Abstract
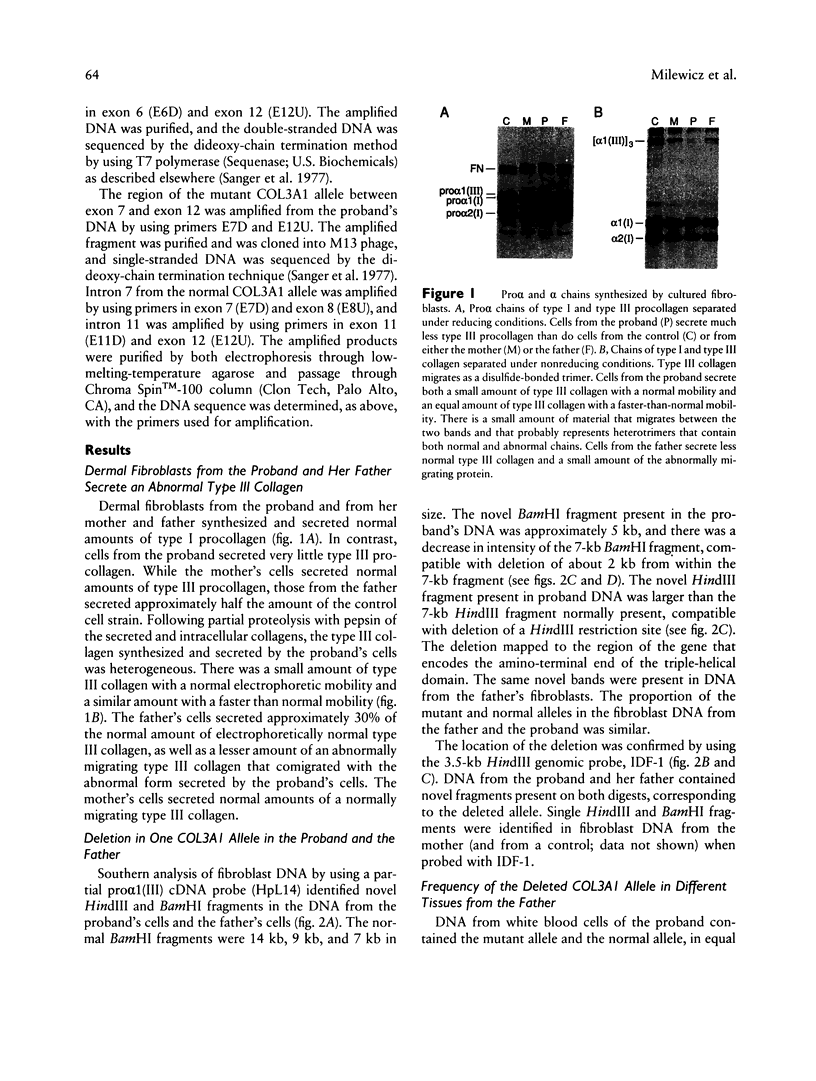
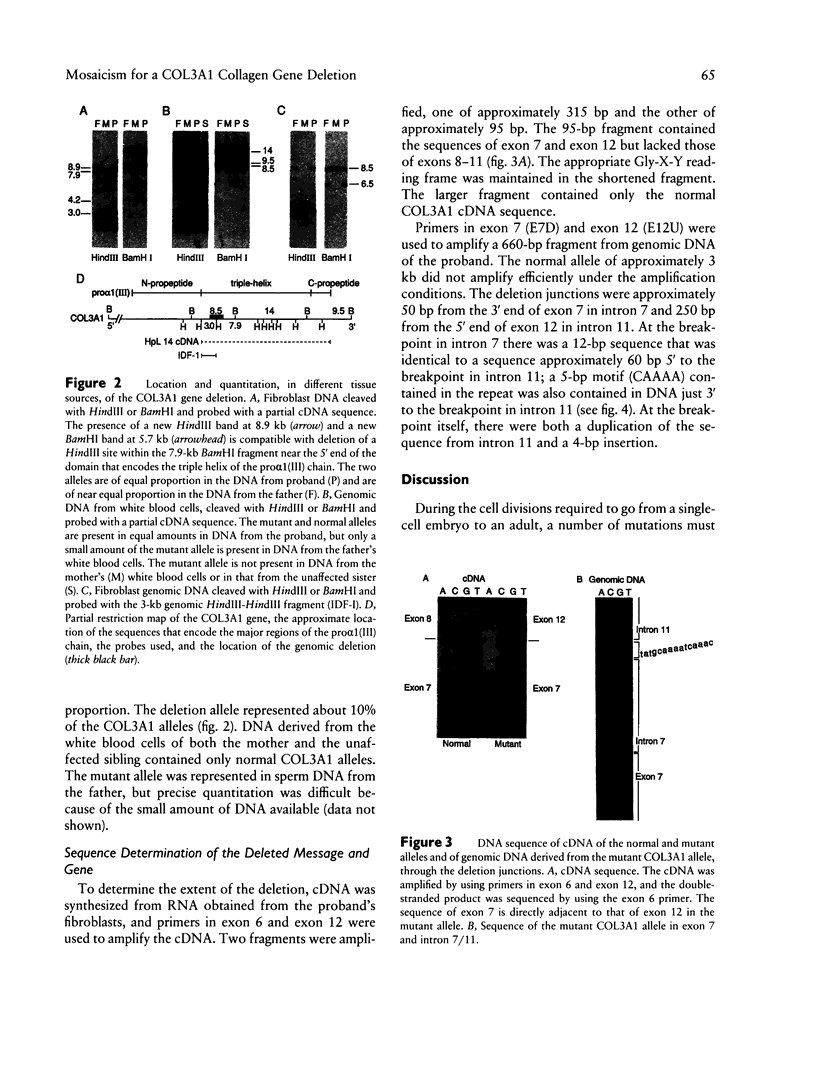
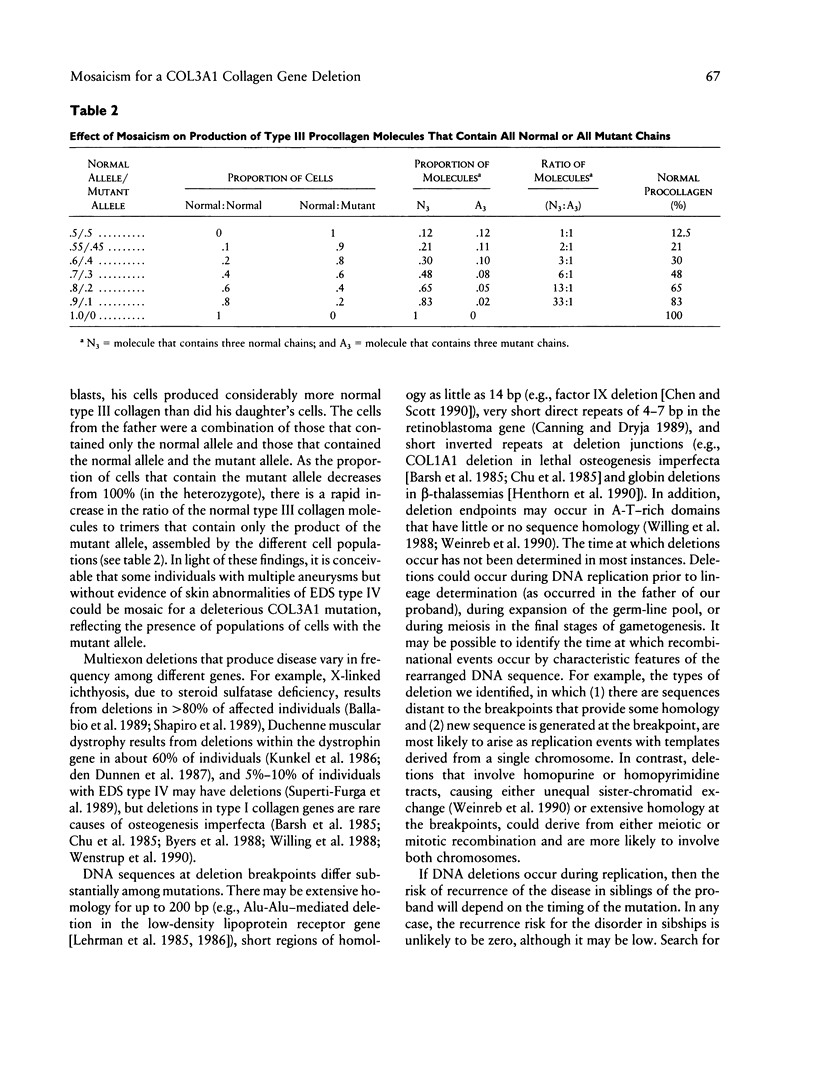
Ehlers-Danlos syndrome (EDS) type IV is a dominantly inherited disorder that results from mutations in the type III collagen gene (COL3A1). We studied the structure of the COL3A1 gene of an individual with EDS type IV and that of her phenotypically normal parents. The proband was heterozygous for a 2-kb deletion in COL3A1, while her father was mosaic for the same deletion in somatic and germ cells. In fibroblasts from the father, approximately two-fifths of the COL3A1 alleles carried the deletion, but only 10% of the COL3A1 alleles in white blood cells were of the mutant species. The deletion in the mutant allele extended from intron 7 into intron 11. There was a 12-bp direct repeat in intron 7 and intron 11, the latter about 60 bp 5' to the junction. At the breakpoint there was a duplication of 10 bp from intron 11 separated by an insertion of 4 bp contained within the duplicated sequence. The father was mosaic for the deletion so that the gene rearrangement occurred during his early embryonic development prior to lineage allocation. These findings suggest that at least some of the deletions seen in human genes may occur during replication, rather than as a consequence of meiotic crossing-over, and that they thus have a risk for recurrence when observed de novo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Kontusaari S., Baldwin C. T., Kuivaniemi H., Prockop D. J. Structure of cDNA clones coding for the entire prepro alpha 1 (III) chain of human type III procollagen. Differences in protein structure from type I procollagen and conservation of codon preferences. Biochem J. 1989 Jun 1;260(2):509–516. doi: 10.1042/bj2600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allanson J. E. Germinal mosaicism in Apert syndrome. Clin Genet. 1986 May;29(5):429–433. doi: 10.1111/j.1399-0004.1986.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Bakker E., Van Broeckhoven C., Bonten E. J., van de Vooren M. J., Veenema H., Van Hul W., Van Ommen G. J., Vandenberghe A., Pearson P. L. Germline mosaicism and Duchenne muscular dystrophy mutations. Nature. 1987 Oct 8;329(6139):554–556. doi: 10.1038/329554a0. [DOI] [PubMed] [Google Scholar]
- Ballabio A., Carrozzo R., Parenti G., Gil A., Zollo M., Persico M. G., Gillard E., Affara N., Yates J., Ferguson-Smith M. A. Molecular heterogeneity of steroid sulfatase deficiency: a multicenter study on 57 unrelated patients, at DNA and protein levels. Genomics. 1989 Jan;4(1):36–40. doi: 10.1016/0888-7543(89)90311-x. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Bonadio J., Byers P. H., Gelinas R. E. Intron-mediated recombination may cause a deletion in an alpha 1 type I collagen chain in a lethal form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1985 May;82(9):2870–2874. doi: 10.1073/pnas.82.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson-Chanda V., Su M. W., Weil D., Chu M. L., Ramirez F. Cloning and analysis of the 5' portion of the human type-III procollagen gene (COL3A1). Gene. 1989 May 30;78(2):255–265. doi: 10.1016/0378-1119(89)90228-x. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Holbrook K. A., Gelinas R. E., Jacob J., Byers P. H. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985 Feb 10;260(3):1734–1742. [PubMed] [Google Scholar]
- Bradley T. B., Wohl R. C., Petz L. D., Perkins H. A., Reynolds R. D. Possible gonadal mosaicism in a family with hemoglobin Köln. Johns Hopkins Med J. 1980 Jun;146(6):236–240. [PubMed] [Google Scholar]
- Bröcker-Vriends A. H., Briët E., Dreesen J. C., Bakker B., Reitsma P., Pannekoek H., van de Kamp J. J., Pearson P. L. Somatic origin of inherited haemophilia A. Hum Genet. 1990 Aug;85(3):288–292. doi: 10.1007/BF00206748. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Canning S., Dryja T. P. Short, direct repeats at the breakpoints of deletions of the retinoblastoma gene. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5044–5048. doi: 10.1073/pnas.86.13.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Scott C. R. Recombination between two 14-bp homologous sequences as the mechanism for gene deletion in factor IX Seattle 1. Am J Hum Genet. 1990 Dec;47(6):1020–1022. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Gargiulo V., Williams C. J., Ramirez F. Multiexon deletion in an osteogenesis imperfecta variant with increased type III collagen mRNA. J Biol Chem. 1985 Jan 25;260(2):691–694. [PubMed] [Google Scholar]
- Cohn D. H., Starman B. J., Blumberg B., Byers P. H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am J Hum Genet. 1990 Mar;46(3):591–601. [PMC free article] [PubMed] [Google Scholar]
- Cole W. G., Chiodo A. A., Lamande S. R., Janeczko R., Ramirez F., Dahl H. H., Chan D., Bateman J. F. A base substitution at a splice site in the COL3A1 gene causes exon skipping and generates abnormal type III procollagen in a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1990 Oct 5;265(28):17070–17077. [PubMed] [Google Scholar]
- Constantinou C. D., Nielsen K. B., Prockop D. J. A lethal variant of osteogenesis imperfecta has a single base mutation that substitutes cysteine for glycine 904 of the alpha 1(I) chain of type I procollagen. The asymptomatic mother has an unidentified mutation producing an overmodified and unstable type I procollagen. J Clin Invest. 1989 Feb;83(2):574–584. doi: 10.1172/JCI113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C. D., Pack M., Young S. B., Prockop D. J. Phenotypic heterogeneity in osteogenesis imperfecta: the mildly affected mother of a proband with a lethal variant has the same mutation substituting cysteine for alpha 1-glycine 904 in a type I procollagen gene (COL1A1). Am J Hum Genet. 1990 Oct;47(4):670–679. [PMC free article] [PubMed] [Google Scholar]
- Darras B. T., Francke U. A partial deletion of the muscular dystrophy gene transmitted twice by an unaffected male. Nature. 1987 Oct 8;329(6139):556–558. doi: 10.1038/329556a0. [DOI] [PubMed] [Google Scholar]
- David T. J. Dominant ectrodactyly and possible germinal mosaicism. J Med Genet. 1972 Sep;9(3):316–320. doi: 10.1136/jmg.9.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. H. The population genetics of Duchenne: natural and artificial selection in Duchenne muscular dystrophy. J Med Genet. 1986 Dec;23(6):521–530. doi: 10.1136/jmg.23.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryns J. P., Kleczkowska A., Verresen H., van den Berghe H. Germinal mosaicism in achondroplasia: a family with 3 affected siblings of normal parents. Clin Genet. 1983 Sep;24(3):156–158. doi: 10.1111/j.1399-0004.1983.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Levinson B., Lehesjoki A. E., De La Chapelle A. Mosaicism and sporadic haemophilia: implications for carrier determination. Lancet. 1989 Feb 4;1(8632):273–274. doi: 10.1016/s0140-6736(89)91279-8. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Dorst J. P., Rotta J., McKusick V. A. Gonadal mosaicism in pseudoachondroplasia. Am J Med Genet. 1987 Sep;28(1):143–151. doi: 10.1002/ajmg.1320280121. [DOI] [PubMed] [Google Scholar]
- Hall J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet. 1988 Oct;43(4):355–363. [PMC free article] [PubMed] [Google Scholar]
- Hartl D. L. Recurrence risks for germinal mosaics. Am J Hum Genet. 1971 Mar;23(2):124–134. [PMC free article] [PubMed] [Google Scholar]
- Henthorn P. S., Smithies O., Mager D. L. Molecular analysis of deletions in the human beta-globin gene cluster: deletion junctions and locations of breakpoints. Genomics. 1990 Feb;6(2):226–237. doi: 10.1016/0888-7543(90)90561-8. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Kochhan L., Olek K. A somatic mosaic for haemophilia A detected at the DNA level. Mol Biol Med. 1988 Feb;5(1):23–27. [PubMed] [Google Scholar]
- Johnson P. H., Richards A. J., Pope F. M., Hopkinson D. A. A COL3A1 glycine 1006 to glutamic acid substitution in a patient with Ehlers-Danlos syndrome type IV detected by denaturing gradient gel electrophoresis. J Inherit Metab Dis. 1992;15(3):426–430. doi: 10.1007/BF02435995. [DOI] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Ladda R. L., Prockop D. J. Inheritance of an RNA splicing mutation (G+ 1 IVS20) in the type III procollagen gene (COL3A1) in a family having aortic aneurysms and easy bruisability: phenotypic overlap between familial arterial aneurysms and Ehlers-Danlos syndrome type IV. Am J Hum Genet. 1990 Jul;47(1):112–120. [PMC free article] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Romanic A. M., Prockop D. J. A mutation in the gene for type III procollagen (COL3A1) in a family with aortic aneurysms. J Clin Invest. 1990 Nov;86(5):1465–1473. doi: 10.1172/JCI114863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Stolle C., Pope F. M., Prockop D. J. Substitution of aspartate for glycine 1018 in the type III procollagen (COL3A1) gene causes type IV Ehlers-Danlos syndrome: the mutated allele is present in most blood leukocytes of the asymptomatic and mosaic mother. Am J Hum Genet. 1992 Sep;51(3):497–507. [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Kontusaari S., Tromp G., Zhao M. J., Sabol C., Prockop D. J. Identical G+1 to A mutations in three different introns of the type III procollagen gene (COL3A1) produce different patterns of RNA splicing in three variants of Ehlers-Danlos syndrome. IV. An explanation for exon skipping some mutations and not others. J Biol Chem. 1990 Jul 15;265(20):12067–12074. [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Hejtmancik J. F., Caskey C. T., Speer A., Monaco A. P., Middlesworth W., Colletti C. A., Bertelson C., Müller U., Bresnan M. Analysis of deletions in DNA from patients with Becker and Duchenne muscular dystrophy. Nature. 1986 Jul 3;322(6074):73–77. doi: 10.1038/322073a0. [DOI] [PubMed] [Google Scholar]
- Lebo R. V., Olney R. K., Golbus M. S. Somatic mosaicism at the Duchenne locus. Am J Med Genet. 1990 Oct;37(2):187–190. doi: 10.1002/ajmg.1320370206. [DOI] [PubMed] [Google Scholar]
- Lee B., D'Alessio M., Vissing H., Ramirez F., Steinmann B., Superti-Furga A. Characterization of a large deletion associated with a polymorphic block of repeated dinucleotides in the type III procollagen gene (COL3A1) of a patient with Ehlers-Danlos syndrome type IV. Am J Hum Genet. 1991 Mar;48(3):511–517. [PMC free article] [PubMed] [Google Scholar]
- Lee B., Vitale E., Superti-Furga A., Steinmann B., Ramirez F. G to T transversion at position +5 of a splice donor site causes skipping of the preceding exon in the type III procollagen transcripts of a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1991 Mar 15;266(8):5256–5259. [PubMed] [Google Scholar]
- Legius E., Baten E., Stul M., Marynen P., Cassiman J. J. Sporadic late onset ornithine transcarbamylase deficiency in a boy with somatic mosaicism for an intragenic deletion. Clin Genet. 1990 Aug;38(2):155–159. doi: 10.1111/j.1399-0004.1990.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Exon-Alu recombination deletes 5 kilobases from the low density lipoprotein receptor gene, producing a null phenotype in familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3679–3683. doi: 10.1073/pnas.83.11.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Südhof T. C., Brown M. S., Goldstein J. L., Russell D. W. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985 Jan 11;227(4683):140–146. doi: 10.1126/science.3155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalena A., Sosnoski D. M., Berry G. T., Nussbaum R. L. Mosaicism for an intragenic deletion in a boy with mild ornithine transcarbamylase deficiency. N Engl J Med. 1988 Oct 13;319(15):999–1003. doi: 10.1056/NEJM198810133191507. [DOI] [PubMed] [Google Scholar]
- Murray E. W., Giles A. R., Lillicrap D. Germ-line mosaicism for a valine-to-methionine substitution at residue 553 in the glycoprotein Ib-binding domain of von Willebrand factor, causing type IIB von Willebrand disease. Am J Hum Genet. 1992 Jan;50(1):199–207. [PMC free article] [PubMed] [Google Scholar]
- Navarrete C., Peña R., Peñaloza R., Salamanca F. Germinal mosaicism in Crouzon syndrome. A family with three affected siblings of normal parents. Clin Genet. 1991 Jul;40(1):29–34. doi: 10.1111/j.1399-0004.1991.tb03065.x. [DOI] [PubMed] [Google Scholar]
- Pope F. M., Nicholls A. C., Narcisi P., Temple A., Chia Y., Fryer P., De Paepe A., De Groote W. P., McEwan J. R., Compston D. A. Type III collagen mutations in Ehlers Danlos syndrome type IV and other related disorders. Clin Exp Dermatol. 1988 Sep;13(5):285–302. doi: 10.1111/j.1365-2230.1988.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Richards A. J., Lloyd J. C., Narcisi P., Ward P. N., Nicholls A. C., De Paepe A., Pope F. M. A 27-bp deletion from one allele of the type III collagen gene (COL3A1) in a large family with Ehlers-Danlos syndrome type IV. Hum Genet. 1992 Jan;88(3):325–330. doi: 10.1007/BF00197268. [DOI] [PubMed] [Google Scholar]
- Richards A. J., Lloyd J. C., Ward P. N., De Paepe A., Narcisi P., Pope F. M. Characterisation of a glycine to valine substitution at amino acid position 910 of the triple helical region of type III collagen in a patient with Ehlers-Danlos syndrome type IV. J Med Genet. 1991 Jul;28(7):458–463. doi: 10.1136/jmg.28.7.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. J., Ward P. N., Narcisi P., Nicholls A. C., Lloyd J. C., Pope F. M. A single base mutation in the gene for type III collagen (COL3A1) converts glycine 847 to glutamic acid in a family with Ehlers-Danlos syndrome type IV. An unaffected family member is mosaic for the mutation. Hum Genet. 1992 Jun;89(4):414–418. doi: 10.1007/BF00194313. [DOI] [PubMed] [Google Scholar]
- Rollnick B. R. Germinal mosaicism in Crouzon syndrome. Clin Genet. 1988 Mar;33(3):145–150. doi: 10.1111/j.1399-0004.1988.tb03429.x. [DOI] [PubMed] [Google Scholar]
- Rudd N. L., Nimrod C., Holbrook K. A., Byers P. H. Pregnancy complications in type IV Ehlers-Danlos Syndrome. Lancet. 1983 Jan 1;1(8314-5):50–53. doi: 10.1016/s0140-6736(83)91577-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. J., Yen P., Pomerantz D., Martin E., Rolewic L., Mohandas T. Molecular studies of deletions at the human steroid sulfatase locus. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8477–8481. doi: 10.1073/pnas.86.21.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solera J., Magallón M., Martin-Villar J., Coloma A. Factor IXMadrid 2: a deletion/insertion in factor IX gene which abolishes the sequence of the donor junction at the exon IV-intron d splice site. Am J Hum Genet. 1992 Feb;50(2):434–437. [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga A., Steinmann B., Ramirez F., Byers P. H. Molecular defects of type III procollagen in Ehlers-Danlos syndrome type IV. Hum Genet. 1989 May;82(2):104–108. doi: 10.1007/BF00284038. [DOI] [PubMed] [Google Scholar]
- Taylor S. A., Deugau K. V., Lillicrap D. P. Somatic mosaicism and female-to-female transmission in a kindred with hemophilia B (factor IX deficiency). Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):39–42. doi: 10.1073/pnas.88.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Shikata H., Prockop D. J. A single base mutation that substitutes serine for glycine 790 of the alpha 1 (III) chain of type III procollagen exposes an arginine and causes Ehlers-Danlos syndrome IV. J Biol Chem. 1989 Jan 25;264(3):1349–1352. [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Stolle C., Pope F. M., Prockop D. J. Single base mutation in the type III procollagen gene that converts the codon for glycine 883 to aspartate in a mild variant of Ehlers-Danlos syndrome IV. J Biol Chem. 1989 Nov 15;264(32):19313–19317. [PubMed] [Google Scholar]
- Tsipouras P., Byers P. H., Schwartz R. C., Chu M. L., Weil D., Pepe G., Cassidy S. B., Ramirez F. Ehlers-Danlos syndrome type IV: cosegregation of the phenotype to a COL3A1 allele of type III procollagen. Hum Genet. 1986 Sep;74(1):41–46. doi: 10.1007/BF00278783. [DOI] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Byers P. H., Steinmann B., Superti-Furga A. Multiexon deletion in the procollagen III gene is associated with mild Ehlers-Danlos syndrome type IV. J Biol Chem. 1991 Mar 15;266(8):5244–5248. [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Zinn A. B., Byers P. H. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990 Jun;46(6):1034–1040. [PMC free article] [PubMed] [Google Scholar]
- Weinreb A., Collier D. A., Birshtein B. K., Wells R. D. Left-handed Z-DNA and intramolecular triplex formation at the site of an unequal sister chromatid exchange. J Biol Chem. 1990 Jan 25;265(3):1352–1359. [PubMed] [Google Scholar]
- Weinstein L. S., Shenker A., Gejman P. V., Merino M. J., Friedman E., Spiegel A. M. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991 Dec 12;325(24):1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- Wenstrup R. J., Willing M. C., Starman B. J., Byers P. H. Distinct biochemical phenotypes predict clinical severity in nonlethal variants of osteogenesis imperfecta. Am J Hum Genet. 1990 May;46(5):975–982. [PMC free article] [PubMed] [Google Scholar]
- Wijsman E. M. Recurrence risk of a new dominant mutation in children of unaffected parents. Am J Hum Genet. 1991 Apr;48(4):654–661. [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Byers P. H. Frameshift mutation near the 3' end of the COL1A1 gene of type I collagen predicts an elongated Pro alpha 1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest. 1990 Jan;85(1):282–290. doi: 10.1172/JCI114424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Starman B., Holbrook K. A., Greenberg C. R., Byers P. H. Heterozygosity for a large deletion in the alpha 2(I) collagen gene has a dramatic effect on type I collagen secretion and produces perinatal lethal osteogenesis imperfecta. J Biol Chem. 1988 Jun 15;263(17):8398–8404. [PubMed] [Google Scholar]
- Wood S., McGillivray B. C. Germinal mosaicism in Duchenne muscular dystrophy. Hum Genet. 1988 Mar;78(3):282–284. doi: 10.1007/BF00291677. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Bakker E., Breteler E. G., Pearson P. L., van Ommen G. J. Direct detection of more than 50% of the Duchenne muscular dystrophy mutations by field inversion gels. Nature. 1987 Oct 15;329(6140):640–642. doi: 10.1038/329640a0. [DOI] [PubMed] [Google Scholar]