975 Sep;7(9):643–653. doi: 10.1016/0022-2828(75)90141-8. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBORG B. L., WARRINER J. The isolated chick biventer cervicis nerve-muscle preparation. Br J Pharmacol Chemother. 1960 Sep;15:410–411. doi: 10.1111/j.1476-5381.1960.tb01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner T. K., Land B., Podleski T. R. Genetic and physiological evidence concerning the development of chemically sensitive voltage-dependent inophores in L6 cells. J Neurobiol. 1976 Nov;7(6):537–549. doi: 10.1002/neu.480070607. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E., Cheng-Raude D. Central neurotoxicity of apamin, crotamin, phospholipase A and alpha-amanitin. Toxicon. 1975 Dec;13(6):465–473. doi: 10.1016/0041-0101(75)90176-2. [DOI] [PubMed] [Google Scholar]
- Laure C. J. Die Primärstruktur des Crotamins. Hoppe Seylers Z Physiol Chem. 1975 Feb;356(2):213–215. [PubMed] [Google Scholar]
- Narahashi T., Seyama I. Mechanism of nerve membrane depolarization caused by grayanotoxin I. J Physiol. 1974 Oct;242(2):471–487. doi: 10.1113/jphysiol.1974.sp010718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens U. Electromechanical studies of an Anemonia sulcata toxin in mammalian cardiac muscle. Naunyn Schmiedebergs Arch Pharmacol. 1976 Dec;296(1):73–78. doi: 10.1007/BF00498842. [DOI] [PubMed] [Google Scholar]
- Rochat H., Rochat C., Sampieri F., Miranda F., Lissitzky S. The amino-acid sequence of neurotoxin II of Androctonus australis Hector. Eur J Biochem. 1972 Jul 24;28(3):381–388. doi: 10.1111/j.1432-1033.1972.tb01924.x. [DOI] [PubMed] [Google Scholar]
- SCHENBERG S. Geographical pattern of crotamine distribution in the same rattlesnake subspecies. Science. 1959 May 15;129(3359):1361–1363. doi: 10.1126/science.129.3359.1361. [DOI] [PubMed] [Google Scholar]
- Seyama I. Effect of grayanotoxin 1 on the electrical properties of rat skeletal muscle fibers. Jpn J Physiol. 1970 Aug;20(4):381–393. doi: 10.2170/jjphysiol.20.381. [DOI] [PubMed] [Google Scholar]
- Warnick J. E., Albuquerque E. X., Diniz C. R. Electrophysiological observations on the action of the purified scorpion venom, tityustoxin, on nerve and skeletal muscle of the rat. J Pharmacol Exp Ther. 1976 Jul;198(1):155–167. [PubMed] [Google Scholar]
- Wunderer G., Machleidt W., Wachter E. Toxin II from Anemonia sulcata-the first sequence of a coelenterate toxin. Hoppe Seylers Z Physiol Chem. 1976 Feb;357(2):239–240. [PubMed] [Google Scholar]