Abstract
The phosphoenolpyruvate(PEP):carbohydrate phosphotransferase system (PTS) is found only in bacteria, where it catalyzes the transport and phosphorylation of numerous monosaccharides, disaccharides, amino sugars, polyols, and other sugar derivatives. To carry out its catalytic function in sugar transport and phosphorylation, the PTS uses PEP as an energy source and phosphoryl donor. The phosphoryl group of PEP is usually transferred via four distinct proteins (domains) to the transported sugar bound to the respective membrane component(s) (EIIC and EIID) of the PTS. The organization of the PTS as a four-step phosphoryl transfer system, in which all P derivatives exhibit similar energy (phosphorylation occurs at histidyl or cysteyl residues), is surprising, as a single protein (or domain) coupling energy transfer and sugar phosphorylation would be sufficient for PTS function. A possible explanation for the complexity of the PTS was provided by the discovery that the PTS also carries out numerous regulatory functions. Depending on their phosphorylation state, the four proteins (domains) forming the PTS phosphorylation cascade (EI, HPr, EIIA, and EIIB) can phosphorylate or interact with numerous non-PTS proteins and thereby regulate their activity. In addition, in certain bacteria, one of the PTS components (HPr) is phosphorylated by ATP at a seryl residue, which increases the complexity of PTS-mediated regulation. In this review, we try to summarize the known protein phosphorylation-related regulatory functions of the PTS. As we shall see, the PTS regulation network not only controls carbohydrate uptake and metabolism but also interferes with the utilization of nitrogen and phosphorus and the virulence of certain pathogens.
From a few peaks rising above the fog we try to imagine what the hidden landscape underneath might look like.
—Pieter W. Postma, during a hiking trip in the Beaujolais region in October 1997.
INTRODUCTION
Carbon Catabolite Repression
Given a certain extracellular environment, a bacterium needs only a subset of the enzymes encoded by the genome to propagate, and therefore, it regulates gene expression differentially. For instance, in case a particular substrate is absent, the genes encoding the enzymes required for uptake and subsequent metabolism of the substrate are often repressed. Availability of the substrate then leads to the relief of repression. The classical example of this mode of transcription regulation is the lac operon of Escherichia coli (367). More complex responses of cells can occur when they are exposed to multiple nutrients at the same time. More than a hundred years ago, it was observed that growth on glucose can lower the activity of certain enzymes in bacteria and yeast. This phenomenon became known as the glucose effect (see also reference 744). One of the first descriptions of the glucose effect stems from the work of Dienert and was published in 1900 in Paris, France. He observed that Saccharomyces cerevisiae cells, which had been adapted to galactose (i.e., they were able to utilize galactose), rapidly lost the adaptation when the cells were exposed to glucose or fructose (188). This observation was later confirmed, for example, by Söhngen and Coolhaas, in Wageningen, The Netherlands, who measured the influence of temperature on the glucose effect in yeast (820). One of the first quantitative analyses of the glucose effect was carried out by Stephenson and Gale in Cambridge, England, who measured the activity of E. coli galactozymase. Galactozymase was understood as being the entity of enzymes necessary for the degradation of galactose by a specific organism. Those authors found that glucose exerted a strong repressive effect on galactozymase activity (more than sevenfold), while the utilization of glucose was not affected by galactose (836). In the early 1940s, Jacques Monod observed that when cultivated in a synthetic medium containing sucrose and dextran, the gram-positive bacterium Bacillus subtilis first utilized sucrose and then stopped growing for a certain period (lag phase or phase of adaptation) before the cells resumed growth by utilizing dextran (572). Owing to the biphasic growth he had observed, Monod called this phenomenon diauxie. He extended his studies with B. subtilis and found that diauxie was a more general phenomenon. When this organism was provided with sucrose or glucose, for example, and a second carbohydrate such as maltose, mannitol, inositol, or sorbitol, it also showed diauxic growth; i.e., sucrose or glucose was utilized first before the bacterium started to transport and metabolize the “less favorable” carbon source. He finally extended his studies to the gram-negative bacterium E. coli, which exhibited diauxie when grown in the presence of glucose or fructose, for example, as the preferred carbon source and xylose, arabinose, rhamnose, maltose, lactose, or glucitol as the less favorable sugar (572). It appeared that in each organism, a specific hierarchy existed for the utilization of carbon sources, with glucose usually being on top of it. In subsequent years, it was found that preferred sugars such as glucose, fructose, or sucrose, as long as they are present in sufficient amounts in the growth medium, repress the synthesis of the enzymes necessary for the transport and metabolism of less favorable carbon sources. The phenomenon therefore became known as carbon catabolite repression (CCR) (137). When the preferred carbon source is exhausted, bacteria first need to synthesize the enzymes necessary for the transport and metabolism of the less favorable carbon source (lag phase) before they can resume growth. The glucose effect reported at the beginning of the last century can therefore be considered CCR.
The regulatory mechanisms underlying CCR have since been intensively studied, and it was found that the preferential use of one carbon source over the other involves either the prevention of transcription activation or the repression of transcription. Here, we define CCR, somewhat more broadly, as the inhibitory effect of a certain carbon source in the growth medium on gene expression and/or the activity of enzymes involved in the catabolism of other carbon sources. Gene expression and enzyme activity are regulated via protein-DNA, protein-protein, and protein-metabolite interactions, and these interactions are in turn modulated via protein modification. Interestingly, the bacterial phosphoenolpyruvate (PEP):carbohydrate phosphotransferase system (PTS), which catalyzes the uptake and concomitant phosphorylation of numerous carbohydrates, plays a major role in bacterial CCR. However, quite different mechanisms have evolved in gram-negative and gram-positive organisms, and different PTS proteins are implicated in the two types of bacteria (for earlier reviews on the subject, see references 90, 756, and 845). In some bacteria, CCR is governed by other preferences and is possibly mediated by additional mechanisms. For instance, α-proteobacteria of the genera Sinorhizobium, Rhizobium, and Bradyrhizobium (83, 889) and γ-proteobacteria of the Pseudomonas family (135) prefer acetate or tricarboxylic acid (TCA) cycle intermediates such as succinate over carbon sources such as glucose, fructose, or lactose. The underlying mechanisms have not yet been unraveled, but in pseudomonads, they involve the catabolite repression control (Crc) protein (22, 331, 332, 573, 747, 982), and rhizobacteria, they involve inducer accumulation (83).
In this review, we will focus on mechanisms that control carbohydrate transport and metabolism in PTS-containing bacteria, and we will concentrate on the regulatory roles played by the components of the system. We start by discussing the individual components of the PTS and their properties. Subsequently, we will describe their role in catabolite repression and in other regulatory mechanisms governing carbon metabolism. We will pay particular attention to the mechanisms that have developed in the enteric bacteria E. coli and Salmonella enterica serovar Typhimurium (γ-Proteobacteria) and in low-G+C gram-positive bacteria (also known as Firmicutes) such as B. subtilis and Lactobacillus casei, but we will also refer to other proteobacteria and to gram-positive bacteria with high G+C content (also known as Actinobacteria). The two groups of gram-positive bacteria can be distinguished not only on the basis of the different G+C content of their DNA but also based on many other different characteristics. For example, actinobacteria have been shown to contain numerous proteins that have been detected only in members of this phylum so far (corynebacteria, streptomyces, mycobacteria, nocardiae, propionibacteria, bifidobacteria, leifsoniae, and rhodococci, to name just a few) (253). Important for this review is that most high-G+C gram-positive bacteria possess PTS components and transport sugars via this system but seem to be devoid of adenylate cyclase and HPr kinase/phosphorylase (41). Nevertheless, other PTS-related control mechanisms of carbon metabolism are operative.
The Phosphoenolpyruvate:Carbohydrate Phosphotransferase System
The PTS was discovered in E. coli by Kundig, Ghosh, and Roseman as a system that uses PEP to phosphorylate a number of hexoses, including N-acetylmannosamine, glucose, mannose, glucosamine, and N-acetylglucosamine (434). Subsequently, it was recognized that the PTS is in fact a transport system that catalyzes the uptake of numerous carbohydrates and their conversion into their respective phosphoesters during transport. After its discovery in E. coli, the PTS was found in many other bacterial species. The coupled transport and phosphorylation of carbohydrates were originally described as a two-step reaction catalyzed by two enzymes, enzyme I (EI) and enzyme II (EII), with the protein HPr as an intermediate phosphoryl donor:
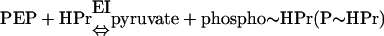 |
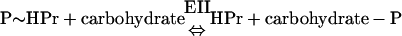 |
However, this representation is a bit misleading in the sense that EI, HPr, and EII behave in a functionally identical manner. They accept a phosphoryl group from a donor and donate it to an acceptor (i.e., they transfer a group), thus cycling between the phosphorylated and unphosphorylated states (depicted in Fig. 1). It is precisely this aspect of the mechanism that is exploited by the cell in PTS-mediated regulation.
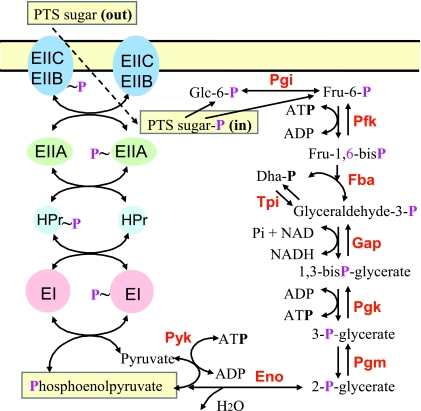
FIG. 1.
Carbohydrate transport and phosphorylation by the PTS and their coupling to glycolysis. Carbohydrates are transported and concomitantly phosphorylated by the PTS. The phosphorylated carbohydrate feeds into glycolysis, normally at the glucose-6-P or fructose-6-P level. Two phosphoenolpyruvate molecules are usually formed in glycolysis, one of which is used to drive the transport and initial phosphorylation of the carbohydrate. As a result, the phosphorylation state of the PTS proteins depends on both the concentration of extracellular carbohydrates and the ratio of internal phosphoenolpyruvate and pyruvate. Abbreviations for enzymes (in boldface type) are as follows: Pgi, phosphoglucose isomerase; Pfk, phosphofructokinase; Fba, fructose-1,6-bisphosphate aldolase; Tpi, triose-phosphate isomerase; Gap, glyceraldehyde-3-phosphate dehydrogenase; Pgk, phosphoglycerate kinase; Pgm, phosphoglycerate mutase; Eno, enolase; Pyk, pyruvate kinase.
The basic composition of the PTS is in fact similar in all species studied so far (for reviews, see references 678 and 730). It is comprised of two “general” cytoplasmic components, EI and HPr, which are common to all PTS carbohydrates. Carbohydrate specificity resides in EII, and hence, bacteria usually contain many different EIIs. Each EII complex consists of one or two hydrophobic integral membrane domains (domains C and D) and two hydrophilic domains (domains A and B), which together are responsible for the transport of the carbohydrate across the bacterial membrane as well as its phosphorylation. In a sense, the EII complexes constitute parallel transport pathways connected to a common PEP/EI/HPr phosphoryl transfer pathway. E. coli contains at least 15 different EII complexes, and the existence and properties of these enzymes have been established by genetic, biochemical, and physiological studies. A similar number of PTSs is present in B. subtilis (178, 706). EII complexes are formed either by distinct proteins or by a single multidomain protein. Likewise, fusion proteins that contain EI and/or HPr domains exist. A prominent example of the latter is FPr, which consists of HPr and an EIIA domain and mediates the phosphotransfer in the uptake of fructose by E. coli and Salmonella enterica serovar Typhimurium (266). In Rhodobacter capsulatus, a similar fusion protein also contains an EI domain (958).
Genome sequencing projects have uncovered many proteins that are similar to either one of the two general PTS proteins or to parts of carbohydrate-specific EII complexes (345, 867, 935). For example, in E. coli, the protein DhaM is composed of an EIIAMan-like regulatory domain followed by an HPr and a truncated EI domain (304). In other cases, the newly discovered proteins are formed by fusions between domains that originate from both PTS and non-PTS proteins, e.g., a Clostridium acetobutylicum response regulator (built from HPr and an NtrC-like protein) (721) and the Streptococcus thermophilus LacS protein (built from a melibiose carrier and EIIAGlc) (673). The functions of most of these chimeric proteins remain unknown, but for some proteins, like LacS and DhaM, the function has been elucidated. Indeed, one of the present challenges is finding the function of the newly discovered PTS-like proteins, most of which have not been detected by any biochemical or mutant study. A very intriguing group consists of the paralogs of EI and HPr. In E. coli, at least five paralogs of each general PTS protein were uncovered (345, 720). We will discuss some of these proteins below (see “SOME UNUSUAL PTS PATHWAYS AND PROTEINS”).
The general PTS proteins EI and HPr.
EI is encoded by the ptsI gene. Sequence comparisons reveal that the EIs (about 570 residues; 63 kDa) from various gram-positive and gram-negative bacteria exhibit significant identity (345, 678). EI is autophosphorylated in the presence of Mg2+ (940) at the N-3 position of the imidazole ring of a conserved histidine (His-189 in E. coli EI) (16, 940), which is located on the N terminus of the protein. The C terminus contains the PEP binding site and is necessary for dimerization (114, 254, 798). The two-domain structure of the protein was discovered by high-sensitivity differential scanning calorimetry (478). Limited proteolysis of E. coli EI in vitro results in a specific split and provides a stable peptide representing the N-terminal domain (455). The N-terminal domain of EI (EI-N) (115) as well as the C-terminal domain (EI-C) (234) were cloned and characterized. EI-C was shown to complement EI-N both in vivo and in vitro; i.e., EI-N was phosphorylated in the presence of EI-C, PEP, and Mg2+ (234). A truncated protein composed of the first 259 amino acids has been synthesized and purified, and both the crystal (475) and solution (258) structures were resolved. It appears that phosphorylation does not drastically change the conformation of the N-terminal domain per se. Recently, the crystal structure of the C-terminal domain of EI of the thermophile Thermoanaerobacter tengcongensis was also elucidated (619). EI exhibits about 30% sequence similarity with pyruvate phosphate dikinase (PPDK) (328, 605, 670), and the various domains in both proteins have similar structures (619). PPDK, which converts ATP, Pi, and pyruvate into AMP, pyrophosphate (PPi), and PEP; PEP synthase, an enzyme that catalyzes the conversion of pyruvate and ATP into PEP, AMP, and Pi and that is also phosphorylated on a histidyl residue (590); and EI have related functions. For PPDK, a structural model with three domains has been proposed: a C-terminal PEP/pyruvate binding domain, an N-terminal nucleotide binding domain, and a P∼His domain linked to the other two domains via two flexible polypeptide segments (328). Although the nucleotide and PEP/pyruvate binding sites are approximately 45 Å apart, the P∼His domain is thought to bridge the distance by swiveling between the two other domains to enable the phosphoryl transfer. Recent data obtained from a comparison of several PPDK crystal structures are consistent with the proposed model (587). Similar to PPDK, a large distance (>20 Å) between the PEP/pyruvate binding site and the phosphorylatable histidine was observed in a model of EI in which the N-terminal and C-terminal EI structures were fused (619). Swiveling of the N-terminal domain by changing the position of the domains with respect to each other but retaining the structure of the individual domains reduces the distance significantly (to 7.4 Å). In the case of EI, the N-terminal domain contains the HPr binding site rather than the nucleotide binding site of PPDK and PEP synthase. This model was confirmed by recent studies in which the structure of full-length EI of Staphylococcus carnosus was determined (516). The phosphohistidine and HPr-binding domain are clearly separated from the C-terminal dimerization and PEP binding domain. The structure also revealed the extensive interaction surface related to the dimer. Thermodynamic analyses of the influence of several effectors on the conformational stability of EI of Streptomyces coelicolor showed that at a low pH, EI is partly unfolded and that the presence of PEP causes structural changes (356). Two other recent studies on the structure of EI (639) and its C-terminal domain (638) suggest a relatively large structural variability for monomeric EI, which progressively diminishes when EI dimerizes and binds Mg2+ and PEP. The observed effects are explained by a swiveling mechanism, as described for PPDK. The structure thus provides additional evidence for the proposed conformational change necessary to facilitate phosphotransfer.
HPr (about 90 residues; 9 to 10 kDa) is encoded by the ptsH gene and has been purified from a variety of organisms (678). Similarity is most pronounced around the active-site histidyl residue, His-15 in the HPr of most enteric bacteria and firmicutes (183, 681, 941). HPr is phosphorylated at the N-1 position of the imidazole ring of His-15. The crystal and solution structures of HPrs from E. coli and B. subtilis as well as a few other species are known (for reviews, see references 33, 123, 329, and 936). The molecule forms an open-faced β-sandwich consisting of a four-stranded antiparallel β-sheet that is covered at one side by one short and two long α-helices. The active-site His-15 is located in the N-terminal part of the first long α-helix and is exposed to the solvent.
The solution structures of the complex between HPr and the N-terminal domain of EI (260) and between HPr and EIIAGlc of E. coli (929) have been determined. The absence of large changes of the chemical shift values for the backbone atoms upon complexation indicates that the interactions of HPr with EI and EIIAGlc do not require considerable conformational changes in any of the domains of the PTS binding partners. The binding interface of HPr and glycogen phosphorylase (see “REGULATION OF CARBON METABOLISM IN GRAM-NEGATIVE ENTERIC BACTERIA”) was also mapped, and the interaction surface of the three structures was compared (930). EI and EIIAGlc interact with the same narrow region of HPr, whereas the region of interaction with glycogen phosphorylase is somewhat wider (929, 930). This is consistent with the higher binding affinity reported for HPr with glycogen phosphorylase than for HPr with EI or EIIAGlc (259, 799). A similar interaction surface was reported for E. coli HPr binding either EIIAMtl (139, 908) or EIIAMan (949) and B. subtilis HPr binding EIIAGlc (123). The key interacting residues are located in helices 1 and 2 and the loops preceding helix 1 and following helix 2. Studies with a large number of ptsH mutants, which show lowered affinity for binding to EI, suggest that the same residues are important for the interaction between EI and HPr (85).
In most low-G+C gram-positive bacteria and a few gram-negative organisms, HPr can also be phosphorylated by an ATP-dependent protein kinase on a seryl residue, e.g., Ser-46 in B. subtilis. We will discuss the importance of the second regulatory phosphorylation in detail below (see REGULATORY FUNCTIONS OF P-Ser-HPr). The regulatory phosphorylation is not part of the phosphoryl transfer to carbohydrates, but phosphorylation of the seryl residue slows the phosphoryl transfer from P∼EI to HPr at least 100-fold. E. coli HPr contains a Ser-46 residue but lacks an HPr kinase. Nevertheless, replacement of Ser-46 in E. coli HPr by an aspartyl residue lowers the affinity of P∼EI for HPr almost 1,000-fold (589). This effect is most likely caused by electrostatic repulsion, as the mutation caused by the replacement of Ser-46 with an aspartyl residue (Ser46Asp) leads to only weak structural changes (952).
Enzyme II complexes.
The carbohydrate specificity of the PTS resides in the EIIs, which consist of an integral membrane domain facing both the periplasmic space and the cytoplasm and cytoplasmic domains or proteins. All EIIs are built basically in a similar modular manner (764) and can consist of up to four separate proteins (for reviews, see references 464, 678, 730, and 813). The PTSs were classified into four (super)families with distinct evolutionary origins on the basis of the phylogenies of the EIIs (759) as follows: (i) the glucose-fructose-lactose superfamily, comprised of the glucose family (e.g., E. coli EIIAGlc/EIICBGlc or B. subtilis EIICBAGlc [CB, CBA, etc., indicate the domain order in multidomain EIIs]), the fructose-mannitol family (e.g., E. coli EIICBAMtl), and the lactose family (e.g., L. casei EIIALac/EIICBLac); (ii) the ascorbate-galactitol superfamily, comprised of the ascorbate family (e.g., E. coli SgaA/SgaB/SgaT) (358, 989) and the galactitol family (e.g., E. coli EIIAGat/EIIBGat/EIICGat) (610); (iii) the mannose family (e.g., E. coli EIIABMan/EIICMan/EIIDMan or B. subtilis EIIALev/EIIBLev/EIICLev/EIIDLev [a mannose- and fructose-specific PTS]); and (iv) the dihydroxyacetone family (e.g., E. coli EIIA-HPr-EIDha [DhaM] [304] or EIIADha in firmicutes).
The presence of a common preserved domain in the latter two families (411) might indicate a similar evolutionary origin of the two families and thus the existence of a third superfamily. The sequence-based classification of the various PTSs is supported by X-ray crystallography and nuclear magnetic resonance (NMR) studies, which clearly show that the structures of the EIIA (474, 617, 818, 858, 906, 955) and EIIB (1, 2, 98, 459, 623, 778, 906) domains/proteins belonging to the various classes are quite different. Information on the structure of the integral membrane domain EIIC (and EIID) is limited. The membrane topologies of these proteins have been studied using Cys replacement mutagenesis and chemical modification by thiol reagents for EIIBCABgl (960) and EIICBAMtl (918).
Extensive work on the intricate properties of the EII complexes and the detailed mechanisms by which they transport and phosphorylate carbohydrates has been performed in the laboratories of G. T. Robillard, B. Erni, and G. R. Jacobsen. The results that focus predominantly on EIICBAMtl, EIICMan/EIIDMan, and EIICBGlc have been reviewed extensively (369, 730, 813), and we will therefore discuss only a few important aspects. Some more recent papers included work on the properties of a cyclized protein formed by the soluble EIIAGlc and the membrane-bound EIICBGlc (812), the dimerization of EIICBAMtl (903, 904), sugar recognition by the EIICMan/EIIDMan and EIICBGlc of E. coli (256), the membrane topologies of EIIBCABgl (960) and EIICBAMtl (918), and transient-state kinetics of enzyme EIICBGlc (544).
The glucose-specific EII complex of enteric bacteria consists of two distinct proteins, the cytoplasmic protein EIIAGlc (gene crr) and the membrane-associated protein EIICBGlc (gene ptsG), in which the EIIB domain is hydrophilic and in contact with the cytoplasm and the EIIC domain is buried within the membrane. The EIIA and EIIB domains are defined as the domains that receive the phosphoryl group from P∼HPr and P∼EIIA, respectively. Whereas the phosphorylated residue in the EIIA domain/protein is a histidyl residue, that in the EIIB domain/protein can be either a histidyl residue (mannose family) or a cysteyl residue (all other families). The latter was first demonstrated in E. coli EIIMtl (634), and the specific phospho-cysteyl residue was thereafter identified in a number of cases by analytical methods (547, 633). The phosphoryl group of the EIIB domain is transferred to the carbohydrate after translocation of the carbohydrate by the EIIC domain across the membrane and delivery at the inside face of the cytoplasmic membrane. In the case of the mannitol-specific E. coli EIIMtl or the glucose-specific B. subtilis EIIGlc complex, the EII is a single polypeptide that contains the two hydrophilic domains EIIA and EIIB as well as the transmembrane domain EIIC. Again, in other EII complexes, such as the mannose-specific E. coli EIIMan, phosphoryl groups are carried by two histidyl residues present in the A and B domains of the cytoplasmic EIIABMan, whereas translocation of the carbohydrate requires the two transmembrane subunits EIIC and EIID.
Organization of the PTS.
Phosphoryl transfer between the PTS proteins proceeds by an in-line associative mechanism (48). The transfer is accommodated by the formation of heterodimeric transition complexes, and considering the kinetics of the transfer, the heterodimeric association should be transient. The solution structures of different transition complexes, including EI and HPr (260), HPr and EIIAGlc (124, 929), HPr and EIIAMtl (139), HPr and EIIAMan (949), EIIAGlc and EIIBGlc (98, 270), and, finally, P∼EIIAChb and Cys10Ser mutant EIIBChb (398), could be determined. The formation of the heterodimeric complexes has a significant effect on the flux control properties of the individual components of the PTS (738, 896, 901).
Based on the observation that E. coli EI and HPr are attached to membrane fragments of E. coli (757), it was speculated that the PTS components associate into a metabolon (611), which would improve PTS function. However, it is not clear how phosphotransfer from one component to the next could take place in a fully associated system without linkers to provide room for the domains to move, and furthermore, as it was calculated that diffusion should not limit glucose influx (239, 240), there should be no functional driving force to fully associate into an EI-HPr-EII complex. Formation of larger complexes by EI linked to yellow fluorescent protein has been observed in cells grown on PTS as well as non-PTS carbohydrates when they reached stationary phase and high cell densities (637). The observed distribution of the fluorescence over the cells was punctate and sometimes even bipolar (after induction of yellow fluorescent protein-EI with IPTG [isopropyl-β-d-thiogalactopyranoside]). The aggregation appeared to be reversible, as it disappeared after the addition of a carbohydrate, which led to renewed growth of the cells and dispersion of the fluorescence. Thus, phosphotransfer activity within the PTS, as invoked by the addition of a carbohydrate, in fact seems to promote the overall dissociation of the PTS components rather than their association into a metabolon.
Notwithstanding, several PTS proteins were experimentally proved to associate to functional homodimers. The first PTS protein that was found to functionally dimerize was EI (432, 560; also reviewed in reference 114). Later, it was shown that the N-terminal domain does not dimerize (115) but that the C-terminal domain is responsible for dimerization (86). In fact, the association properties of the isolated C-terminal domain are similar to that of entire EI, although the binding constants are orders of magnitude larger (638). It was proposed that the monomer/dimer transition of EI could potentially regulate uptake flux (940), and as a result, the transition has been extensively studied. The idea was supported by the fact that PEP phosphorylates only dimeric EI, and the rate of association/dissociation is very slow, especially compared to sugar uptake (114, 539). The monomer/dimer transition has been studied by various analytical techniques, and many physiological conditions have been tested. It was observed that P∼EI has a 10-fold increased dimerization constant with respect to unphosphorylated EI and that the dimerization of EI is promoted by PEP and Mg2+ and inhibited by pyruvate (190-192). It was concluded that the stimulatory effect of Mg2+ and PEP on EI association results from a change in conformation. Association of both normal EI and the nonphosphorylatable mutants H189E, H189A, and H189Q was studied by carrying out sedimentation equilibrium experiments (639). The association constant appears to be very sensitive to pH, temperature, and ionic strength and may vary by as much as 700-fold. It was concluded that the ligands Mg2+ and PEP are the major determinants for dimerization and that their binding leads to conformational changes. A model that accommodates the known facts about the dimerization was presented. A potential regulatory function of EI dimerization with regard to the role of PEP is discussed below.
Several membrane-spanning EIIs have also been shown to dimerize, such as EIICBAMtl (730) and EIICBGlc (213, 214, 548). Proteome analysis of stable oligomeric protein complexes confirmed the existence of EIICBAMtl and EIICBGlc oligomers in the membrane of E. coli (826). In addition, oligomers of EIINag and EIITre were detected, which complies with a generalized picture in which the PTS permeases function as dimers (or oligomers). In contrast, it is generally assumed that HPr and EIIA do not form functional homooligomers. However, several experimental observations suggest the opposite. Biochemical evidence strongly suggested that EIIALac forms homotrimers (176, 319). This suggestion was supported by the crystal structure of EIIALac from Lactococcus lactis, which also revealed a homotrimer (818). In addition, it was reported that four molecules of EIIAGlc associate with one EIICBGlc homodimer (213). Moreover, multimeric EIIAGlc was observed during gel filtration (786) and in crystals (224). Dimeric EIIAChb and P∼EIIAChb were observed when ultracentrifugation was carried out (398). EIIABMan was found in the dimeric form both in the cytoplasm and linked to the membrane (216, 826). Also, EIIANtr, a protein homologous to EIIAs that are functional in sugar transport, crystallizes as a dimer (73), although it seems to be a monomer in solution (927). Crh, an HPr-like protein of B. subtilis, was shown to form homodimers in crystals (644), and F29W HPr from Geobacillus stearothermophilus (previously Bacillus stearothermophilus) forms similar domain-swapped dimers in crystallization experiments (827). On the other hand, several measurements performed with the protein in solution suggested that F29W HPr forms monomers. Recently, in vitro experiments provided kinetic evidence that all components of the E. coli glucose PTS form functional dimers (R. Bader, J. G. Blom, K. J. Hellingwerf, M. A. Pelletier, H. V. Westerhoff, and C. Francke, unpublished results).
Although the supposed primary function of the PTS is the transfer of the phosphoryl group from PEP to carbohydrates, all reactions up to and including the EIIs are reversible. The equilibrium constant, Keq, for the reaction PEP⇔P∼EI⇔P∼HPr is about 80 for the E. coli enzymes (539), whereas the Keq for the phosphoryl group transfer between HPr and EIIAGlc from E. coli has a value of between 1 and 1.5 (540). Thus, the overall Keq for the phosphoryl group transfer from PEP to EIIAGlc is in the order of 80 to 120. Furthermore, the Keq for the reaction P∼EIIAGlc + EIICBGlc⇔EIIAGlc + P∼EIICBGlc was determined to be 12 (539), very close to the estimated value of 7 (737). The number signifies that the phosphotransfer potential of the thiophospho bond in P∼Cys-EIICBGlc is very close to that of the phosphoamidate bond in phosphohistidine of the other PTS proteins. Only the final step, P∼EIIBGlc + glucose⇔EIIBGlc + glucose-6-phosphate (glucose-6-P), is virtually irreversible, with a measured forward rate of 3.2 × 106 M−1 s−1 (539). These values indicate that under physiological conditions, the phosphoryl transfer reactions catalyzed by the PTS between PEP and P∼EIIBGlc can run in both directions, from one high-energy P∼enzyme intermediate to the next or back to the previous reactions. This differs from most eukaryotic signal transduction systems in which the seryl, tyrosyl, and threonyl residues that are often phosphorylated represent low-energy phosphoenzyme intermediates (837). The reversibility of the phosphoryl group transfer in the PTS has regulatory consequences because it allows the metabolic network to control the phosphorylation state of the PTS proteins in a number of ways, and we will discuss how numerous cellular processes are regulated by the phosphorylation states of certain PTS proteins below (see Table 1 for a summary).
TABLE 1.
The PTS components and their various non-PTS interaction and/or phosphorylation partners
| PTS component | Non-PTS phosphorylation/interaction partner(s) | Phosphorylation or interaction | Effect(s) of phosphorylation or interaction |
|---|---|---|---|
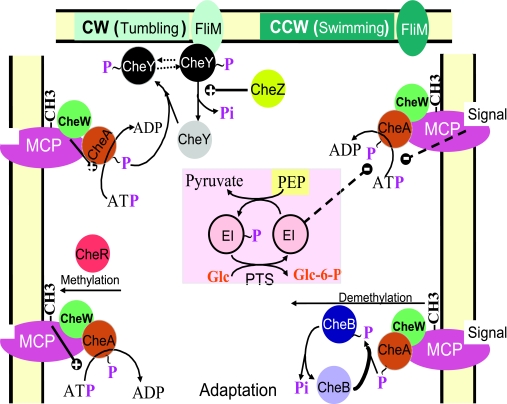
| EI (E. coli) | CheA, chemotaxis protein | Interaction | Stimulates CheA autophosphorylation |
| P∼EI (B. subtilis) | CheA, chemotaxis protein | Interaction | Inhibits CheA autophosphorylation |
| HPr (E. coli) | Glycogen phosphorylase | Interaction | Stimulates glycogen phosphorylase activity |
| P∼His-HPr (E. coli) | Glycogen phosphorylase | Interaction | Prevents binding of HPr to glycogen phosphorylase |
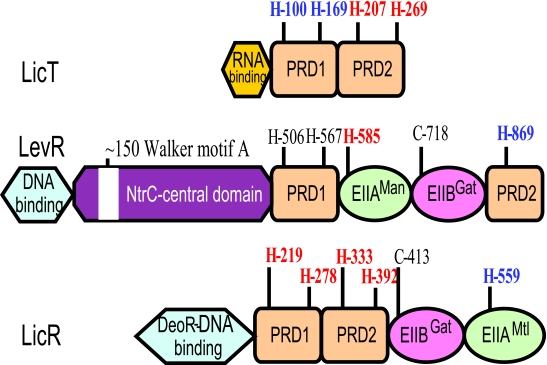
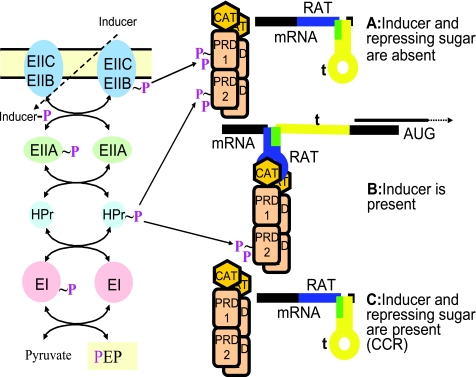
| P∼His-HPr | Antiterminators, BglG, SacY, LicT, etc.a | Phosphorylation in PRD2 | Stimulates antitermination,b alternate CCR mechanism |
| Transcription activators, LevR-like | Phosphorylation in the EIIAMan domain | Stimulates transcription, alternate CCR mechanism | |
| Transcription activators, MtlR-like | Phosphorylation in PRD2 | Stimulates transcription, alternate CCR mechanism | |
| Transcription activators, LicR-like | Phosphorylation in PRD1 and PRD2 | Stimulates transcription, alternate CCR mechanism | |
| P∼His-HPr (firmicutes) | Glycerol kinase GlpK | Phosphorylation, His in the N-terminal half | Stimulates GlpK activity, inducer exclusion |
| LacS, RafP; transporters for lactose, raffinose | Phosphorylation in the EIIAGlc domain | Stimulates substrate/galactose exchange | |
| P-Ser-HPr (firmicutes) | CcpA | Interaction | CCR or CCA, catabolite corepressor |
| Non-PTS transporters for maltose, ribose, etc. | Interaction | Inducer exclusionc | |
| RbsR | Interaction | Physiological role not yet established | |
| P-Ser-Crh (bacilli) | CcpA | Interaction | CCR or CCA, catabolite corepressor |
| EIIAGlc (enterobacteria) | Non-PTS transporters LacY, MalK, MelB | Interaction | Inducer exclusion |
| Glycerol kinase, GlpK | Interaction with the C-terminal domain | Inducer exclusion | |
| Fermentation respiration switch protein, FrsA | Interaction | Probably causes increased respiration | |
| P∼EIIAGlc (enterobacteria) | Adenylate cyclase | Interaction | CCR, activation of adenylate cyclased |
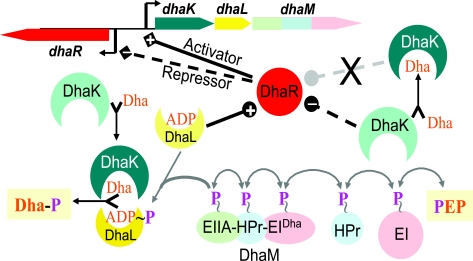
| P∼EIIADha | DhaL, L subunit of dihydroxyacetone kinase | Phosphoryl transfer | ADP bound to DhaL is converted into ATP |
| EIIBGlc (enterobacteria) | Mlc | Interaction | Derepression of genes of the Mlc regulon |
| P∼EIIBs, Glc/Sac/Lac class | Antiterminators, BglG, SacY, LicT, etc.a | Phosphorylation in PRD1e | Inhibits antitermination, induction mechanism |
| P∼EIIBs, Man/Lac class | Transcription activators, LevR-like | Phosphorylation in PRD2e | Inhibits transcription, induction mechanism |
| P∼EIIBs, Mtl/Gut class | Transcription activators, MtlR-like | Phosphorylation in the EIIAMtl domaine | Inhibits transcription, induction mechanism |
| P∼EIIBs, Lac/Cel class | Transcription activators, LicR-like | Phosphorylation in the EIIAMtl domaine | Inhibits transcription, induction mechanism |
For a more detailed summary of well-studied antiterminators, see Table 4.
Certain antiterminators (such as SacY and GlcT of B. subtilis) are phosphorylated in vitro by P∼His-HPr in PRD2, but their activity is not stimulated by this modification.
An interaction of P-Ser-HPr with the maltose or ribose transport systems in L. casei and L. lactis has so far not been demonstrated but is suggested from genetic experiments.
An additional cellular factor seems to be necessary for the activation of adenylate cyclase by P∼EIIAGlc (632).
It is not clear whether phosphorylation of the antiterminators/transcription activators occurs via P∼His-HPr and P∼EIIBs stimulate only the phosphoryl transfer or whether the phosphoryl group is transferred from P∼EIIBs to the antiterminators/transcription activators. It is possible that both modes of regulation exist.
REGULATION OF CARBON METABOLISM IN GRAM-NEGATIVE ENTERIC BACTERIA
In the following sections, we will discuss the numerous regulatory functions carried out by EIIAGlc in enteric bacteria. This protein not only is involved in the regulation of adenylate cyclase, and therefore in CCR, but also interacts with several non-PTS permeases and glycerol kinase to inhibit their activity. The latter phenomenon is described by the term inducer exclusion. We will present new insights into the complex regulation of the intracellular cyclic AMP (cAMP) level and the binding of EIIAGlc to adenylate cyclase and to non-PTS permeases.
EIIAGlc Is the Central Processing Unit of Carbon Metabolism in Enteric Bacteria
The integration of hundreds of enzymatic reactions into a metabolic network and the complex response of such a network to environmental changes require global regulation at the level of enzyme activity and gene transcription (89, 592, 917). A bacterium that is confronted with changes in the supply of nutrients can adapt its metabolic potential through the induction of specific catabolic operons (most often induced by the substrate) (90, 94). More drastic changes may require global adaptation and therefore the induction of complete regulons (89, 592, 917). Transcriptome data indicate that when E. coli cells that have been growing on a rich carbon source such as glucose are exposed to a carbon source allowing only slow growth, the number of induced genes increases progressively (485). Low growth rates result in the expression of many transport systems and catabolic genes for nutrients that might be absent but that would stimulate growth if present. Results obtained by high-throughput nutrient screening of glucose-grown cells and cells grown under carbon limitation confirmed the effects observed by transcriptome analysis: cells grown under carbon limitation do utilize a large variety of carbon sources, whereas cells growing on glucose do not (the result of CCR) (360). The large-scale transcriptional responses in E. coli are mediated via a limited number of global regulators (527). In enteric bacteria, global regulation of carbon metabolism involves several σ and transcription factors such as Crp (cyclic AMP receptor protein) (reviewed in references 76, 95, 96, 416, and 636), Mlc (666), FruR (145, 267, 268, 762), CsrA (carbon storage regulator) (451, 742, 743, 752, 861), and σ54 (25, 150, 843). The activities of some of these regulators are directly affected by the PTS.
The importance of the PTS in the regulation of carbon metabolism in enteric bacteria became apparent when ptsHI mutants containing defective EI and/or HPr were isolated. These mutants failed to grow not only on PTS carbohydrates but also on a large number of non-PTS carbon sources like lactose, melibiose, glycerol, and maltose (for reviews, see references 677, 678, and 763). Analysis of suppressor mutations, which restored growth of ptsHI mutants on the non-PTS carbon sources (765, 767), revealed that these mutations were in the crr gene (carbohydrate repression resistant), which was later shown to be the structural gene for EIIAGlc (542, 543, 786). While ptsHI-crr triple mutants grew on the non-PTS carbon sources, they did not resume growth on PTS carbohydrates. These results indicated that not only is EIIAGlc involved in glucose transport and phosphorylation via the glucose PTS, it also regulates the transport and/or metabolism of several non-PTS carbon sources.
The changes of structure or charge induced by phosphorylation or dephosphorylation determine the regulatory role of EIIAGlc. P∼EIIAGlc is required for the activation of adenylate cyclase. S. enterica serovar Typhimurium and E. coli crr mutants exhibit a low residual adenylate cyclase activity, generally 5 to 20% of the activity found in the parental strain (227, 469, 595). Unphosphorylated EIIAGlc can bind to and inhibit numerous non-PTS proteins, including the permeases for lactose (LacY) and melibiose (MelB), the ATP-hydrolyzing component of the maltose transport system (MalK), and glycerol kinase (GlpK). As a result, induction of the genes involved in the uptake and metabolism of these substrates is prevented, and hence, the inhibitory process was called inducer exclusion.
Both control mechanisms, inducer exclusion and modulation of adenylate cyclase activity, have a physiological function and allow the cell to choose between different carbon sources as long as the uptake of glucose and other PTS carbohydrates leads to the dephosphorylation of EIIAGlc. The connections between carbohydrate uptake, the phosphorylation state of EIIAGlc, adenylate cyclase activity, and the intracellular concentration of metabolites such as PEP and pyruvate relate metabolic flux directly to the regulation of solute uptake and gene expression. In Fig. 2, a summary of the mechanisms underlying CCR in enteric bacteria is given. Although EIIAGlc-mediated regulation has been found only in enteric bacteria so far, this signal transduction system has served and serves as a model for other regulatory processes that involve reversible phosphorylation, such as PTS-regulated transcription activation or antitermination.
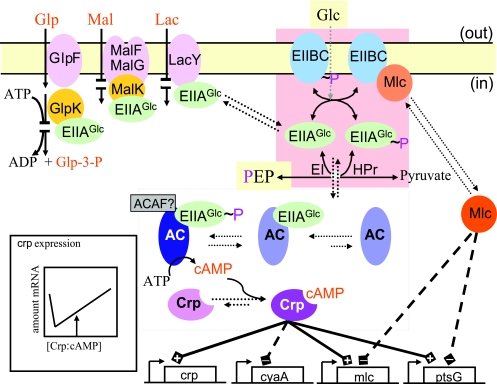
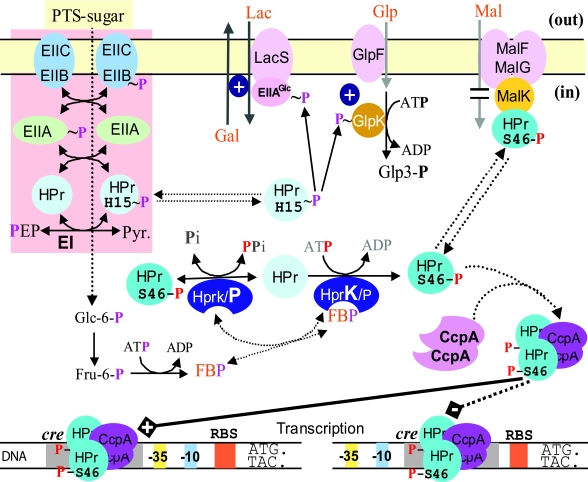
FIG. 2.
Mechanisms underlying CCR and inducer exclusion in enteric bacteria. The import of glucose and other PTS carbohydrates leads to net dephosphorylation of the PTS proteins (including EIIAGlc and the B domain of EIIBCGlc) and thereby to inducer exclusion and recruitment of the transcription regulator Mlc to the membrane. The upper left part of the figure shows that unphosphorylated EIIAGlc blocks the import of lactose, maltose, and melibiose and the phosphorylation of glycerol by binding to the respective transporter or kinase. The upper right part of the figure shows recruitment of Mlc by unphosphorylated EIIBCGlc, which prevents the regulator from binding to its target sites on the DNA. In the absence of glucose and in the presence of phosphoenolpyruvate, the PTS proteins are found mainly in the phosphorylated state. The central part of the figure shows that phosphorylated EIIAGlc activates adenylate cyclase (AC) but probably only in the presence of an unknown adenylate cyclase activation factor (ACAF). Adenylate cyclase binds phosphorylated as well as unphosphorylated EIIAGlc (see reference 632). The bottom part of the figure shows the effect of the activated transcription factors (free Mlc and Crp:cAMP) on the transcription of the genes encoding Crp, adenylate cyclase, Mlc, and EIIBCGlc, respectively. The inset shows the Crp:cAMP concentration dependence of crp transcription (deduced from data reported in references 310 and 311). The arrow indicates the physiological concentration of activated Crp in exponentially growing cells in the absence of PTS carbohydrates.
Transcription Regulation by Crp/cAMP and Role of P∼EIIAGlc
In enteric bacteria, Crp is one of the few truly global regulators, as it controls the expression of a vast number of genes/operons (34, 291, 527, 990). Crp is activated by binding cAMP, which is synthesized from ATP by adenylate cyclase (gene cyaA) (for recent papers on the structure of the Crp/cAMP regulator, see references 316, 471, 671, and 888). Different genes and operons require different levels of Crp/cAMP for full expression because the Crp/cAMP complex can adopt several conformationally active states and because the affinity for the recognition sites on the DNA varies (149, 316, 417, 484, 853, 888).
The concentration of Crp/cAMP is tightly regulated by the PTS. Soon after the discovery of cAMP in E. coli, it was observed, for example, that the addition of glucose to cells growing on lactate lowers the concentration of cAMP (508). Later results confirmed this finding (212, 384), and experiments with toluenized cells established that adenylate cyclase activity is inhibited by glucose (318, 648-652). Other PTS carbohydrates were also shown to inhibit cAMP synthesis, provided that the cognate EII complex was intact and induced (317, 767). Studies with ptsHI and crr mutants demonstrated that adenylate cyclase activity is low in both types of mutants (227, 469, 595). Based on these and other studies (see references 678 and 763), a regulatory mechanism for the activation of adenylate cyclase by P∼EIIAGlc was proposed. In this scheme, transport and phosphorylation of glucose or other PTS carbohydrates decrease the extent of phosphorylation of the PTS proteins, including EIIAGlc, and thus lower the activity of adenylate cyclase, whereas growth on non-PTS carbohydrates, particularly poor carbon sources like lactate and succinate, results in fully phosphorylated PTS proteins and the activation of adenylate cyclase. However, this model does not explain how growth on non-PTS carbon sources such as glucose-6-phosphate or gluconate can lead to intracellular cAMP levels similar to, or sometimes lower than, those observed in glucose-grown cells (212, 337). Moreover, the inhibitory effect of glucose-6-phosphate is found only in intact but not in toluenized cells (318), and we will discuss this important point later.
Regulation of cyaA and crp transcription.
Most of our current knowledge regarding Crp/cAMP-mediated regulation is derived from the study of cyaA, crp, and/or ptsHI-crr mutants (76, 416, 636, 677, 678, 763, 890). The effect of different carbon sources on growth, gene expression levels, cAMP concentrations, and Crp levels has been tested with these mutants. The addition of exogenous cAMP was used to distinguish between the effects caused by concentration changes of either cAMP or Crp. One class of mutants, designated crp* or crp(In), produced Crp, which is active without cAMP. Unfortunately, an interpretation of the mutant data is not easy, as the factors involved in cAMP-mediated regulation are interdependent, as depicted in Fig. 2.
Inactivation of the cyaA gene disables bacteria like E. coli and S. enterica serovar Typhimurium from growing on a large number of carbon sources (76), while cyaA overexpression appears to be detrimental (699). Inactivation of crp also affects the cAMP concentration by leading to a drastic increase in the rate of cAMP synthesis (75, 170, 241, 363, 679). The increase in the cAMP concentration depends on an intact crr gene. In crp crr double mutants, cAMP overproduction is markedly reduced (from about 60-fold of the wild-type level in the crp strain to only 4-fold of the wild-type level in the double mutant) (143, 170).
The phenomena described above are related to feedback regulation on the level of transcription. Expression of cyaA is negatively regulated by Crp/cAMP in both E. coli (10, 387, 395, 576) and S. enterica serovar Typhimurium (221), whereas expression of crp is both positively (310) and negatively (9, 140, 311) affected by Crp/cAMP. As a result, strong crp expression occurs at low and high Crp/cAMP concentrations (due to reduced inhibition and increased stimulation, respectively), whereas low crp expression is observed at intermediate Crp/cAMP concentrations (310). The concentration of Crp/cAMP in exponentially growing cells is an order of magnitude higher than that allowing minimal crp transcription (310, 365). Consequently, a decrease in the cAMP concentration caused by the addition of glucose to these cells should lower the transcription of crp and hence the concentration of Crp. Glucose-grown E. coli cells indeed contain less Crp, and this was shown not to be due to faster degradation of the crp mRNA (364, 365). The glucose effect on crp expression is absent in mutants lacking either adenylate cyclase or Crp, consistent with the postulated role of Crp/cAMP. The growth stage of E. coli cultures also has an influence on the amount of Crp (365). These variations might explain why some authors reported complete relief from glucose repression after the addition of cAMP (647), whereas others observe only partial relief (932).
Expression of the EIIAGlc-encoding crr gene is almost completely independent of Crp/cAMP (857). In contrast, expression of the EIICBGlc-encoding ptsG gene is almost fully repressed in cyaA or crp mutants (727). Owing to the low level of the glucose transporter EIICBGlc in these mutants, the addition of glucose to cyaA or crp mutants does not lead to the same extent of dephosphorylation of P∼EIIAGlc as in the wild-type strain. The regulation of crp transcription by factors other than Crp/cAMP adds further complexity to catabolite repression data. crp transcription is negatively regulated by both the DNA binding protein Fis (280), the transcription of which is in turn regulated by Crp/cAMP (591), and SpoT (378), the protein that mediates the stringent response and that catalyzes the synthesis of ppGpp (guanosine tetraphosphate).
Regulation of adenylate cyclase activity.
Whether P∼EIIAGlc exerts its regulatory effect on adenylate cyclase directly or indirectly has been an open question for a long time. Early experiments with permeabilized cells containing an intact PTS showed that adenylate cyclase activity is stimulated by potassium and phosphate and inhibited by α-methylglucoside (α-MG) and pyruvate (476, 477, 701), which is in line with a stimulatory effect by P∼EIIAGlc. However, in vitro experiments with purified adenylate cyclase were not conclusive (963). The effect of EIIAGlc phosphorylation on the activation of adenylate cyclase was studied in crr mutants producing EIIAGlc in which the phosphorylatable His-90 (197) and the catalytically important His-75 had been changed. In comparison to wild-type EIIAGlc, His75Gln EIIAGlc synthesized in a crr mutant caused an approximately fourfold activation of adenylate cyclase. In contrast, neither His90Gln nor His90Glu EIIAGlc activated adenylate cyclase (700, 852). The latter two mutant proteins are not phosphorylated by P∼His-HPr, while His75Gln EIIAGlc is. Takahashi and coworkers also found that His75Glu EIIAGlc, which is barely phosphorylated in vitro (700), can slightly activate adenylate cyclase (852). In wild-type EIIAGlc, His-75 and His-90 are in very close proximity, and the replacement of His-75 with a negatively charged Glu possibly mimics phosphorylated His-90. Although these results indicate that phosphorylation of His-90 of EIIAGlc is important for the activation of adenylate cyclase, they do not prove a direct interaction. Recent results with adenylate cyclase tethered to the membrane showed that although both P∼EIIAGlc and unphosphorylated EIIAGlc bind with similar affinity to the protein, it is activated by P∼EIIAGlc only when an additional, not-yet-identified factor from cell extracts is present (632) (Fig. 2).
From studies with truncated adenylate cyclase, it can be concluded that the catalytic activity resides in the N-terminal domain, with the C-terminal domain required for EIIAGlc-mediated regulation (632, 699, 746). Starting from an E. coli ptsHI-crr deletion strain containing, in addition, an Asp414Asn mutation in cyaA, which prevents the large increase in adenylate cyclase activity in a crp mutant (143), suppressor mutants affected in the cyaA gene could be isolated. Most suppressor mutations were located in the region around Asp-414 and resulted in a truncated adenylate cyclase that was about half the size of the wild-type enzyme (144). The truncated molecules produce about 10 times more cAMP in a crr strain than the wild-type enzyme. These results suggest that the N-terminal domain of adenylate cyclase is active in the absence of P∼EIIAGlc and that the C-terminal domain acts as an inhibitor that may be removed/inactivated by P∼EIIAGlc.
Mutation of crp leads to a drastic increase of extracellular cAMP. This increase is 50-fold diminished when the C-terminal 48 amino acids of adenylate cyclase are cut off. In contrast, the increase is diminished by only fourfold when the CRP/cAMP-mediated transcriptional regulation of the cya gene is prevented by replacing the cya promoter with the constitutive bla promoter (363). Therefore, it was concluded that the regulation of cAMP production occurs mainly posttranslationally (at the level of enzyme activity) and not at the level of transcription. However, because the regulation of cyaA transcription is affected by the Crp/cAMP concentration, which in turn is affected by CyaA activity, that conclusion does not seem to be justified. This can be illustrated by the changes in adenylate cyclase activity caused by a crr null mutation. Adenylate cyclase activity measured in crr mutants amounts to only 5% to 20% of the activity measured in wild-type strains (227, 469, 595), and the reintroduction of plasmid-borne crr increases it four- to fivefold, thus approaching levels of wild-type activity (477, 700). If EIIAGlc was the only factor interacting with and stimulating adenylate cyclase, crr mutants and strains producing adenylate cyclase missing the last 48 amino acids should exhibit the same phenotype. However, it is evident that the positive effect on adenylate cyclase caused by the expression of plasmid-borne crr in a crr mutant is much smaller than the negative effect (50-fold) caused by the deletion of the 48 C-terminal amino acids of adenylate cyclase in a crp mutant. This difference probably owes to the absence of the Crp-dependent effect of cAMP on transcription in a crp mutant.
Regulation by Crp/cAMP.
Despite the enormous amount of experimental data, the control of cAMP and Crp levels in enteric bacteria is still not completely understood. Regulation occurs not only at the level of transcription but also at the level of enzyme activity. These two processes affect each other, making it difficult to quantify their individual contributions. Nevertheless, the general properties of the regulatory mechanism are clear. The immediate response of cells to the addition of a rapidly metabolizable carbohydrate involves regulation only at the level of enzyme activity. The addition of glucose or another PTS sugar causes the dephosphorylation of P∼EIIAGlc. This deactivates adenylate cyclase and hence lowers the concentration of cAMP and Crp/cAMP inside the cell. On a longer time scale, regulation on the transcriptional level becomes important. At normal physiological levels of cAMP and Crp, the crp gene is negatively regulated by Crp/cAMP. Thus, a reduced activity of adenylate cyclase leads to lower expression of crp. As a result, the concentration of Crp/cAMP drops even further. On the other hand, a lower Crp/cAMP concentration leads to an increased expression of cyaA, and more adenylate cyclase will accumulate, thereby counteracting the decrease in activity and raising the Crp/cAMP concentration. In principle, the elevated pool of adenylate cyclase provides a higher potential for cAMP production once the glucose is depleted, although the Crp concentration will be lower.
Some results cannot be explained by the proposed mechanism. For instance, in S. enterica serovar Typhimurium crp* (595) and E. coli crp* cya (851) mutant strains, the addition of glucose lowers the concentration of Crp* and hence repression. However, because Crp* is constitutively active, i.e., it does not need cAMP for activity, its concentration should not be affected by the presence or absence of glucose. The occurrence of a component that is able to interact with Crp in the presence of glucose could explain the observed decrease in the Crp concentration. In fact, such a mechanism regulates the activity of the repressor Mlc (588): when glucose is present, EIICBGlc is dephosphorylated and binds the transcription factor Mlc, thus relieving repression. It is tempting to speculate that unphosphorylated EIICBGlc might also bind Crp, but as yet, there is no experimental proof to support this assumption.
Factors other than those mentioned above have been reported to influence the activity of adenylate cyclase, but their physiological relevance remains uncertain. A decrease in adenylate cyclase activity, for instance, was observed upon lowering the membrane electrochemical potential (649). Mutation of fruB (formerly fruF) can have a negative (272) or positive (225) effect on adenylate cyclase activity. Also, when GTP or the elongation factor Tu, a GTP-binding protein (702), was added to purified adenylate cyclase, it stimulated its activity almost twofold (829).
Growth rate effects on cAMP concentration.
Although early studies reported hardly any effect of the growth rate on the cAMP concentration under glucose limitation (529, 957), more recent studies report a clear relationship, with elevated cAMP concentrations occurring at low growth rates (613, 614). During growth on glucose in continuous culture, expression of lacZ appears to be independent of ppGpp but directly related to the cAMP concentration and inversely related to the growth rate (438); i.e., growth rate and cAMP concentration are also inversely related. The effect of the growth rate on the cAMP concentration can be explained in terms of the stimulation of adenylate cyclase activity by P∼EIIAGlc. The phosphorylation state of the PTS components in these experiments should depend on the growth rate because the cells were grown in a chemostat under carbohydrate-limiting conditions, and the growth rate was varied by changing the rate of carbon source supply. As a result, at high growth rates, less P∼EIIAGlc and hence less cAMP should be formed than that at slow growth rates.
Secretion and breakdown of cAMP.
Makman and Sutherland (508) observed that following the addition of glucose, starved E. coli cells halt cAMP synthesis and excrete cAMP into the medium. In a later study, the presence of other metabolizable sugars was shown to elicit the same effect (758). Interestingly, crp mutants excrete much higher amounts of cAMP than wild-type cells (75, 241, 679). A putative low-affinity cAMP exporter (Km of approximately 10 mM) was identified (275). However, as the internal cAMP concentration is generally in the micromolar range, excretion is not thought to be relevant to cAMP-mediated regulation.
The breakdown of cAMP by the endogenous cAMP phosphodiesterase (80) provides another possible way to affect the cAMP concentration. However, cAMP phosphodiesterase has a low affinity for cAMP (Km of between 0.5 and 0.8 mM) (80, 604), making it unlikely that this enzyme exerts much, if any, control over cAMP levels. The findings that, in mutants lacking cAMP phosphodiesterase, glucose still lowers the cAMP level (508) and that CCR exerted by various carbon sources is as strong as that in wild-type cells (337) also argue against a physiological role of the cAMP phosphodiesterase.
Inducer Exclusion and Role of EIIAGlc
Soon after the discovery of the PTS, it became clear that ptsHI mutants have a pleiotropic phenotype; i.e., they fail to grow not only on PTS carbohydrates but also on non-PTS carbon sources such as lactose, melibiose, maltose, and glycerol. Based on a number of growth and transport studies with several ptsHI-crr mutants, a model in which unphosphorylated EIIAGlc inhibits systems catalyzing the transport and subsequent metabolism of these non-PTS sugars in enteric bacteria was proposed (765-767, 770; also see reference 678 for more references). An important observation strengthening the model was made in so-called “leaky” ptsH or ptsI mutants of S. enterica serovar Typhimurium (773), where “leaky” means that these mutants retain low EI or HPr activity. The “leaky” mutants do not grow on PTS sugars, but they do grow on the non-PTS sugars melibiose, maltose, and glycerol. Moreover, mutants growing on the latter sugars are hypersensitive to repression by the glucose analog α-MG. This phenotype can be explained by the fact that a low amount of EI or HPr strongly reduces the phosphorylation flux via the general PTS proteins EI and HPr, causing rapid and nearly complete dephosphorylation of EIIAGlc in the presence of α-MG. The phenomenon was called inducer exclusion because the presence of unphosphorylated EIIAGlc prevents either the import of these sugars or their subsequent metabolism, and as a consequence, bacterial cells are devoid of the inducer for the corresponding operons (see reference 502).
Inducer exclusion is mediated by unphosphorylated EIIAGlc.
During inducer exclusion, unphosphorylated EIIAGlc binds directly to either the respective permease (lactose or melibiose), the ATP-binding subunit of the ATP binding cassette (ABC) transporter (maltose), or, in the case of glycerol, GlpK, the cytoplasmic enzyme catalyzing the formation of the inducer (inducer exclusion is depicted in Fig. 2). Initial evidence for a direct interaction between EIIAGlc and the inhibited proteins came from studies showing that the lactose transport activity of LacY in E. coli vesicles is strongly reduced when the vesicles contain partially purified unphosphorylated EIIAGlc (189, 559). Similar results were obtained with whole cells synthesizing either LacY or the melibiose transporter (MelB) (561). Direct binding of unphosphorylated EIIAGlc to membrane preparations of an E. coli strain overproducing LacY was demonstrated by Osumi and Saier (624), who also found that the binding of EIIAGlc correlates with the amount of transporter and is enhanced by the presence of lactose, while the phosphorylation of EIIAGlc abolishes it. These results were confirmed and quantified by Nelson et al. (593, 597). Direct binding and inhibition by unphosphorylated EIIAGlc were also established for the MalK component of the maltose transport system (163, 675) and for GlpK (166, 676).
The uptake systems for raffinose (876), galactose (559), and arabinose (338) are also subject to inducer exclusion. Raffinose uptake via the plasmid-encoded raffinose permease RafB is inhibited by α-MG, and the effect is enhanced in “leaky” ptsI mutants (876). It was suggested that galactose transport in E. coli would also be under the control of inducer exclusion (7, 385). Indeed, the uptake of galactose by membrane vesicles containing galactose permease was reported to be diminished by the addition of unphosphorylated EIIAGlc (559). Nevertheless, other experiments from the same group (562, 767) did not reveal an effect of the PTS on galactose fermentation by intact cells. The authors of those reports argued that the absence of an effect probably results from the full induction of the galactose permease in intact cells, thereby reducing the effectiveness of inhibition by EIIAGlc, as inducer exclusion is mediated by a 1:1 stoichiometric interaction (559). Nevertheless, E. coli gal operon expression is submitted to CCR, as was demonstrated by the early experiments of Stephenson and Gale (836).
Efficient binding of unphosphorylated EIIAGlc to the target protein occurs only when the substrate of the target is present. For example, EIIAGlc binds strongly to the lactose transporter LacY only in the presence of a β-galactoside (597, 624, 800, 825). This mechanism ensures that EIIAGlc, the concentration of which is fairly constant in E. coli and S. enterica serovar Typhimurium cells, will not be wasted on useless binding, i.e., under conditions in which the substrate is not present in the environment. The necessity of substrate binding indicates that the target proteins probably have to undergo a conformational change before unphosphorylated EIIAGlc can bind. Evidence for conformational changes associated with substrate binding has been presented for LacY (245, 390), MelB (586), MalK (71, 581), and GlpK (355, 654, 874).
Interactions of EIIAGlc
Structure of EIIAGlc.
EIIAGlc directly interacts with many different proteins as it serves in PTS phosphoryl transfer, inducer exclusion, and probably activation of adenylate cyclase (Table 1). Although most interaction partners are structurally unrelated, it appears that the binding surface of EIIAGlc is always similar. The structure of EIIAGlc is hardly affected by binding to its target proteins (98, 124, 270, 355, 929) or by phosphorylation (642). E. coli EIIAGlc forms an antiparallel β-sandwich in which His-90 and His-75 of the active site are located off center on one face of the sandwich (224, 643, 955). Besides His-90, the residue that carries the phosphoryl group in E. coli P∼EIIAGlc (197), His-75, appears to be very important for the phosphocarrier activity. Replacement of His-75 with Gln results in strongly diminished phosphoryl group transfer (686). A structural study of His75Gln mutant EIIAGlc indicated no significant structural changes around the active site compared to the wild-type protein (643). Two possible explanations were proposed: (i) His-75 stabilizes the negative charge of P∼His-90, or (ii) the active site contains a proton relay network also involving Thr-73. The latter hypothesis was recently falsified by replacing Thr-73 with Ser, Ala, or Val and measuring the effect on the phosphotransfer rates to and from HPr (545). These rates were barely affected by the mutations.
The active site of E. coli EIIAGlc is surrounded by a ring of solvent-exposed hydrophobic residues flanked by some polar and charged residues. A similar arrangement was found for the EIIAGlc of Mycobacterium capricolum (349) and the EIIA domain of B. subtilis EIICBAGlc (122, 474). The NMR structure of E. coli P∼EIIAGlc shows that the phosphoryl group bound to His-90 (197) protrudes from the ring of hydrophobic residues that surround the active site (642). Supposedly, the disturbance of the hydrophobic binding surface of EIIAGlc prevents the interaction between P∼EIIAGlc and the target proteins. Furthermore, the introduction of a negative charge on the protein surface contributes to the diminished interaction.
Interaction with HPr and EIICBGlc.
The structures of B. subtilis HPr (330) and the EIIA domain of B. subtilis EIICBAGlc (474) were used to construct a model of the HPr:EIIAGlc transition complex (327). Herzberg concluded that (i) upon the formation of the transition complex, no major conformational change occurs and (ii) the central part of the binding surface consists mainly of hydrophobic residues. These assessments are sustained by the NMR solution structures of the HPr:EIIAGlc transition complexes of B. subtilis (124) and E. coli (929). The transition complex perfectly accommodates the binding of a phosphoryl group by both the active-site histidine of EIIAGlc and that of HPr in either an associative or dissociative mechanism. The active-site histidine of EIIAGlc (His-90 in E. coli) is located in a shallow depression of a slightly concave surface (224, 643, 955), whereas the active-site histidine of HPr is located at the end of the first α-helix on a convex protrusion of the protein (330). The binding of the phosphoryl group in B. subtilis EIIAGlc is stabilized by His-68 (His-75 in E. coli) (474) and by Arg-17 in HPr (330). In addition, two aspartyl residues are perfectly positioned to form alternate ion pairs with Arg-17 of HPr. The hydrophobic binding surface containing the active site of EIIAGlc includes several valine and phenylalanine residues (Phe-41, -71, and -88 and Val-40, -46, and -96 in E. coli) (929).
It appears that the binding surfaces of EIIAGlc for HPr and EIIBGlc are almost identical (Table 2). The soluble EIIB domain of E. coli EIICBGlc was isolated, and the solution structure of the EIIAGlc:EIIBGlc transition complex was determined (98, 270). The interaction surface consists of a central hydrophobic patch composed of the phenylalanine and valine residues mentioned above, and the same two aspartyl residues form alternate ion pairs with arginine residues. P∼EIIAGlc transfers its phosphoryl group to Cys-421 of the glucose permease EIICBGlc (92, 547, 618). This residue is located at the C terminus of β1 of the EIIB domain on a hydrophobic protrusion (203). Two arginine residues neutralize the negative charge added to Cys-421 upon phosphorylation of EIIBGlc (98). Like Cys-421 (92), the presence of these residues was reported to be compulsory for effective glucose transport and phosphorylation (449). Although the central interaction parts are very similar, the residues forming the edges of the binding surfaces are different between the transition complexes HPr:EIIAGlc and EIIAGlc:EIIBGlc (98, 929). The edges of the binding surface of HPr contain only positively charged residues, whereas those of EIICBGlc include both positively and negatively charged amino acids. Consequently, the edges of the binding surface of EIIAGlc for HPr include many negatively charged and some polar residues, while those of EIIAGlc for EIIBGlc include a polar residue and both negatively and positively charged amino acids.
TABLE 2.
Residues involved in the interaction of EIIAGlc with partner proteinsa
| Partner protein | Interface residue(s) of:
|
Reference(s) | |
|---|---|---|---|
| Partner protein | EIIAGlc | ||
| HPr active site | His-15, Arg-17 | His-90,b His-75b | 330, 941 (HPr); 197, 686 (EIIAGlc) |
| Interaction surface | Arg-17, Lys-24, Lys-27, Lys-49 | Val-40, Phe-41, Val-46, Phe-71, Phe-88, Val-96, Lys-69 (aliphatic side chain), Asp-38,c Glu-72, Glu-80, Glu-86; Asp-94,c Glu-97, Glu-109, Asp-144, Ser-78, Ser-141 | 929 |
| EIICBGlc active site | Cys-421, Arg-424, Arg-426 | 449, 547 | |
| Interaction surface | Arg-424,d Arg-426,d Lys-467, Asp-419; Asp-464 | Val-40, Phe-41, Val-46, Phe-71,e Phe-88, Val-96, Lys-69,e Lys-99,e Asp-38, Glu-72; Asp-94, Glu-97; Ser-141 | 98 |
| GlpK (E. coli) interaction surface | Residues 472-481 | Val-40, Phe-41, Val-46, Phe-71, Phe-88, Val-96, Lys-69, Lys-99, Asp-38, Glu-72; Glu-92, Asp-94, Asp-97 | 355 |
| Cofactor Zn2+ | Glu-478 | His-75, His-90 | 223 |
| LacY interaction surface | Val-132, Pro-192, Val-197, Ala-198, Arg-135, Arg-142, Ser-133, Thr-196, Asn-199, Ser-202, Asn-204, Ser-206 | Gly-47, Phe-71, Ala-76, Lys-69, Glu-86; Asp-94, Ser-78 | 338, 823, 951 (LacY); 987 (EIIA) |
| MelB interaction surface | Ile-445, Asp-438, Asp-441; Asp-449, Arg-452 | 439, 440 | |
| MalK interaction surface | Ala-124, Phe-241, Gly-278, Gly-284, Gly-302, Arg-228, Glu-119, Ser-322 | 167, 431 | |
The active-site residues are indicated by boldface type.
His-68 and His-83 in B. subtilis (124).
Asp-31 and Asp-87 in B. subtilis (124).
Arg-38 and Arg-40 of the E. coli EIIBGlc domain (270).
Point mutations that block P transfer to HPr and/or EIICBGlc are Lys69Leu, Lys69Glu, Lys99Glu, Phe71Leu, and Phe71Ser (822).
Sequence comparisons of EIIAGlc and EIIBGlc from various bacteria reveal that the interfacial residues are strongly conserved (98). The few changes are mostly conservative, thus preserving the central network of intermolecular hydrophobic interactions. A higher variability is observed for the peripheral polar and charged residues. There are, for instance, several positively charged residues in E. coli EIIAGlc replaced with polar or negatively charged residues in the B. subtilis protein. Although these substitutions abolish some salt bridges, their contribution to the stability of the complex appears to be moderate, as illustrated by the similar apparent Km values for heterologous (PTS proteins from different organisms) and homologous phosphoryl transfer (98, 723). Point mutations in several well-conserved residues of EIIAGlc also lower the phosphoryl transfer activity but only by between 20 and 70% (822). It was concluded from these observations (98) that multiple interactions contribute to the affinity between PTS protein partners.
The N terminus of EIIAGlc is disordered in the structures of both the HPr:EIIAGlc and EIIAGlc:EIIBGlc transition complexes and is therefore probably not involved in the protein/protein interactions. Nevertheless, Meadow et al. (538, 541) established that although the naturally occurring N-terminal truncation (the first seven residues are absent, and the truncated EIIAGlc therefore migrates faster on nondenaturing gels and was called EIIAfastGlc) has no effect on phosphoryl transfer between HPr and EIIAGlc, it reduces the phosphoryl transfer to EIICBGlc by about 97%. Recent measurements by the same group confirm these observations with genetically truncated EIIAGlc (deletion of the first 7 and 16 residues) (545). Simultaneously, they showed that phosphoryl group transfer to the soluble EIIBGlc domain remains unaffected. Misko et al. (559) found that compared to intact EIIAGlc, EIIAfastGlc has a lower affinity for LacY-containing membranes. These findings suggest that the N terminus plays a role in the recruitment of EIIAGlc to the E. coli inner membrane. Indeed, Wang et al. (926, 928) showed that the N terminus interacts with anionic lipids isolated from E. coli, suggesting that the N terminus serves as a kind of membrane anchor.
Interaction with GlpK.
The crystal structure of the E. coli EIIAGlc:GlpK complex has been determined (355). GlpK with bound glycerol and ADP forms tetramers in which each GlpK subunit interacts with one EIIAGlc molecule. Indeed, complete inhibition of glycerol kinase activity occurs only when at least four molecules of EIIAGlc are present per GlpK tetramer (900). The structure of EIIAGlc in the complex is very similar to that of EIIAfastGlc (955), except that residues 1 to 11 are in contact with the surface of GlpK. Like EIIAfastGlc, the EIIA domain of B. subtilis EIICBAGlc lacks the seven N-terminal residues. Nevertheless, the protein complements an E. coli ptsHI-crr mutant defective in GlpK regulation (723). Therefore, the N-terminal residues cannot be very important for the inhibition of glycerol kinase (355). The inhibitory contact between the two proteins is mediated by a 310 helix of GlpK (residues 472 to 481), which protrudes into the concave surface of the active site of EIIAGlc. The contact surface of EIIAGlc is very similar to that in the EIIAGlc:EIIBGlc complex (Table 2). The hydrophobic residues surrounding the active-site histidine are important, and binding involves negatively and positively charged residues.
The organization of the residues around the active site of EIIAGlc in the EIIAGlc:GlpK complex suggests a possible role for Zn2+ in the association. Indeed, crystals soaked in a 20 mM ZnCl2 solution adopted a Zn2+ ion at the expected position (223). Subsequent in vitro studies revealed that the presence of the metal ion lowers the Ki of EIIAGlc for GlpK by about 60-fold (223, 616). The Zn2+ ion is liganded by His-75, His-90, and a water molecule in EIIAGlc and by Glu-478 in GlpK. The stimulatory effect of Zn2+ on EIIAGlc-mediated GlpK inhibition disappeared when Glu-478 was replaced with Cys, Asp, His, or Gln (657).
It is not known how the interaction with unphosphorylated EIIAGlc inhibits glycerol phosphorylation. The more than 30-Å distance between the sites of EIIAGlc binding and glycerol phosphorylation rules out a direct effect. A conformational change of glycerol kinase is part of the catalytic cycle, and so it was proposed that the binding of EIIAGlc could lock the protein in a closed state (355). The lower inhibition by EIIAGlc observed for some mutant GlpKs affected in the domain associated with “opening” and “closing” GlpK is consistent with this concept (655, 658).
Interaction with LacY.
LacY is an H+ symporter that uses electrochemical potential to drive the uptake of several galactosides such as lactose and melibiose (390, 391, 942). The recently resolved LacY structure confirms previous predictions that the protein contains 12 hydrophobic transmembrane domains connected by relatively hydrophilic loops (4). The major determinants of substrate binding are located at the interfaces of helices IV, V, and VIII (300, 444, 754). In contrast, the cytoplasmic loop between helices IV and V together with the flanking helical domains and some residues in the large cytoplasmic loop connecting helices VI and VII are important for the interaction of LacY with EIIAGlc (338, 800, 823). Based on the analysis of various lacY mutants, a model for LacY function in galactoside transport has been proposed (3, 391, 942). LacY is assumed to have a conformation in which the sugar binding site is facing the periplasm (“outward”). The binding of the sugar induces a structural change, which exposes the substrate binding site to the cytoplasm (“inward”). To explain the observation that EIIAGlc binds to LacY with higher affinity when its substrate is present (597, 624, 800, 825), the “outward”/“inward” transition is assumed to make the EIIAGlc interaction site more easily accessible (800, 823). Evidence supporting this concept comes from studies of a Cys154Gly mutant LacY. The mutation abolishes both lactose transport and binding of EIIAGlc and was originally thought to lock the protein in the “outward” conformation (819, 825, 902). However, the crystal structure of Cys154Gly LacY indicates that the protein is locked in an “inward” conformation (4). It was then proposed that EIIAGlc binds to the “outward” conformation of LacY, thus preventing the structural change to the “inward” conformation and, consequently, the entry of the bound sugar. Other observations support this interpretation. When galactosides are present at saturating amounts, 125I-labeled unphosphorylated EIIAGlc binds in a 1:6 stoichiometry to wild-type LacY (in inside-out vesicles) (824, 825). The replacement of residues Ile-129 and Lys-131 (these residues are not located in the EIIAGlc interaction site but are located on central helix IV, which is involved in substrate binding) with Cys allows more EIIAGlc molecules to bind to LacY but does not affect the apparent equilibrium dissociation constant, KD, of the EIIAGlc/LacY complex. In addition, different sugars lead to different binding stoichiometries, with the preferred substrates melibiose and lactose causing maximum binding of EIIAGlc. The data were explained by assuming that LacY exists in two (or more) populations: one population that binds EIIAGlc (“outward”) and one that doesn't (“inward”). The observed EIIAGlc binding stoichiometry was thought to reflect the ratio of these populations, which should be affected by the transported sugar (and in mutant LacYs by the conformational flexibility). According to the model, the binding stoichiometry should not affect the dissociation constant of the EIIAGlc/LacY complex, which is consistent with the observations. It is noteworthy that in contrast to the data reported by Sondej et al. (824, 825), Nelson et al. (597) reported an EIIAGlc/LacY binding stoichiometry of 1:1. The reason for the discrepancy is not clear.
The interaction surface of LacY and EIIAGlc has been probed by analyzing the effect of various mutations. Residues of the central loop of LacY were found to be involved in the interaction (338, 951). Changing Thr-7 or Met-11 to Ile prevents EIIAGlc from binding (597, 625). However, a deletion of residues 2 to 8 hardly affects the regulation of LacY by EIIAGlc (338). Other residues of LacY involved in binding EIIAGlc were identified by Cys-scanning mutagenesis (823). Although some of the LacY residues important for EIIAGlc binding are conserved in MalK, MelB, and RafB, no consensus EIIAGlc binding motif could be proposed (823). In contrast to the binding surfaces of other EIIAGlc target proteins (HPr, EIIBGlc, and GlpK), the LacY binding surface seems to contain only a small number of hydrophobic residues. Several E. coli EIIAGlc (crr) mutants defective in inducer exclusion of LacY substrates have been identified (822, 987). Some of these residues are also important for the interaction of EIIAGlc with HPr, EIIBGlc, and GlpK (Table 2).
Interaction with MelB.
Similar to LacY, the melibiose permease MelB is an ion (Na+/H+) sugar symporter with 12 membrane-spanning segments (74, 586, 966). S. enterica serovar Typhimurium melB mutants synthesizing an inducer exclusion-resistant melibiose permease have been isolated (439) (Table 2). The mutated residues all lie on one side of an α-helical stretch situated in the cytoplasmic C-terminal region of MelB. Other substitutions located on the same α-helical stretch diminished inhibition of the permease by EIIAGlc. Cutting off the last 22 amino acids of MelB reduces inducer exclusion by fourfold, and mutants lacking more residues exhibit low melibiose and methyl-α-thiogalactoside transport even in the absence of inducer exclusion.
Interaction with MalK.
Maltose uptake in E. coli requires the malE-encoded periplasmic maltose binding protein and the multisubunit ABC transporter MalFGK2 (72, 226, 447). The soluble MalK subunits are tightly associated with the two permease subunits MalF and MalG (354, 580). Binding of maltose-carrying maltose binding protein to MalFGK2 stimulates ATP hydrolysis by MalK and therefore maltose transport (118, 161, 187). Stimulation of ATP hydrolysis is cooperative, suggesting that the MalK subunits interact with each other (159, 160, 226). MalK possesses an N-terminal ATPase domain (present in all ATP-binding proteins of ABC transporters) and, in certain bacteria including E. coli and S. enterica serovar Typhimurium, a specific C-terminal regulatory domain (66, 187, 782, 828). In the presence of maltose and glucose, EIIAGlc is assumed to bind to the regulatory domain, thus preventing maltose import. In addition, if no maltose is present, the C-terminal MalK domain is proposed to bind and inhibit the transcription activator MalT (380, 627, 828), which controls the expression of the mal regulon (65, 70).
Several malK mutants exhibiting weak inducer exclusion of maltose but normal inhibition of MalT by MalK and vice versa have been isolated (163, 431) (Table 2). In addition, the binding of monoclonal antibodies directed against linear epitopes extending from either amino acids 113 to 123 or 352 to 361 in the MalK sequence is diminished by the presence of EIIAGlc (828). The scattered location of the mutations (Table 2) and the EIIAGlc/antibody competition sites made it difficult to propose a binding site for EIIAGlc (66, 828). In contrast to Thermococcus litoralis MalK (187), biochemical studies suggest that in the functional MalFGK2 complex of E. coli, the C termini of the MalK subunits are in close contact (774). This assumption was confirmed by resolving the structures of three different forms of the MalK dimer (117). Several amino acids, the replacement of which leads to weak maltose exclusion, lie on the same face of the dimer, thus identifying a potential binding site for EIIAGlc. The binding of EIIAGlc to the nucleotide binding domain of one subunit and the regulatory domain of the other could well prevent the “tweezers-like motion” necessary to accommodate the cycle of hydrolysis and subsequent transport (117).
Other interactions.
Using ligand fishing, yet another interaction partner of unphosphorylated E. coli EIIAGlc was recovered (419). The protein was called fermentation/respiration switch protein (FrsA; formerly YafA) because frsA disruption causes increased respiration on several sugars including glucose, and overexpression results in increased fermentation. EIIAGlc forms a 1:1 complex with FrsA. Thus, in E. coli, the balance between fermentation and respiration seems to be regulated by the PTS. Furthermore, the E. coli genome has been screened for proteins containing a potential EIIAGlc binding site using sequence patterns deduced from mutagenesis studies of LacY (823). The search has yielded over 35 potential binding partners including HPr and MalK but excluding MelB and FsrA. Therefore, whether the recovered list of proteins is significant or not remains an open question.
Diauxic Growth
As mentioned in the introduction, diauxic growth was first described by Monod (572), who observed that when two carbon sources are supplied simultaneously, one (e.g., glucose or fructose) is often preferred over the other (e.g., glucan, maltose, lactose, or galactose). When the preferred carbon source is exhausted, growth is arrested. After a certain time (lag time), the genes involved in the catabolism of the second carbon source are expressed, and growth resumes. Inducer exclusion and/or poor expression of catabolic genes due to low Crp/cAMP levels is responsible for this phenomenon (Fig. 2).
As outlined above, EIIAGlc-mediated inducer exclusion prevents the uptake of carbohydrates such as lactose, melibiose, glycerol, and maltose and, consequently, the induction of the related catabolic genes. Expression of these genes is also regulated by Crp/cAMP, and growth on glucose reduces the cAMP levels by lowering the concentration of P∼EIIAGlc. Even when induced, non-PTS sugar transporters are present at relatively low concentrations compared to those of EIIAGlc. The preferential uptake of glucose during the first part of diauxie is probably ensured by the inducer exclusion mechanism. In contrast, the induction of gene transcription during the lag phase should be caused by the entry/formation of the inducer as well as by rising concentrations of Crp/cAMP. This concept complies well with the available experimental data on glucose-lactose, glucose-maltose, and glucose-melibiose diauxie. An example is glucose-melibiose diauxie in S. enterica serovar Typhimurium, where inducer exclusion of melibiose is responsible for the preferential use of glucose (621). The addition of extracellular cAMP eliminates the lag period, an observation also reported for glucose-lactose diauxie (365, 502). However, in no case could the addition of extracellular cAMP prevent the preferred utilization of glucose over the second carbon source (242).
The concentrations of PEP and P∼EIIAGlc, which are low during growth on glucose, rise strongly when glucose is exhausted (335), which switches off the inducer exclusion mechanism and allows the second sugar to enter and eventually leads to the induction of the respective catabolic genes. In the case of glucose-lactose diauxie, the lac operon is initially repressed by LacI, as the presence of glucose does not allow the entry of lactose. When glucose is depleted, lactose is taken up via the small amount of LacY formed despite repression, the disaccharide is converted by β-galactosidase into allolactose, which binds to and inhibits LacI, and transcription commences (6). The effect of the entry of the inducer on transcription can be mimicked by the inactivation of lacI or by the addition of the nonmetabolizable inducer IPTG. Indeed, both abolish repression of the lac operon and the lag period in glucose-lactose diauxie but not the preferential uptake of glucose (362, 409).
While these results suggest that the repression of the catabolic lac genes is mainly a consequence of inducer exclusion, other data indicate that Crp/cAMP is also involved in the regulation of the lac operon. Four observations have lent support to the latter concept. First, the complete repression of the lac operon in cyaA and crp mutants shows that its expression requires a basal level of Crp/cAMP (6, 438). Second, the addition of extracellular cAMP abolishes the lag phase when switching from glucose to lactose utilization (362, 365, 621). Third, the addition of lactose to a lacI mutant or to IPTG-induced cells leads to a 1.5-fold increase in LacZ production, although inducer exclusion plays no role in this case (362, 409). An up to sixfold increase of LacZ is observed when the E. coli lacI strain KB53 switches from glucose to lactose utilization (425). Fourth, Crp/cAMP and LacI both bind in a cooperative manner to the lac promoter (40, 351, 470).
It is commonly assumed that the lag phase in diauxie is related to the time period required by the cells to synthesize the proteins necessary for the catabolism of the second sugar once glucose is exhausted. The addition of external cAMP abolishes the lag phase. It was therefore not surprising that a strong (sevenfold) transient increase in cAMP levels was observed during the switch from glucose to lactose metabolism (362). In addition, during the lag phase, Crp levels increased slightly before dropping again. We mentioned above that the low concentration of P∼EIIAGlc present in cells growing on glucose stimulates the synthesis (but not activity) of adenylate cyclase due to a relief from repression by Crp/cAMP (763). At the onset of the lag phase, the rise in the P∼EIIAGlc concentration together with the relatively high amount of adenylate cyclase should cause a transient surge in cAMP before it should drop again as a result of the down-regulation of the cyaA gene due to the increased concentration of Crp/cAMP and possibly by a decrease in EIIAGlc phosphorylation.
As described above, external cAMP is expected to increase the synthesis of the proteins necessary for lactose catabolism despite the presence of glucose, which can still prevent lactose catabolism via inducer exclusion. However, the addition of cAMP to E. coli cells growing on glucose leads to only a slight increase in β-galactosidase activity (362). This makes it difficult to explain why the addition of extracellular cAMP abolishes the lag phase but not the repression of β-galactosidase. It is possible that the slight increase of LacY and LacZ synthesis in the presence of high (extracellular) concentrations of cAMP might be sufficient to shorten the lag phase by allowing a more rapid induction of the relevant genes as soon as glucose is exhausted. However, this assumption does not explain why a lag phase is still observed in a crp* mutant in which Crp activity is independent of cAMP (362). In this case, the activity of Crp* is possibly too low in the presence of glucose due to inhibition by an as-yet-unidentified mechanism (365, 851). This would be in line with the observed lowered Crp concentration in the presence of glucose. If glucose regulates the interaction of Crp with an effector and if the regulation would also depend on the concentration of cAMP, the addition of cAMP would, at least partly, relieve repression.
The experimental data discussed in this section concern mostly glucose-lactose diauxie. Although it is likely that the same concept holds for diauxie observed with other sugars that are sensitive to inducer exclusion, i.e., maltose and melibiose, there is little experimental evidence supporting this assumption. Indeed, experiments by Holtman et al. (340) indicate that inducer exclusion plays only a minor role in glucose-glycerol diauxie. The proteins involved in glycerol metabolism are encoded by the genes of the glp regulon. Similar to the lac operon, it is positively controlled by Crp/cAMP and negatively controlled by a repressor (GlpR) (479, 480, 944). The inducer sn-glycerol-3-phosphate is produced from glycerol by glycerol kinase (GlpK), the activity of which is regulated by the allosteric inhibitors fructose-1,6-bisphosphate (FBP) and EIIAGlc (487, 616, 676, 994). Reverse genetics has been used to distinguish between FBP- and EIIAGlc-mediated allosteric regulation of GlpK (340). Surprisingly, glpK mutants that are insensitive to regulation by FBP exhibit no lag phase, consume glucose and glycerol simultaneously, and have only a transient induction of GlpK activity after the cells have depleted glucose. By contrast, glpK mutants that are insensitive to inducer exclusion exhibit diauxie similar to wild-type strains. Therefore, it was concluded that allosteric regulation by metabolic intermediates and poor expression due to low Crp/cAMP levels are probably the dominant mechanisms in glucose-glycerol diauxie. It was proposed that this unexpected behavior might be related to the fact that the inducer of the glp operon is also an intermediate of lipid metabolism.
The supposed direct link between the phosphorylation state of EIIAGlc, glucose uptake, and adenylate cyclase activity has been questioned (614). In a glucose-limited chemostat, it was observed that the cAMP concentration rises sharply (8- to 10-fold) when the external glucose concentration drops below 300 μM, a concentration more than 20-fold higher than the Km of glucose transport (5 to 15 μM). Notley-McRobb and Ferenci claimed that this result ruled out a direct role of the PTS in cAMP production and therefore that another signaling molecule would be required. However, Kremling et al. (425) reported that the lac operon is induced when the glucose concentration drops below 100 μM, which is much closer to the Km. In addition, we found that in glucose-consuming E. coli cells, the cAMP concentration rises steeply between 120 and 30 μM glucose (M. Hoorneman, R. Bader, and P. W. Postma, unpublished results). At the same time, EIIAGlc, which is predominantly unphosphorylated at higher glucose concentrations, becomes mostly phosphorylated. The latter observation complies with the concept that P∼EIIAGlc stimulates adenylate cyclase activity.
Transcription Regulation by Mlc
When E. coli grows on PTS carbohydrates, the levels of both the general PTS proteins and the respective EIIs are often increased. The synthesis of EII complexes is usually controlled by sugar-related mechanisms. Operons encoding sugar-specific PTS components are often flanked by or contain a gene encoding either a specific repressor (operons encoding hexitol-specific PTS) (461, 465, 466), an antiterminator (the E. coli bgl operon [287] or the L. casei lac operon [288]), or a transcription activator (the B. subtilis lev operon) (518). These are examples of single operons controlled by their respective inducers. In enteric bacteria, the expression of several operons encoding either sugar-specific PTS components or the general PTS proteins is controlled by an additional mechanism responding to the phosphorylation state of a PTS protein (Fig. 2).
Repression by Mlc and its interaction with unphosphorylated EIICBGlc.
The utilization of a PTS substrate, such as glucose, by enteric bacteria increases the amount of not only EIICBGlc and the mannose-specific EIIs (215, 420, 769, 839) but also EI and HPr (172, 727, 771, 839). Unfortunately, a detailed analysis of transcription regulation of glucose-activated genes is complicated by the fact that most of them are also under the control of Crp/cAMP, i.e., partly repressed by the presence of glucose. For instance, cyaA or crp mutants have lower levels of EI, HPr, and several EIIs (727). On the other hand, E. coli wild-type cells grown on glucose exhibit increased levels of these proteins compared to cells grown on lactate. The relatively elevated synthesis in the presence of glucose appeared to require the activity of the repressor Mlc. Overexpression of the E. coli mlc gene leads to an increased colony size during growth on glucose-containing solid medium (hence the name mlc, for making large colonies), which is due to retarded glucose uptake and the reduced formation of acetate (343). This growth behavior led to the discovery of mlc in the first place.
Earlier studies of E. coli mutants that were affected in growth had already suggested that ptsG and ptsM expression might be controlled via a repressor. Suppressor mutants that were able to grow anaerobically on glucose, mannose, and glucosamine were obtained from E. coli ptsG strains, which cannot utilize the above-mentioned carbon sources in the absence of oxygen (732). The suppressor mutants were also 2-deoxy-d-glucose (2DG) sensitive under anaerobic conditions, and hence, the locus/gene was called dgsA. The toxic 2DG is transported mainly via EIICMan/EIIDMan, and indeed, after anaerobic growth, dgsA mutants exhibited elevated mannose and 2DG transport activities compared to the parental strain. DgsA was therefore assumed to be a repressor for the man operon. In fact, mlc was later found to be allelic with dgsA (662).
Strains overproducing Mlc (DgsA) were constructed, and the protein was purified (407, 857). It was found that the presence of a Zn2+ ion is essential for binding to the DNA (780). The amino acid sequence of the 44-kDa Mlc is about 40% identical to that of NagC (343, 579). NagC is a repressor/activator involved in N-acetylglucosamine uptake and metabolism (646, 667, 669, 922), and its activity is induced by N-acetylglucosamine-6-phosphate (668). Both Mlc and NagC belong to the ROK family, which contains repressors, open reading frames (ORFs), and kinases (314, 877). The DNA binding sequences of Mlc and NagC (663) and the putative sugar binding sites of Mlc and the structurally similar ROK family members glucokinase (GlcK) of E. coli and a putative fructokinase (FrcK) of B. subtilis (780) appear to be very similar. Nevertheless, in contrast to NagC, GlcK, and FrcK, Mlc does not seem to bind glucose or metabolites that are derived from it. Gel mobility shift assays showed that these molecules do not affect the interaction of Mlc with its various DNA target sites (167, 407, 408, 664). In addition, activation/deactivation of Mlc by phosphorylation seems unlikely, as no phosphorylation of Mlc has been detected (407).
While studying the expression of ptsHI-crr and ptsG, which are both regulated by Mlc, it became clear that the PTS controls Mlc activity (171, 172, 664, 665, 857). de Reuse and Danchin (171) concluded that transcriptional regulation of the pts operon is a consequence of an increase in the level of unphosphorylated EIICBGlc. Plumbridge (665) then suggested that unphosphorylated EIICBGlc might interact with Mlc (Table 1). Affinity chromatography demonstrated that Mlc indeed binds to unphosphorylated, but not to phosphorylated, EIICBGlc (856). Mlc was found to be sequestered to the membrane when P∼EIICBGlc was dephosphorylated during the uptake of glucose (458, 588). In addition, the overproduction of EIICBGlc resulted in the derepression of the Mlc-controlled operons ptsG and ptsHI-crr (588). Finally, surface plasmon resonance experiments and mobility shift assays confirmed that Mlc binds to EIICBGlc but not to EI, HPr, or EIIAGlc (588). The affinity of Mlc for unphosphorylated EIICBGlc (KD = 10−7 M) and for its various DNA target sites (KD = 10−8 to 10−7 M) is similar (588).
Initial studies with mutant EIICBGlc carrying various deletions suggested that Mlc binds to the EIIB domain only when the EIIC-EIIB linker (residues 320 to 390) is present (458). Certain mutations in the EIIC domain interfere with Mlc-mediated regulation (615, 661, 988). In addition to dgs mutants, another class of mutants that also exhibited a phenotype resembling that of mlc strains was isolated. E. coli manXYZ strains grow only poorly on glucosamine, which is also slowly transported via EIICBGlc. Starting from a manXYZ strain, several mutants that were able to grow efficiently on glucosamine were obtained. The mutants, which synthesized EIICBGlc constitutively, were called umgC (uptake of α-methylglucoside control), and the umgC mutations were mapped close to the ptsG gene (383). UmgC was hence originally postulated to be a repressor for ptsG. Investigation of the original mutants and some new umgC mutants revealed that these mutations actually map within ptsG (615, 661, 988). Interestingly, some of these mutations affect the putative cytoplasmic loops in the EIICGlc domain and cause an up to fivefold increase in α-MG uptake rates compared to wild-type cells as well as elevated ptsG mRNA levels under noninducing conditions. The effects of umgC mutations resemble those found in mlc null mutants (615), and it was therefore assumed that they increase the affinity of EIIBGlc for Mlc. The binding of Mlc to EIIB is very sensitive to changes in the surface-exposed residues in the vicinity of the phosphorylatable Cys (795), and specific mutations in the EIIC domain possibly affect the arrangement of these residues in the EIIB domain, thereby affecting the binding of the transcription factor. However, the purified EIIB domain alone was found to be sufficient to bind Mlc, but anchoring to the membrane is necessary to prevent Mlc-mediated repression (795, 855). Nevertheless, other effects resulting from the membrane sequestration, like the induction of conformational changes or the masking of binding domains, might contribute to the regulation of Mlc (855).
Mlc has been crystallized, and its structure has been solved (780). The protein appears in two distinct homodimeric forms in the crystallographic unit, and the difference in the distances of the helix-turn-helix (HTH) DNA binding domains between the two forms suggests that only one should be able to bind to the DNA. The helix-turn-helix DNA binding domain of Mlc is stabilized by the C-terminal helix of the protein, and this structural feature seems to be crucial for Mlc function (780). Although deletion of the first 9 C-terminal amino acids does not affect Mlc activity, deletion of the first 18 C-terminal amino acids prevents repression and EII binding (795). The loss of function is accompanied by a transition from the tetrameric form, which is found in dilute solutions (588), to a dimeric form (795).
The Mlc regulon.
DNase I footprinting experiments uncovered Mlc binding sites in the regulatory regions of five operons/genes in E. coli. They include ptsHI (407, 665, 857); ptsG (408, 664); manXYZ (662); malT, the positive regulator of the maltose regulon (167); and mlc itself (167). Transcription of these operons/genes is repressed by the binding of Mlc to a site overlapping the downstream promoter (except for the pts operon, where Mlc binds close to P0) and is stimulated by Crp/cAMP, which binds upstream from Mlc. To find out to what extent Mlc and Crp/cAMP contribute to the regulation of the operons, expression of lacZ fusions in wild-type strains was compared to that in strains devoid of adenylate cyclase, Crp, Mlc, or EIICBGlc.
A null mutation of the mlc gene caused a threefold increase in manX expression in glycerol-grown cells, and a similar increase was observed for malT (167). Synthesis of a ManX-LacZ fusion protein is very low in a cyaA mutant and is increased 14-fold by the addition of cAMP. The control exerted by Crp/cAMP appears to be stronger than that of Mlc, as a fivefold-higher amount of ManX-LacZ is present in cells grown on glycerol than in cells grown on glucose (662).
Glucose-grown cells also exhibit expression of mlc that is about twofold lower than that of cells grown on glycerol (167, 808). Whereas transcription regulators are often associated with one of the transcription units that they control, mlc is monocistronic. The coding sequence of the gene is preceded by a Crp/cAMP binding site, two promoters (579), and an Mlc binding site overlapping the second promoter (167). Although both promoters are recognized by RNA polymerase containing the housekeeping sigma factor σ70 (Eσ70), P2 is also recognized by the heat shock factor σ32 complex (Eσ32) (808). In vitro transcription assays corroborated the finding that transcription is initiated from both promoters and that Crp/cAMP has a positive effect on P2 in the presence of Eσ70 but a negligible effect in the presence of Eσ32. Crp/cAMP inhibits transcription from P1, and Mlc inhibits transcription from P2. The synthesis of σ32 in response to a heat shock leads to the overexpression of mlc. Nevertheless, the Mlc-to-EIICBGlc ratio remains fairly constant because ptsG transcription is also induced by Eσ32.
The EIICBGlc-encoding ptsG gene is expressed from two promoters, which are under the control of Mlc (408, 664). One Mlc binding site overlaps P1 and precedes a Crp/cAMP target site, and the other is upstream from P2. Crp/cAMP-dependent expression from P1 prevails, as P2 transcripts amount to only 10% of the total ptsG mRNA (664). crp or cyaA null mutants do not express ptsG (408, 409, 727), while ptsG is up to 18-fold overexpressed in mlc null mutants (depending on the growth conditions) (373, 664). In wild-type cells, growth on glucose causes an eightfold induction of ptsG expression compared to growth on non-PTS carbon sources (215, 664). It follows that although affected by Crp/cAMP, the expression of ptsG is regulated mainly by Mlc. In addition, other factors have been implicated. It appears that the repression of ptsG transcription from promoters P1 and P2 by Mlc and the activation of transcription from promoter P1 by Crp-cAMP are enhanced by Fis (807), one of the major histone-like proteins of E. coli (279). In addition, under conditions of nutrient limitation, ptsG expression is repressed by Eσs (RpoS), and this repression seems to act in synergy with that of Mlc (792). Furthermore, using ligand fishing with the promoter region of E. coli ptsG, it was found that the two-component response regulator ArcA, when phosphorylated, binds the promoter region at three positions: two between P2 and P1 overlapping the Crp/cAMP binding sites and one next to P1 overlapping the Mlc binding site (373). P∼ArcA also binds to the promoter region of the ptsHI-crr operon but with lower affinity. The binding of P∼ArcA leads to a twofold reduction in ptsG transcription. The following link between the Arc two-component system and the glucose PTS might exist. An elevated glycolytic flux might cause a redox imbalance and hence lead to the activation of the response regulator ArcA, which is phosphorylated when reducing equivalents accumulate under aerobic conditions (271). P∼ArcA then binds to the ptsG promoter region and represses the transcription of ptsG (373), thus reducing the uptake of glucose and the glycolytic flux.
A different mode of regulation of the EIICBGlc concentration is related to the stability of the RNA message. It was established that ptsG mRNA stability is reduced in an RNase E-dependent manner by metabolic blocks early in glycolysis (accumulation of glucose-6-P or fructose-6-P) (209, 410, 577, 578). Destabilization of the mRNA depends on enolase (578), a major component of the RNase E-containing degradosome, and the RNA chaperone Hfq (394). The chaperonin Hfq promotes the annealing of specific small RNA (sRNA) to target mRNA, thereby affecting the translation of the message (269, 842, 895). The ptsG mRNA is destabilized by an Hfq-binding sRNA called SgrS (898). Furthermore, expression of sgrS (formerly ryaA) is controlled by the transcription activator SgrR (formerly YabN) and becomes activated under conditions of α-MG-6-P (glucose-6-P) accumulation. However, Hfq-catalyzed annealing of the SgrS sRNA to ptsG mRNA alone is not enough to explain the observed instability of the ptsG mRNA. Kawamoto et al. (394) showed that the presence of the first two transmembrane helices of translated PtsG (i.e., localization near the membrane) is essential for an effective ptsG mRNA breakdown under conditions of hexose-6-P accumulation.
Transcription of the ptsHI-crr operon is initiated from three promoters (172, 173, 237, 753). P0 and P1 each yield two different transcripts: a short transcript coding for HPr only and a long transcript encompassing all three genes (172). The third promoter is located at the 3′ end of ptsI and allows crr transcription. Transcription from P2 is independent of Crp/cAMP and Mlc (857) and accounts for about 80% of the total crr-containing mRNA (172). As a result, growth on glucose hardly affects the concentration of EIIAGlc, whereas it increases the number of ptsH and ptsI transcripts three- to fourfold (171, 172, 727, 857). This induction is due to an increase in transcription from P0. In the absence of glucose, Mlc binds to the P0 promoter region and prevents the binding of the RNA polymerase (407, 665, 857). Although the presence of Crp/cAMP is essential for expression from P0 (173, 665, 750, 857), this promoter is controlled mainly by Mlc (407, 857). The P1 promoter is preceded by a second Crp/cAMP binding site, and expression from this promoter is weakly controlled by Crp/cAMP (173) but is not affected by Mlc (407, 857). Next to the Crp/cAMP is a FruR binding site (751), the inactivation of which has no effect on ptsHI-crr expression (665, 857). Recently, it was found that crr expression from P2 is affected by the histone-like nucleoid-structuring protein (427), but the physiological relevance of this finding remains to be established.
Repression by Mlc and activation by Crp/cAMP are directly related to the phosphorylation state of EIICBGlc and EIIAGlc, respectively. As explained above, during growth on glucose, unphosphorylated EIIAGlc and EIICBGlc prevail, with the latter sequestering the repressor Mlc to the membrane. As a result, EIICBGlc will be synthesized, whereas the amount of Mlc will decrease slightly due to the low amount of Crp/cAMP, which will further increase EIICBGlc synthesis. Repression by Mlc is relieved not only by growth on glucose but also by growth on other rapidly metabolized PTS substrates such as N-acetylglucosamine and mannitol and to a lesser extent by growth on fructose and mannose (407, 661). Lactose, melibiose, and sucrose have no effect. Mlc repression is also affected by maltose, although maltose is not a PTS sugar. However, maltose degradation yields intracellular glucose and glucose-1-P. The import of maltose can therefore relieve Mlc repression inasmuch as intracellular glucose causes P∼EIICBGlc dephosphorylation (678).
Role of Phosphoenolpyruvate
The effects of PTS carbohydrates on the transport of non-PTS carbon sources and on cAMP synthesis seem to result mainly from changes in the phosphorylation state of EIIAGlc. Early data on the phosphorylation state of PTS proteins showed that in galactose-grown S. enterica serovar Typhimurium cells, EIIAGlc was mainly phosphorylated, whereas after the addition of α-MG, P∼EIIAGlc rapidly became dephosphorylated (596). In the meantime, the phosphorylation state of EIIAGlc in E. coli cells growing on a number of PTS carbohydrates has been determined. When grown on glucose, more than 95% of the EIIAGlc is unphosphorylated. A lower percentage of unphosphorylated EIIAGlc was detected in cells grown on other PTS carbohydrates such as mannose, fructose, and mannitol (335). Interestingly, EIIAGlc is also mainly dephosphorylated in wild-type cells grown only in rich medium, whereas in cya or crp mutants, EIIAGlc is almost completely phosphorylated (852).
An unexpected finding was that EIIAGlc is also partly unphosphorylated in cells grown on several non-PTS carbon sources, including lactose, melibiose, maltose, arabinose, and glucose-6-phosphate (334, 335). For instance, in cells growing on lactose or glucose-6-phosphate, approximately 70% and 60% of the EIIAGlc is unphosphorylated, respectively. A correlation was found between the phosphorylation state of EIIAGlc and the ratio of the intracellular concentrations of PEP and pyruvate, the substrate and product of EI phosphorylation, respectively. A decrease in this ratio leads to a decrease in EIIAGlc phosphorylation. In harvested and washed E. coli cells, the intracellular PEP concentration ranges from 2 to 4 mM, and that of pyruvate ranges from 0.2 to 1.2 mM. The addition of glucose changes the concentration to about 0.3 mM PEP and 2.5 mM pyruvate within 15 s (335). For other carbohydrates, the changes are weaker and take slightly longer (30 to 60 s). The low PEP-to-pyruvate ratio observed during the metabolism of several carbon sources is expected to lead to poor phosphorylation of the PTS proteins, as the first phosphoryl transfer steps are reversible (540, 737, 940). An important corollary of these results is that carbon sources, whose transport/metabolism lowers the PEP-to-pyruvate ratio and is catalyzed by enzymes that are sensitive to inhibition by EIIAGlc, should slow their own uptake. This has been confirmed for lactose. Cells lacking EIIAGlc or producing a mutated LacY permease that is insensitive to inducer exclusion (inhibition by EIIAGlc) exhibit elevated lactose influx (336).
It has been observed that when nonmetabolizable PTS substrates were added to starved cells of S. enterica serovar Typhimurium (839) or L. lactis (871), their uptake is initially fast and slopes off quickly. The phenomenon has been used as an argument for a possible regulatory role of the monomer/dimer transition of EI (639, 940). However, the observation can be explained by the rapid drop of the PEP concentration upon the addition of sugar (335, 527, 871), which will cause a continuous decrease of the uptake rate until the PEP concentration reaches steady-state levels. In addition, model calculations (C. Francke, unpublished results) suggest that the initial uptake (first seconds) by starved cells might be extremely fast owing to a surplus of phosphoryl groups that are attached to PTS proteins in the nonfluxing state (equilibrium) compared to the state where phosphoryl group transfer towards the incoming sugar has commenced. The strongly diminished uptake rate that is sometimes observed can be explained by the accumulation of phosphorylated (nonmetabolizable) products and equilibrium, which will finally be reached under these conditions. In conclusion, although the extremely slow monomer/dimer transition rate of EI certainly shows regulatory potential, so far, uptake experiments can be perfectly explained by mechanistic models (239, 737) that do not take into account the dimerization of EI. In contrast, these models indicate that the PEP-to-pyruvate ratio is an important factor in regulation.
CCR Mediated by Non-PTS Sugars and Catabolic Intermediates
To determine the effect of Crp/cAMP on the expression of catabolic genes without the interference of inducer exclusion, lacZ expression was studied in the presence of the nonmetabolizable inducer IPTG (337), and mal operon expression was studied in the absence of its inducing sugar (210, 211). Lactose, gluconate, and glucose-6-P exert strong CCR on lacZ expression, even in a crr mutant, and a clear correlation between cAMP/Crp levels and lacZ expression was observed (337). Glycerol-3-P, glycerol, and other non-PTS sugars (i.e., arabinose, rhamnose, and especially xylose) cause strong repression of malT and malK, although phosphorylation of EIIAGlc is only slightly lowered (210, 211). Repression by glycerol is absent in a glpK mutant and enhanced in a glpD mutant (GlpD transforms glycerol-3-P into dihydroxyacetone-P), suggesting that repression is caused by elevated glycerol-3-P levels. Similar to glycerol-3-P, glucose-6-P and gluconate also lower malT expression (211). While growing a pgi mutant (Pgi, phosphoglucose isomerase, converts glucose-6-P into fructose-6-P) in the presence of glucose-6-P has only a weak effect on the phosphorylation state of EIIAGlc (334, 335), malT expression is still repressed under these conditions (211). If glucose-6-P is normally metabolized, mainly unphosphorylated EIIAGlc is present (334), and the repression of catabolic genes can be achieved via both inducer exclusion and low adenylate cyclase activity. However, if gluconate-6-P or glycerol-3-P accumulates, these metabolites seem to exert CCR even at high levels of P∼EIIAGlc. Although the phosphorylation state of EIIAGlc is barely altered, the cAMP concentration drops severalfold upon the addition of glucose-6-P or glycerol-3-P, whereas the concentration of Crp remains fairly constant (210, 337). A low cAMP level during growth on glucose-6-P has also been reported (201). It was therefore concluded that these metabolites interfere with the activation of adenylate cyclase by P∼EIIAGlc (211).
2-Ketobutyrate, a precursor of leucine, is another metabolite involved in CCR. The addition of 2-ketobutyrate causes a strong transient decrease of the cAMP concentration in E. coli wild-type cells but not in ptsI or crr null mutants (152). The link between the PTS and the metabolism of 2-ketobutyrate is not clear. However, it has previously been reported that 2-ketobutyrate (which resembles pyruvate) accepts the phosphoryl group from P∼EI (768). This could reduce the phosphorylation state of EIIAGlc and thereby reduce the activity of adenylate cyclase, especially when 2-ketobutyrate accumulates inside the cell. A transient accumulation of 2-ketobutyrate and a concomitant drop in cAMP concentration were indeed observed when cells growing on glucose were shifted from anaerobic to aerobic conditions (152).
Reverse Inducer Exclusion or Retroregulation
It is generally believed that PTS sugars, particularly glucose, are the preferred substrates in enteric bacteria and that they are on top of the hierarchy of carbohydrate utilization. However, one would expect that the overproduction of non-PTS target proteins of EIIAGlc, such as GlpK or LacY, would lower the amount of free EIIAGlc. Because the EIIAGlc phosphorylation site is usually part of the interface in the EIIAGlc/non-PTS protein complexes, the phosphoryl group transfer through the glucose PTS will be diminished. This should lower the uptake of glucose via the PTS and at the same time diminish or even prevent inducer exclusion. The latter phenomenon has indeed been observed and was called escape from inducer exclusion (595) or desensitization (760). About 25% of the amount of EIIAGlc present in S. enterica serovar Typhimurium wild-type cells is sufficient for maximal uptake of α-MG (901). Lowering the amount of EIIAGlc below this level causes a decrease in the rate of α-MG transport. Interestingly, in cells producing high levels of GlpK, the rate of α-MG transport decreases by adding glycerol (734, 735), which reinforces the interaction between GlpK and EIIAGlc. The competition of various target proteins for EIIAGlc and the resulting consequence for regulation in intact cells have also been studied. If different EIIAGlc target proteins are simultaneously present in the cell, they could compete for EIIAGlc, especially when they outnumber the EIIAGlc molecules. Competition between the lactose carrier and GlpK for EIIAGlc was indeed observed in vitro (166). Similarly, inducer exclusion of glycerol or maltose in intact cells is relieved after the induction of the lac operon and the addition of thio-β-galactoside, which strengthens the interaction between LacY and EIIAGlc (761). In the same vein, inducer exclusion of maltose is relieved after the induction of GlpK synthesis and the addition of glycerol. Increasing the amount of EIIAGlc by introducing a crr-containing plasmid restores inducer exclusion (594).
Regulation by EIIAGlc-Like Proteins
The crr mutation was isolated as a suppressor mutation in ptsHI strains, which restored growth on many non-PTS carbohydrates. No other mutations giving rise to this general suppressor phenotype have been reported, suggesting that EIIAGlc is unique in being able to regulate PTS-mediated inducer exclusion and to activate adenylate cyclase in enteric bacteria. Therefore, it came as a surprise that a residual level of inducer exclusion was observed in S. enterica serovar Typhimurium crr deletion strains (595). This effect is probably mediated via EIIs containing an EIIAGlc domain such as EIICBANag and EIIBCABgl. In bacteria grown on a carbohydrate that induces one of the EIIs, antibodies against EIIAGlc cross-react with membrane-associated proteins (786). In addition, E. coli EIIBCABgl and Klebsiella pneumoniae EIICBANag completely restore α-methyl-glucoside transport in a crr mutant (921), and EIIAGlc complements a C-terminal deletion in EIICBANag (923). The EIIA domain of EIICBANag restores inducer exclusion in a strain lacking both EIIAGlc and EIICBANag (899). In addition, several EIIAGlc-like domains of proteins from gram-positive organisms, such as EIIAGlc of B. subtilis EIICBAGlc or Streptococcus thermophilus LacS, restore inducer exclusion in E. coli (302, 723).
Is EIIAGlc-mediated regulation limited to enteric bacteria?
Many bacteria contain an EIIAGlc-like protein or domain, but whether EIIAGlc plays a regulatory role has been investigated for only a few organisms, such as B. subtilis, M. capricolum, Haemophilus influenzae, S. thermophilus, and S. coelicolor.
Similar to E. coli ptsI mutants, B. subtilis ptsI mutants are not able to grow on the non-PTS carbon source glycerol (265). However, in contrast to E. coli, inactivation of EIIAGlc does not restore growth of the B. subtilis ptsI strain on glycerol (282). It will be discussed below that in gram-positive bacteria, EIIAGlc does not interact with GlpK but that this enzyme is regulated via P∼His-HPr-mediated phosphorylation (158). In M. capricolum, glycerol kinase is also not inhibited by EIIAGlc (992).
Sequencing of the H. influenzae genome revealed genes encoding EI, HPr, and EIIAGlc (ptsHI-crr operon) as well as genes encoding an EIIBCFru-like protein (fruA) and a protein in which two FPr domains, each containing a phosphorylatable histidine (His-300 and His-424), are fused to EIIAFru (EIIAFru-FPr-FPr; fruB) (500, 717). In H. influenzae, only fructose, but not glucose, is taken up via a PTS, and a ptsI mutant can therefore ferment glucose (499). Interestingly, the development of competence, which in H. influenzae requires cAMP and Crp (105, 196), was lowered about 70-fold in a crr mutant (305) and 250- to 500-fold in a ptsI mutant (305, 499). The addition of extracellular cAMP restored the development of competence. It is likely that H. influenzae P∼EIIAGlc stimulates adenylate cyclase activity in a manner similar to that observed in enteric bacteria. No EIICBGlc exists in H. influenzae, and thus, the presence of glucose does not lead to direct dephosphorylation of P∼EIIAGlc. Nevertheless, the uptake of fructose will lower the amount of P∼EI and P∼HPr and consequently will also lower the amount of P∼EIIAGlc.
Although the main uptake system for glucose in S. coelicolor is GlcP, a non-PTS permease of the major facilitator superfamily (911), CCR by glucose was observed (21, 217, 530, 912). Glucose kinase (443, 911) and the accumulation of glycolytic intermediates (694) are implicated in mediating the effect. Although S. coelicolor contains EI, HPr, EIIAGlc (designated EIIAcrr), and EIIBCGlc homologs as well as other sugar-specific EII complexes, PTS proteins do not seem to be involved in CRR in this high-G+C gram-positive organism (58, 629). In contrast to E. coli, the crr gene of S. coelicolor precedes ptsI, and ptsH is located somewhere else on the chromosome. EIIAcrr restores growth of an E. coli crr strain on glucose (392). HPr from S. coelicolor is efficiently phosphorylated by B. subtilis EI and PEP but is barely modified by B. subtilis HprK/P and ATP (97, 630). In fact, S. coelicolor is missing HPrK/P, and the deletion of ptsH has no effect on the glucose repression of galactokinase (97) or glycerol kinase (612). The absence of fluctuations in the cAMP concentration (112) restricts the potential role of the PTS to inducer exclusion-mediated mechanisms. However, although expression of the S. coelicolor crr gene in an E. coli ptsHI-crr deletion strain significantly reduces maltose uptake and although a specific interaction between E. coli MalK and S. coelicolor EIIAcrr was established by using surface plasmon resonance spectroscopy (392), no evidence showing that the PTS protein exerts a similar function in S. coelicolor has been obtained.
Mathematical Modeling of the PTS and Its Role in CCR
The study of complex metabolic and regulatory networks is facilitated by the availability of mathematical models (463). As a consequence of the vast amount of reliable experimental data on individual reaction rates, it has become possible to make such models for the PTS. A kinetic model for the glucose PTSs of E. coli and S. enterica serovar Typhimurium has been constructed (737) and converted spatiotemporally to unravel the possible effects of diffusion on PTS function (239, 240). The flux data obtained earlier in vivo (901) and in vitro (738) are described reasonably accurately by these models. When using the measured cellular protein concentrations, the computations indicate that most PTS components should be present as heterodimeric phosphoryl transfer complexes. Lowering the total EIIAGlc concentration in the calculation, for example, by binding to non-PTS components (735), has no significant effect on either the flux of phosphoryl groups or the concentration of unphosphorylated EIIAGlc. Consequently, inducer exclusion will hardly have an effect on glucose influx, which was also observed experimentally (734). During the uptake of a rapidly metabolizable PTS sugar, the concentration of unphosphorylated EIIAGlc is predicted to be low. The low concentration of unphosphorylated EIIAGlc implies that the affinities between non-PTS transporters or GlpK and unphosphorylated EIIAGlc should be stronger than originally predicted. In fact, this prediction is in line with the interaction data for GlpK and EIIAGlc. Initially, the Ki was reported to be 16.6 μM, but in the presence of 0.1 mM Zn2+, it was found to be as low as 0.28 μM (223). When assuming a simple one-to-one association mechanism between GlpK and unphosphorylated EIIAGlc and a predicted concentration range for unphosphorylated EIIAGlc of 2.5 to 0.25 μM (239), the ratio of active/inactive GlpK can vary from 1.1 to 0.11. The prediction is also supported by the reported KDs for EIIAGlc from LacY (1.0 μM) (825) and FrsA (0.2 μM) (419).
A remarkable outcome of the spatiotemporal modeling is that although diffusion is not limiting for glucose uptake, it prevents an equal distribution of unphosphorylated EIIAGlc throughout the cell, with a significantly (30%) higher concentration near the cytoplasmic membrane (239, 240). LacY, MelB, MalK, and probably also GlpK (920) are associated with the cytoplasmic membrane, which implies that diffusion limitation enhances inducer exclusion. The calculations also clearly indicate that the PTS is a dual sensing system. In accordance with the experimental data, both the glucose and the PEP concentrations are predicted to affect the phosphorylation state of EIIAGlc (335, 736). The same conclusion can be drawn from the response towards carbohydrate pulses of two other detailed kinetic models of the PTS (including glycolysis) (426, 777). The first model (426) was analyzed further with respect to the time hierarchy of the responses, and the second model (777) was analyzed further with respect to the regulatory roles of the various PTS components.
A mathematical model providing a different view of the PTS was presented by Thattai and Shraiman (868). Those authors attempted to describe the competition of different sugar-specific PTSs for the phosphoryl groups provided by the common PTS components EI and HPr and derived phase diagrams for the uptake rates of the “competing” PTS sugars. Their procedure revealed that there is only one “nontrivial switching” phenotype generated by the PTS. This phenotype corresponds to diauxic growth (winner-takes-it-all behavior). However, the calculations did not include the regulation of enzyme activity by catabolic intermediates and transcription regulation via Crp/cAMP and Mlc. In addition, the model is based on the assumption that the concentrations of EIIAs and EIIBCs are correlated and that the sugar uptake rate is maximized, while the phosphoryl group flux is limiting. In the case of glucose, these assumptions are problematic, as the expression of EIIAGlc and that of EIICBGlc are not correlated (see the section on the mlc regulon) and transport controls the flux of phosphoryl groups (240, 737, 901). Nevertheless, hierarchical utilization of PTS carbohydrates is a common feature of both gram-negative and gram-positive bacteria (see also reference 678).
Several mathematical models that describe the expression of the lac operon exist (626, 776, 802, 953, 978, 979). The model described by Wong et al. (953) contains regulation by inducer exclusion, cAMP, and LacI but lacks regulation of cyaA and crp expression. The expression of lacI is assumed to be constitutive, and the cAMP level is linearly proportional to the glucose transport rate. Intracellular phosphorylation of glucose by EIICBGlc is not considered. Nevertheless, for cells growing on glucose and lactose, the experimentally observed preferential utilization of glucose, the short lag phase when shifting to lactose utilization, the accompanying sharp rise in the cAMP concentration, and the induction of lacZYA transcription can be reproduced. However, in contrast to the experimental data (362), the concentration of cAMP remains high after the lag phase, and the time course predictions are incorrect. The models by the group of Mackey focus on the proposed bistable nature of the lac operon. A bistable system can convert a graded signal into a switch-like response; i.e., when the inducer concentration passes a certain threshold, the system is switched on. At the same time, to switch off and return to the initial state, the inducer concentration has to drop below a smaller threshold value (hysteresis). The initial models, which did not include the mechanisms responsible for CCR (978, 979), described the induction of the lac operon during growth on lactose well. By including experimental data on β-galactosidase expression, the models predicted bistability for the lac operon depending on the concentration of extracellular lactose and the growth rate. When activation/repression by Crp/cAMP and inducer exclusion were included (776), bistability was maintained. The potential for bistable behavior of the lac operon was further proved by the observed history dependence (i.e., a hysteretic dependence) of the induction of fluorescent reporter proteins synthesized from genes expressed from the lac or gat promoter (626). Based on these induction experiments, a mathematical model was constructed, which separates the effects of inducer exclusion (acting on LacI activity) and Crp/cAMP, as E. coli MG1655, which was used in these studies, lacks GatR. Its gat promoter is regulated solely by Crp/cAMP and can thus be used as a specific reporter of Crp/cAMP effects. It was found that repression by glucose is mediated by both inducer exclusion and Crp/cAMP and that the effect of the latter is stronger. Similarly, the calculations reported by Santillán and Mackey (776) indicate that the effect of activation/repression by Crp/cAMP and inducer exclusion on the induction of the lac operon are cumulative so that both the sensitivity of the system towards glucose and the concentration of lactose required to induce the system are elevated. However, in agreement with the conclusions drawn from “wet” experiments, the calculated effective β-galactosidase concentration appears to be more sensitive to inducer exclusion than to Crp/cAMP (see the section on diauxic growth).
A comprehensive model of diauxic growth was presented by Kremling et al. (425). Repression of ptsG by Mlc, inducer exclusion, and non-PTS transport of glucose were included, but the formation of heterodimeric phosphoryl group transfer complexes was not taken into account. Parameters were taken from the literature or otherwise estimated and optimized on the basis of growth experiments with isogenic mutants (cyaA, lacI, and ptsG) obtained from E. coli strain LJ110, a well-characterized derivative of the E. coli K-12 reference strain W3110. The model perfectly reproduces biomass yield, glucose and lactose consumption, the preferential use of glucose, and the induction of lacZ expression. In agreement with the experimental data, the model predicts high concentrations of unphosphorylated EIIAGlc during growth on glucose, followed by a rise in the P∼EIIAGlc and cAMP concentrations when glucose is exhausted. When intracellular glucose is produced from lactose and phosphorylated by the PTS, P∼EIIAGlc is predicted to drop again. A similar model describes the transport and metabolism of the non-PTS sugar glycerol and the PTS substrate sucrose as well as glp and scr gene expression (931). Again, the model reproduces the experimental data obtained with E. coli K-12 derivatives well. The models described by Wang et al. and Kremling et al. (425, 931) were used to construct a large-scale stochastic model of glucose, lactose, and glycerol metabolism (up to pyruvate), including transcriptional regulation (688). The model reveals potential effects of history and stochasticity, the latter especially in transcription, on reaction network behavior towards the changes in available nutrients.
Other Regulatory Mechanisms Involving the PTS in Enteric Bacteria
Phosphorylation of EI by ATP.
PEP-dependent phosphorylation of EI was originally thought to be the only route to generate P∼His-HPr (435, 436). However, two decades after the discovery of the PTS, PEP-independent EI phosphorylation was discovered (236). E. coli acetate kinase (AckA) and [γ-32P]ATP phosphorylate EI in a reversible reaction (236). When catalyzing the formation of acetyl∼P from acetate and ATP, AckA is transitorily phosphorylated on a glutamyl residue (238, 881), probably at Glu-387 in E. coli AckA (817). PEP and P∼AckA most likely phosphorylate the same histidyl residue in EI (16), as the phosphoryl group of P∼AckA can be passed to EIIAGlc via EI and HPr. A second protein kinase that also phosphorylates EI at the expense of ATP was found in E. coli (154, 155). It was partially purified, but its activity vanished during purification due to the loss of a cofactor, which was shown to be NAD(P)+. The reaction catalyzed by this EI kinase is reversible; i.e., it can transfer the phosphoryl group of P∼EI back to ADP, and the backward reaction requires the presence of NAD+. In its absence, EI kinase acts as a phosphatase and converts P∼EI to EI and Pi. It is not yet clear whether phosphorylated EI kinase is transitorily formed during P∼EI dephosphorylation. Although AckA- and EI kinase-mediated phosphorylation of EI have been demonstrated in vitro, whether they have physiological importance remains to be shown.
Chemotactic response to carbohydrates.
Motile bacteria such as E. coli and B. subtilis can be attracted to or repelled from various chemical and physical environmental signals. These signals can be processed via different pathways (see reference 11), but ultimately, they affect the frequency with which the rotation direction of the flagella changes. An increase in counterclockwise rotation results in smooth, straight movement of the bacterium (e.g., towards the attractants), whereas an increase in clockwise rotation leads to “tumbling” and a change in direction (e.g., away from the attractant) (see reference 501). The most important chemotaxis signal processing pathway comprises chemoreception in the cytoplasmic membrane by methyl-accepting chemotaxis proteins (MCPs) (for reviews on chemotaxis, see references 77, 82, 838, and 850). Although the number of proteins involved in the chemotactic signaling pathway varies in different bacteria, the main mechanism follows similar principles (850). The chemotaxis pathway of enteric bacteria is displayed in Fig. 3. An incoming chemotactic signal is relayed to the flagellar motor via the CheA/CheY two-component system. The autophosphorylation activity of the ATP-dependent histidine kinase CheA is stimulated by its CheW-mediated recruitment to the MCPs. P∼CheA transfers its phosphoryl group to an aspartyl residue of CheY, the response regulator. P∼CheY binds to the flagellar motor protein FliM, and as a consequence, the tumbling frequency increases. To prevent the accumulation of P∼CheY, the protein has autophosphatase activity, and in various bacteria (including E. coli), dephosphorylation is stimulated by a protein called CheZ. This signal transduction pathway is controlled by an adaptive feedback mechanism involving MCP methylation by the methyltransferase CheR, which stimulates CheA activity, and demethylation by the methylesterase CheB, which inhibits CheA activity. CheB is activated by phosphoryl transfer from P∼CheA. Upon chemoreception, the MCP lowers the activity of CheA considerably, and as a result, the concentration of P∼CheY decreases. The bacterium therefore tumbles less frequently and thus effectively moves up a concentration gradient of chemoattractant. Simultaneously, receptor demethylation diminishes due to lower levels of P∼CheA/P∼CheB, and CheA will be reactivated, leading finally to an adaptation of the system.
FIG. 3.
Mechanism of chemotaxis in E. coli and role of the PTS in carbohydrate chemotaxis. In the absence of chemotactically active molecules (top left), CheA autophosphorylates at a histidyl residue. This reaction is stimulated by CheW, which recruits CheA to the MCP receptor protein. P∼CheA transfers its phosphoryl group to CheY, and P∼CheY binds the flagellar motor FliM, thereby evoking clockwise (CW) rotation of the flagella, which leads to tumbling of the bacterium. In the presence of chemotactically active molecules (top right), the autophosphorylation activity of CheA is inhibited, and as a result, the concentration of P∼CheY drops. FliM molecules will no longer be complexed with CheY, which favors counterclockwise (CCW) rotation of the flagella. This results in smooth swimming towards increasing concentrations of the chemotactically active substance. The presence of an efficiently metabolizable PTS carbohydrate (or the absence of PEP) induces a similar response, as under these conditions, EI is present mainly in an unphosphorylated form. In fact, dephospho-EI inhibits the autophosphorylation of CheA (center) and therefore also favors smooth swimming. The sensitivity of the system towards the signal is regulated through methylation and demethylation of the MCPs (bottom), which are catalyzed by CheR and CheB, respectively. MCP methylation stimulates the autophosphorylation of CheA, and demethylation inhibits it.
E. coli shows chemotaxis towards many nutrients, including several PTS sugars (8, 462). By using a variety of null mutants, it was established that the chemotrophic response towards PTS sugars was elicited by their transport and not by their binding or metabolism (8, 297, 462, 641, 948). It was established that chemotaxis towards PTS sugars at relatively high concentrations (>1 mM) is also mediated by the chemoreceptors Aer and Tsr (296), which sense redox state and proton motive force, respectively (865). In E. coli, chemotaxis by PTS sugars can be abolished by knocking out CheA, CheY, CheW, EI, HPr, or the corresponding sugar-specific EII complexes, whereas the removal of the MCPs, CheR, or CheB has no significant effect (8, 297, 460, 462, 498, 550, 609, 641, 745, 863, 864; see also reference 678). Despite the fact that PTS-mediated sugar uptake and Che protein-mediated chemotaxis involve similar phosphoryl transfer reactions, no phosphoryl transfer between PTS components and Che proteins has been demonstrated (379). Rather, in vitro experiments established that unphosphorylated EI interacts with CheA and thereby inhibits the autokinase activity of CheA by up to 10-fold (497). In addition, CheA activity drops about threefold upon shifts of the PEP concentration in the physiologically relevant range (from 2 to 0.3 mM). Further evidence for an effect of the phosphorylation state of PTS proteins on CheA activity was provided by kinetic analyses of the chemotactic response in E. coli wild-type cells and several che null mutants (498). The response, which was induced by flash photolysis of caged d-glucose and α-MG, shows rapid kinetics and high signal sensitivity (Km for d-glucose is about 10 nM). Considering the rapidity and sensitivity of the response, those authors proposed that transport-induced dephosphorylation of P∼EI affects CheA activity but has little influence on the PEP concentration (the intracellular concentration of PEP is about 200-fold higher than that of EI). This hypothesis holds only when phosphorylation of EI by PEP would be a slow process controlling glucose influx. However, because even at high glucose concentrations, nearly all control over the flux resides in the glucose transporter (901), it is highly unlikely that EI phosphorylation exerts control at low glucose concentrations. Rather, PTS model calculations predict that the extent of EI phosphorylation rapidly responds to changes in the PEP concentration (239, 240, 777). At high intracellular PEP concentrations, mainly P∼EI and little EI have been calculated to be present. Thus, even a slight reduction in the PEP concentration (as anticipated for the chemotaxis experiments) (497) could cause a relatively significant increase of unphosphorylated EI, which would lower CheA activity. This could in turn slightly lower the amount of P∼CheY and lead to a strong chemotactic response, as the binding of P∼CheY to the flagellar motor is highly cooperative (133).
In contrast to enteric bacteria, chemotaxis towards PTS carbohydrates in B. subtilis is mediated by the methyl-accepting chemotaxis protein McpC, which is also a sensor for several amino acids (582). Deletion of mcpC abolishes chemotaxis towards the carbohydrates d-glucose, d-mannitol, d-fructose, trehalose, N-acetylglucosamine, and α-methyl-d-glucoside (261, 428). Nevertheless, chemotaxis in B. subtilis is affected by the PTS, and the cytoplasmic domain of McpC is thought to receive a transport-related signal from the PTS (428). As a consequence, EIIMtl, EIIFru, and EIITre null mutations resulted in a loss of chemotaxis towards the related carbohydrate. Deletion of ptsH also prevents B. subtilis from moving up a sugar gradient of mannitol and fructose (261). The response towards glucose is more complex and involves McpA (313). A ptsH or mcpA deletion strain shows chemotaxis towards glucose (which is taken up via a non-PTS transporter by the ptsH mutant), whereas a double mutant does not (261). Binding studies with purified CheA and EI indicate that P∼EI, but not EI or PEP, binds CheA and thereby inhibits its autophosphorylation. In B. subtilis, P∼CheY exerts an effect opposite that in E. coli (61, 62, 945) (i.e., P∼CheY leads to smooth swimming), and therefore, the inhibition of CheA activity by phosphorylated EI in B. subtilis is consistent with CheA inhibition by unphosphorylated EI in E. coli. However, experimental findings with tethered cells raise doubts about the proposed mechanism. For instance, the addition and removal of mannitol had effects similar to those of the addition of glucose on the frequency of counterclockwise rotation in a ptsH mutant (261), although this mutant does not transport mannitol. It is not clear how the phosphorylation state of the PTS proteins could be affected by a sugar that is not transported and metabolized. In addition, conflicting results were obtained when the effect of glucose on the rotation of tethered cells was studied (261, 428).
Glycogen storage.
Many bacteria, including E. coli and S. enterica serovar Typhimurium, store carbohydrates in the form of glycogen. They produce it from glucose-1-P by the sequential action of ADP-glucose pyrophosphorylase (GlgC) and glycogen synthase (GlcA) (see reference 683). When needed, glycogen is reconverted to glucose-1-phosphate by glycogen phosphorylase (GlgP) (962). The related E. coli genes are located in the glgCAP operon, which is positively regulated by Crp/cAMP (684) and negatively regulated by CsrA (38, 486, 962). B. subtilis possesses a glgBCDAP operon (glgB codes for a glycogen branching enzyme, and glgD codes for a second ADP glucose pyrophosphorylase), which is presumably negatively regulated by CcpA/P-Ser-HPr (178). Surface plasmon resonance-based ligand fishing revealed a strong interaction between E. coli glycogen phosphorylase (GlgP) and HPr; indeed, GlgP was the only protein “fished” out of a cell extract by immobilized HPr (799). The interaction between the two proteins was confirmed by mobility shift assays and sedimentation equilibrium centrifugation. Subsequent competition experiments established that the binding was highly specific. Neither B. subtilis and Mycobacterium capricolum HPrs nor E. coli NPr and diphosphoryl transfer proteins (FPr) bind to E. coli GlgP, and vice versa, E. coli maltodextrin phosphorylase, which is very similar to GlgP (352, 797), does not bind HPr (418, 799). The affinity of GlgP for P∼His-HPr is four times higher than that for unphosphorylated HPr. Binding of HPr stimulates the formation of GlgP dimers and tetramers. Only the binding of HPr, but not of P∼His-HPr, leads to a significant increase in GlgP activity (2.5-fold) (799). The concentration of HPr (531) is much higher than that of GlgP (116), and therefore, a major part of cellular GlgP will be complexed with HPr or P∼His-HPr. As a result, GlgP activity will be determined by the phosphorylation state of HPr. Conversely, overproduction of GlgP should lead to the sequestration of most HPr and thereby to the inhibition of the PTS, as was indeed observed (418).
Based on these results, the following model for the regulation of glycogen breakdown was proposed for E. coli. Cells growing on glucose start to accumulate glycogen during the late exponential growth phase and the onset of stationary phase (418, 683, 684). Under these conditions, the concentration of phosphorylated PTS proteins is relatively high (797). cAMP levels will therefore rise (653), and expression of the glgCAP operon will be stimulated. The activity of GlgP is low, because mainly P∼His-HPr is present, and glycogen will accumulate. Later, when growth of the cells has advanced far into stationary phase, the PEP concentration drops, PTS proteins will become dephosphorylated, and the cAMP concentration and expression of the glgCAP operon will decrease. However, GlgP is now activated by binding unphosphorylated HPr, and glycogen breakdown will therefore commence and dominate over glycogen synthesis.
REGULATION OF CARBON METABOLISM IN LOW-G+C GRAM-POSITIVE BACTERIA: REGULATORY FUNCTIONS OF P-Ser-HPr
HPr, the Central Processing Unit of Carbon Metabolism in Gram-Positive Bacteria
Most low-G+C gram-positive organisms do not possess adenylate cyclase, and the cAMP-dependent EIIAGlc-controlled CCR mechanism of gram-negative bacteria can therefore not be operative in these organisms. In fact, the general PTS protein HPr turned out to be the master regulator of carbon metabolism in gram-positive bacteria. Similar to EIIAGlc in gram-negative bacteria, HPr carries out its diverse regulatory functions in response to changes in its phosphorylation state. In low-G+C gram-positive bacteria, HPr becomes phosphorylated not only by PEP at His-15 but also by ATP at Ser-46. As a consequence, four different forms of HPr exist in these organisms: dephospho-HPr, HPr phosphorylated at either His-15 (P∼His-HPr) or Ser-46 (P-Ser-HPr), and doubly phosphorylated HPr (571, 893). The rapid metabolism of various sugars affects the activities of a bifunctional protein kinase/P-protein phosphorylase, which responds to changes of the ATP, Pi, PPi, and FBP concentrations, and of EI, which responds to alterations of the PTS phosphotransfer activity and the PEP-to-pyruvate ratio (335). These two enzymes control the concentration of the various forms of HPr, which regulate carbon metabolism via protein-protein interactions (HPr and P-Ser-HPr) or the phosphorylation of non-PTS proteins (P∼His-HPr) (Table 1). In gram-positive bacteria, HPr therefore functions as the “central processing unit” for carbon metabolism, as its phosphorylation state is determined by various signals (input), which in turn allow it to phosphorylate or to interact with numerous other proteins (output). In the following sections, we discuss the characteristics of the enzyme catalyzing the phosphorylation/dephosphorylation of HPr at Ser-46, the role of P-Ser-HPr in CCR and inducer exclusion, and the regulation of non-PTS proteins (antiterminators, transcription activators, carbohydrate transporters, and catabolic enzymes) via phosphorylation by P∼His-HPr and/or P∼EIIBs.
Characteristics of ATP-Dependent HPr Phosphorylation
In the following sections, we will describe the enzyme HPr kinase/phosphorylase (HprK/P), which catalyzes the ATP-dependent phosphorylation of HPr (and certain HPr paralogs) at Ser-46 as well as the dephosphorylation of P-Ser-HPr. We will include structural studies of HprK/P and the complexes with HPr and P-Ser-HPr and discuss the regulation of its two opposing activities by metabolites. The organization of hprK with specific genes, some of unknown function, in gram-positive bacteria will be discussed, and finally, results that suggest a function of P-Ser-HPr in the regulation of PTS activity by a feedback mechanism will be provided.
Phosphorylation of HPr at Ser-46.
Isocitrate dehydrogenase from E. coli was the first bacterial protein shown to be phosphorylated by an ATP-dependent seryl protein kinase (257). In the early 1980s, when Deutscher, Reizer, and Saier investigated a possible implication of protein phosphorylation in inducer expulsion (185, 710, 712), a process where certain sugar phosphates that have accumulated in bacteria are dephosphorylated and expelled as soon as the cells are exposed to rapidly metabolizable carbon sources such as glucose or mannose, a second seryl-phosphorylated bacterial protein was detected in Streptococcus pyogenes and was identified as being HPr of the PTS (185). Unlike PEP-dependent EI-catalyzed phosphorylation at the catalytic His-15 of HPr, which occurs in both gram-negative and gram-positive organisms (Fig. 1) (262, 941), phosphorylation of HPr at a seryl residue, which requires a specific protein kinase and ATP (185) or GTP (709), was originally believed to be restricted to gram-positive organisms with low G+C content.
The site of ATP-dependent phosphorylation in HPr of Enterococcus faecalis was identified as being Ser-46 (183). An equivalent of Ser-46 is present in HPr of all low-G+C gram-positive bacteria, and the surrounding sequence is usually well conserved (Fig. 4). However, in the HPr of some organisms, the phosphorylatable serine is in a slightly different position (position 45 in HPr of Bacillus thuringiensis subsp. israelensis [400] and position 47 in HPr of Mycoplasma genitalium [713]). Replacement of Ser-46 in B. subtilis HPr with an alanine, tyrosine, or aspartate prevents the FBP-stimulated ATP-dependent phosphorylation of HPr (206, 722), whereas HPr carrying a threonine at position 46 is still slowly phosphorylated (722). Mutations at Ser-46 also affect the PEP-dependent, EI-catalyzed phosphorylation at His-15. In a mutant complementation assay carried out with rate-limiting amounts of B. subtilis HPr (20 μM), the phosphoryl carrier activity was lowered to 35, 30, and 20% when Ser46Ala, Ser46Tyr, or Ser46Thr HPr, respectively, was used in place of wild-type HPr (206) and was lowered to 10% when Ser46Asp HPr was used (722). Phosphorylation of HPr at Ser-46 lowered the rate of PEP-dependent phosphorylation by 2 orders of magnitude (177). In addition, the apparent Km value for the PEP-dependent phosphorylation of P-Ser-HPr was 15-fold higher than the apparent Km value determined with HPr (723). In E. coli HPr, Ser-46 is located within a hydrophobic patch implicated in the interaction with EI (259) and EIIAs (139, 691, 949). The fact that Ser-46 of HPr faces the well-conserved Glu-84 of EI in the HPr:EI complex (260) probably explains why HPrs of gram-positive bacteria carrying a negative charge at Ser-46, due to either phosphorylation or mutation, interact poorly with EI. Vice versa, phosphorylation of HPr by EI at His-15 slowed its phosphorylation at Ser-46 by the ATP-dependent HPr kinase (177). In the HPr:HPr kinase complex, helix α4 of HPr kinase interacts with the region around His-15 (228, 533). Phosphorylation at His-15 probably diminishes the affinity between the kinase and its protein substrate. Indeed, in two-hybrid experiments, an interaction of B. subtilis HPr kinase with Ser46Ala mutant HPr could be detected. This interaction was prevented when EI was additionally produced in the yeast cells, suggesting that EI efficiently competes with HprK/P for binding to HPr and/or that EI phosphorylates HPr at His-15, which lowers its affinity for HprK/P (P. Noirot, S. Poncet, and J. Deutscher, unpublished results).
FIG. 4.
Alignment of the first 55 amino acids of HPr proteins from firmicutes (B.s., B. subtilis; E.f., E. faecalis; L.c., L. casei; S.s., S. salivarius; S.c., S. carnosus), from a spirochete (T.p., T. pallidum), from proteobacteria with a Ser-46 region strongly resembling the corresponding sequence in HPr of firmicutes (X.f., Xylella fastidiosa; N.m., N. meningitidis), and from other gram-negative bacteria (E.c., E. coli; H.i., H. influenzae; V.c., V. cholerae) (V. cholerae possesses a second HPr in which the region around Ser-46 more strongly resembles the corresponding region in the HPr of firmicutes). The arrows indicate the amino acids His-15 and Ser-46. In HPrs from organisms, in which these residues are phosphorylated, they are shown in boldface type. Conserved regions around the phosphorylation sites are boxed.
Gram-negative enteric bacteria do not possess HPr kinase, although their HPrs usually contain Ser-46 and exhibit about 45% overall sequence identity to HPrs from gram-positive organisms. Nevertheless, the region around Ser-46 is quite different. This difference is probably responsible for the failure of HPr kinase from gram-positive bacteria to phosphorylate E. coli HPr (177, 722). HPrs of Mycoplasma capricolum, in which amino acids next to Ser-46 were replaced with the corresponding amino acids of E. coli HPr, were poorly phosphorylated with ATP (991). Based on the solution and crystal structures of HPrs from E. coli, E. faecalis, and B. subtilis, the presence of a lysine in position 49 of E. coli HPr, which is a glycine in HPrs of most gram-positive bacteria (Fig. 4), was assumed to represent a major obstacle for the interaction of HPr kinase with HPr from E. coli and presumably with HPrs from other gram-negative bacteria (374).
Interestingly, several gram-negative nonenteric organisms possess an HPr kinase, and the sequence around Ser-46 of their HPrs resembles that of HPr from low-G+C gram-positive bacteria (64) (Fig. 4). In contrast, Streptomyces coelicolor, a gram-positive organism with high G+C content in its DNA, does not possess a protein exhibiting significant sequence similarity to HPr kinase (55), and the sequence around Ser-47 of its HPr barely resembles the sequence around Ser-46 in HPrs of low-G+C gram-positive bacteria. Nevertheless, HPr from S. coelicolor can be phosphorylated by the HPr kinase from B. subtilis (97, 630), suggesting that despite the sequence differences, the structure around Ser-47 sufficiently resembles the structure around Ser-46 in B. subtilis HPr to allow its phosphorylation.
HPr kinase also dephosphorylates P-Ser-HPr by producing PPi.
A soluble enzyme that specifically dephosphorylated P-Ser-HPr was purified to near homogeneity from E. faecalis (181). It had a molecular mass of 30 kDa (on sodium dodecyl sulfate-polyacrylamide gels), but it escaped the authors' attention that the purified enzyme must have also exhibited HPr kinase activity. In fact, it was later discovered that similar to the first described bacterial serine protein kinase, isocitrate dehydrogenase kinase/phosphatase (450), HPr kinase is also bifunctional and possesses P-Ser-HPr dephosphorylation activity (423). HPr kinase of E. faecalis has a molecular mass of 33 kDa, which is close to the molecular mass detected for the major protein in the P-Ser-HPr phosphatase preparation. Inorganic phosphate (Pi) was reported to stimulate P-Ser-HPr dephosphorylation (181). This was surprising, as Pi was assumed to be one of the products of P-Ser-HPr dephosphorylation and was therefore rather expected to inhibit this reaction. The paradox was resolved by demonstrating that P-Ser-HPr dephosphorylation is not a hydrolysis reaction. Dephosphorylation of either 32P-Ser-HPr in the presence of Pi or P-Ser-HPr in the presence of 32Pi leads to the formation of radioactive 32PPi. Pi thus functions as a substrate, and lowering the Pi concentration below the μM range almost completely prevents the dephosphorylation of P-Ser-HPr (557). In analogy to the usual phosphohydrolysis reaction, this novel protein dephosphorylation mechanism was called phospho-phosphorolysis, and the responsible enzyme was termed HPr kinase/P-Ser-HPr phosphorylase (HprK/P). Because P-Ser-HPr dephosphorylation is reversible, PPi can replace ATP as the phosphoryl donor for HPr phosphorylation. The kinetic parameters indicate that PPi-dependent HPr phosphorylation is actually the energetically favored reaction (557). To allow the efficient dephosphorylation of P-Ser-HPr in the cell, the resulting PPi therefore needs to be hydrolyzed. In B. subtilis and other bacilli, PPi hydrolysis seems to be achieved by YvoE, which exhibits pyrophosphatase activity (557). The yvoE gene is located in the hprK operon of bacilli (Fig. 5), and since YvoE exhibits sequence similarity to P-glycolate phosphatases and stimulates P-Ser-HPr dephosphorylation, it was mistakenly assumed to be P-Ser-HPr phosphatase (251). It is likely that organisms missing a yvoE gene in the hprK operon use one of usually several YvoE homologs present in bacteria to hydrolyze PPi formed during P-Ser-HPr dephosphorylation.
FIG. 5.
The gene context of hprK in bacteria of the phylum Firmicutes. The hprK gene is followed by lgt in all sequenced genomes of the firmicutes except in L. mesenteroides, Oenococcus oeni, and some clostridiae. In addition, there are other genes associated with hprK that appear to be conserved. They include two genes without a known function (in L. lactis, E. faecalis, and several streptococci [S. agalactiae, S. mutans, S. pneumoniae, S. pyogenes, S. suis, S. thermophilus, and S. uberis]) and a glycerol-3-phosphate dehydrogenase gene and a UTP-glucose-1-phosphate uridylyltransferase gene (in E. faecalis) followed by a thioredoxin reductase gene (in L. acidophilus, L. johnsonii, L. gasseri [these species lack the uridylyltransferase], L. brevis, L. plantarum, P. pentosaceus, L. mesenteroides, and O. oeni [the latter two species lack the lgt gene]). Staphylococci contain YvoF, a protein with a hexapeptide transferase motif (S. aureus, S. epidermidis, S. haemolyticus, and S. saprophyticus). YvoF, together with YvoE (a pyrophosphatase), is also present in B. anthracis, B. cereus, B. thuringiensis, Exiguobacterium species, L. innocua, and L. monocytogenes, while YvoD, YvoE, and YvoF are found in the bacilli B. clausii, B. halodurans, B. licheniformis, B. stearothermophilus, B. subtilis, G. kaustophilus, and O. iheyensis.
Cold-chase ATP labeling of HPr in Listeria monocytogenes crude extracts suggested that HprK/P of this organism cannot dephosphorylate P-Ser-HPr (129). However, later experiments with purified HprK/P established that L. monocytogenes HprK/P functions as P-Ser-HPr phosphorylase, similar to the B. subtilis enzyme (326).
Interestingly, although Mycoplasma pneumoniae hprK mutant cells cannot form P-Ser-HPr, crude extracts prepared from this mutant were still able to dephosphorylate P-Ser-HPr. The dephosphorylation activity could be attributed to a PP2C-type P-protein phosphatase, which, in accordance with its B. subtilis homolog, was called PrpC (306). M. pneumoniae HprK/P is unusual, as it can phosphorylate HPr at relatively low ATP concentrations (831). In addition, in vivo P-Ser-HPr formation is stimulated by the presence of glycerol in the growth medium (307). Although the M. pneumoniae enzyme was reported to dephosphorylate P-Ser-HPr in vitro in a Pi-dependent manner, similar to HprK/Ps from other organisms (831), the P-Ser-HPr dephosphorylation activity exhibited by PrpC seems to be of physiological relevance. While an M. pneumoniae wild-type strain grown in the presence of glucose and glycerol contained only low amounts of P-Ser-HPr and doubly phosphorylated HPr, more than half of the HPr was found to be present as P-Ser-HPr and doubly phosphorylated HPr in the prpC mutant (306). Because a B. subtilis strain synthesizing Val267Phe HprK/P, which still functions as a kinase but has lost its phosphorylase activity (see “P-Ser-HPr Regulates PTS Transport Activity by a Feedback Mechanism”), contains more than 95% of its HPr as P-Ser-HPr (571), it is unlikely that PrpC of this organism plays a major role in P-Ser-HPr dephosphorylation. In B. subtilis, PrpC and the corresponding protein kinase, PrkC, were reported to play important roles in stationary-phase cells and to control the phosphorylation state of the translation elongation factor EF-G (248). It is interesting that HprK/Ps of certain gram-negative bacteria such as Neisseria meningitidis (S. Poncet, M.-K. Taha, M. Larribe, and J. Deutscher, unpublished results) and Brucella melitensis (S. Poncet, M. Dozot, X. de Bolle, J. J. Letesson, and J. Deutscher, unpublished results) exhibit no or very low P-Ser-HPr dephosphorylation activity, and although these organisms do not contain a homolog of PrpC, it is possible that they possess another P-Ser-HPr phosphatase.
Structure determination of HprK/P.
The crystal structures of truncated HprK/P from L. casei (missing the N-terminal 120 amino acids) (229) and of full-length HprK/Ps from Staphylococcus xylosus (515) and M. pneumoniae (13) have been determined. The crystallized proteins form hexamers composed of two layers of trimers. Size exclusion chromatography and equilibrium sedimentation showed that L. casei HprK/P also forms hexamers in solution (229). B. subtilis HprK/P was reported to form hexamers at a neutral pH but was reported to form monomers and dimers at pH 9.5 (697). HprK/Ps do not exhibit similarity to eukaryotic protein kinases (312, 866) or P-protein phosphatases (43, 805) but resemble PEP carboxykinase and nucleosidediphosphate kinases (229, 252, 748). A P loop (or Walker motif A [GXXGXGKS]) that is usually located between amino acids 150 and 170 serves as a nucleotide binding site. The crystal structures of L. casei and S. xylosus HprK/Ps revealed that Pi binds to the same position in Walker motif A as the β-phosphate of the nucleotide. This explains the inhibitory effect of Pi on the kinase activity, as ATP and Pi compete for the same binding site. Vice versa, ATP inhibits the phosphorylase activity of HprK/P (181). The inhibitory effect of ATP was stronger at low Mg2+ concentrations. Mutant studies with hprK from various organisms suggested that Walker motif A is important not only for the kinase but also for the phosphorylase function (315, 571, 831).
The dual role of Walker motif A was confirmed by solving the crystal structures of HprK/Ps complexed with HPr or P-Ser-HPr, which also allowed the prediction of a detailed mechanism for HPr phosphorylation and P-Ser-HPr dephosphorylation (228). His-140 of L. casei HprK/P forms a hydrogen bond to Asp-179, which in turn acts as a base during HPr phosphorylation by forming a hydrogen bond to the hydroxyl group of Ser-46 in HPr. The structure of the HprK/P:P-Ser-HPr complex, which was obtained by cocrystallizing HPr, HprK/P, and PPi, allowed the identification of the amino acids cooperating in the nucleophilic attack of Pi on the P-Ser bond and revealed that the binding of HPr and P-Ser-HPr to HprK/P involves nearly identical interfaces. Compared to the HprK/P:HPr complex, an additional contact is made between the phosphoryl group in P-Ser-HPr and Arg-245 of a neighboring HprK/P subunit (228). The loop containing Arg-245 is too flexible to be traced in the HprK/P and HprK/P:HPr structures, but it is stabilized in the HprK/P:P-Ser-HPr complex due to the interaction of Arg-245 with the phosphoryl group (228, 229). During P-Ser-HPr dephosphorylation, Asp-179 (present in protonated form) and His-140 (donates a proton to Ser-46 of HPr) (228) play an inverse role compared to the kinase reaction. The interaction site of S. aureus HPr with S. xylosus HprK/P was also determined by NMR, and the HPr interface appears to be similar in solution and in the crystal (533).
The crystal structure of HprK/P from S. xylosus revealed that a Pi ion binds not only to Walker motif A but also to the N-terminal domain (515). It was fixed by three conserved arginines located in two different subunits, one in the upper and one in the lower HprK/P trimer. The second Pi therefore bridges the two trimers (515), but the Pi bridge is not essential for hexamer formation, as truncated L. casei HprK/P (229) and M. pneumoniae HprK/P without bound Pi (13) also form hexamers. The function of the N-terminal domain, which protrudes from the central part like a propeller, is unknown. Truncated L. casei HprK/P that is missing this domain is active as a kinase and phosphorylase and responds to all known effectors (229). As will be discussed below, the N-terminal domain is naturally truncated in HprK/Ps of α-proteobacteria. Surprisingly, the N-terminal domain of HprK/P exhibits a fold similar to that of the N-terminal part of MurE (13), an enzyme implicated in the formation of cytoplasmic precursors for cell wall synthesis (284).
The structure of HprK/P with bound ATP or GTP has not yet been solved. If the nucleotide would occupy the same position as that in the closely related adenylate kinase, it would clash with the central K3 loop of a neighboring HprK/P subunit. Structural changes of ATP and/or HprK/P must therefore accompany nucleotide binding (13, 229, 515). Likewise, structural changes of HprK/P might be responsible for the observed cooperative binding of ATP (372).
Organization of the hprK operon.
About 15 years after the discovery of the ATP-dependent phosphorylation of HPr at Ser-46 (185), at almost the same time, three laboratories succeeded in identifying the HprK/P-encoding hprK gene (named ptsK in one publication) from three different organisms. In the first report, purification of E. faecalis HprK/P and sequencing of its N terminus and internal peptides allowed the amplification of the 5′ part of the hprK gene by PCR. This information was subsequently used to identify hprK of B. subtilis within the sequenced genome (251) and to obtain the complete hprK gene of E. faecalis (423). In the second report, purification and N-terminal sequencing of B. subtilis HprK/P was used to directly identify hprK of B. subtilis (709). Finally, purification of HprK/P and probing of a DNA library with a degenerate oligonucleotide derived from the N-terminal amino acid sequence allowed the identification of hprK from Streptococcus salivarius (84). More recently, the hprK genes from other low-G+C gram-positive bacteria including S. xylosus (357), L. casei (198), M. pneumoniae (830), and G. stearothermophilus (127) have been cloned and sequenced, and the corresponding HprK/Ps were purified and characterized. Genome sequencing revealed the presence of hprK in most low-G+C gram-positive organisms.
In the firmicutes, hprK is usually the first gene within an operon (Fig. 5), which is composed of five genes in B. subtilis. In most gram-positive bacteria, the second gene is the prolipoprotein diacylglyceryl transferase-encoding lgt gene, which carries out the diacylglyceryl lipidation of lipoproteins at a cysteyl residue. The frequent association of hprK with lgt might indicate that the functions of the two enzymes are somehow related, although no experimental evidence for this assumption has been obtained so far. A possible link between the PTS and Lgt is further suggested by the observation that in Enterobacteriaceae (E. coli, S. enterica serovar Typhimurium, Yersinia pestis, etc.), lgt is preceded by ptsP (T. Doan, personal communication), which encodes an EI with an N-terminal extension resembling the GAF domain in NifA. The hprK operon of G. stearothermophilus, Oceanobacillus iheyensis, L. monocytogenes, Listeria innocua, and the seven bacilli, whose genomes have been sequenced, contains the pyrophosphatase-encoding yvoE gene followed by yvoF downstream of lgt. B. subtilis, Bacillus clausii, Bacillus halodurans, Bacillus licheniformis, and G. stearothermophilus contain an additional gene, yvoD, inserted between lgt and yvoE. In S. aureus and Staphylococcus epidermidis, yvoE is missing, and yvoF directly follows lgt. The yvoDEF genes are absent from most other low-G+C gram-positive organisms. O. iheyensis possesses two genes encoding HprK/Ps, with one followed by lgt. It is not known whether the HprK/P ortholog encoded by the other hprK gene can phosphorylate HPr. In the genus Clostridium, hprK and lgt are located in different regions of the genome. In Clostridium acetobutylicum, hprK is preceded by a gene encoding a protein with significant sequence similarity to the glycerol-inducible E. coli GlpX, which resembles fructose-1,6-bisphosphatases (859).
Quite unique is a presumed hprK gene in Fusobacterium nucleatum ATCC 25586 and F. nucleatum subsp. vincentii, which encodes two complete HprK/P proteins fused together in tandem. The two HprK/P domains exhibit 22% sequence identity to each other and about 30% identity to HprK/Ps from gram-positive bacteria. However, a conserved Walker motif A is present only in the C-terminal domain. The corresponding sequence in the N-terminal domain is strongly altered and does not resemble Walker motif A. A prerequisite for HPr phosphorylation is the deprotonation of the hydroxyl group of Ser-46 of HPr by the concerted action of conserved histidyl and aspartyl residues (His-140 and Asp-179 in L. casei HprK/P). In F. nucleatum HprK/P, these residues are present in the C-terminal domain but are replaced with Glu and Lys, respectively, in the N-terminal domain. In contrast, Arg-245, which interacts with the phosphoryl group of P-Ser-HPr and probably plays a major role in P-Ser-HPr dephosphorylation, is present only in the N-terminal domain. It is therefore tempting to assume that in this duplicated enzyme, the two activities have been separated and that the C-terminal domain carries the kinase activity, whereas the N-terminal domain might function as P-Ser-HPr phosphorylase. The two sequenced F. nucleatum strains also contain HPr (FN1782) with conserved His-15 and Ser-46 regions and EI (FN1793), but although the genes are located close to each other on the chromosome, they do not seem to be organized in an operon.
The organization of the hprK gene in certain gram-negative bacteria will be discussed below in more detail.
Metabolites regulate the antagonistic activities of HprK/P.
In vitro experiments suggested that the two antagonistic activities associated with HprK/P are regulated in the cell in response to changes in the concentrations of FBP, ATP, and Pi (177). FBP clearly stimulates the kinase activity of B. subtilis HprK/P (372), whereas its effect is less pronounced with the L. casei enzyme (198). The effect of FBP is usually stronger when low, nonphysiological concentrations of ATP are used. In addition, the stimulatory effect of FBP on HPr phosphorylation was more evident when the experiments were carried out in the presence of a few mM Pi (177, 198, 423), which lowers the HPr kinase activity (84, 181, 251). The main physiological function of FBP might therefore be to prevent the inhibition of HprK/P by Pi. For the S. xylosus enzyme, activation by FBP was observed only when HprK/P was present at low concentrations (357). Surprisingly, the Streptococcus salivarius enzyme was not at all stimulated by FBP (84). Similarly, the M. pneumoniae enzyme is fully functional at relatively low ATP concentrations, and its activity is also not stimulated by FBP (831). The intracellular concentrations of FBP, ATP, and Pi vary largely depending on whether the cells utilize a favored carbon source or not. During the uptake of a rapidly metabolizable carbon source such as glucose, fructose, or mannose by L. lactis, the concentrations of Pi, ATP, and FBP are about 5, 8, and 30 mM, respectively (528, 598, 873). Under these conditions, HprK/P functions as an HPr kinase and not as P-Ser-HPr phosphorylase. In contrast, in the absence of a rapidly metabolizable carbon source, the concentration of Pi increases to about 50 mM, whereas the concentrations of FBP and ATP drop to 1 to 3 mM (528, 598, 873). The effect of growth on a preferred or less favorable carbon source on P-Ser-HPr formation was confirmed with S. salivarius cells. By carrying out crossed immunoelectrophoresis experiments, the fractions of the various forms of intracellular HPr in S. salivarius cells grown in glucose-containing medium were found to be 45% for P-Ser-HPr and 50% for doubly phosphorylated HPr, whereas only little P∼His-HPr (5%) and almost no dephospho-HPr could be detected (263). Cells grown on galactose, a less efficiently metabolized carbon source, also exhibited a high amount of doubly phosphorylated HPr (44%) and little P∼His-HPr (3%), whereas P-Ser-HPr was lowered to 18%, and dephospho-HPr increased to 35%. A similar observation was made for Streptococcus bovis, where an inverse correlation between P-Ser-HPr formation and the Pi concentration was observed. Cells growing on glucose contained more than half of the HPr as P-Ser-HPr, and the Pi concentration was in the low mM range. When glucose was exhausted, P-Ser-HPr disappeared, and the Pi concentration went up to 30 mM (26). In in vitro experiments, 20 mM Pi completely prevents the phosphorylation of HPr by 5 mM ATP, even when 25 mM FBP is present (177).
The observation that PPi is formed during P-Ser-HPr dephosphorylation and used as a phosphoryl donor by HprK/P suggested that the PPi concentration affects the intracellular amount of P-Ser-HPr. B. subtilis cells grown in the presence of glucose contain 10-fold more FBP than cells grown on succinate (from 1.4 to 14 mM). A similar increase was observed for PPi (from 1.2 to 6 mM) (557). This metabolite distribution is probably the reason why about 65% of the HPr in glucose-grown B. subtilis cells is present as P-Ser-HPr, 35% is present as HPr, and little is present as P∼His-HPr and doubly phosphorylated HPr (571). In contrast, in succinate-grown cells, mainly dephospho-HPr was present, together with a few percent of P∼His-HPr. In in vitro experiments, HPr phosphorylation with B. subtilis HprK/P was maximal above 5 mM PPi but negligible below 1 mM PPi. The approximately 6 mM PPi present in cells growing on glucose is therefore expected to be partly responsible for the increase of P-Ser-HPr, whereas in the absence of a rapidly metabolizable carbon source, the low PPi and high Pi concentrations probably allow efficient P-Ser-HPr dephosphorylation. ATP-dependent HPr phosphorylation by B. subtilis HprK/P was maximal in the presence of 10 mM FBP, whereas it almost disappeared below 2 mM FBP (372). On the contrary, FBP was not required for efficient PPi-dependent HPr phosphorylation (557).
An endogenous low-molecular-weight HprK/P inhibitor was detected when soluble extracts of Lactobacillus brevis were separated by size exclusion chromatography (715). However, no further details about the nature of the bacterial HPr kinase inhibitor were reported. Because the HPr kinase activity was detected by autoradiography after phosphorylation with [γ-32P]ATP, it is possible that the reported HPr kinase inhibitor was PPi. If the PPi concentration in the assay mixtures exceeded the employed [γ-32P]ATP concentration (10 μM) by severalfold, primarily nonradioactive (and therefore undetected) P-Ser-HPr would have been formed. Ironically, in the same publication, it was reported that the presence of PPi can completely inhibit [γ-32P]ATP-dependent HPr phosphorylation (715). Inhibition of [γ-32P]ATP-dependent HPr phosphorylation by PPi was also reported for HprK/P from Treponema denticola and was suggested to be probably due to the competition of the two phosphoryl donors for binding to HprK/P, as P-Ser-HPr was again detected by autoradiography (278). A synthetic inhibitor of HprK/P has also been identified. It is a heterocyclic bis-cationic compound, which was reported not to bind to the Walker motif (696).
In conclusion, the two opposing activities of HprK/P and consequently the ratio of the various HPr forms seem to be regulated mainly via metabolites. In agreement with this assumption, the amount of HprK/P present in B. subtilis cells was reported to have no influence on the ratio of the different HPr forms (59). The amount of HprK/P probably determines only how fast cells can adapt to changes in carbohydrate availability but apparently has no influence on the equilibrium reached under a certain condition.
Interestingly, in oral streptococci (glucose or lactose grown) (660, 893) and S. thermophilus (lactose grown) (134), a major part (up to 75%) of the HPr is converted into doubly phosphorylated HPr. A possible role of (P∼His,P-Ser)-HPr in the regulation of the lactose transporter LacS of S. thermophilus will be discussed below (see “Regulation of EIIA-Containing Non-PTS Transporters by P∼His-HPr-Mediated Phosphorylation”).
P-Ser-HPr Regulates PTS Transport Activity by a Feedback Mechanism
P-Ser-HPr is a poor substrate for PEP-dependent phosphorylation by EI, although phosphorylation at Ser-46 causes only minor changes in the HPr structure (29, 952). One of these changes leads to an extension of the short β-helix, which is capped by Ser-46 at the N-terminal site. This alteration was assumed to affect the interaction of Met-48 with a hydrophobic pocket in EI, thus partly explaining why phosphorylation at Ser-46 drastically slows the EI-catalyzed phosphorylation at His-15. However, the main reason for the poor phosphorylation of His-15 in P-Ser-HPr is probably an electrostatic repulsion between the phosphoryl group at Ser-46 and residue Glu-84 of EI (260). As discussed in the preceding section, in glucose-grown cells, a major fraction of HPr is present as P-Ser-HPr or doubly phosphorylated HPr, which, compared to P∼His-HPr, has a much lower affinity for EIIAs (723), but there is little P∼His-HPr.
If P∼His-HPr is present at low concentrations, it is expected to control the PTS sugar uptake rate. The efficient phosphorylation of HPr at Ser-46 during growth on glucose might thus reduce PTS-catalyzed sugar uptake. A ptsH1 mutant (Ser46Ala replacement in HPr) was therefore expected to show deregulated PTS transport activity. However, a B. subtilis ptsH1 mutant did not exhibit elevated uptake of glucose or other rapidly metabolizable PTS sugars (184), indicating either that additional regulatory mechanisms are operative or that in B. subtilis, the small amount of P∼His-HPr detected in cells growing on glucose is sufficient to fully catalyze sugar transport via the PTS. In contrast, an S. xylosus hprK mutant, also unable to phosphorylate HPr at Ser-46, exhibited reduced growth in the presence of glucose, although glucose-grown hprK mutant cells showed a two- to fourfold-elevated initial glucose transport rate compared to the wild-type strain. It was therefore concluded that the growth deficiency of the S. xylosus hprK mutant is probably due to uncontrolled glucose uptake leading to the accumulation of deleterious amounts of metabolites (357).
In various gram-positive bacteria, the presence of glucose exerts an inhibitory effect on the uptake of other PTS sugars. This inhibitory effect could be due partly to the low amounts of P∼His-HPr detected in glucose-grown cells, which probably aggravates the competition between the EIIAs for their common phosphoryl donor. Competition was expected to be less severe in a ptsH1 mutant that is unable to form P-Ser-HPr. The effect of the ptsH1 mutation on the simultaneous uptake of the two PTS substrates mannitol and glucose was measured with B. subtilis cells. If phosphorylation at Ser-46 of HPr and competition for the remaining P∼His-HPr were involved in glucose-mediated inhibition of mannitol uptake, elevated mannitol transport should occur in the ptsH1 mutant. Indeed, mannitol uptake in the presence of glucose was two- to fivefold higher in the ptsH1 mutant than in the wild-type strain (184, 974).
Evidence for a participation of P-Ser-HPr in the regulation of PTS transport activity was also provided by experiments with L. lactis vesicles. During the preparation of L. lactis vesicles, larger proteins such as HprK/P and EI remain entrapped in the vesicles, whereas almost all HPr is lost (970). An influence of the ATP-dependent phosphorylation of HPr at Ser-46 on PTS-catalyzed sugar transport could be demonstrated by 2DG uptake measurements using L. lactis vesicles into which B. subtilis HPr and various glycolytic intermediates had been electroporated (971). 2DG is transported via a PTS, and electroporation of HPr or coelectroporation of Ser46Ala mutant HPr with ATP and FBP into the vesicles stimulated 2DG uptake. In contrast, coelectroporation of wild-type HPr with ATP and FBP had no stimulatory effect. In addition, only slow 2DG uptake was detected with the P-Ser-HPr-resembling Ser46Asp mutant HPr (952), irrespective of whether it was electroporated alone or together with ATP and FBP. Similar results were obtained when the uptake of methyl-β-d-thiogalactoside (TMG) by L. lactis vesicles was studied (970). In addition to FBP, several other metabolites including gluconate-6-P, glycerate-2-P, and fructose-6-P were reported to inhibit TMG uptake when electroporated into L. lactis vesicles together with B. subtilis HPr. Moreover, when wild-type HPr was electroporated into L. lactis vesicles, the uptake of fructose was inhibited by the presence of glucose, while only slight inhibition occurred when Ser46Ala HPr was electroporated in place of HPr (974).
Although providing no direct proof, these in vitro results suggested that the formation of P-Ser-HPr during the utilization of a rapidly metabolizable carbon source can lower the amount of P∼His-HPr to such an extent that it is insufficient to sustain the simultaneous uptake of two PTS carbohydrates. This hypothesis was supported by in vivo experiments using the hprK(Val265Phe) allele. The Val265Phe replacement causes an almost complete loss of the P-Ser-HPr phosphorylase activity but has little effect on the FBP-activated HPr kinase function (571). When grown in the absence of a rapidly metabolizable carbohydrate, a B. subtilis wild-type strain contains little P-Ser-HPr, whereas in strains carrying the Val265Phe hprK allele, almost all HPr is accumulated as P-Ser-HPr. The Val265Phe hprK strain grows barely or not at all on media containing the PTS sugar glucose, fructose, maltose, or mannitol as the sole carbon source. Similar results were obtained with a B. subtilis strain in which the hprK gene had been replaced with the L. casei Val267Phe hprK allele (571). A strain carrying the Val267Phe hprK allele transports glucose and mannitol at rate that is more than 10 fold lower than that of an integrant expressing wild-type L. casei hprK (571). The inability of B. subtilis strains expressing the B. subtilis Val265Phe or the L. casei Val267Phe hprK allele to grow on PTS sugars is due to strongly reduced PTS transport activity and, as will be explained below, to a kind of “permanent CCR.” Expression of the EIICBAGlc-encoding ptsG, which is not submitted to CCR, was not repressed by the presence of the L. casei Val267Phe hprK allele. The assumption that the accumulation of P-Ser-HPr in the Val267Phe hprK strain results in slow PTS phosphoryl group transfer, which in turn prevents PTS sugar uptake, was confirmed by introducing a ptsH1 mutation in the Val267Phe hprK strain (no P-Ser-HPr formation). It restored growth on PTS sugars, whereas introducing a ccpA mutation, which prevents CCR, had only a slight effect on PTS transport (571).
As mentioned above, glucose-grown S. salivarius (263, 893) and B. subtilis (571) cells contain very little P∼His-HPr. Nevertheless, the small fraction present as P∼His-HPr (about 5%) seems to be sufficient to fully catalyze PTS-mediated uptake of glucose and probably other rapidly metabolizable PTS sugars in gram-positive bacteria. Similarly, less than 20% of the HPr present in wild-type S. enterica serovar Typhimurium cells can fully sustain PTS-catalyzed glucose uptake (901). However, P∼His-HPr probably starts exerting flux control when two or more PTS sugars are simultaneously transported or when the natural balance of the different forms of HPr is disturbed by, for example, the conversion of an additional fraction of HPr to P-Ser-HPr, as is the case in B. subtilis strains expressing the Val265Phe hprK allele (571).
Role of P-Ser-HPr in CCR
The following sections will deal with the role of P-Ser-HPr and P-Ser-Crh in CCR/carbon catabolite activation (CCA). We will discuss some early results that suggested a connection between CCR and P-Ser-HPr formation and the first confirmation of this connection by construction of a B. subtilis ptsH(Ser46Ala) mutant. The ptsH(Ser46Ala) mutation has a pleiotropic effect and leads to a relief from CCR for numerous genes and operons. We will mention the interaction of P-Ser-HPr and P-Ser-Crh with the catabolite repressor/activator CcpA (Table 1) and how these interactions allow CcpA, a LacI/GalR-type DNA binding protein, to interact with the catabolite response elements (cre's), operator sites which precede genes that are sensitive to CCR/CCA. The occurrence of Crh, an HPr paralog in which His-15 is replaced with a Gln, in bacilli, oceanobacilli, and geobacilli will also be discussed.
Loss of ATP-dependent HPr phosphorylation prevents CCR.
Heterofermentative lactobacilli such as L. brevis and Lactobacillus buchneri were reported to contain HPr and HprK/P but to be devoid of EI and EIIs (715). Owing to the presumed absence of a functional PTS, there seemed to be no obvious regulatory role for the ATP-dependent phosphorylation of HPr at Ser-46 in these organisms. Although the report about the absence of EI and EIIs from heterofermentative lactobacilli later turned out to be wrong (an L. brevis ptsHI operon has been cloned and sequenced) (193) and the presence of a fructose-specific PTS induced under anaerobic conditions has been established (772), it initiated the search for additional functions of P-Ser-HPr, one of them being the regulation of CCR.
A connection between the phosphorylation of HPr at Ser-46 and CCR was suggested by the observation that CCR in B. subtilis requires the metabolism of the repressing sugar (490, 606). In gram-negative bacteria, the transport of a carbohydrate via the PTS is sufficient to elicit CCR. By studying CCR in mutants defective at various steps of glycolysis, the number of glycolytic intermediates potentially serving as a signal for CCR in B. subtilis could be narrowed to four candidates: dihydroxyacetone phosphate, glyceraldehyde-3-phosphate, 1,3-diphosphoglycerate, and FBP (606). As FBP, the main activator of HPr kinase, was among these metabolites, ATP-dependent phosphorylation of HPr was suspected to play a role in CCR (184).
The ptsH1 mutation has a pleiotropic effect on CCR and carbon catabolite activation.
To test the proposed hypothesis that P-Ser-HPr plays a role in CCR, Deutscher and coworkers studied CCR in a B. subtilis ptsH1 mutant (Ser46Ala replacement). Indeed, several enzymes, including gluconate kinase, mannitol-1-P dehydrogenase, inositol dehydrogenase, and glucitol dehydrogenase, which are all sensitive to CCR in a wild-type strain, were not or only partly repressed by glucose and other rapidly metabolizable sugars in the ptsH1 mutant (184). Moreover, while the expression of a translational fusion (integrated at the amyE locus) of the promoter region and the 5′ part of gntK (encodes gluconate kinase) to lacZ was sensitive to CCR in a wild-type strain, it was not repressed by glucose in the ptsH1 mutant (184). In addition, if a B. subtilis strain carrying a deletion of the ptsGHI genes, which encode EIICBAGlc, HPr, and EI, respectively, was transformed with a plasmid that allowed the production of the P-Ser-HPr-resembling Ser46Asp HPr (952), partial repression of the gnt operon was observed even in the absence of a repressing sugar (184). These results clearly established the crucial role of the phosphorylation of HPr at Ser-46 in CCR of B. subtilis.
The ptsH1 mutation turned out to be an important tool to study the implication of P-Ser-HPr in CCR in B. subtilis in more detail (184, 206). All B. subtilis ptsH1 mutants used in various laboratories are derivatives of ptsH1 strain SA003. They were obtained by either introducing additional mutations into SA003 or transforming specific mutants with chromosomal DNA of ptsH1 strains carrying an antibiotic resistance cassette inserted about 6 kb downstream from ptsH (184). The ptsH-linked antibiotic resistance cassette was recovered from strain MO481 (P. Stragier, unpublished results). The use of the ptsH1 mutant demonstrated that many other B. subtilis genes and operons are partly or completely relieved from CCR, such as the acetyl coenzyme A (acetyl-CoA) synthetase-encoding acsA gene (984) as well as the lev (523, 526), xyl (148), xyn (249, 250), and glp (158) operons (for a review, see reference 178). In a few cases, the effect of the ptsH1 mutation on CCR depends on the growth conditions. CCR of the B. subtilis hut operon, for example, is not altered in a ptsH1 mutant grown in minimal medium, whereas CCR in this strain completely disappears when it is grown in complex medium (985). A similar but less pronounced growth medium-dependent effect on CCR was observed with the iol operon (184). The mechanisms underlying the growth-dependent differences in CCR in ptsH1 mutants are not understood but might be related to the different nitrogen sources present in the media (220).
Synthesis of the plasmid-encoded B. thuringiensis larvicidal protoxin Cry4A by stationary-phase cells is also sensitive to CCR. The repressive effect of glucose on Cry4A production disappeared in a ptsH1 mutant (Ser45Ala replacement in B. thuringiensis HPr) (399). In L. casei, the ptsH1 mutation causes a relief from CCR of 6-P-β-galactosidase and N-acetyl glucosaminidase and from diauxic growth in media containing glucose and either lactose, maltose, or ribose (919). An L. lactis ptsH1 mutant is relieved from CCR of the ptbA-bglH operon (569). These results confirm that P-Ser-HPr plays an important role in CCR of not only bacilli but also other gram-positive organisms.
A chromosomal B. subtilis ptsH2 mutant (Ser46Thr replacement) has also been constructed. Although the purified mutant HPr is slowly phosphorylated in ATP-dependent phosphorylation assays (722), the B. subtilis ptsH2 mutant exhibits a relief from CCR of the gnt operon identical to that of the ptsH1 mutant (M. Steinmetz and J. Deutscher, unpublished results). In contrast, an L. casei ptsH2 mutant shows only partial relief from CCR and diauxic growth (919). It is therefore likely that Ser46Thr HPr from L. casei is more efficiently phosphorylated than the B. subtilis mutant protein.
Some genes and operons are more strongly expressed in the presence of glucose or other rapidly metabolizable carbohydrates. This phenomenon is known as CCA. In B. subtilis, the expression of several genes such as alsS (α-acetolactate synthase) (726, 983), ackA (acetate kinase) (886), and pta (phosphotransacetylase) (685) is stimulated by the presence of glucose. CCA of these B. subtilis genes is absent from ptsH1 mutants. Expression of the L. lactis las operon containing the genes encoding the glycolytic enzymes phosphofructokinase, pyruvate kinase, and lactate dehydrogenase is also activated by the presence of glucose in the growth medium. The stimulatory effect of glucose on las operon expression disappeared in an L. lactis ptsH-disrupted strain transformed with a plasmid allowing the synthesis of L. lactis Ser46Ala mutant HPr (493), whereas transformants containing a plasmid carrying L. lactis wild-type ptsH exhibited normal glucose-mediated las activation.
The involvement of P-Ser-HPr in CCR and CCA was further supported by studying mutants that were unable to form P-Ser-HPr due to hprK inactivation. Such mutants have been obtained, for example, with B. subtilis (251, 315, 709), S. xylosus (357), and L. casei (198). In all three organisms, the inactivation of hprK causes a relief from CCR, but for several B. subtilis catabolic operons, the releasing effect in hprK mutants is markedly stronger than that in ptsH1 mutants (249, 523, 685). This phenomenon was observed for many other genes when transcriptome analysis was carried out with B. subtilis hprK and ptsH1 mutants (491). As will be explained below, the difference between ptsH1 and hprK mutants is due to the presence of Crh, a B. subtilis HPr paralog, which is also phosphorylated by HprK/P (250) and which can partly substitute for P-Ser-HPr in CCR and CCA.
The Catabolite Response Element cre, an Operator Site for CCR and CCA
A cis-acting element regulating CCR and CCA.
Groundbreaking work in the laboratory of Glenn Chambliss led to the discovery of two other components involved in CCR in B. subtilis. Both were identified by isolating mutants in which the α-amylase-encoding amyE gene was relieved from CCR. Two independently isolated mutants were affected in a 14-bp imperfect palindromic sequence called amyR1, which is located just downstream from the amyE promoter and which exhibits similarity to the lac operator site of E. coli (601, 603). In both strains, replacement of the G:C base pair in position 8 of amyR1 with an A:T base pair was responsible for the observed CCR resistance (603, 939). Expression of a plasmid-borne cat gene under the control of the amyE promoter followed by mutated amyR1 was insensitive to CCR, while the simultaneous expression of the chromosomal amyE (promoter followed by wild-type amyR1) was repressed by glucose (602). These findings suggested that amyR1 is a cis-acting element. A series of mutants in which one or two positions of amyR1 were altered was constructed, and most of these mutants were partly or fully relieved from CCR of amyE. Based on mutational analysis and on the occurrence of similar imperfect palindromic sequences in the promoter regions of many other catabolite-repressed B. subtilis genes, the following consensus sequence was proposed for the catabolite response operator: TGWNANCGNTNWCA (939). Slightly different consensus sequences have been proposed by others (353, 563). The general term catabolite response element (cre) has been introduced for these operator sites (421).
Distribution of cre's.
Imperfect palindromic sequences resembling the proposed consensus cre sequence are located in the promoter region or at the 5′ ends of many catabolite-repressed transcription units. For some of these cre's, their implication in CCR or CCA has been experimentally confirmed (178). By searching the B. subtilis genome with a degenerate cre sequence as a query, Miwa and coworkers detected a total of 126 tentative cre's (565). Out of these 126 putative cre sites, 32 were inserted into a plasmid between the spac promoter and the lacZ gene, and the effect of these sequences on the glucose repression of β-galactosidase synthesis was tested. In 22 constructs, expression of the lacZ gene was repressed by glucose (565). By using a slightly different search method, 61 additional potential B. subtilis cis elements for CCR could be detected, including several cre's preceding operons known to be submitted to CCR. A list of the about 160 identified or presumed B. subtilis cre's was presented by Deutscher et al. (178). As the transcription units regulated by these cre's contain, on average, two to three cistrons, about 400 genes, which represent 10% of the B. subtilis genome, are estimated to be regulated by CCR or CCA. A similar number was obtained when transcriptome analyses were carried out using a ccpA mutant (see below for ccpA) (575, 980). The number of genes presumed to be regulated by cAMP/Crp-mediated CCR in E. coli is in the same order (34).
A unique potential cre precedes the B. subtilis uxaC gene from the yjm operon, which codes for the enzymes necessary for glucuronide/galacturonate transport and metabolism (549, 729). While most cre's are composed of an imperfect palindromic sequence, the putative cre of the yjm operon is a perfect palindrome. Moreover, the 14-bp-long putative yjm cre is part of a longer perfect palindrome composed of a total of 26 bp (TCAAAATGTTAACGTTAACATTTTGA [the 14-bp consensus cre is in boldface type]). To date, it is not known whether this extended palindrome plays a special role in CCR. The β-galactosidase-encoding mbgA gene of Bacillus megaterium contains two cre's overlapping by 1 bp. Mutations in either one affect CCR of the mbgA gene (803). Two potential cre's overlapping to a much larger extent (by 4 bp) begin at positions 278 and 287 within the B. subtilis gudB (former ypcA) gene, which encodes one of two glutamate dehydrogenases present in this organism and the expression of which is sensitive to the presence of glucose. The second B. subtilis glutamate dehydrogenase, RocG, is also submitted to CCR (53). The first 8 bp in the two presumed gudB cre's are identical (GTGAAGGCGgtgaaggcgCTTTCA [the first potential cre is in boldface type, the second is in italics, and the repeated sequences are underlined and in lowercase letters]). Interestingly, a rocG mutant is not able to utilize proline, ornithine, or arginine as the sole carbon source (growth on these carbon sources requires an active glutamate dehydrogenase), establishing that wild-type B. subtilis contains only low GudB activity. However, spontaneous revertants that were able to grow on these compounds appeared with high frequency. Surprisingly, in four independently isolated revertants, the GTGAAGGCG direct repeat was deleted, providing a gudB1 allele with only a single cre (TGAAGGCGCTTTCA) (54). It is not clear whether the elevated GudB activity in the spontaneous mutants is due to the change in the two overlapping cre's or to the deletion of the -V-K-A- repeat in the protein. The B. subtilis iol (563) and ara (361) operons also contain two functional cre's, which, however, are not located next to each other. One cre precedes the first gene (mmsA and araA, respectively); the other is located within the second gene (iolB and araB). Several other catabolite-repressed or -activated genes or operons contain more than one potential cre in the promoter region or within the 5′ end (93, 290, 564, 886), but in several cases, only one of the cre's is operative in CCR or CCA.
Two divergently transcribed genes or operons can potentially be regulated by a single cre. This seems to be the case for the putative L. casei lev cre, which overlaps the transcription start site of the levABCDX operon and the −10 box of the levR gene, which encodes the transcription activator for the lev operon. Simultaneous repression of levABCDX and the transcription activator gene levR presumably leads to very efficient CCR (534).
The L. casei lev operon is only one of many examples establishing the presence of functional cre sites in bacteria other than B. subtilis (Table 3). In S. xylosus, deletion of the cre located in front of the malRA operon (for maltose/maltotriose utilization) causes a complete relief from CCR for α-glucosidase (204). Similarly, deletion of a cre located right behind the transcription initiation site of the fructan hydrolase-encoding fruA gene of Streptococcus mutans causes a relief from glucose repression (93, 946). CCR of the β-amylase-encoding bamM gene from B. megaterium is mediated by a cre located in the DNA region encoding the signal peptide of this extracellular enzyme (457). A cre site is also present in front of the P-β-glucosidase-encoding bglH gene of Lactobacillus plantarum (511, 512) and the pepQ gene of Lactobacillus delbrueckii (779). These examples confirm that the cre-dependent CCR/CCA mechanism is widely distributed within gram-positive bacteria with low G+C content. Interestingly, the HPr-encoding ptsH gene of several streptococci is also preceded by a potential cre site, and expression of the ptsHI operon in S. salivarius and Streptococcus bovis is up-regulated in the presence of glucose (CCA) (894).
TABLE 3.
Genes and operons in gram-positive bacteriaa containing functional cre's or being regulated by the P-Ser-HPr:CcpA complex
| Organism | Gene/operon | Function | cre sequenceb | CcpA effectc | Reference(s) |
|---|---|---|---|---|---|
| B. megaterium | bamM | β-Amylase | +48TGTTAACGCTTTCA+61 | − | 457 |
| B. megaterium | mbgA | β-Galactosidase | −34TGATAAGGTTTTCA−21 | − | 803 |
| B. megaterium | mbgA | β-Galactosidase | −21AGAAAACGTTATCA−8 | − | 803 |
| B. megaterium | xyl | Xylose metabolism | +124TGAAAGCGCAAACA+137 | − | 290 |
| B. thuringiensis | cry4A | Cry4A toxin | NI | − | 399 |
| C. perfringens | cpe | Enterotoxin | NI | + | 913 |
| C. saccharobutylicum | staA | Starch degradation | NI | − | 162 |
| E. faecalis | bkd | β-Keto acid metabolism | NI | − | 933 |
| E. faecalis | galKETR | Galactose metabolism | NI | − | 453 |
| E. faecalis | gdh dhaK | Glycerol dehydrogenase/dihydroxyacetone kinase | NI | − | 453 |
| E. faecalis | ? | Glucose starvation-induced proteins | NI | − | 453 |
| E. faecalis | ldh | Lactate dehydrogenase | NI | − | 453 |
| E. faecalis | pfk | Phosphofructo kinase | NI | − | 453 |
| E. faecalis | pyk | Pyruvate kinase | NI | − | 453 |
| E. faecalis | ? | Serine dehydratase | NI | − | 453 |
| L. casei | nag | N-Acetylglucosaminidase | NI | − | 568, 796 |
| L. casei | lac | Lactose metabolism | NI | − | 568 |
| L. delbrueckii subsp. bulgaricus | ccpA | Catabolism repression | NI | − | 574 |
| L. delbrueckii subsp. lactis | pepQ | Peptidase | −61TGCAATCGCTTACA−48 | + | 799 |
| L. lactis | las | Glycolysis | NI | + | 494 |
| L. lactis | gal | Galactose metabolism | NI | − | 494 |
| L. lactis | ptbA bglH | β-Glucoside metabolism | NI | − | 569 |
| L. lactis | fruRCA | Fructose metabolism | NI | − | 45 |
| L. monocytogenes | ? | β-Glucosidase | NI | − | 50 |
| L. pentosus | xpkA | Xylulose-5-P phosphoketolase | NI | − | 674 |
| L. pentosus | xylAB | Xylose metabolism | NI | − | 489 |
| L. plantarum | bglH | P-β-glucosidase | −3TGTAAGGGCTATCA+11 | − | 511, 512 |
| S. aureus | pckA | PEP carboxy kinase | NI | − | 791 |
| S. bovis | ccpA | CcpA | −68TGAAAAGGTTTTCA-54 | + | 27 |
| S. bovis | ldh | Lactate dehydrogenase | NI | − | 27 |
| S. bovis | pfl | Pyruvate formate lyase | NI | + | 27 |
| S. gordonii | abpA | Amylase binding | NI | − | 733 |
| S. gordonii | arcA | Arginine deiminase | NI | − | 195 |
| S. mutans | fruA | Fructan hydrolase | −164AGATAGCGCTTACA−150 | − | 93, 946 |
| S. mutans | ftf | Fructosyltransferase | NI | + | 88 |
| S. mutans | gtfBC | Glucosyltransferase | NI | + | 88 |
| S. pneumoniae | cps | Capsular polysaccharide | NI | + | 273 |
| S. rattus | arcA | Arginine deiminase | NI | − | 298 |
| S. thermophilus | lacSZ | Lactose metabolism | NI | − | 897 |
| S. thermophilus | ldh | Lactate dehydrogenase | NI | − | 897 |
| S. xylosus | ? | β-Glucoronide | NI | − | 204 |
| S. xylosus | ccpA | Catabolic repressor | NI | − | 204 |
| S. xylosus | lacPH | Lactose metabolism | NI | − | 46 |
| S. xylosus | mal | Maltose metabolism | −58TGCAAACGCTTGCA−45 | − | 204 |
| Consensus sequence | TGWNANCGNTNWCA | 939 |
cre's can either overlap the promoter, overlap the transcription start site, or be located between the transcription start site and the initiation codon or within the 5′ part of catabolite-repressed genes (Table 3). Binding of a target protein to cre sites located within the promoter region is thought to interfere with the binding of the RNA polymerase holoenzyme, while cre's situated downstream from the promoter are assumed to be regulated via a road block. As will be discussed below, most transcription units submitted to CCA contain a cre located in front of the promoter region.
Catabolite Control Protein A Functions as a Catabolite Repressor or Activator
A LacI/GalR-type trans-acting factor mediates CCR and CCA.
Since cre's exhibit similarity to the E. coli lac and gal operators, it was tempting to assume that a LacI/GalR-type repressor might be involved in CCR of B. subtilis and other gram-positive bacteria (603). By carrying out transposon mutagenesis, a B. subtilis strain in which amyE expression was resistant to catabolite repression could be isolated (322). Indeed, this strain carried the transposon inserted into a gene encoding a LacI/GalR-type repressor, which was called CcpA (catabolite control protein A) (938). Interestingly, genetic mapping revealed that the ccpA gene is located close to the previously described B. subtilis alsA1 mutation, which prevented the production of acetoin in glucose-grown cells (983). The failure of the alsA1 mutant to produce acetoin during growth on glucose is due to the almost complete absence of the alsS-encoded acetolactate synthase. The alsS gene forms an operon together with the acetolactate decarboxylase-encoding alsD (726) and is induced by a CCA mechanism. Similar to the alsA1 mutation, the ccpA mutation abolishes acetoin production. Introduction of an SPβ prophage carrying intact ccpA restored acetoin production in the ccpA and alsA1 mutants, strongly suggesting that ccpA and alsA are identical loci (322). Indeed, the alsA1 mutation was identified as a G-to-A exchange within the Shine-Dalgarno box of the ccpA gene (at position −14 with respect to the ccpA start codon) (566). This mutation lowers the synthesis of CcpA about 10-fold. The concentration of CcpA in wild-type B. subtilis was calculated to be 0.6 μM (566).
Disruption of ccpA not only prevented the inhibitory effect of glucose on α-amylase activity and the induction of acetolactate synthase but also affected CCR of many other B. subtilis enzymes (178, 184, 299, 566), thus establishing the pleiotropic character of the ccpA mutation. Whole-genome analysis turned out to be a powerful tool for detecting CcpA-regulated transcription units (575, 980). Interestingly, three B. subtilis ccpA mutants that specifically affected CCA of the alsSD operon, while CCA or CCR of other transcription units was not or only slightly altered, were isolated (887). Two of the mutations affect conserved regions in the DNA binding domain of CcpA (A18V and R48C replacements), whereas the third mutation was in a less conserved region in the core domain (Pro286Leu replacement).
The effect of ccpA inactivation on the synthesis of catabolic enzymes could also be demonstrated by comparing the protein patterns of two-dimensional gels obtained with crude extracts from B. subtilis (880) or E. faecalis (453) wild-type and ccpA strains. E. faecalis proteins isolated from a few spots exhibiting an altered intensity in the ccpA mutant compared to the wild-type strain were identified by microsequencing. They include a protein with strong similarity to the α subunit of l-serine dehydratase, an enzyme catalyzing the conversion of serine to pyruvate, as well as glycerol dehydrogenase and the two subunits of dihydroxyacetone kinase (DhaKL). The genes encoding the three latter proteins are present in the dha operon (453), which also contains the gene for an EIIA of the mannose class PTS (see “SOME UNUSUAL PTS PATHWAYS AND PROTEINS”). In addition, galKETR operon expression as well as the synthesis of 13 proteins identified as glucose starvation proteins were no longer sensitive to CCR in the E. faecalis ccpA mutant. Two-dimensional gel electrophoresis with B. subtilis crude extracts showed that in a ccpA mutant, seven tricarboxylic acid cycle enzymes (CitC, CitG, CitH, CitZ, OdhA, SdhA, and SucC/SucD) were relieved from CCR (880). The citZ (403) and citG genes and the odhAB, sdhCAB, and sucCD operons are preceded by potential cre's (178, 565). Interestingly, E. faecalis and B. subtilis ccpA mutants contain a much larger amount of P-Ser-HPr than wild-type strains (453, 492). The increased percentage of P-Ser-HPr slows glucose uptake by the B. subtilis ccpA mutant when grown on synthetic medium (492), which in turn seems to prevent CCA of the gapA operon, which encodes the enzymes of the lower part of glycolysis (492, 880). When a ptsH1 mutation, which prevents the formation of P-Ser-HPr, was introduced into the ccpA strain, the activating effect of glucose on gapA operon expression (230) was restored (492). Expression of gapA is controlled by the repressor CggR, which is inactivated by elevated amounts of FBP (194). A similar repressive effect of the elevated amount of P-Ser-HPr in a ccpA mutant was observed for the B. subtilis glutamate synthase-encoding gltAB operon. Introduction of a ptsH1 mutation restored gltAB expression in a ccpA background (925). The mechanism leading to enhanced P-Ser-HPr formation in ccpA mutants is not known. In addition, when grown in complex medium, ccpA mutants seem to efficiently transport PTS sugars, because glucose utilization by a B. subtilis ccpA mutant represses the synthesis of several enzymes submitted to a second, CcpA-independent CCR mechanism. For example, CCR of the α-glucosidase- and glycerol kinase-encoding genes is not diminished in a B. subtilis ccpA mutant (184).
Genes regulated by CcpA include not only carbon catabolic genes but also genes related to carbon metabolism in a larger sense. The L. delbrueckii subsp. lactis CcpA ortholog PepR1 stimulates the expression of the prolidase-encoding pepQ gene and seems to affect the expression of pepX, pepI, and brnQ, which encode two other peptidases and a branched-chain amino acid transporter, respectively (779). A connection between CCR and amino acid metabolism also exists in Streptococcus gordonii and Streptococcus rattus, where the arginine deiminase operon is repressed by CcpA (195, 298), and in B. subtilis, where CcpA stimulates the expression of the ilv-leu operon, which encodes the enzymes for the synthesis of branched-chain amino acids (810, 882). A connection between CCR and the utilization of nitrogen sources was provided by the finding that the expression of rocG, which encodes the main glutamate dehydrogenase of B. subtilis, is subject to CcpA-mediated CCR (53). A connection between carbon and nitrogen metabolism in B. subtilis was further supported by the finding that the σ54-encoding sigL gene is regulated by CcpA (128). Transcriptome analysis with B. subtilis ccpA, hprK, and ptsH1 mutants revealed that numerous nitrogen and phosphorus metabolic genes are submitted to CCR or CCA (491). CcpA of Lactobacillus pentosus represses the activity of xylulose 5-phosphate phosphoketolase, the central enzyme of the phosphoketolase pathway (674). In Clostridium perfringens, inactivation of ccpA diminishes the expression of the enterotoxin-encoding cpe gene during entry into the stationary growth phase (913). S. mutans ccpA mutants exhibit diminished fructosyltransferase and glucosyltransferase gene expression (88) and biofilm formation (947).
CcpA-specific sequences.
CcpA belongs to the family of LacI/GalR-type repressors but contains characteristic sequences located at the end of the DNA binding domain and within the core protein and can therefore easily be distinguished from other members of this family. These regions are important for CcpA function, because mutations affecting amino acids in the CcpA-specific sequences prevent or lower CCR of a xylA-lacZ fusion in B. megaterium (422). Interestingly, polyclonal antibodies directed against CcpA from B. megaterium do not recognize E. coli LacI (441). Phylogenetic analysis shows that all low-G+C gram-positive bacteria for which the genomes have been sequenced contain a ccpA ortholog (J. Deutscher and C. Francke, unpublished observation). The gene is probably always expressed, as a protein exhibiting the approximate size of CcpA and cross-reacting with the B. megaterium CcpA antibody was present in the crude extracts of all gram-positive bacteria tested (441). For several of these bacteria, including E. faecalis, L. casei, Lactobacillus pentosus, L. lactis, L. monocytogenes, Clostridium saccharobutylicum (formerly C. acetobutylicum P262) (162), and S. xylosus, a role of their CcpAs in CCR was demonstrated experimentally. Either their ccpA genes complement a B. subtilis ccpA mutant or B. subtilis ccpA complements ccpA mutants of these organisms. Genes affected by ccpA mutations in bacteria other than B. subtilis are listed in Table 3.
CcpA needs a corepressor.
Interestingly, overexpression of the C. saccharobutylicum ccpA ortholog regA in an E. coli strain harboring the C. saccharobutylicum staA gene inhibited the expression of staA, which encodes a starch-degrading enzyme. As the corepressor of CcpA is absent from E. coli (see the next section), these results imply that, if overproduced, CcpA can bind to cre's without a corepressor. Therefore, regulation of ccpA expression represents one way to control the binding of CcpA to cre's. Indeed, in a few organisms (S. xylosus, L. pentosus, and L. delbrueckii), ccpA is preceded by a cre, and its expression is submitted to autoregulation (204, 507, 574). In L. monocytogenes, the synthesis of CcpA was elevated when the cells were exposed to prolonged salt stress (200). By contrast, expression of B. subtilis ccpA is constitutive and is not influenced by the presence or absence of glucose (566). CCR in B. subtilis is therefore not regulated via ccpA expression. Instead, additional factors must be involved in the CCR signal transduction pathway. Because CcpA at physiological concentrations was not able to bind to the cre of the catabolite-repressed B. subtilis gnt operon (566), it was likely that these additional factors would function as corepressors of CcpA.
P-Ser-HPr Functions as a Catabolite Corepressor
Interaction of P-Ser-HPr with CcpA.
When the effects of the B. subtilis ptsH1 mutation on CCR were compared to those of a ccpA mutation, it was found that all enzymes that are partly or completely relieved from CCR in the ptsH1 mutant are also relieved from CCR in the ccpA mutant (184). In addition, two of the enzymes that remained repressed by glucose in the ptsH1 mutant (GlpK and α-glucosidase) remained repressed in the ccpA mutant (184). The similar pleiotropic phenotypes observed for ptsH1 and ccpA mutants suggested that P-Ser-HPr and CcpA participate in the same CCR mechanism and might interact. Binding of P-Ser-HPr to CcpA was indeed demonstrated by elution retardation experiments (182). In the presence of FBP, elution of P-Ser-HPr from a Ni-nitrilotriacetic acid column saturated with His-tagged CcpA was clearly retarded compared to the elution of HPr or P∼His-HPr, while doubly phosphorylated (P∼His,P-Ser)-HPr was only slightly retarded.
The specific interaction between P-Ser-HPr and CcpA was confirmed by NMR measurements (381). The formation of the P-Ser-HPr:CcpA complex could be observed in the absence of FBP, probably owing to the high protein concentrations used in the NMR studies. The NMR measurements also allowed the determination of the residues of P-Ser-HPr interacting with CcpA. In addition to the regions around the active-center His-15 and the regulatory Ser-46 (amino acids 43, 44, and 46 to 56), the interface includes amino acids 21 to 27. The regions at amino acids 21 to 27 and 46 to 56 are well conserved within the HPrs of gram-positive bacteria but are quite different in the HPrs of most gram-negative organisms (Fig. 4).
The size of CcpA (37 kDa) is too large to allow a determination of its interface in the P-Ser-HPr:CcpA complex by NMR measurements. However, based on the crystal structure of PurR (788), a LacI/GalR-type repressor missing the CcpA-specific sequences, and the failure of various mutant CcpAs to form a complex with P-Ser-HPr, a presumed surface-exposed area including Tyr-89, Tyr-295, Ala-299, and Arg-303 was proposed to be part of the interface in the P-Ser-HPr:CcpA complex (422). The four amino acids presumed to participate in P-Ser-HPr binding are located in CcpA-specific regions that are absent from other members of the LacI/GalR repressor family. The crystal structure of the B. megaterium P-Ser-HPr:CcpA complex revealed that residues Tyr-295, Ala-299, and Arg-303 are located on helix IX of the CcpA N subdomain (which directly follows the DNA binding domain) and indeed interact with P-Ser HPr (787). Val-300 and Leu-304 of CcpA are also part of this tight interface, which includes Ile-47, Met-48, and Met-51 of P-Ser-HPr. Replacement of Ile-47 with Thr in S. salivarius HPr leads to the disappearance of diauxic growth and the CCR observed with the wild-type strain during lactose and maltose utilization (263). However, crossed immunoelectrophoresis with HPr antibodies revealed that the amount of P-Ser-HPr in the ptsH(Ile47Thr) mutant was similar to that observed in the wild-type strain. The ptsH(Ile47Thr) mutation was therefore assumed to diminish the affinity of the mutant P-Ser-HPr for CcpA (263). The surface-exposed hydrophobic patch formed by Ile-47, Met-48, and Met-51 is also involved in the interaction of HPr with EI (260) and EIIAs (139, 691, 949).
NMR studies revealed an affinity of CcpA for P-Ser-HPr that was about 50-fold higher than that for HPr (381). This effect must be due mainly to electrostatic repulsion, as no significant structural changes accompany the phosphorylation of HPr at Ser-46 (29, 381). The electrostatic effects were assumed to involve the negatively charged phosphorylated Ser-46 of HPr and a positively charged counterpart in CcpA. According to the crystal structure, Arg-303 and Lys-307 indeed form hydrogen bonds with the phosphoryl group in P-Ser-HPr. Tyr-89, the fourth CcpA residue predicted to be involved in the interaction with P-Ser-HPr, is located on helix I of CcpA. Its close contact to helix IX probably explains why a mutation affecting Tyr-89 prevents the interaction with P-Ser-HPr (422). The P-Ser-HPr:CcpA example therefore proves that based on genetic and biochemical studies, reliable predictions about protein-protein interfaces can be made.
Similar to gram-negative bacteria, where PEP-dependent phosphorylation of EIIAGlc is important for CCR (see “REGULATION OF CARBON METABOLISM IN GRAM-NEGATIVE ENTERIC BACTERIA”), phosphorylation of HPr at His-15 affects CCR in gram-positive bacteria, probably by weakening the P-Ser-HPr:CcpA interaction. Indeed, the region around His-15 of P-Ser-HPr makes contacts to helix IX of subunit 1 and helices I and II of subunit 2 in the regulatory domains of the CcpA dimer (787). One P-Ser-HPr molecule therefore interacts with both subunits of the CcpA dimer. Asp-69 and Asp-99 on helices I and II in the regulatory domain of subunit 2 of the CcpA dimer form hydrogen bonds with Arg-17 in P-Ser-HPr. The crystal structure provides an explanation as to why CcpA exhibits only a weak affinity for (P-Ser,P∼His)-HPr (184) and why replacing His-15 with Glu or Ala caused an almost complete relief from CCR (707). When a ptsH1 mutant strain was transformed with a plasmid carrying the His15Ala ptsH allele, the Ser46Ala mutant HPr synthesized from the chromosomal ptsH1 allele allowed the uptake of glucose via the PTS, while the His15Ala mutant HPr synthesized from the plasmid-located ptsH allele could be phosphorylated at Ser-46 and was therefore expected to be active in CCR. However, only a weak repressive effect of glucose on gluconate kinase activity was observed in the ptsH1 transformant (707), suggesting that seryl-phosphorylated His15Ala mutant HPr has only a low affinity for CcpA (707). Indeed, in the P-Ser-HPr:CcpA complex, Asp-296 forms hydrogen bonds with the Nδ1 of His-15 and the backbone amide nitrogen of Ala-16, which enhances the affinity between the two proteins. Phosphorylation at Nδ1 of His-15 in P-Ser-HPr by PEP and EI or replacing His-15 with Glu or Ala probably prevents the interaction of Asp-296 with HPr and, in the case of (P-Ser,P∼His)-HPr and His15Glu mutant P-Ser-HPr, probably even leads to electrostatic repulsion. The participation of His-15 in the interaction with CcpA provides a link between CCR and PEP-dependent phosphorylation of PTS proteins (182).
Several mutations leading to “permanent” CCR have been described. Replacing Ser-46 of HPr with a negatively charged Asp provides a mutant protein resembling P-Ser-HPr (952), which was therefore able to bind to CcpA immobilized on a Ni-nitrilotriacetic acid column (707). In agreement with this finding, overexpression of the ptsH(Ser46Asp) allele from a plasmid led to partial CCR of the B. subtilis gnt operon even in the absence of a repressing sugar (184). Two other classes of mutations that caused similar “permanent” CCR have been described. Several B. megaterium ccpA mutants that exhibit partial CCR of the xylose utilization genes in the absence of a repressing sugar have been isolated (442). In contrast to wild-type CcpA, the mutant CcpAs are thought to interact with the xyl cre even in the absence of a corepressor (442). The mutations affected the DNA binding domain (Glu77Leu replacement) or the core domain of CcpA. Permanent CCR was also observed with a B. subtilis mutant producing Val265Phe mutant HprK/P, which exhibits normal kinase but strongly reduced P-Ser-HPr phosphorylase activity, which leads to the accumulation of P-Ser-HPr (571). Similar to the HPr(Ser46Asp)-producing strain, CCR in the hprK(Val265Phe) mutant occurs even in the absence of a repressing sugar. Expression of a xynB′-lacZ fusion in the hprK(Val265Phe) mutant grown in a medium containing xylose and succinate as the sole carbon sources is 100 times lower than that in a wild-type strain (571). This permanent repressive effect is responsible for the failure of the hprK(Val265Phe) mutant to grow on CCR-sensitive sugars such as gluconate, glucitol, and ribose. Inactivation of ccpA or the insertion of a ptsH1 mutation in the hprK(Val265Phe) strain restored growth on the above-mentioned sugars (571). These results confirm that P-Ser-HPr formation represents the main signal for CCR in gram-positive bacteria. When present at elevated concentrations in the cell, P-Ser-HPr is sufficient to lead to strong CCR, while glycolytic intermediates do not seem to be essential.
Binding of the P-Ser-HPr:CcpA complex to cre's.
The above-described results indicate that in gram-positive bacteria with low G+C content, P-Ser-HPr functions as a corepressor by allowing the repressor CcpA to bind to cre sites. This concept was first tested with the B. subtilis gnt operon, which possesses a cre located within the 5′ end of gntR, the first gene of this operon (247). Footprint experiments revealed that CcpA or P-Ser-HPr alone does not bind to the gnt cre. By contrast, if P-Ser-HPr and CcpA were present together in the reaction mixture, a specific protection of a region corresponding to the gnt cre was observed, while no protection occurred with CcpA and dephospho-HPr (247). Mutations affecting the gnt cre and causing a relief from CCR prevented binding of the P-Ser-HPr:CcpA complex. Similar results were obtained with cre's from many other catabolite-repressed or -activated transcription units. In addition, by isolating the DNA:protein complex and separating its components on a denaturing gel, it was demonstrated that P-Ser-HPr and CcpA bind together to cre sites (30).
The binding affinity of the P-Ser-HPr:CcpA complex for a cre site was first studied with circular dichroism spectroscopy. Based on association/dissociation-related changes in the spectrum, an apparent KD of 4.5 μM was determined for the formation of the ternary complex P-Ser-HPr:CcpA:optimized B. megaterium xyl cre (A:T in position 10 replaced with T:A) (381). A 1:1:2 (cre:CcpA dimer:P-Ser-HPr) binding stoichiometry was proposed. Because no interaction with the cre could be observed when 0.5 mM HPr and CcpA was used, binding of P-Ser-HPr to CcpA was estimated to increase the affinity of the repressor for its DNA targets by more than 2 orders of magnitude (381). Besides, equilibrium constants for the binding of the P-Ser-HPr:CcpA complex to the B. megaterium xyl cre and the B. subtilis amyE cre were determined by electrophoretic mobility shift experiments and were in the orders of 5 × 106 and 3.5 × 108 M−1, respectively (30, 404). A KD (0.6 nM) that was much lower than the one determined by circular dichroism spectroscopy (4.5 μM) was reported for the formation of the ternary complex of B. subtilis CcpA, P-Ser-HPr, and the xylA cre (794).
The crystal structure of the triple complex composed of the CcpA dimer and two P-Ser-HPr molecules from B. megaterium and a cre has been solved (787). It revealed that the N-terminal HTH motifs and the hinge helices of the CcpA dimer make 32 phosphate contacts to the DNA. They interact with 10 bp out of the 16 bp forming the pseudopalindromic cre, an artificial operator site not present in B. megaterium. However, a reverse complementary sequence identical to bp 2 to 15 of the employed cre is present in Streptococcus iniae and probably controls expression of the lactose permease- and lactose oxidase-encoding lctPO operon (cre located 52 bp upstream from the start codon) (J. Deutscher, unpublished observation). In the ternary complex, important contacts are made with the central C:G and G:C base pairs via Leu-55, the “leucine lever.” These contacts are responsible for DNA kinking and minor groove expansion. DNA bending by the P-Ser-HPr:CcpA complex (35°) is smaller than what was observed for LacI (40°) and PurR (50°). The HTH motif exhibits sufficient flexibility to allow CcpA binding to the variable half sites of pseudopalindromic cre's. The plasticity of the HTH motif also explains the capacity of CcpA to interact with the various cre's of an organism, which generally exhibit significant sequence differences.
A comparison of the structure of the triple complex with that of unliganded CcpA (apoCcpA) revealed that the interaction of P-Ser-HPr with CcpA causes significant structural changes, primarily in the regulatory N subdomain, which allow CcpA to interact with cre's (787). The N subdomain in both subunits of “activated” CcpA is rotated on average by 6° compared to apoCcpA. In addition, the position of helix IX (contains Arg-303) in “activated” CcpA is different from its location in apoCcpA. The interaction of Arg-303 of CcpA with the phosphoryl group in P-Ser-HPr requires the rotation of the side chain of Arg-303, which initiates a series of structural changes culminating at Thr-61, which is located at the core/DNA binding junction. The rotation of Tyr-91, which in apoCcpA interacts with Thr-306 via a hydrogen bond, towards the CcpA dimer interface plays an important role in this process, which ultimately allows high-affinity binding of CcpA to cre sites (787).
The P-Ser-HPr:CcpA interaction mode seems to be independent of the absence or presence of a cre site. The crystal structure of a complex formed between B. subtilis P-Ser-HPr and CcpA missing the first 57 amino acids was very similar to the corresponding structure in the B. megaterium P-Ser-HPr:CcpA complex (106). Interestingly, the B. subtilis protein complex contains two sulfate ions located close to each other. Both sulfate ions interact with Asp-276, and because an FBP molecule could easily be modeled into the structure with its two phosphoryl groups superimposed on the sulfate ions, it was predicted that the region around Asp-276 represents the FBP binding site.
Based on the above-described results, a general CCR and CCA mechanism, as outlined in Fig. 6 can be proposed for low-G+C gram-positive bacteria. The increase in FBP in cells growing on a rapidly metabolizable carbohydrate such as glucose stimulates the HprK/P-catalyzed formation of P-Ser-HPr, which forms a complex with CcpA and allows the LacI/GalR-type repressor to bind to the cre's. For all genes of gram-positive organisms known to be submitted to CCA, the cre is located upstream from the promoter, and binding of the P-Ser-HPr:CcpA complex stimulates transcription, probably by a mechanism similar to that reported for Crp- or Cra-mediated activation of gene expression (142, 645). cre's located upstream from the promoter are found in the catabolite-activated B. subtilis ackA (886), glg (178), pta (685), and ilv-leu (810, 882) transcription units and the pepQ and las genes of L. delbrueckii and L. lactis, respectively (494) (Table 3). CcpA-mediated CCA of the B. subtilis als operon occurs probably via an indirect mechanism, as no obvious cre is located in front of the als promoter (882). The cre of the CCR-sensitive S. xylosus malRA operon is also located in front of the promoter (205). However, the mal cre and the mal promoter are separated by only 8 bp. This might be close enough to enable the P-Ser-HPr:CcpA complex bound to the cre to prevent binding of the RNA polymerase holoenzyme. In carbon catabolite-activated genes (ackA, pta, ilv-leu, and pepQ), the cre and the corresponding promoter are separated by at least 12 bp.
FIG. 6.
Mechanisms underlying CCR in firmicutes. The uptake of glucose and other rapidly metabolizable PTS sugars (top left) leads to a net dephosphorylation of the PTS proteins. The center and bottom of the figure show that the high concentration of FBP present in cells growing on a rapidly metabolizable carbohydrate stimulates the HPr kinase activity of the bifunctional HprK/P and the formation of P-Ser-HPr. P-Ser-HPr interacts with CcpA, and the protein complex binds to the cre operator sites on the DNA. The promoter regions are indicated as −10 and −35. CCA occurs when the cre is located upstream from the promoter, while CCR requires a cre located within or downstream from the promoter. High concentrations of Pi present in resting cells favor the pyrophosphate-producing dephosphorylation of P-Ser-HPr by HprK/P. The top right part of the figure shows that P-Ser-HPr probably interacts with certain non-PTS carbohydrate transport systems, such as the maltose transporter from L. casei, and thereby inhibits their transport activity. Phosphorylation of LacS by P∼His-HPr stimulates the lactose/galactose exchange reaction (in S. thermophilus). In the presence of rapidly metabolizable PTS substrates, the low level of P∼His-HPr does not allow sufficient phosphorylation of GlpK, which leads to the inactivation of GlpK and to inducer exclusion. Pyr., pyruvate.
Deviations from the general CCR mechanism in gram-positive bacteria.
When Ramseier et al. studied the in vitro binding of B. megaterium CcpA to an “optimized” B. subtilis xyl cre, in which the G:C and A:T base pairs in positions 3 and 10 were replaced with T:A, an interaction was observed in the absence of P-Ser-HPr (695). Moreover, the presence of P-Ser-HPr or the structurally related Ser46Asp mutant HPr (952) inhibited the interaction of CcpA with the modified xyl cre. In addition to the two mutations introduced intentionally in the B. subtilis xyl cre, there is also an ambiguity for position 9, which was previously reported to be a T (421) but is a C in the published genome sequence (437). In the nearly identical B. megaterium xyl cre (only one base pair difference) (290), there is a C at position 9. As the experiments reported by Ramseier et al. (695) were carried out with the sequence carrying a T in position 9, the unexpected results obtained in the CcpA binding studies might be due to this sequence difference. Indeed, when footprint experiments were carried out with B. megaterium CcpA and an optimized B. megaterium xyl cre, which differs from the optimized B. subtilis xyl cre used previously (695) only at position 9 (C:G instead of T:A), results similar to those reported for the B. subtilis gnt cre were obtained; i.e., binding of B. megaterium CcpA to the xyl cre was observed only when P-Ser-HPr was present (290).
CcpA has been reported to bind in vitro to the B. subtilis ccpC (401) and ackA cre's (886) in the absence of a corepressor. Nevertheless, the stimulating effect of glucose on ackA expression disappeared in a ptsH1 crh double mutant (for crh, see the next section) (886). The discrepancy between in vivo and in vitro results could not be explained.
Glucose-6-P allowed P-Ser-HPr-independent binding of CcpA to the B. subtilis gnt cre (564) and the optimized B. megaterium xyl cre (290), albeit only under nonphysiological conditions. For the B. subtilis gnt cre, an effect could be detected only when glucose-6-P was used at more than 30 mM, which is far above the 4 mM glucose-6-P detected in glucose-grown B. subtilis cells (233). For the B. megaterium xyl cre, an effect of glucose-6-P on the binding of CcpA could be observed only at pH values below 5.4 (290), i.e., far below the cytoplasmic pH of 7 to 8 reported for B. megaterium cells (503). Glucose-6-P was therefore not considered to play a role in regulating binding of CcpA to the cre's but was suggested to mimic a possibly physiologically relevant unknown effector (290).
In footprint experiments, CcpA was found to bind weakly to the B. subtilis amyE cre in the absence of a corepressor (405). CcpA binding was stimulated by P-Ser-HPr and further enhanced by FBP (404). In in vitro transcription studies, CcpA in the 500 nM range was able to specifically inhibit amyE expression. In the presence of 2.7 μM P-Ser-HPr, inhibition of amyE expression occurred at slightly lower CcpA concentrations (around 100 nM) and was more efficient (90% inhibition). However, the P-Ser-HPr effect was considered to be nonspecific, as expression from control promoters was also inhibited. In addition, the ptsH1 mutation had no influence on CCR of the amyE gene (924). Surprisingly, when NADP or NADPH was present, specific inhibition of in vitro transcription from the amyE promoter was observed at very low CcpA concentrations (in the 5 nM range), but NADP(H) was less efficient (70% inhibition) than P-Ser-HPr. Moreover, in footprint experiments, the addition of NADP(H) had only a weak effect on the protection of the amyE cre by CcpA. It was therefore concluded that NADP(H) does not function as a corepressor by enhancing the binding of CcpA to the cre but, rather, that it allows CcpA to inhibit the transcription machinery, thus leading to reduced amyE expression (404). CcpA has been proposed to directly interact with and to inhibit RNA polymerase, and NADP/NADPH was thought to stimulate the interaction of CcpA with RNA polymerase (406). The suggested role of NADP and NADPH cannot be related to the redox state of the cells, as both molecules were similarly efficient in the stimulation of CcpA-mediated inhibition of amyE expression, while NAD and NADH had no effect. With the exception of dormant spores, the concentration of the NADP(H) pool was reported to remain fairly constant in B. megaterium cells under various growth conditions, including growth in the absence and presence of glucose (801). It is therefore not clear to which signal the interaction of NADP(H) with CcpA responds.
In several instances, CCR of catabolic genes is mediated by an indirect mechanism. For example, cre sites can be located in front of genes encoding transcription regulators of catabolic genes. This is the case for B. subtilis CcpC, which controls the expression of citZ and citB (401) and for LevR of L. casei, which regulates the expression of the levABCDX operon (534). Similarly, the acoABCL operon of B. subtilis is controlled by the transcription activator AcoR, the gene of which is preceded by a cre recognized by CcpA (12). While a cre site also precedes citZ and the L. casei levABCDX operon, a cre seems to be absent from acoABCL, which is therefore only indirectly regulated by CcpA. A cre site has also been identified within the sigL gene of B. subtilis, which codes for σ54, which is necessary for the transcription from several “−12, −24” promoters (128).
The regM gene of S. mutans encodes a homolog of CcpA. Surprisingly, the disruption of regM increased the glucose repression of α-galactosidase, mannitol-1-P dehydrogenase, and P-β-galactosidase, suggesting that RegM functions as a positive regulator for the expression of the corresponding genes. In addition, regM inactivation affected neither diauxic growth in media containing glucose and a less efficiently metabolizable sugar (816) nor expression of the fructanase-encoding fruA gene, although deletion or mutation of the cre preceding fruA led to a relief from CCR (946). Moreover, FBP, which stimulates HPr kinase activity in most bacteria, slightly inhibited HPr kinase from S. mutans (869). It therefore seems that S. mutans follows a CCR mechanism different from that operative in most other gram-positive organisms.
P-Ser-Crh, a Second Catabolite Corepressor in Bacilli, Geobacilli, and Oceanobacilli
A B. subtilis HPr-like protein without His-15.
Although most catabolite-repressed genes and operons of B. subtilis are partly or completely relieved from CCR in both ccpA and ptsH1 mutants, the degree of relief can be quite different for the two mutants. For example, CCR of the amyE gene and the hut, cta, xyn, and iol operons is only slightly affected by the ptsH1 mutation (less than 20%), whereas these five transcription units are completely relieved from CCR in ccpA mutants (184, 250, 488, 924, 985). Similarly, CCA of the phosphotransacetylase-encoding pta gene is not affected in a ptsH1 mutant but is completely lost in a ccpA strain (685). Less pronounced differences in CCR or CCA between ptsH1 and ccpA mutants were observed for the glucitol dehydrogenase-encoding gutB (184), the acetyl-CoA synthetase-encoding acs (984), and the acetate kinase-encoding ackA (886) genes as well as for the lev operon (250, 526). These results suggested that in B. subtilis, the binding of CcpA to certain cre's is regulated not only by P-Ser-HPr but that a second corepressor might exist.
Evidence for a potential second corepressor came from the B. subtilis genome sequencing project, which led to the discovery of a gene encoding an HPr-like protein (219). Although this protein exhibits 45% sequence identity to HPr, there is a striking difference: His-15, the site of PEP-dependent phosphorylation in HPr, is replaced with a Gln in the HPr paralog. As a consequence, the HPr-like protein is not phosphorylated by PEP and EI (250). Nevertheless, the sequence around Ser-46 resembles that in HPr of gram-positive bacteria, and HprK/P-mediated, ATP-dependent, FBP-stimulated phosphorylation at Ser-46 in Crh could be demonstrated (250). These results suggested that the phosphorylated HPr paralog might play a role in CCR similar to that of P-Ser-HPr, and it was therefore called catabolite repression HPr (Crh).
However, when the crh gene is disrupted, no effect on CCR is observed. The participation of P-Ser-Crh in CCR can be detected only in a ptsH1 background. The residual CCR of the lev, iol, xyn (250), and hut (985) operons as well as the remaining CCA of the pta (685) and ackA (886) genes observed in ptsH1 mutants disappeared almost completely when crh was inactivated. Complete relief of the acsA (984), xyn (249), and lev (522) operons from CCR was also observed in a ptsH1 crh1 double mutant in which Ser-46 of both HPr and Crh was replaced with Ala.
In vitro, Crh forms a mixture of monomers and dimers (644), which, similar to EI, are in slow equilibrium. The structure of the monomer and the interface of the dimer were determined by NMR spectroscopy (222). The NMR data suggested that domain swapping of the β1 strand is implicated in B. subtilis Crh dimer formation, and this was confirmed by the crystal structure (389). The Crh monomer strongly resembles HPr, except for a few specific differences. Their structural similarity explains why Gln15His mutant Crh can be phosphorylated by PEP and EI (524). When the Gln15His mutant Crh is overproduced in a B. subtilis ptsH deletion strain, it partly restores PTS transport activity and growth on PTS substrates or glycerol and allows the full activation of several transcription regulators possessing a PTS regulation domain (PRD) (156, 524). GlpK and PRD-containing transcription regulators (see “REGULATORY FUNCTIONS MEDIATED BY P∼His-HPr AND P∼EIIBs”) are stimulated by PEP-dependent phosphorylation via EI and HPr (158, 844).
Binding of the P-Ser-Crh:CcpA complex to cre's.
To determine the binding characteristics of the P-Ser-Crh:CcpA complex, footprint experiments were carried out with cre's from transcription units, which are not completely relieved from CCR in ptsH1 mutants. The xyn cre site was indeed specifically protected by the P-Ser-Crh:CcpA complex, but the effect was slightly weaker than that with P-Ser-HPr:CcpA (249). The use of various mixtures of P-Ser-HPr and P-Ser-Crh did not increase the protective effect compared to the protection obtained with only a single corepressor. For a few catabolic genes, the binding of P-Ser-Crh:CcpA to the cre's was stimulated by FBP, and in some cre's, certain positions became hypersensitive towards DNase I digestion after binding the repressor:corepressor complex. P-Ser-HPr:CcpA and P-Ser-Crh:CcpA protected identical DNA regions, and FBP always had comparable effects on the DNA binding affinity of the two protein complexes. For example, FBP increased the protective effect of the P-Ser-HPr:CcpA and P-Ser-Crh:CcpA complexes against DNase I digestion of the xyn cre (249) and the pta cre (685). In contrast, FBP did not enhance the protective effect of both CcpA:corepressor complexes in footprint experiments with the lev cre (523). Both protein complexes rendered several nucleotides located outside the lev cre hypersensitive towards DNase I digestion. Expression of the lev operon is regulated by the transcription activator LevR, which binds to an upstream activating sequence (UAS) centered at about 130 bp upstream from the lev promoter. LevR was therefore assumed to exert its stimulating effect on lev operon expression by DNA bending (523). The lev cre is located between the UAS and the σ54-dependent “−12, −24” promoter. Binding of the P-Ser-HPr:CcpA or P-Ser-Crh:CcpA complexes to the lev cre supposedly prevents LevR-induced DNA bending by altering the DNA structure, which might be responsible for the hypersensitivity towards cleavage during footprint experiments. Although in the case of the pta cre, only weak protection by P-Ser-HPr:CcpA or P-Ser-Crh:CcpA was observed, several nucleotides located outside the cre site became hypersensitive to DNase I digestion. When FBP was included in the reaction mixtures, the hypersensitive bands disappeared, and the protective effect on the pta cre was enhanced (685). The physiological significance of these in vitro observations remains to be determined.
In addition to the fact that certain cre's are not recognized by the P-Ser-Crh:CcpA complex (when the ptsH1 mutation leads to a complete relief from CCR or CCA), P-Ser-Crh was reported to have a 10-fold-lower affinity for CcpA than P-Ser-HPr. These differences are surprising, as, with the exception of two amino acids (His-15 and Thr-20), all P-Ser-HPr residues involved in the interaction with CcpA are conserved in Crh, and the crystal structures of the two proteins revealed a very similar fold. A possible explanation was that the dimerization of Crh (389) might have an influence on the formation of the P-Ser-Crh:CcpA complex and its interaction with cre sites. However, the crystal structure of the triple complex P-Ser-Crh(B. subtilis):CcpA(B. megaterium):cre (same cre as that used for the P-Ser-HPr:CcpA structure) (787) revealed that the two P-Ser-Crh molecules bound to the CcpA dimer are present exclusively in monomeric form (789). The binding of P-Ser-Crh:CcpA to the cre induces a kink (DNA bend angle of 31°) in the central conserved C-G that is almost identical to that observed in the triple complex with P-Ser-HPr (DNA bend angle of 35°). Nevertheless, specific differences between P-Ser-HPr:CcpA and P-Ser-Crh:CcpA occur in the contact region including His-15/Gln-15 and Ala-20/Thr-20 of HPr/Crh, respectively. In the complex with P-Ser-HPr, His-15 hydrogen bonds with the side-chain carboxyl of Asp-296 of CcpA. In contrast, Gln-15 of Crh makes only a weak contact to Asp-296 (distance of 4 Å), but instead, hydrogen bonds with the side chain of Arg-324, which, compared to the P-Ser-HPr:CcpA structure, needs to be rotated to come sufficiently close to the interface to allow the contact with Gln-15. Interestingly, an equivalent of Arg-324 seems to be present in CcpAs of only those organisms possessing Crh (789). If this assumption holds, Bacillus pseudofirmus, Bacillus sphaericus, and Exiguobacterium sibiricum 255-15 should contain a Crh ortholog, as their CcpAs contain an equivalent of Arg-324, whereas G. stearothermophilus, in contrast to Geobacillus kaustophilus, should be devoid of Crh, as its CcpA is missing an equivalent of Arg-324 (replaced with a Glu). The presence of Crh in the above-mentioned four organisms is uncertain, as no or only incomplete genome sequences are presently available.
In conclusion, the structures of the complexes of P-Ser-HPr and P-Ser-Crh with CcpA and a cre exhibit extensive similarity, and the few differences that were observed do not explain why the B. subtilis P-Ser-HPr:CcpA complex binds to several cre sites, which, according to genetic data, are not or only barely targeted by the P-Ser-Crh:CcpA complex. The corresponding transcription units are partly or completely relieved from CCR in ptsH1 and hprK mutants (gnt, mtl, etc.) (184). To better understand the different affinities of the two corepressor:CcpA complexes for certain cre's, it might be useful to determine the structure of a triple complex of P-Ser-HPr, CcpA, and a cre that does not interact with the P-Ser-Crh:CcpA complex.
Distribution of Crh in gram-positive organisms.
In addition to B. subtilis (250) and B. megaterium (794), Crh has been detected in most other bacilli (Bacillus anthracis, B. clausii, B. cereus, B. halodurans, B. licheniformis, B. thuringiensis, and B. weihenstephanensis) as well as in G. kaustophilus and O. iheyensis, which are both closely related to bacilli. The various Crhs exhibit a high degree of sequence identity (between 61 and 83%), whereas they show only 41 to 47% sequence identity to the HPrs of their respective organisms. Interestingly, B. licheniformis possesses two HPr-like proteins containing a Gln in place of His-15. One of them has a Ser-46 and therefore corresponds to Crh (83% sequence identity with Crh of B. subtilis). The other protein exhibits only 44% sequence identity to B. subtilis Crh, and its Ser-46 is replaced with an Asp, which probably mimics permanent phosphorylation at position 46 (Deutscher, unpublished). The function of the latter protein is unknown. crh is the last gene (or penultimate gene in B. subtilis) of the yvc operon. The last gene of the B. subtilis yvc operon (250), yvcN, is absent from the other organisms, and O. iheyensis also misses the first gene, yvcI.
The genome sequences of other gram-positive bacteria revealed that staphylococci, clostridia, lactobacilli, lactococci, enterococci, etc., also have a yvc operon, but none of them contains a crh gene. Although the in vivo and in vitro results unequivocally established that P-Ser-Crh can contribute to CCR in B. subtilis, the fact that crh is present only in bacilli and a few close relatives and that crh inactivation in wild-type B. subtilis has no effect on CCR raises serious doubts about whether CCR is the primary function of Crh. P-Ser-HPr seems to be sufficient to mediate CCR in gram-positive organisms, independently of whether they possess Crh or not. In addition, numerous B. subtilis operons are completely relieved from CCR in a ptsH1 mutant, suggesting that their cre is not recognized by the P-Ser-Crh:CcpA complex. So far, only one P-Ser-Crh-specific function could be established. Expression of the Mg2+-citrate transporter-encoding B. subtilis citM gene is strongly repressed during growth on glucose but is also slightly repressed when grown on glutamate/succinate as the sole carbon source. Whereas repression of citM by glucose is mediated via CcpA in complex with either P-Ser-HPr or P-Ser-Crh, repression by glutamate/succinate completely disappeared in a crh-disrupted strain (934). Interestingly, crh expression, which is much weaker than ptsH expression, is highest in cells grown on one of the two TCA cycle intermediates, citrate or succinate (286).
It is possible that additional functions of Crh are related to the genes cotranscribed with crh. It is noteworthy that the yvcJ gene organized in an operon with crh resembles yhbJ from the E. coli rpoN operon. YhbJ possesses Walker motifs A and B, but its function is not known. Because in many hprK-containing gram-negative bacteria, yhbJ and other genes of the E. coli rpoN region are organized in an operon with hprK, it is tempting to assume that Crh might somehow play a role in the regulation of SigL (σ54), the RpoN ortholog of B. subtilis.
An HPr paralog containing a conserved Ser-46 but missing His-15 (His-15 is replaced with a Phe) is also present in the mollicute Ureaplasma urealyticum (also called Ureaplasma parvum serovar 1) (308). The sequence around Phe-15 (amino acids 12 to 22) completely differs from all known HPrs, while the other parts of the protein resemble HPr from gram-positive and gram-negative bacteria. In contrast to other mollicutes, U. urealyticum lacks EI as well as an HPr with His-15. Mollicutes generally lack CcpA but contain an hprK gene, as is also the case for U. urealyticum (308). The role of P-Ser-HPr formation in these organisms therefore remains unknown (307).
What Are the Functions of CcpB and CcpC?
A second trans-acting factor implicated in CCR in B. subtilis was identified. This protein, which was called catabolite control protein B (CcpB), exhibits 30% sequence identity to CcpA, and especially, the helix-turn-helix motifs of the two proteins are very similar. Indeed, ccpB disruption leads to a partial relief from CCR of the gnt and xyl operons in cells grown on solid medium (113). However, when the cells are grown in liquid media at a high agitation rate, no effect of the ccpB mutation is observed. In contrast, mutant cells grown at a low agitation rate exhibit partial relief from CCR. Based on these results, it was proposed that CcpB would affect CCR in response to changes in environmental conditions, such as oxygen supply, cell density, etc. However, the DNA targets and the factors controlling the binding of CcpB to these targets are not known. The structures of the P-Ser-HPr:CcpA and P-Ser-Crh:CcpA complexes (787, 789) revealed that those sequences that are specific for the CcpA repressor subfamily (422) are important for the interaction with P-Ser-HPr. The fact that these sequences are absent from CcpB argues against the assumption that it might use P-Ser-HPr as a corepressor (113).
A third trans-acting factor called CcpC is involved in CCR of the B. subtilis citB and citZ genes (which code for aconitase and citrate synthase, respectively) (386) and in CCR of the L. monocytogenes genes encoding a CitB ortholog and a presumed glutamine transporter (lmo0847) (402). However, CcpC is not a member of the LacI/GalR family but is a member of the LysR family of transcription regulators. In the absence of an effector, B. subtilis CcpC functions as a repressor by binding to both a dyad symmetry element centered at position −66 with respect to the transcription start site of citB and a repeat of only the downstream arm of the dyad symmetry element at position −27. In the presence of citrate, the putative inducer for citB and citZ, CcpC, binds only to the complete dyad symmetry element (at position −66), which leads to transcription activation. Mutations affecting the CcpC binding sites in front of citB cause derepressed expression of only citB, whereas ccpC inactivation stimulates the expression of both citB and citZ (386). The dyad symmetry element shows no similarity to cre's. Nevertheless, expression of the CcpC-regulated citB and citZ genes is elevated in a ccpA mutant (880), probably owing to two indirect effects. Firstly, a cre site is present in front of citZ (403). Inactivation of ccpA therefore leads to enhanced synthesis of the citrate synthase CitZ and probably to elevated amounts of citrate, the inducer of citB (403). Secondly, CcpA regulates ccpC expression by binding to a cre site located between the two promoters of the ccpC gene (401). Disruption of ccpA leads to elevated ccpC expression and, in the presence of high concentrations of citrate, to enhanced citB and citZ expression.
It seems contradictory that B. subtilis cells growing on rapidly metabolizable carbon sources such as glucose activate the expression of glycolytic enzymes but repress enzymes of the TCA cycle. However, during growth on glucose, this organism secretes large amounts of acetate into the medium (685) and also produces acetoin when approaching stationary phase (726). To divert the carbon flux towards acetate/acetoin production, the expression of the genes encoding the enzymes necessary for the synthesis of these metabolites (acetate kinase, phosphotransacetylase, acetolactate synthase, and acetolactate decarboxylase) is stimulated, whereas TCA cycle enzymes, cytochromes, and cytochrome oxidases are repressed. Once the rapidly metabolizable carbon source is exhausted, B. subtilis begins to utilize the previously secreted acetate and acetoin. Under these conditions, the synthesis of the enzymes for acetate and acetoin production is no longer activated, and TCA cycle enzymes, cytochromes, and cytochrome oxidases are derepressed. This coordinated regulation probably provides B. subtilis with a growth advantage, as not all bacteria are able to efficiently utilize acetate and acetoin.
CcpB and CcpC do not seem to be directly regulated by P-Ser-HPr. Nevertheless, the phosphoprotein has been shown to interact with B. subtilis RbsR (Table 1), a LacI/GalR-type repressor, which controls the expression of the ribose operon (583). When electrophoretic mobility shift assays were carried out, only P-Ser-HPr, but not HPr, allowed RbsR to interact with a DNA fragment containing the putative RbsR binding site, which also represents a perfect cre site. It was therefore proposed that this site might be the target for both RbsR and CcpA (731). The positively charged residues of RbsR that were predicted to interact with the phosphoryl group of P-Ser-HPr according to a structure model differ from the positively charged residues located in the interface of the P-Ser-HPr:CcpA complex (787). So far, no in vivo data that would attribute a physiological role to the observed in vitro P-Ser-HPr/RbsR interaction have been published.
Role of P-Ser-HPr in the Virulence of Certain Pathogens
P-Ser-HPr and the regulation of virulence genes in gram-positive pathogens.
Surprisingly, in certain pathogens, P-Ser-HPr formation affects the activity of transcription regulators of virulence genes. The best-studied example is the Crp/Fnr-type transcription activator PrfA of L. monocytogenes (207). It regulates the expression of several virulence genes, including the hemolysin-encoding hly gene and the phospholipase-encoding plcA gene, which together with the prfA gene are clustered within a 10-kb region on the chromosome (129). Growth of L. monocytogenes on rapidly metabolizable carbon sources such as glucose and fructose leads not only to CCR of catabolic genes (50) but also to repression of the PrfA-controlled genes (558). While repression of catabolic genes requires CcpA, repression of the virulence genes is CcpA independent, since it persists in ccpA mutants (50). Nevertheless, P-Ser-HPr, the corepressor of CcpA, seems to play a role in the regulation of PrfA activity. A B. subtilis strain containing L. monocytogenes prfA under the control of the spac promoter and the lacZ reporter gene fused to the PrfA-activated hly promoter exhibits lacZ expression only in the presence of IPTG (804). Expression of lacZ and thus PrfA activity are inhibited by glucose and fructose, indicating that CCR of PrfA-controlled genes is also operative in B. subtilis (179, 326). Interestingly, introduction of the hprK(Val267Phe) mutation in the B. subtilis strain, which leads to the conversion of more than 95% of the HPr into P-Ser-HPr (571), strongly inhibits PrfA activity even in the absence of a repressing sugar (326). Replacement of Ser-46 in HPr with an alanine (ptsH1 mutation) prevented the inhibitory effect of the hprK(Val267Phe) mutation, suggesting that P-Ser-HPr is directly or indirectly involved in PrfA regulation. In contrast, inactivation of ccpA or introduction of the crh1 mutation in the hprK(Val267Phe) strain did not stimulate PrfA activity (326). The effect of P-Ser-HPr on PrfA activity seems to be indirect and based primarily on its poor phosphorylation by enzyme I, which probably leads to barely phosphorylated EIIAs and EIIBs. In agreement with this concept, deletion of ptsH or the enzyme I-encoding ptsI also led to strong inhibition of PrfA activity (326). PrfA therefore seems to require a fully functional PTS and might be either stimulated by P∼His-HPr, P∼EIIAs, or P∼EIIBs or inhibited by dephosphorylated EIIAs or EIIBs. A connection between the PTS components and PrfA was also suggested by the observation that the overproduction of PrfA inhibits glucose utilization via the PTS (517). In contrast, the utilization of glucose-6-P via the hexose phosphate transport protein (UhpT) was not affected by PrfA overproduction. In addition, in contrast to glucose, fructose, cellobiose, etc., glucose-1-P did not inhibit PrfA activity, although it is probably converted to glucose-6-P and metabolized via glycolysis (728). The failure of glucose-1-P, which is not transported via the PTS, to inhibit PrfA activity might therefore be related to its different mode of transport. In conclusion, these results suggest a direct or indirect effect of PTS components necessary for the uptake of glucose, fructose, cellobiose, etc., on PrfA activity.
In contrast to PrfA, the S. pyogenes virulence gene regulator Mga seems to be controlled by the classical CcpA-dependent CCR mechanism. Mga functions as a transcription activator for the expression of numerous genes encoding mainly surface-associated proteins involved in pathogenesis. Expression of the Mga regulon is growth phase dependent, and the presence of rapidly metabolizable carbon sources such as glucose stimulates the synthesis of members of the M-protein family (emm, mrp, arp, and enn) (424, 535). In addition, Mga controls genes encoding fibronectin-binding proteins (sof and fbx) and a collagen-like protein (sclA) (14, 15). Mga also controls its own expression by interacting with two 59-bp binding motifs located within the mga promoter region (536). In addition, the −35 region of the first of the two mga promoters is preceded by a DNA sequence that almost perfectly matches the cre consensus sequence. Purified CcpA was found to bind to the presumed cre site (K. S. McIver, personal communication). Its location upstream from the −35 promoter region suggests that mga is submitted to CCA. This is in agreement with the previous observation that the presence of glucose stimulates the synthesis of the M protein.
The Streptococcus pneumoniae CcpA ortholog RegM represses α-glucosidase and β-galactosidase activities, but the inactivation of ccpA (regM) also attenuates the virulence of this organism. The mutation has no impact on the expression of several established pneumococcal virulence genes but, rather, affects the expression of genes from the capsular polysaccharide synthesis (cps) locus (273). Capsular polysaccharides are known to be important factors for host cell adhesion. Indeed, the ccpA mutant is severely attenuated for nasopharyngeal colonization and lung infection in mice (366). This effect might partly be related to the differences in surface protein composition observed between the wild type and the ccpA mutant.
P-Ser-HPr and the regulation of virulence genes in gram-negative pathogens.
Genes encoding proteins with significant similarity to HprK/P are present in numerous gram-negative bacteria (α-, β-, γ-, and δ-proteobacteria), spirochetes, green sulfur bacteria, Fibrobacteres, and fusobacteria (41, 64). The existence of HprK/P homologs in gram-negative bacteria came as a surprise. Due to the absence of Ser-46 phosphorylation in the HPrs of E. coli and other Enterobacteriaceae, HprK/P was originally thought to exist only in gram-positive organisms. Interestingly, HprK/P-containing gram-negative bacteria possess an HPr in which the sequence around Ser-46 differs from the corresponding sequence in HPrs of Enterobacteriaceae and instead resembles those in HPrs from gram-positive organisms (64) (Fig. 4). For the gram-negative bacteria Neisseria gonorrhoeae and N. meningitidis (64), the spirochete T. denticola (278), and the α-proteobacteria Agrobacterium tumefaciens (S. Poncet, A. Khemiri, I. Mijakovic, A. Bourand, and J. Deutscher, unpublished results) and B. melitensis (S. Poncet, M. Dozot, A. Bourand, J. Deutscher, J. J. Letesson, and X. de Bolle, unpublished results), ATP-dependent phosphorylation of HPr by their HprK/Ps has been demonstrated in vitro. In several of the above-mentioned bacteria, ptsH and sometimes also ptsI are located in the hprK operon. Nevertheless, most of these bacteria seem to contain neither CcpA nor an EIIB or EIIC, excluding the possibility that P-Ser-HPr plays a role in the regulation of PTS transport activity or CcpA-mediated CCR in these bacteria.
In addition to ptsH and ptsI, the hprK operons of gram-negative bacteria frequently contain a gene encoding an EIIA of either the fructose/mannitol or the mannose class PTS (64, 345). The organization of the hprK operon in certain gram-negative pathogens and symbionts suggests a role of HprK/P in cell adhesion and virulence (64). In these proteobacteria, the hprK operon usually contains between 1 and 10 genes of the E. coli rpoN region or genes encoding a two-component system. Genes coding for an EIIA-like protein (PtsN) and an HPr-like protein (PtsO or NPr) are also present in the rpoN operon of E. coli and other enterobacteria (553) (for the regulatory functions of RpoN, PtsN, and PtsO, see “SOME UNUSUAL PTS PATHWAYS AND PROTEINS”). In hprK-containing β-, γ-, and δ-proteobacteria, hprK is usually located between rpoN and yhbJ (64). In a few α-proteobacteria, the hprK and yhbJ genes are also located next to each other. Interestingly, the crh gene of B. subtilis is also organized in an operon with a yhbJ homolog (64), while in Ruminococcus albus, hprK is followed by murB and a yhbJ-like gene (Deutscher, unpublished). These observations strongly suggest a functional connection between the PTS and the nucleotide binding protein YhbJ.
For β- and γ-proteobacteria containing a combined hprK/rpoN operon, HprK/P was proposed to control the phosphorylation state of the EIIAFru-like PtsN (64, 724), the gene of which often precedes hprK. In these organisms, signals causing enhanced HPr kinase and diminished P-Ser-HPr phosphorylase activity were assumed to lead to elevated amounts of P-Ser-HPr, which would slow the phosphorylation of the EIIA (180, 723). In N. meningitidis, such a phosphorelay system seems to somehow control the interaction with host cells, as the inactivation of hprK strongly reduces cell adhesion. The inactivation of hprK possibly leads to the enhanced formation of P∼PtsN. Dephospho-PtsN was therefore proposed to participate in a signal transduction pathway controlling cell adhesion (64).
Similarly, in several α-proteobacteria, the colocalization of hprK with genes encoding a two-component system suggests a role of HprK/P in the regulation of cell adhesion (64, 345). The A. tumefaciens EnvZ/OmpR-like two-component system ChvG/ChvI, which is encoded by the genes preceding hprK, controls the expression of the virulence genes virB and virE (472), which encode components of a type IV secretion system delivering oncogenic DNA to susceptible plant cells (100). Inactivation of chvG or chvI prevents tumor formation on the host plants (108). Similarly, inactivation of the chvG/chvI-like bvrR and bvrS genes of the pathogenic animal endosymbiont Brucella abortus affects cell invasion and virulence of this organism (821). Inactivation of the ChvG-like sensor kinase ExoS in the plant symbiont Sinorhizobium meliloti prevents the production of succinoglycan, the main exopolysaccharide necessary for the successful invasion of nodules on the host plant alfalfa (126). It has been proposed that HprK/P and the PTS proteins might somehow control the activity of the two-component systems (64). In α-proteobacteria, the hprK operon usually contains a gene encoding an EIIA of the mannose class PTS and sometimes also the genes for EI and HPr, whereas the gene for an EIIA of the fructose class PTS is located next to the rpoN gene (S. Poncet, unpublished observation). HprK/P was thought to control the phosphorylation state of the EIIAs. An EIIA or P∼EIIA was in turn suggested to interact with the two-component system (64), or alternatively, a P∼EIIA might phosphorylate one of the proteins of the two-component system. Interestingly, during a search for genes affecting the virulence of Brucella melitensis, inactivation of the EIIAFru-encoding gene was found to lower the pathogenicity of this organism (169).
α-Proteobacteria as well as Coxiella burnetii contain a truncated HprK/P missing the N-terminal domain, which resembles the N-terminal part of MurE (13), the enzyme catalyzing the formation of cytoplasmic precursors for cell wall synthesis (284). Similar to the artificially truncated L. casei HprK/P (229), the natural absence of the first 130 amino acids of A. tumefaciens HprK/P does not affect the known HprK/P functions. Nevertheless, the A. tumefaciens enzyme is not able to efficiently use PPi as a phosphoryl donor and to dephosphorylate P-Ser-HPr in the presence of Pi (I. Mijakovic, A. Khemiri, A. Bourand, S. Poncet, and J. Deutscher, unpublished results). However, the absence of these functions from A. tumefaciens HprK/P is likely due to sequence differences in the central loop compared to HprK/P from gram-positive organisms. Mutations affecting this loop in L. casei or B. subtilis HprK/P strongly diminish the phosphorylase activity (571).
Interestingly, all known bacteria with a truncated HprK/P possess an EI with an N-terminal extension resembling the N-terminal DNA binding domain of NifA, a transcription activator for certain σ54-dependent promoters. EINtr (PtsP) of E. coli, which, together with the HPr paralog PtsO, is assumed to catalyze the phosphorylation of PtsN, also contains this NifA domain (see “SOME UNUSUAL PTS PATHWAYS AND PROTEINS”).
Is P-Ser-HPr Involved in Inducer Expulsion?
In the next few sections, we will deal with the regulatory phenomena inducer expulsion and inducer exclusion in low-G+C gram-positive bacteria, which were both suggested, based mainly on in vitro data, to be related to P-Ser-HPr formation. However, while in vivo experiments confirmed a participation of P-Ser-HPr in inducer exclusion, inducer expulsion also occurs when P-Ser-HPr cannot be formed due to the inactivation of hprK or the replacement of the Ser-46 of HPr with an alanine.
Simultaneous occurrence of inducer expulsion and P-Ser-HPr formation.
Inducer expulsion is a regulatory phenomenon that has been known for at least 40 years. Halpern and Lupo observed that the presence of certain carbon sources accelerates the exit of α-MG from E. coli cells that had accumulated this nonmetabolizable PTS substrate (309). Glucose had the strongest effect and accelerated α-MG expulsion about sixfold compared to cells to which no carbon source was added. When discussing the expulsion of nonmetabolizable carbohydrates, at least two different forms need to be distinguished, as they follow different mechanisms: the expulsion of non-PTS sugars and PTS sugars. The latter are accumulated as phospho derivatives inside bacterial cells and are first dephosphorylated before they are expelled. We will discuss the expulsion of PTS sugars, because ATP-dependent HPr phosphorylation was discovered in connection with TMG expulsion from S. pyogenes cells (185, 710).
Streptococci and lactococci transport nonmetabolizable sugar derivatives such as TMG or 2DG via the PTS and accumulate these carbohydrates as P derivatives. Interestingly, these nonmetabolizable P-sugars are rapidly expelled when the cells are exposed to a well-metabolizable carbon source such as glucose, sucrose, or mannose (712, 872). Expulsion turned out to be a two-step process: the accumulated P-sugars are first intracellularly dephosphorylated before the dephosphorylated sugars are expelled (710). The expulsion could be prevented when the ATP synthesis was inhibited by poisoning the cells with arsenate, fluoride (712), or iodoacetate (872). It has subsequently been established that in poisoned cells, the dephosphorylation of the accumulated P-sugars is inhibited (710). Arsenate- or fluoride-poisoned S. pyogenes cells synthesize little ATP, but they can metabolize arginine via the deiminase pathway and thereby generate ATP. The presence of arginine indeed restored sugar-P dephosphorylation and inducer expulsion in arsenate- or fluoride-poisoned S. pyogenes cells (710). It was therefore concluded that the first step of inducer expulsion depends on the presence of intracellular ATP and that the sugar-P phosphatase catalyzing this step might be regulated directly or indirectly via ATP-dependent protein phosphorylation. The search for this presumed P-protein led to the discovery of P-Ser-HPr: under conditions provoking inducer expulsion, a low-molecular-weight protein became phosphorylated by ATP (185, 710). This protein was identified as P-Ser-HPr (185), and it was therefore assumed that P-Ser-HPr might play a role in inducer expulsion, probably by controlling the activity of the sugar-P phosphatase (708, 710).
Experiments aimed at studying in vitro TMG-6-P expulsion by L. lactis vesicles supported this concept. When we discussed the effect of P-Ser-HPr formation on PTS-catalyzed carbohydrate uptake, we mentioned that vesicles prepared from L. lactis cells contain EI, HPr kinase, and a sugar-P phosphatase but had lost most of the HPr (970). As a result, L. lactis vesicles exhibit only about half the TMG uptake rate measured with intact cells (970). The full TMG uptake rate was restored when 100 μM B. subtilis HPr was electroporated into the L. lactis vesicles. Coelectroporation of wild-type or mutant HPrs with various metabolites was used to test whether phosphorylation of HPr at Ser-46 affects expulsion of TMG from the vesicles. L. lactis vesicles were able to accumulate TMG-6-P, which, similar to in intact cells, was expelled when glucose was added. Interestingly, vesicles into which HPr had been electroporated exhibited glucose-induced TMG expulsion that was about three times faster than that of vesicles that had been electroporated in the absence of HPr or in the presence of Ser46Ala mutant HPr (970). In addition, a cytoplasmic sugar-P phosphatase was activated about sevenfold when glucose was added to toluenized vesicles into which HPr had been electroporated prior to toluenization. Coelectroporation of FBP and HPr similarly enhanced the sugar-P phosphatase activity, and stimulation was even stronger when ATP was included. Because glucose and FBP stimulate the ATP-dependent phosphorylation of HPr at Ser-46 in L. lactis vesicles, it was assumed that the sugar-P phosphatase catalyzes the first step in inducer expulsion and that it is allosterically regulated by P-Ser-HPr. In agreement with this concept, electroporation of the P-Ser-HPr-resembling Ser46Asp HPr stimulated the intravesicular sugar-P phosphatase even in the absence of FBP (970), whereas only a weak increase of the phosphatase activity was observed with FBP and Ser46Ala mutant HPr. In addition to this cytoplasmic phosphatase, a membrane-associated L. lactis sugar-P phosphatase was also reported to be activated by P-Ser-HPr (973). Similarly, S. bovis contains a cytoplasmic and a membrane-associated sugar-P phosphatase (138). However, the activity of only the latter enzyme was stimulated by Ser46Asp mutant HPr. Membrane-associated, Ser46Asp HPr-activated sugar-P phosphatases were also detected in S. pyogenes and E. faecalis, two other organisms exhibiting inducer expulsion (712, 967). Inducer expulsion in all these bacteria was therefore assumed to be triggered by P-Ser-HPr-mediated activation of the membrane-associated sugar-P phosphatase. However, as heterologous systems were used for the in vitro electroporation experiments (HPr from B. subtilis and vesicles from streptococci, lactococci, or enterococci), these results needed to be confirmed by in vivo experiments.
Inducer expulsion in L. casei and L. lactis does not require P-Ser-HPr.
Similar to L. lactis ML3, L. casei strains 64H and BL23 transport TMG via a lactose-specific PTS, accumulate it as TMG-6-P, and expel it from the cells as TMG when glucose or another rapidly metabolizable carbon source is added (111, 198). An L. casei mutant synthesizing Ser46Ala HPr (ptsH1) was constructed from strain BL23. Although P-Ser-HPr cannot be formed in this strain, it expelled accumulated TMG-6-P similarly to the wild-type strain when it was exposed to glucose or mannose. Identical results were obtained with ptsH mutants in which Ser-46 or Ile-47 had been replaced with a threonine (198). These results established that the expulsion of TMG in L. casei does not depend on P-Ser-HPr. To exclude the possibility that an HPr-like protein capable of substituting for P-Ser-HPr in inducer expulsion might exist in L. casei, TMG expulsion was also studied in an L. casei hprK mutant (198). Compared to inducer expulsion by a wild-type strain, no effect of the hprK mutation on glucose- or mannose-triggered expulsion of accumulated TMG-6-P was observed.
Because most experiments suggesting a role for P-Ser-HPr in inducer expulsion were carried out with L. lactis vesicles, a ptsH1 mutant of this organism was constructed and tested for the expulsion of preaccumulated TMG-6-P and 2DG-6-P. Expulsion of both nonmetabolizable sugar derivatives was observed in the ptsH1 mutant. However, whereas 2DG expulsion was nearly identical in the wild type and the mutant, TMG expulsion was slowed by about 2.5-fold in the ptsH1 strain (569). Because L. lactis possesses only HPr and no HPr-like proteins (68), these results unequivocally established that inducer expulsion in this organism does not depend on the presence of P-Ser-HPr. Nevertheless, HPr seems to play an indirect role in TMG expulsion in L. lactis, which might partly explain the vesicle results, which led to the conclusion that P-Ser-HPr participates in inducer expulsion. Differences in the phosphorylation state of the EIIBCLac, which was proposed to catalyze not only TMG uptake but also TMG expulsion (719), in the wild-type and the ptsH1 strains were suggested to be responsible for the indirect effect of the ptsH1 mutation on TMG expulsion (569).
Is P-Ser-HPr Involved in Inducer Exclusion?
In vitro interaction of Ser46Asp mutant HPr with non-PTS permeases.
As explained above (see REGULATION OF CARBON METABOLISM IN GRAM-NEGATIVE ENTERIC BACTERIA), the inducer exclusion mechanism in enteric bacteria is mediated by dephospho-EIIAGlc, which binds to several target proteins (permeases and catabolic enzymes), thereby inhibiting their activity and preventing the entry or the intracellular formation of the corresponding inducer. Several lines of evidence indicated that P-Ser-HPr might play a similar role in inducer exclusion in gram-positive bacteria (Fig. 6). In L. brevis, the galactose/H+ symporter GalP also catalyzes the uptake of TMG (193, 739). The addition of glucose arrests TMG uptake and causes the rapid efflux of preaccumulated TMG from the cells. Both effects of glucose were ascribed to P-Ser-HPr, as its structural homolog, Ser46Asp HPr (from B. subtilis) (952), labeled with 125I was able to bind to the galactose/H+ symporter of L. brevis (972). Galactose as well as galactose derivatives stimulated the binding of 125I-labeled Ser46Asp HPr to the galactose/H+ symporter about 10-fold. Coelectroporated metabolites known to stimulate the kinase activity of HprK/P (FBP, gluconate-6-P, and glycerate-2-P) as well as extravesicular glucose inhibited TMG uptake by L. brevis vesicles preloaded with B. subtilis HPr by electroporation about 10-fold (969). No inhibitory effect of glucose or its metabolites on TMG uptake was observed when Ser46Ala mutant HPr was used in place of wild-type HPr. Moreover, Ser46Asp mutant HPr slowed TMG uptake even in the absence of extravesicular glucose or intravesicular metabolites. Studies of 2DG uptake by L. brevis vesicles electroporated with wild-type or mutant HPrs and with glycolytic intermediates provided results that were almost identical to those obtained for TMG uptake (968). In addition, by expressing the L. brevis galP gene (encodes the galactose/H+ symporter) in a B. subtilis wild-type strain and an hprK mutant, both B. subtilis strains gained the capacity to efficiently take up TMG. When the B. subtilis strains also synthesized L. brevis wild-type or Ser46Ala or Ser46Asp mutant HPr, a strong inhibition of TMG uptake was observed with the strain producing Ser46Asp HPr (193), suggesting that the P-Ser-HPr-resembling Ser46Asp HPr causes inducer exclusion. However, when the strain producing L. brevis wild-type HPr was grown in the presence of glucose, which stimulates the formation of P-Ser-HPr, no inducer exclusion occurred. In addition, since the experiments with L. brevis vesicles were carried out in vitro with B. subtilis wild-type or mutant HPrs instead of the physiologically relevant L. brevis P-Ser-HPr, the proposed implication of P-Ser-HPr in inducer exclusion needed to be confirmed in vivo.
Mutations preventing P-Ser-HPr formation abolish inducer exclusion.
The importance of ATP-dependent HPr phosphorylation for inducer exclusion could be established by studying this regulatory process in L. casei and L. lactis mutants that are unable to form P-Ser-HPr. To detect a potential influence of the ptsH1 and hprK mutations on inducer exclusion in L. casei, transport studies with maltose and ribose were carried out (198, 919). In L. casei, maltose and ribose are taken up by ABC transport systems (570). When wild-type cells are grown in a medium containing glucose and either ribose or maltose, diauxic growth with a long-lasting lag phase is observed. In addition, maltose uptake is immediately arrested when glucose is added to maltose-transporting L. casei cells (198, 919), suggesting that glucose inhibits maltose uptake via an inducer exclusion mechanism. Interestingly, in ptsH1 (919) and hprK (198) mutants, glucose no longer exerts its inhibitory effect on maltose transport. These results imply that the phosphorylation of HPr at Ser-46 plays a role in inducer exclusion in L. casei. Inactivation of the ccpA gene had no effect on inducer exclusion (919), confirming that the P-Ser-HPr-dependent inhibitory effect of glucose on maltose uptake is due exclusively to an inhibition of the transport step and not to CCR of the maltose operon mediated via the P-Ser-HPr:CcpA complex. Additional evidence for P-Ser-HPr-mediated maltose exclusion from L. casei cells came from direct measurements of sugar consumption in the growth medium. In wild-type cells, maltose consumption is instantaneously arrested when glucose is added to the incubation mixture and does not restart before glucose is exhausted. In contrast, in the hprK and ptsH1 mutants, the addition of glucose causes only a short transient stop of maltose consumption, and the two sugars are subsequently simultaneously utilized (198, 919).
Interestingly, ribose transport was stimulated by the presence of glucose. Glucose stimulation of ribose uptake has also been reported for Lactobacillus sakei. Dephosphorylation of the PTS proteins in the presence of glucose was suggested to be responsible for the stimulating effect, as the inactivation of ptsI also led to enhanced ribose uptake (834, 835).
The repressive effect of glucose on maltose uptake and maltose consumption had also disappeared in an L. casei strain producing Ser46Thr mutant HPr, which is a poor substrate for HprK/P. Unexpectedly, inducer exclusion in L. casei was also prevented by the ptsH(Ile47Thr) mutation, although the amount of P-Ser-HPr detected in the S. salivarius ptsH(Ile47Thr) mutant was similar to that in the parental strain (263). The hydrophobic patch on the surface of HPr, which is formed by Ile-47, Met-48, and Met-51 in gram-positive bacteria (375), is involved in the interaction of P-Ser-HPr with CcpA (381, 787) and in the interaction of HPr with EI (260) and various EIIAs (139, 691, 949). The finding that the L. casei ptsH(Ile47Thr) mutant does not exhibit inducer exclusion suggests that this hydrophobic patch might also be important for the interaction of P-Ser-HPr with non-PTS permeases regulated by inducer exclusion.
Inducer exclusion studies were also carried out with an L. lactis ptsH1 mutant. Similar to L. casei, this organism takes up maltose and ribose via specific ABC transport systems (68). The inhibitory effect of glucose on the transport of the two non-PTS sugars in the wild-type strain had completely disappeared in the ptsH1 mutant (569).
An approximately twofold decrease in the P-Ser-HPr level was reported to lead to an almost complete loss of inducer exclusion in S. salivarius cells (870). The lower amount of P-Ser-HPr was due to specific point mutations in the ptsH gene or the ptsH leader sequence, which did not affect the uptake rate of PTS substrates, although the metabolism of PTS sugars was slowed. Wild-type S. salivarius cells immediately stop the uptake of the non-PTS sugar lactose or galactose when glucose or fructose is added to the growth medium and resume the metabolism of the non-PTS sugars only when glucose or fructose is exhausted (660). In contrast, the uptake of lactose or galactose by the ptsH mutants containing twofold-lower amounts of P-Ser-HPr was not affected by the presence of the PTS sugar glucose or fructose (870). To exert inducer exclusion, gram-positive bacteria therefore seem to require high levels of P-Ser-HPr.
In summary, the above-described results support the concept that inducer exclusion in gram-positive bacteria is regulated via the metabolite-activated formation of P-Ser-HPr. Considering the similarities between the maltose uptake systems from E. coli and those from the lactic acid bacteria L. casei and L. lactis, it is tempting to assume that the exclusion of maltose follows a mechanism analogous to that in gram-negative organisms, except that P-Ser-HPr instead of dephospho-EIIAGlc binds to MalK (Fig. 6) (Table 1). This assumption is supported by a comparison of the sequences of the nucleotide binding and regulatory domains of MalKs from E. coli (117) and L. casei BL23 (570). The two proteins are composed of very similar N-terminal nucleotide binding domains (amino acids 1 to 241) (60% identity), while the C-terminal regulatory domains (amino acids 242 to 371) exhibit only 19% sequence identity (Deutscher, unpublished). The sequence difference in the regulatory domain might reflect the binding of different effectors: EIIAGlc in gram-negative bacteria and P-Ser-HPr in gram-positive bacteria.
REGULATION OF CARBON METABOLISM IN LOW-G+C GRAM-POSITIVE BACTERIA: REGULATORY FUNCTIONS MEDIATED BY P∼His-HPr AND P∼EIIBs
PEP-Dependent Phosphorylation of Non-PTS Proteins
Besides their role as phosphocarriers during PEP-dependent phosphorylation of carbohydrates transported via the PTS, P∼His-HPr and several P∼EIIBs also phosphorylate non-PTS proteins and regulate their activities. Based on the characteristics of the phosphorylation site, three different types of non-PTS proteins phosphorylated by P∼His-HPr and/or P∼EIIB can be distinguished. In the simplest case, an EIIA domain, the usual phosphoryl acceptor of P∼His-HPr within the PTS phosphorylation cascade, or an HPr domain is fused to the target protein. These PTS protein domains are not active in sugar transport but, rather, regulate the activity of the fusion proteins in response to their phosphorylation state. For instance, an HPr domain is fused to the response regulator HprR of a C. acetobutylicum two-component system (721). EIIA domains are fused to non-PTS sugar transporters (673) and to transcription activators such as MtlR of G. stearothermophilus (324) or LevR of B. subtilis (178), which control the expression of operons encoding sugar-specific PTS components (Table 1). The transcription regulators also contain an EIIB domain (295) for which neither phosphorylation nor a regulatory function could be demonstrated so far.
The second class of proteins phosphorylated by P∼His-HPr and/or P∼EIIBs contains a PRD (844). The PRDs seem to have specifically evolved to control the RNA binding activity of transcription antiterminators and the DNA binding function of transcription activators in response to phosphorylation by PTS proteins (Table 1). PRDs exhibit no obvious similarity to PTS proteins. Nevertheless, they usually contain two histidyl residues, which are phosphorylated by P∼His-HPr or a P∼EIIB. The sequences around the two phosphorylatable histidyl residues are conserved. Most PRD-containing proteins possess two PRDs either organized in tandem or sometimes separated by EIIA and EIIB domains (178, 295) (Fig. 7). Depending on which of the usually four conserved histidyl residues in the two PRDs is phosphorylated, the regulatory domains stimulate or inhibit the activity of their antiterminator or transcription activator (520, 884).
FIG. 7.
Domain structure of the transcription antiterminator LicT and the transcription activators LicR and LevR of B. subtilis. The N-terminal RNA or DNA binding domain and the two regulatory domains PRD1 and PRD2 together with their conserved histidyl residues are indicated. Histidyl residues, which have been shown to become phosphorylated, are in boldface type. When the phosphorylation exerts a positive effect on the activity of the regulator, they are shown in red, whereas blue indicates the sites of negative regulation. For LicT, its four conserved histidyl residues become phosphorylated. Phosphorylation in PRD1 inhibits, while phosphorylation in PRD2 stimulates, LicT activity. Most other antiterminators of the BglG/SacY family also contain four conserved histidines, but sometimes, not all of them can be phosphorylated. LevR contains an NtrC-like central domain and EIIAMan- and EIIBGat-like domains inserted between the complete PRD1 and a truncated PRD2. The positive site of phosphorylation is His-585, and the negative site is His-869. The conserved histidyl residues His-506 and His-567 in PRD1 and the EIIBGat phosphorylation site (Cys-718) do not seem to become phosphorylated. An identical organization can be observed in most other NifA/NtrC-type PRD-containing transcription activators. However, a few LevR-like regulators contain a complete PRD2 with an additional potentially phosphorylatable histidyl residue. The DNA binding domain of LicR resembles that of DeoR, and PRD1 and PRD2 are followed by an EIIBGat-like and EIIAMtl-like domain. An identical domain organization can be observed in other DeoR-type PRD-containing transcription activators. All four conserved histidines in PRD1 and PRD2 are sites of positive regulation, while LicR activity is inhibited by phosphorylation at His-559 in the EIIAMtl-like domain.
The third class of non-PTS proteins phosphorylated by P∼His-HPr contains GlpKs from low-G+C gram-positive bacteria (Table 1). These GlpKs contain neither a PTS protein domain nor a PRD but developed a novel P∼His-HPr phosphorylation site, i.e., a histidine that is usually surrounded by three aromatic amino acids (Tyr and Phe) (109). The phosphorylation site is located on a surface-exposed loop.
Here, we will discuss the complex regulation of PRD-containing antiterminators and transcription activators in both gram-positive and gram-negative bacteria. The activity of these transcription regulators is controlled by multiple (up to fivefold) PEP-dependent PTS-catalyzed phosphorylations. The phosphorylation events can have antagonistic effects on the antitermination and transcription activation function (Table 1). The observations that these transcription regulators occur mainly in low-G+C gram-positive bacteria and that there are only a few representatives in proteobacteria and high-G+C gram-positive organisms will also be discussed. We will subsequently describe the P∼His-HPr-mediated phosphorylation of lactose and raffinose transporters with an EIIAGlc domain and of a conserved histidyl residue in GlpK, regulatory processes so far observed only in gram-positive organisms (Table 1).
Regulation of Transcription Antiterminators by PTS-Mediated Phosphorylation
Regulation of gene expression by transcription attenuation.
In addition to the most common mechanisms regulating gene expression, i.e., transcription activation or repression, bacteria also developed a mechanism based on transcription termination/antitermination, i.e., transcription attenuation, which was first described for the E. coli trp operon (60). Two principal mechanisms of transcription termination have been reported for prokaryotes: factor-dependent termination and intrinsic termination (965). In the first case, the RNA polymerase is halted at a terminator located at the end of a transcription unit. Interaction with a ρ factor is necessary to dissociate the DNA:RNA polymerase complex and to terminate transcription. In contrast, transcription units submitted to intrinsic termination contain a ρ-independent terminator. When the ρ-independent terminator is located between the transcription start site and the start codon (Fig. 8), the expression of the corresponding gene or operon usually occurs from a constitutive promoter. However, under noninducing conditions, transcription stops when the terminator is formed on the nascent mRNA. Formation of the terminator leads to the disruption of the elongation complex and to the release of short, incomplete transcripts. Under inducing conditions, an antiterminator binds to the mRNA in front of the terminator or to a region overlapping the terminator, thereby preventing the formation of the disruptive stem-loop structure and allowing the RNA polymerase to complete transcription of the corresponding gene or operon (Fig. 8). Antiterminators can be either proteins or oligonucleotides. When tRNAs function as antiterminators, binding to their target site on the mRNA is usually prevented when they are charged with their corresponding amino acid (136). Polypeptide antiterminators frequently respond to changes in the concentration of intracellular metabolites, which serve as cofactors and allow the corresponding antiterminator to bind to its target on the mRNA. For example, glycerol-3-P binds to and activates the B. subtilis antiterminator GlpP (749). Another mode of regulation was reported for antiterminators controlling the expression of genes and operons encoding either sugar-specific PTS components or enzymes catalyzing the extracellular degradation of polysaccharides to mono- or oligosaccharides, which are subsequently taken up via the PTS. This group of polypeptide antiterminators is regulated by reversible PTS-catalyzed phosphorylation. They possess an N-terminal RNA binding domain (first 55 amino acids) usually followed by two regulatory domains, each containing two conserved histidyl residues (Fig. 7), which are phosphorylated or dephosphorylated depending on whether the corresponding carbohydrate is present in the growth medium.
FIG. 8.
Transcription regulation by the PTS via PRD-containing transcription antiterminators. (A) In the absence of the corresponding inducer, full-length transcription of several PTS-encoding genes/operons is inhibited owing to the formation of a terminator structure (t, yellow) on the nascent mRNA upstream from the start codon. Under these conditions, the corresponding antiterminator cannot bind to its RNA target, RAT (blue), because the EIIB is mainly phosphorylated and transfers its phosphoryl group to PRD1 of the antiterminator. The absence of a repressing sugar is expected to also allow phosphorylation at the activating domain (PRD2) by P∼His-HPr. However, the negative effect of phosphorylation at PRD1 is dominant. The RAT sequence can also form a stem-loop, which, however, was calculated to free less energy than the terminator t. Interestingly, in most antiterminator-controlled PTS operons, the two sites RAT and t overlap (green), and the formation of the terminator therefore prevents the formation of the RAT stem-loop and vice versa. (B) If an inducer is present, the EIIB as well as PRD1 of the corresponding antiterminator will be present mainly in an unphosphorylated form. Because antiterminator-controlled PTSs are usually low-capacity sugar transporters, there will be sufficient P∼His-HPr to guarantee activating phosphorylation in PRD2. The activated antiterminator binds to its RAT and thus favors the formation of the RAT stem-loop, thereby preventing the formation of the terminator stem-loop, as part of it (in green) is already used for the RAT stem-loop. (C) If, in addition to the inducing sugar, a repressing carbohydrate is present, the amount of P∼His-HPr will be low in the cells. In firmicutes, P-Ser-HPr will also be formed, which further lowers the amount of P∼His-HPr. These conditions prevent activating phosphorylation at PRD2, and most antiterminators are therefore inactive, although the presence of the inducer probably prevents the phosphorylation in PRD1. Dephosphorylation of PRD2 in the presence of a rapidly metabolizable PTS sugar therefore represents a CcpA-independent, P-Ser-HPr-dependent CCR mechanism. ptsH1 as well as licT(Pia) but not ccpA mutants are relieved from this type of CCR.
ρ-Independent terminators.
The B. subtilis levansucrase-encoding sacB gene (31, 806) and the E. coli bgl operon, which encodes a β-glucoside-specific EIIBCA (bglF), a 6-P-β-glucosidase (bglB) (504) (Table 4), and a carbohydrate-specific outer membrane porin (20), were the first two PTS-related transcription units for which experimental evidence for regulation by antitermination has been obtained. The B. subtilis sacB leader region contains an imperfect 28-base-long inverted repeat called sacR (833). When part of sacR was deleted or when a point mutation was introduced, sacB was expressed constitutively, and sacR was therefore proposed to function as a transcription terminator (31, 806). A sucrose-inducible sacB′-′lacZ fusion also became constitutive when sacR was mutated, which further supported the concept of transcription termination (806). Finally, S1 nuclease mapping revealed that in the absence of sucrose, mainly small transcripts terminating within sacR were formed. The presence of the inducer sucrose did not change the total number of transcripts but increased the number of long transcripts extending beyond the terminator sequence sacR (806).
TABLE 4.
Description of some well-studied PRD-containing antiterminators
| Antiterminator | Organism | Controlled gene(s) | Inducer(s) | RAT sequence |
|---|---|---|---|---|
| LicT | B. subtilis | bglPH | β-Glucosides | GGATTGTTACTGCGAAAGCAGGCAAAACC (bglP) |
| bglS | GGATTGTTACTGATAAAGCAGGCAAAACC (bglS) | |||
| SacT | B. subtilis | sacPA (sacB)a | Sucrose | GGATTGTGACTGGTAAAGCAGGCAAGACC (sacP) |
| SacY | B. subtilis | sacB (sacPA)a | Sucrose | GGTTTGTTACTGATAAAGCAGGCAAGACC (sacB) |
| sacXY | GGATTGTGACTGGGCAGGCAGGCAAGACC (sacX) | |||
| GlcT | B. subtilis | ptsG(HI) | Glucose | ACGTGTTACTGATTCGATCAGGCATCAGT (glcT) |
| GlcT | S. carnosus | glcA, glcB | Glucose, β-glucosides | ACGTGTAACTAATTCGATTAGGCATGAGT (glcA) |
| BglG | E. coli | bglFB | β-Glucosides | GGATTGTTACCGCACTAAGCGGGCAAAACC (bglF) |
| bglG | GGATTGTTACTGCATTCGCAGGCAAAACC (bglG) | |||
| LacT | L. casei | lacTEGF | Lactose | TGGATTGTGACTATTTAATTAGGCGACC (lacT) |
The similarity of the RAT sequences preceding sacB and sacPA probably explains the cross talk observed with the two antiterminators SacY and SacT.
An inverted repeat between the transcription initiation site and the start codon of the bgl operon of E. coli was also detected (505). In the noninduced state, more than 90% of the transcripts, which were initiated at the bgl promoter, were already terminated at the inverted repeat. Expression of a bgl-lacZ fusion containing the inverted repeat required an intact bglG (formerly bglC) gene, which is the first gene of the bgl operon, while bgl-lacZ fusions devoid of the inverted repeat exhibited constitutive, bglG-independent expression (505). Based on these results, it was proposed that the inverted repeat located between the transcription initiation site and the start codon of the E. coli bgl operon serves as a ρ-independent transcription terminator and that the protein encoded by bglG exerts a positive effect on bgl expression by functioning as an antiterminator. In agreement with this hypothesis, bglG disruption caused drastically lowered expression from the bgl promoter (504). However, a few bglG point mutations, including the one present in the bglC9 strain described previously (682), can lead to the constitutive expression of the bgl operon.
Terminators similar to those preceding B. subtilis sacB and the E. coli bgl operon have been found in front of many other transcription units encoding sugar-specific PTS components of the glucose/sucrose class. Mutation or partial deletion of the terminator sequences preceding the B. subtilis sacPA (24), bglPH (430), or ptsGHI (448) operon also led to the constitutive expression of the corresponding transcription unit and thus confirmed their negative role in mRNA synthesis.
RAT sequences are the binding sites for PTS-regulated antiterminators.
Sequencing of the bgl operon allowed the detection of a second terminator located in the intercistronic region between bglG and bglF. Interestingly, the sequences preceding the two bgl terminators are very similar. They were assumed to form weak stem-loops partly overlapping the downstream terminator (785). Insertion of 6 bp in the region upstream from the terminator preceding bglG indeed diminished the induction of a bgl-lacZ fusion (505), suggesting that the region upstream from this terminator is important for antitermination and might represent the binding site for BglG. The conserved sequences preceding the terminators were called ribonucleotidic antiterminator targets (RATs) (32). In in vitro experiments, BglG was indeed able to specifically bind to an RNA probe containing the entire E. coli bgl RAT sequence but only the 5′ half of the terminator (344). In contrast, if an RNA probe containing the entire RAT sequence and the complete terminator was used, very weak binding of BglG was observed. The formation of the terminator was therefore assumed to prevent the formation of the BglG binding site, i.e., the RAT stem-loop. In agreement with this concept, BglG binding to the longer RNA probe was improved when a DNA fragment, which was complementary to the 3′ part of the inverted repeat and which therefore prevented formation of the terminator, was included in the assay mixtures. These results suggested that there is competition between the formation of the terminator and the RAT stem-loop (344). Under noninducing conditions, BglG cannot bind to its mRNA, and the terminator will therefore be formed. Calculations revealed that the formation of the terminator releases much more free energy than the formation of the RAT stem-loop. Under inducing conditions, the binding of the “activated” BglG is assumed to favor the formation of the RAT stem-loop. BglG thereby prevents the formation of the terminator partly overlapping the RAT sequence and allows the synthesis of long transcripts (344).
In B. subtilis, sequences very similar to those of the E. coli bgl RATs precede the above-mentioned terminator sacR (833) and the terminators located in front of the B. subtilis 1,3-1,4-β-d-glucan endoglucanase-encoding bglS (formerly licS) gene (585, 785), the sacPA operon (164), which encodes a sucrose-specific EIIBC (sacP) and an endocellular sucrase (sacA) (32), and ptsG (847), which encodes the glucose-specific EIIBCA (Table 4). RAT sequences are also present in front of PRD-containing antiterminator-controlled genes from other organisms (964). An extensive genetic analysis of the RAT sequence preceding the sacB gene confirmed its importance for sacB induction. Expression of sacB′-lacZ fusions containing mutations at positions assumed to be important for the formation of the RAT stem-loop was barely inducible with sucrose (32).
The antiterminators regulating the expression of the four above-mentioned B. subtilis transcription units have been identified. SacY regulates sacB transcription (832), SacT controls the expression of sacPA (164), LicT is necessary for bglS but also for bglPH expression (430), and GlcT controls ptsGHI transcription (847) (Table 4). They all exhibit significant sequence similarity to BglG of E. coli. The new family of antiterminators was called the BglG/SacY family, as the B. subtilis sacB gene and the E. coli bgl operon, which are regulated by SacY and BglG, respectively, were the first transcription units for which this termination/antitermination mechanism was established.
The RAT sequences preceding sacB and sacPA differ in only three positions (Table 4), and consequently, cross talk has been observed: SacY also controls sacPA expression, and SacT regulates the transcription of sacB (32, 146). In gel shift experiments, not only SacT but also SacY could bind to RNA fragments containing the RAT sequence of sacPA (23). In vitro interactions of the RNA binding domains of LicT, SacY, and GlcT with their respective RAT sequences on bglPA, sacB (168), and ptsGHI (448) mRNA have been demonstrated by carrying out surface plasmon resonance experiments. The dissociation constants determined in these experiments varied from 3 μM (SacY) to 10 nM (LicT). The S. carnosus GlcT homolog regulates the expression of glcA and glcB, which encode a glucose- and a β-glucoside-specific EIICBA, respectively (131). The RAT sequence in the leader region of glcAB of S. carnosus strongly resembles the RAT sequence of B. subtilis ptsGHI. As a consequence, S. carnosus GlcT interacts with the RAT sequence of B. subtilis ptsGHI, as was shown by mutant complementation assays and surface plasmon resonance experiments (413). The high affinity between LicT and its bglPA RAT allowed the determination of the solution structure of the LicT catalytic domain:mRNA complex (964). The antiterminator interacts mainly with the minor groove of the double-stranded RNA formed by two internal loops and the stem in between. In summary, the above-described results make it clear that RAT sequences are the binding sites for BglG/SacY-type antiterminators.
In addition to regulating sacB expression, SacY controls its own synthesis by regulating the expression of the sacS locus containing the two genes sacX and sacY (993). The sacX gene encodes an EIIBC with strong similarity to sucrose-specific EIIs. It is preceded by a RAT sequence and a terminator, which are separated by about 100 bp, whereas in all other transcription units regulated by BglG/SacY-type antiterminators, the RAT sequence and the terminator are overlapping or located next to each other. Nevertheless, both regulatory elements are functional, as mutations within the RAT sequence prevent the induction of sacXY expression, while a partial or complete deletion of the terminator sequence causes constitutive expression (885). It was therefore proposed that the formation of the RAT stem-loop preceding sacXY is stabilized by binding SacY, which in turn prevents the formation of the distant terminator via not-further-specified long-range effects (885).
Phosphorylation of BglG/SacY-type antiterminators by PTS proteins.
After RAT sequences were identified as target sites for proteins of the BglG/SacY family, the signal controlling the affinity of the antiterminators for the RAT elements remained to be determined. The early studies on SacY-regulated sacB and BglG-controlled bgl operon expression had already suggested that these antiterminators might be controlled by the PTS. In the late 1970s, it was discovered that the inactivation of B. subtilis EI causes “constitutive” expression of sacB (i.e., synthesis of full-length mRNA) (264, 635). In fact, it was this property of the PTS that was used to successfully clone the B. subtilis ptsHI operon (283). A promoterless kanamycin resistance gene put under the control of the sacB promoter conferred sucrose-inducible kanamycin resistance after integration into the B. subtilis genome. After transposon mutagenesis was carried out, a strain expressing the kanamycin resistance gene in the absence of sucrose was isolated. The mutant had the transposon inserted into the ptsHI operon (283), thus confirming that ptsHI inactivation leads to “constitutive” expression from the sacB promoter.
Another study from the same year showed that the inactivation of E. coli bglF, the gene located downstream from bglG, caused “constitutive” expression of the bgl operon (i.e., synthesis of full-length mRNA) (504). Because bglF encodes a β-glucoside-specific EIIBCA (79, 785), it was concluded that BglG/SacY-type antiterminators might be controlled by a phosphorylation cascade formed by the general PTS proteins EI and HPr and those sugar-specific EIIAs and EIIBs, the synthesis of which is regulated by the antiterminator. In vitro phosphorylation of BglG by P∼EIIBCABgl was indeed reported (18). However, the regulation of antiterminators by PTS-catalyzed phosphorylation turned out to be very complex. First, EI and HPr were later found to be able to phosphorylate most antiterminators in the absence of EIIA and EIIB. Second, the activity of some antiterminators depends on functional EI and HPr, and the inactivation of ptsI therefore does not lead to the “constitutive” expression of the genes controlled by these antiterminators but, on the contrary, lowered or prevented their expression. Third, BglG/SacY-type antiterminators are usually phosphorylated at more than one of the four conserved histidyl residues in their PRDs. Extensive genetic, biochemical, and structural studies carried out mainly with the four B. subtilis antiterminators were necessary to understand in detail how BglG/SacY-type antiterminators are regulated.
In principle, two types of phosphorylation with antagonistic effects on the antiterminator activity have to be distinguished. P∼EIIB-requiring phosphorylation in PRD1 inactivates the antiterminator (site of negative regulation). The absence of phosphorylation in PRD1 leads to full-length expression of the corresponding transcription units. By contrast, phosphorylation by EI and HPr in PRD2 stimulates the activity of antiterminators (site of positive regulation). The absence of phosphorylation in PRD2 leads to CCR of the corresponding genes and operons via a CcpA-independent mechanism (Fig. 8).
Antiterminator-dependent induction is regulated via P∼EIIBs.
“Constitutive” expression from the promoter of an antiterminator-controlled operon following inactivation of the related EII has been observed not only for E. coli bglF (504) but also for several B. subtilis transcription units as well as for the lac operon of L. casei (289). In B. subtilis, disruption of sacX (147), bglP (430, 454), or ptsG (847) leads to inducer-independent expression of sacB, bglPH, and ptsGHI, respectively. As mentioned previously, B. subtilis ptsI mutants exhibit “constitutive” sacB transcription (264, 283, 635), and the deletion of ptsH was reported to lead to the synthesis of full-length ptsGHI mRNA (847). It therefore appeared that a functional phosphorylation cascade composed of the general PTS proteins EI and HPr and sugar-specific EIIAs and EIIBs leads to the inactivation of the corresponding antiterminator.
Interestingly, SacX, the inactivation of which also results in “constitutive” sacB expression (147), contains EIIB and EIIC but no EIIA domain, and it was therefore concluded that EIIB, the last link in the PTS phosphorylation chain (Fig. 1), regulates the induction of antiterminator-controlled transcription units. Experiments with the L. casei lac operon confirmed the importance of P∼EIIB for antiterminator-mediated induction, as the inactivation of lacE as well as lacF (encoding EIICBLac and EIIALac, respectively) led to the synthesis of full-length lacTEGF mRNA (289). A different mechanism had originally been proposed for the regulation of E. coli BglG, which was based on the erroneous assumption that His-306 of E. coli EIIBCABgl would be the phosphorylation site in the EIIB domain (784). The replacement of His-306 with nonphosphorylatable amino acids prevents β-glucoside transport but, in contrast to bglF disruption, does not lead to the synthesis of full-length bgl mRNA. It was therefore proposed that EIIA and not EIIB of EIIBCABgl would regulate BglG activity, and EIIAGlc was also suggested to phosphorylate and inhibit BglG (783). This concept proved to be wrong when the phosphorylation site in the EIIB domain of E. coli EIICBGlc was identified as being Cys-421 (547), which is equivalent to Cys-24 in EIIBCABgl. A strain containing a bglF(Cys24Ser) mutation not only was unable to transport β-glucosides but also expressed a bgl-lacZ fusion in the absence of an inducer (β-glucoside) (120), confirming that E. coli BglG is also regulated via the EIIB domain of the corresponding PTS permease.
P∼EIIBs are necessary for the phosphorylation of a histidine in PRD1.
To determine which of the usually four conserved histidyl residues in the PRDs would be the target for P∼EIIB-mediated regulation, extensive genetic analyses were carried out by replacing the histidyl residues in the B. subtilis antiterminators SacY and LicT with various amino acids and by measuring the effect of the mutations on reporter gene fusions in different genetic backgrounds. Replacement of His-99 in PRD1 of SacY with a Tyr (883) or Ala (884) causes constitutive expression of the sacB gene. In contrast, replacing the three other conserved histidyl residues in SacY with Tyr has little effect on the antitermination activity (883). Similarly, replacement of His-100 in B. subtilis LicT (884) or His-101 in L. casei LacT (288), the equivalents of His-99 of SacY, with alanine leads to the synthesis of full-length bglPH or lacTEGF mRNA, respectively. These results strongly suggest that phosphorylation of the first conserved histidyl residue in PRD1 by the corresponding P∼EIIB prevents induction by PRD-containing antiterminators. Attempts to mimic phosphorylated antiterminators by replacing the first phosphorylatable histidine in PRD1 with negatively charged Asp or Glu were only partly successful. Putting a negative charge at position 99 by replacing His-99 with Glu or Asp indeed inactivates SacY and abolishes sacB induction (884). Similarly, the His101Asp replacement in L. casei LacT prevents lac operon expression (288). However, although His100Asp LicT exhibited significantly lower activity than His100Ala LicT, it was not completely inactive (884), indicating that in LicT, the His100Asp replacement is not completely phosphomimetic. Moreover, replacement of His-104 with an Asp in B. subtilis GlcT even led to strongly “constitutive” expression of a ptsG′-′lacZ fusion (35), suggesting that the mutant protein does not at all resemble GlcT phosphorylated at the first conserved histidine in its PRD1.
The original concept for the regulation of BglG/SacY-type antiterminators implied that P∼EIIBs directly phosphorylate and thereby inactivate their corresponding antiterminator (18). The finding that the first conserved histidine in PRD1 of several antiterminators is phosphorylated in vitro by P∼His-HPr therefore came as a surprise (413, 781, 883, 884). For instance, His-99, the site of negative regulation in B. subtilis SacY, is efficiently phosphorylated in vitro by EI and HPr in the absence of SacX (883). Because P∼His-HPr is also necessary for the phosphorylation of EIIBs, it was not clear whether P∼EIIBs directly phosphorylate their corresponding antiterminators or merely stimulate the P∼His-HPr-mediated phosphorylation. It proved difficult to distinguish between the two possibilities, as it was not easy to obtain P∼EIIB preparations that were free of HPr (EIIBs are often fused to their membrane-integrated EIICs, and HPr usually sticks to membranes). Because the inactivation of EI as well as SacX leads to constitutive expression of the SacY-controlled sacB gene, it was proposed that in the cells, efficient P∼His-HPr-mediated phosphorylation of SacY at His-99 would require the presence of P∼SacX. GlcT of S. carnosus also becomes phosphorylated in vitro by EI and HPr in the absence of an EIIB, and mass spectrometry with proteolytic fragments obtained from phosphorylated and dephospho-GlcT revealed that phosphorylation occurs primarily at His-105 (413), the equivalent of His-99 in SacY. In the case of S. carnosus GlcT, phosphorylation in PRD1 was assumed to stimulate the antitermination activity. In contrast, in experiments with GlcT from B. subtilis, P∼His-HPr barely phosphorylated PRD1 but mainly transferred its phosphoryl group to His-210 in PRD2 (781) (for PRD2 phosphorylation, see the next section). In fact, the first conserved histidine in PRD1 of B. subtilis GlcT has been shown to become phosphorylated by P∼EIIBGlc. When the soluble B. subtilis EIIBAGlc domains were synthesized without the membrane-integrated EIICGlc (to which they are normally fused), HPr-free P∼EIIBAGlc could be prepared, which could phosphorylate wild-type GlcT but not GlcT(His104Ala). The antiterminator became phosphorylated in PRD2 only when HPr was added to the phosphorylation mixture containing P∼EIIBAGlc and GlcT(His104Ala), thus confirming the nearly complete absence of HPr from the P∼EIIBAGlc preparation (781). It therefore seems that phosphorylation of some antiterminators (B. subtilis SacY and S. carnosus GlcT) at PRD1 is catalyzed by P∼His-HPr, and this reaction is stimulated by the corresponding P∼EIIB, while phosphorylation in PRD1 of other antiterminators (GlcT of B. subtilis) occurs via the corresponding P∼EIIB.
Based on genetic studies with licT, it was suggested that P∼EIIB domains might also sequester their corresponding antiterminator (884). A similar mechanism is operative for the E. coli transcription regulator Mlc, which is sequestered by the unphosphorylated EIIB domain of EIICBGlc (458, 588, 856) (see “REGULATION OF CARBON METABOLISM IN GRAM-NEGATIVE ENTERIC BACTERIA”). Preliminary evidence for the formation of a complex between an antiterminator and an EII was obtained for S. carnosus GlcT, which exists as an active dimer and an inactive monomer. Dimerization is favored by P∼His-HPr-mediated phosphorylation at His-105. Membrane fragments containing EIICBAGlc (and possibly P∼EIICBAGlc) specifically interacted only with the dimers in a GlcT monomer/dimer mixture (413). Calorimetric studies had established that the binding of sugar molecules to their respective EIIs, which leads to the dephosphorylation of the EIIB domain, causes extensive structural changes (546). In the case of S. carnosus EIICBAGlc, the structural changes accompanying the binding of glucose were assumed to lead to a release of active P∼GlcT dimers, thus allowing the expression of the glcAB operon in response to the presence of a substrate.
Unlike antiterminators from B. subtilis and L. casei, the negative regulation site of BglG from E. coli was originally thought to be located in PRD2, and His-208 was reported to be phosphorylated by P∼EIIBCABgl (121). However, the phosphorylation mixtures for BglG phosphorylation contained not only P∼EIIBCABgl but also EI and HPr. Like most antiterminators, BglG is phosphorylated by EI and HPr in PRD2 (287) (see the next section), and Chen et al. (121) probably misinterpreted the P∼His-HPr-dependent modification as being P∼EIIBCABgl-dependent phosphorylation. In fact, the negative regulation site of E. coli BglG has been identified as His-101, and phosphorylation at this amino acid requires the presence of P∼EIIBCABgl (285).
In summary, it can be concluded that induction via most BglG/SacY-type antiterminators is regulated by P∼EIIB-controlled or -mediated phosphorylation of the first conserved histidyl residue in PRD1, which inhibits the activity of the antiterminator. In the presence of the corresponding inducer, i.e., the carbohydrate transported by (or, in the case of B. subtilis, SacX probably bound to) the EII regulating the antiterminator, the antiterminator will barely be phosphorylated at its first conserved histidine in PRD1, because phosphorylation of the inducer is probably faster than phosphorylation of the antiterminator (482) or rephosphorylation of the EIIB by its corresponding P∼EIIA (Fig. 8). For B. subtilis SacY, the presence of sucrose in the growth medium indeed significantly reduced the extent of its in vivo phosphorylation (359). Antiterminator molecules that are not phosphorylated at the first conserved histidine in PRD1 are able to bind to their RAT sequence, thus preventing the formation of the terminator and allowing the synthesis of full-length mRNA of their target gene or operon. If the inducer is absent, P∼EIIB will be formed, which leads to phosphorylation of the first histidine in PRD1 and/or sequestration of the corresponding antiterminator. Both effects prevent the binding of the antiterminator to its RAT sequence and therefore the induction of the corresponding gene or operon. According to genetic analyses with licT of B. subtilis (884) and bglG of E. coli (285), phosphorylation at the second conserved histidine in PRD1 plays a detectable but minor role in the induction process. By contrast, replacing either His-101 or His-159 in L. casei LacT with alanine leads to “constitutive” expression of the lac operon, suggesting that in this antiterminator, the two conserved histidines in PRD1 contribute equally to the induction process (288).
Some antiterminators need to be activated by phosphorylation in PRD2.
Based on the effect of ptsI or ptsH mutations on the activity of BglG/SacY-type antiterminators, two classes of transcription attenuators can be distinguished. The first class includes B. subtilis SacY and GlcT. Mutations in ptsI or ptsH result in the “constitutive” expression of their target genes sacB (147, 264, 635) and ptsGHI (847), respectively. In these mutants, neither P∼His-HPr nor P∼EIIB is formed, which prevents the inactivating phosphorylation at PRD1 of the antiterminator in the absence of an inducer. The second class of antiterminators includes B. subtilis SacT and LicT and E. coli BglG. The synthesis of the full-length mRNA of their target genes sacPA (24) and bglPH in B. subtilis (429, 430, 454, 482) and bglGFB in E. coli (287) is almost completely prevented when ptsH or ptsI is inactivated. These results were surprising, as we had learned in the previous section that the inactivation of, for example, the EIIBCABgl-encoding bglP leads to the synthesis of full-length mRNA from the LicT-controlled bglPH promoter (430, 454). Because EI and HPr are essential for the phosphorylation of EIIBCABgl, which in turn inactivates LicT by phosphorylating it at His-100 in PRD1, the disruption of ptsH or ptsI should also lead to the synthesis of full-length bglPH mRNA. The observed dependence of LicT, SacT, and BglG activity on functional EI and HPr therefore suggested that the second class of antiterminators is subject to dual control by PTS proteins: positive regulation by P∼His-HPr and negative control by their corresponding P∼EIIBs. Because the specific replacement of His-15, the site of EI-catalyzed phosphorylation in HPr, with an alanine also prevented LicT activity (429), it was likely that EI and HPr exert their positive effect by phosphorylating this class of antiterminators. Indeed, SacT (23), LicT (482), and BglG (287) are phosphorylated by PEP, EI, and HPr. In LicT, all four conserved histidines are phosphorylated by the general PTS proteins, with the main site of phosphorylation being His-207 in PRD2 (482, 884). Because the P∼EIIB-dependent negative effect is mediated via the first conserved His in PRD1 (see the previous section), it was likely that the positive effect of phosphorylation by EI and HPr is mediated via PRD2. Experiments with SacY/LicT hybrid proteins confirmed this assumption. When PRD2 of the EI/HPr-independent SacY was replaced with PRD2 of LicT, the resulting hybrid protein was EI and HPr dependent (884). This concept was further supported by an extensive genetic analysis, during which the conserved histidines in PRD2 of B. subtilis LicT were replaced with various amino acids and the effect of the mutations on bglP′-lacZ expression was measured in different pts genetic backgrounds. Replacement of His-207 or His-269 or both conserved histidines in PRD2 with Ala led to a complete loss of LicT antitermination activity, i.e., prevented the synthesis of full-length mRNA from the bglPH promoter even in the presence of its inducer salicin or in a bglP background. In contrast, in a bglP mutant, if either one of these two histidines or both were replaced with an Asp, which probably mimics a phosphorylated histidine, expression from the bglPH promoter remained “constitutive” irrespective of whether ptsHI were functional or deleted (884). These results demonstrate that PEP-, EI-, and HPr-mediated phosphorylation of PRD2 in antiterminators of the second class stimulates their activity. This is also true for E. coli BglG, where His-208, the only conserved phosphorylatable histidine in PRD2, was first claimed to be the site of negative regulation by P∼EIIBCABgl (121) but was later identified as the site of positive regulation by P∼His-HPr (285, 287). Interestingly, E. coli FruB, a fusion protein composed of HPr- and EIIAFru-like domains (267), can replace HPr in BglG phosphorylation and activation (287).
CCR mediated by dephosphorylation of PRD2 of antiterminators.
Surprisingly, although the activity of B. subtilis SacY and GlcT does not depend on EI and HPr, they are phosphorylated by PEP, EI, and HPr in their PRD2 (781, 883). It seems that during the course of evolution, SacY and GlcT developed a PTS-independent antitermination activity but retained the ability to become phosphorylated in PRD2. On the contrary, GlcT of S. carnosus, which is probably also EI/HPr independent, is not phosphorylated in its PRD2, as only one major P∼His-HPr-dependent phosphorylation site, His-105 in PRD1, could be detected (413). Interestingly, EI/HPr-independent antiterminators seem to regulate the expression of transcription units that are insensitive to CCR, whereas the EI/HPr-dependent LicT and SacT control operons that are preceded by cre's recognized by the P-Ser-HPr:CcpA complex (429). It was therefore assumed that phosphorylation of LicT and SacT by PEP, EI, and HPr might serve as an additional, CcpA-independent CCR mechanism.
The first evidence for antiterminator-mediated CCR came from studies with a B. subtilis ccpA mutant. In this mutant, expression of a bglP′-lacZ fusion was not completely relieved from CCR. The residual CCR (about 20%) disappeared when the terminator preceding the bglPH operon was deleted, suggesting that bglPH is regulated by two CCR mechanisms: one that is CcpA dependent and one that is terminator dependent (429). Interestingly, the residual CCR also disappeared when a ptsH1 mutation (which prevents the formation of P-Ser-HPr) was introduced into the ccpA mutant. It was therefore assumed that the CcpA-independent CCR mechanism operative for the bglPH operon was based on diminished phosphorylation in PRD2 of LicT due to the formation of P-Ser-HPr (a poor substrate for PEP-dependent phosphorylation by EI) during growth on a rapidly metabolizable carbon source. This assumption was supported by studies with B. subtilis mutants producing LicTs in which either one of the two conserved histidines in PRD2 was replaced with Asp. Expression of a bglP′-lacZ fusion remained inducible in both mutants, but β-galactosidase activity was less severely repressed by glucose than in a strain producing wild-type LicT (909). Unequivocal proof for the antiterminator-mediated CCR mechanism came from studies with strains producing EI/HPr-independent mutant LicTs, which were called LicT(Pia) (Pia stands for PTS-independent antitermination). Starting from a strain carrying a bglPH′-aphA3 (aphA3 confers kanamycin resistance to bacteria) and a bglP′-lacZ fusion as well as the LicT-inactivating ptsGHI deletion, several spontaneous mutants that had gained the ability to express the LicT-dependent bgl fusions despite the absence of functional EI and HPr were isolated (483). The isolated mutants were all affected in licT, and the various mutations apparently render LicT activity independent of functional EI and HPr. The licT(Pia) mutants can be divided into two classes: mutations affecting PRD1 (they lead to constitutive expression from the bglPH promoter) and mutations affecting PRD2 (they remain inducible). The latter type of mutant LicTs resembles the naturally EI/HPr-independent SacY and GlcT. Interestingly, the residual CCR of the bglPH operon detectable in ccpA mutants disappeared when wild-type licT was replaced with one of the licT(Pia) alleles (483), thus confirming that it is the poor phosphorylation of PRD2 in LicT during the uptake of rapidly metabolizable carbon sources that triggers CcpA-independent CCR.
Based on the results obtained with the various LicT(Pia) proteins, distinct amino acids were proposed to be important for the transfer of the activating signal from PRD2 via PRD1 to the RNA binding domain (483), which was called CAT (coantiterminator) (964). When the crystal structures of the two PRDs of the constitutively active His207Asp, His269Asp mutant LicT (909) and of inactive wild-type LicT (292) were solved, a more detailed understanding of the structural changes involved in LicT activation was possible. In fact, the structures of the PRD2 of the two crystallized LicT forms are completely different, while the amino acid backbones of their PRD1s are almost identical. In inactive wild-type LicT, the two PRD2s of the LicT dimer make no contact to each other but are in contact with the two PRD1s. The four phosphorylatable histidines in the PRD2s are exposed to the surface and easily accessible for phosphorylation. In the activated His207Asp, His269Asp mutant LicT, a 180° swing movement of the C-terminal domain leads to extensive structural changes, placing the aspartyl residues (which replace the four histidines) in the center of PRD2, thus rendering them inaccessible from the outside. Two major rotations are responsible for the rearrangement of the PRD2s. Both take place in the interdomain region between PRD1 and PRD2, with one occurring at residues Leu-165 and Asn-166 and the other occurring at Met-169. As a consequence, the two PRD2s of His207Asp, His269Asp mutant LicT are in close contact, whereas most interactions with PRD1 are suspended. These alterations are probably responsible for the changes in the interdomain region connecting PRD1 and CAT, which in turn are thought to cause rearrangements of the CAT domain, ultimately leading to the activation of LicT (292). The monomer/dimer transition does not seem to be important for LicT activation, as the RNA binding domain CAT and active His207Asp, His269Asp mutant LicT as well as inactive dephospho-LicT form dimers (964). Nevertheless, the monomer/dimer transition might be important for the regulation of other antiterminators (56).
The structure of the RNA binding domain of the two antiterminators SacY and LicT has been intensively studied by NMR (510) and X-ray crystallography (910). The solution structure of the RNA binding domain of LicT (first 55 amino acids) attached to its mRNA has also been solved (964). However, the structure of an entire BglG/SacY-type antiterminator has so far not been determined.
Based on the above-described results, the following model for CCR mediated by EI/HPr-dependent antiterminators can be proposed. In the presence of a carbon source rapidly transported via the PTS, the antiterminators will have to compete with the corresponding sugar-specific EIIA for the common phosphoryl donor P∼His-HPr. P∼His-HPr probably transfers its phosphoryl group primarily to EIIAs, thus leaving the antiterminators in the unphosphorylated, less active state. In in vitro experiments, P∼His-HPr indeed phosphorylates EIIAs much faster than antiterminators (482). In addition, the ATP-dependent phosphorylation of HPr at Ser-46 in low-G+C gram-positive bacteria, which is triggered by the presence of rapidly metabolizable carbon sources, drastically slows the PEP-dependent phosphorylation of HPr at His-15 and therefore further diminishes the activation of LicT by P∼His-HPr-dependent phosphorylation. The proposed complex mechanism of induction and CCR of operons controlled by EI/HPr-dependent antiterminators is outlined in Fig. 8. The model requires that during PTS sugar uptake, the corresponding EIIB domain is present mainly in the dephospho form and therefore does not phosphorylate the first conserved His in PRD1 of LicT. On the other hand, P∼His-HPr must be present in sufficient amounts to allow the phosphorylation in PRD2, which implies that PTS operons, the expression of which is regulated by an EI- and HPr-dependent antiterminator, encode low-capacity transport systems, as strong autorepression would otherwise occur. Probably in order to prevent autorepression, the operons for the B. subtilis high-capacity glucose and sucrose PTS are regulated by EI- and HPr-independent antiterminators.
Distribution of BglG/SacY-type antiterminators in bacteria.
In a several-years-old review on PRD-containing transcription regulators, the BglG/SacY antiterminator family was reported to contain 13 members (844). In addition to the above-mentioned antiterminators, it included LicT of Bacillus amyloliquefaciens, AbgG of Clostridium longisporum (87), ArbG of Pectobacterium chrysanthemi (formerly Erwinia chrysanthemi) (208), BglR of L. lactis (42), CasR of Klebsiella oxytoca (446), and SurT of G. stearothermophilus (473). Owing to the many genome sequences completed in the meantime, a simple BLAST search yields numerous new potential family members. A few of these novel PRD-containing antiterminators have been studied in more detail. BvrA of L. monocytogenes controls the expression of the bvrABC operon encoding the antiterminator, a β-glucoside-specific EIIBCA, and a presumed ADP-ribosylglycohydrolase (81), respectively. In L. monocytogenes, repression of several PrfA-controlled virulence genes by the β-glucosides cellobiose and salicin requires an intact bvr locus, while repression by arbutin, glucose, or fructose persists in a bvrAB mutant. BglG of L. plantarum was reported to control the expression of the bglGPT operon encoding the antiterminator BglG, a β-glucoside-specific EII, and a 6-P-β-glucosidase (513), while LicT of S. mutans regulates an esculin-specific PTS (141). C. acetobutylicum ScrT was proposed to control the expression of the scrAKB operon encoding a sucrose-specific EII, a fructokinase, and a sucrose-6-P hydrolase, respectively (860). In the actinobacterium Corynebacterium diphtheriae, a BglG/SacY-type antiterminator-encoding gene is located downstream from the EIIBCAGlc-encoding ptsG gene (631). BglT of Pectobacterium carotovorum probably controls the expression of an operon that encodes a β-glucoside-specific PTS and strongly resembles the arb operon of P. chrysanthemi (19). BglG/SacY-type antiterminators are especially abundant in clostridia. With nine BglG/SacY homologs, Clostridium difficile seems to be the organism that contains the highest number of PRD-containing antiterminators, followed by L. plantarum, which contains five such proteins. In fact, BglG/SacY-type antiterminators are present in most firmicutes including bacilli, clostridia, enterococci, lactobacilli, lactococci, listeriae, leuconostocs, staphylococci, streptococci, etc. BglG/SacY-type antiterminators occur less frequently in gram-negative bacteria. So far, in addition to E. coli, PRD-containing antiterminators have been detected in proteobacteria such as P. carotovorum (BglT) (19), P. chrysanthemi (ArbG) (208), Klebsiella oxytoca (CasR) (446), Klebsiella aerogenes (BglG) (690), Shigella flexneri, Shigella sonnei, Shigella boydii, Photorhabdus luminescens, and Yersinia enterocolitica. Interestingly, a gene coding for a BglG/SacY-type antiterminator is also present in the pathogenicity island of a uropathogenic E. coli strain (393). It is preceded by a gene encoding an EIICB resembling the glucose-specific EII (PtsG) of E. coli. However, whether this system is related to the virulence of this organism is not known. BglG/SacY homologs are also present in the actinobacteria Bifidobacterium longum, Corynebacterium diphtheriae, Propionibacterium acnes, and Symbiobacterium thermophilum. The antiterminator-controlled expression of a fructose PTS in Bifidobacterium breve has been studied in more detail (534a).
Regulation of Transcription Activators by PTS-Mediated Phosphorylation
PRDs in transcription activators.
Transcription activators are DNA binding proteins usually containing a helix-turn-helix motif. They bind upstream from their target promoter, interact with a specific RNA polymerase holoenzyme, and thereby stimulate transcription. Based on the σ factor associated with the RNA polymerase, different classes of transcription activators can be distinguished. The first class comprises NifA/NtrC-type transcription activators. They usually interact with RNA polymerase containing σ54 (also called RpoN in E. coli and SigL in B. subtilis). They are composed of an N-terminal regulatory domain, which is usually a receiver domain of a two-component system, a central domain interacting with σ54, and a C-terminal helix-turn-helix motif. A nucleotide binding site (Walker motifs A and B) is present in the central domain. In the case of NtrC, phosphorylation of its receiver domain by the sensor histidine kinase NtrB (396, 608) strongly stimulates its ATPase activity, which allows NtrC to enhance the formation of open complexes by the RNA polymerase/σ54 holoenzyme (943). NifA/NtrC-type transcription activators bind to the DNA at about 100 bp upstream from their target promoters, and DNA bending is necessary to allow an interaction with σ54 (705). In a few cases, the binding site for the transcription activator is located even more than 1 kb away from the target promoter (698, 809). Owing to their mode of action, these transcription activators are also called enhancer DNA binding proteins (EBP).
A second class of transcription activators interacts with the major σ70-containing RNA polymerase holoenzyme. This class is very large and heterogenous and includes regulator proteins, which, depending on the location of their binding site, function as transcription activators (binding upstream from the promoter) and/or repressors (the binding site overlaps the promoter or is located downstream from it). This class includes the DeoR family of transcription regulators containing repressors such as E. coli GlpR (790) and SrlR (formerly GutR) (961), B. subtilis IolR (981), and L. lactis LacR (986) but also transcription activators such as E. coli FucR (125), B. anthracis AcpA (414), and S. pyogenes VirR (537). Often, DeoR-like transcription regulators do not contain a clearly identifiable nucleotide binding site. Recognition of their target sequence is controlled by low-molecular-weight effectors such as sugar phosphates, which interact with the C-terminal ligand-binding domain.
Several transcription activators belonging to one of the above-described classes are regulated neither by ligand-binding (DeoR type) nor via phosphorylation by a sensor kinase (NifA/NtrC type) but instead possess PRDs, suggesting that their activity is controlled by PEP-dependent, PTS-catalyzed phosphorylation. PRDs thus seem to be used not only to regulate the RNA binding activity of antiterminators but also to control the DNA binding function of some members of the above-described classes of transcription activators (Fig. 7). Interestingly, all PRD-containing transcription activators also possess an EIIA and an EIIB domain. The EIIB domain in transcription activators always exhibits similarity to EIIBs of the galactitol class PTS (295). In contrast, the EIIA domain belongs to the mannose class PTS in regulators with an NifA/NtrC-like σ54 binding domain (178) and to the mannitol/fructose class PTS in regulators with a DeoR-like DNA binding domain. The type of EIIA domain present in transcription regulators seems to be related to the specificity of the PTS that they control. Three cases can be distinguished: (i) transcription activators containing NifA/NtrC- and EIIAMan-like domains control the expression of operons coding for PTSs belonging to the mannose or lactose class; (ii) transcription activators with DeoR- and EIIAMtl-like domains regulate the expression of operons coding for PTSs of the mannitol, lactose, or glucitol class; and (iii) PRD-containing antiterminators (see the previous section) are devoid of an EIIA domain and primarily regulate the expression of operons coding for PTSs belonging to the glucose/sucrose class but also several PTSs of the lactose class.
Domain organization in NifA/NtrC-type PRD-containing transcription activators.
The best-studied PTS-controlled transcription activator is B. subtilis LevR, which regulates the expression of the lev operon (165). The levR gene is located just upstream from the lev operon, which encodes the EIIs for a low-capacity fructose/mannose-specific PTS (LevD, LevE, LevF, and LevG) and an extracellular levanase (SacC) capable of degrading fructose polymers such as levan (110, 519, 522). Expression of the lev operon occurs from a σ54 (σL)-dependent −12, −24 promoter, is induced by low concentrations of fructose, and is repressed by rapidly metabolizable carbon sources. Although the Lev PTS transports fructose, high concentrations of this sugar repress lev operon expression, because B. subtilis also possesses a high-capacity fructose PTS encoded by the fruA gene (437).
LevR stimulates the activity of the RNA polymerase/σ54 holoenzyme after binding to a UAS, which was identified by footprint experiments (521) and is centered at about 110 bp upstream from the lev promoter. LevR contains an N-terminal helix-turn-helix motif (positions 34 to 54) (521) and thus differs from NifA/NtrC-type transcription activators with a receiver domain of two-component systems, which possess the DNA binding motif at the C terminus. The second domain of LevR resembles the central domain in NifA/NtrC-type transcription activators (165). This domain probably interacts with RNA polymerase associated with the alternate sigma factor σ54 and contains Walker motifs A (148-GPTGSGKS-155) and B (221-GILFMDEI-228) for ATP binding and hydrolysis. In LevR, the NifA/NtrC-like central domain is followed by a complete PRD. A second, truncated PRD containing only one conserved histidyl residue is located at the C terminus of LevR (see Fig. 7 for the domain structure of LevR).
The region separating the complete and the truncated PRD of LevR (from positions 575 to 820) is much longer than that in BglG/SacY-type antiterminators. A BLAST search with the sequence separating the two PRDs in LevR revealed that the region from amino acids 578 to 707 exhibits significant similarity to EIIAs of the mannose class PTS (178) (Fig. 7). A histidine equivalent to the phosphorylatable histidine of E. coli EIIAMan (110, 840) is present in position 585. The strongest similarity was detected with EIIAMan of Vibrio furnissii. In addition, the sequence between the EIIAMan-like domain and the truncated PRD from positions 708 to 807 was found to exhibit significant similarity to EIIBs from the galactitol class PTS (GatB, SgaB, and SgcB of E. coli) (295). These EIIBs contain a conserved cysteyl residue close to the N terminus, and an equivalent cysteyl residue is present in the EIIB-like domain of LevR at position 718. It therefore seems that LevR evolved by numerous domain fusions and insertions. It is tempting to assume that the two PRDs were added to the central domain of a NifA/NtrC-type regulator before the EIIA and EIIB domains were inserted between the PRDs. LevR contains a total of five potential PTS phosphorylation sites, two in PRD1, one in the EIIA and EIIB domains, and one in PRD2 (Fig. 7).
Interestingly, the L. casei lev operon (levABCDX) is not preceded by a −12, −24 promoter but is preceded by a −10, −35 promoter. It was therefore assumed that L. casei LevR, which controls the expression of the lev operon of this organism, does not interact with σ54. Indeed, while the disruption of levR strongly diminished lev operon expression, inactivation of the σ54-encoding rpoN gene had no effect (534). In addition, the amino acid sequence motif -GAFTGA-, known to be essential for the interaction of NifA/NtrC-type EBPs with σ54, is absent from the central domain of L. casei LevR. Nevertheless, the domain organization of L. casei LevR is identical to that in B. subtilis LevR, and the two proteins exhibit 41% sequence similarity. So far, L. casei LevR is the only known protein of this family that does not interact with σ54 but that interacts with another σ factor (presumably σ70).
Antagonistic effects of PTS-mediated phosphorylation reactions on LevR activity.
The binding of B. subtilis LevR to its UAS and/or its interaction with σ54 was thought to be regulated by PTS-catalyzed phosphorylation (165). This assumption was based on the observation that mutations affecting LevD or LevE, the fructose-specific EIIA and EIIB of the Lev PTS, caused strong constitutive expression from the lev promoter (520, 522). Mutants carrying deletions of the EI- and HPr-encoding ptsI and ptsH genes or synthesizing His15Ala or Ser46Asp mutant HPr (the latter is a poor substrate for EI) also exhibit elevated LevR activity, which, however, is considerably lower than that in levD (EIIALev) and levE (EIIBLev) mutants (526, 846). Deletion of ptsHI in a levD or levE strain diminished the constitutive LevR activity, indicating that EI and HPr exert a positive effect on LevR function (846). These results suggest that similar to the second, PTS-dependent class of BglG/SacY-type antiterminators, LevR might also be regulated by multiple PTS-catalyzed phosphorylations in its PRDs and possibly also in the EIIA and EIIB domains (Table 1).
Indeed, PEP-dependent, EI- and HPr-mediated phosphorylation of B. subtilis LevR occurs at His-585, which was originally thought to be part of PRD1 (520) but was later identified as the phosphorylatable His in the EIIAMan domain (178). His-488 of L. casei LevR is equivalent to His-585 of B. subtilis LevR and was accordingly identified as a phosphorylation site by P∼His-HPr (534). Replacement of this phosphorylatable His in LevR of B. subtilis or L. casei with an alanine diminishes lev operon expression, thus supporting the concept that EI/HPr-catalyzed phosphorylation exerts a positive effect on the transcription activation function of LevR (520, 534).
A second PEP-dependent phosphorylation requiring EIIALev and EIIBLev (in addition to EI and HPr) occurs at the single conserved histidyl residue present in the C-terminally truncated PRD: His-869 in B. subtilis LevR (520) and His-776 in L. casei LevR (534). Experiments with B. subtilis [32P]∼EIIBLev, which had been separated from EI, HPr, and EIIALev after its phosphorylation by these proteins, strongly suggested that P∼LevE directly phosphorylates LevR at His-869 (520). The fact that His869Ala mutant LevR was not phosphorylated by the [32P]∼EIIBLev preparation proved that it was almost free of [32P]∼His-HPr. Inactivation of levD (EIIALev) and levE (EIIBLev) as well as the replacement of His-869 of B. subtilis LevR (or His-776 of L. casei LevR) with an alanine cause constitutive expression from the lev promoter of both organisms. These results confirm that phosphorylation in PRD2 mediated by PEP, EI, HPr, EIIALev, and EIIBLev exerts an inhibitory effect on the transcription activation function of LevR.
Mutation of the first conserved histidine (His-506) in PRD1 also slightly affects B. subtilis LevR activity. However, B. subtilis and L. casei mutant LevRs in which both identified phosphorylatable histidines had been replaced with alanine were not phosphorylated in vitro either by EI and HPr or by EI, HPr, EIIALev, and EIIBLev (520, 534). These results suggest that in wild-type LevR, neither the two conserved histidyl residues in PRD1 nor the conserved cysteyl residue in the EIIBGat domain is phosphorylated.
The results described above establish that the transcription activator LevR in B. subtilis and L. casei is regulated by at least two PTS-catalyzed phosphorylation reactions exerting antagonistic effects on LevR activity. Control of LevR therefore appears to be a complex kinetic phenomenon similar to LicT regulation. Although the kinetics of the phosphoryl group transfer reactions are barely understood, a model can be proposed on the basis of the above-mentioned biochemical and genetic results. In in vitro experiments, B. subtilis P∼EIIBLev phosphorylates not only fructose (110) but also His-869 in PRD2 of LevR (520). According to genetic data, the latter reaction inhibits LevR activity, and it is therefore likely that the induction of the lev operon requires the dephosphorylation of His-869. In fact, the uptake of fructose via the Lev PTS will lead to the dephosphorylation of not only P∼EIIBLev but, as a consequence of the reversibility of the phosphoryl transfer, probably also P∼His(869)-LevR. Because the phosphorylation of HPr by EI is very fast and because the Lev-PTS is a low-capacity fructose transport system, one can assume that cells transporting fructose via the Lev-PTS probably contain relatively high concentrations of P∼His-HPr, which are probably sufficient for the phosphorylation of LevR at His-585 in the EIIAMan-like domain. As a consequence, during Lev-PTS-catalyzed fructose uptake, a major fraction of LevR will probably be present in the fully active form, i.e., phosphorylated at His-585 and dephosphorylated at His-869. Dephosphorylation of His-585 will occur when a rapidly metabolizable carbon source, such as glucose, is present in the growth medium. The uptake of glucose will lower the P∼His-HPr level and, consequently, the activity of LevR, even when it is dephosphorylated at His-869. Indeed, LevR activity in levD ΔptsH or levE ΔptsH double mutants was reported to be about 15-fold lower than that in levD or levE single mutants (846). Poor phosphorylation of His-585 during growth on a rapidly metabolizable carbon source serves as a CcpA-independent CCR mechanism and accounts for the residual CCR, which was observed when a ccpA disruption was introduced into an inducer-independent mutant (levE or levR8). The residual CCR almost completely disappeared in a ccpA ptsH1 double mutant (526), which, due to the inability to form P-Ser-HPr, probably contains a larger amount of P∼His-HPr.
Because LevR contains a NifA/NtrC-like central domain, it is likely that LevR activates transcription by a mechanism similar to that operating in non-PRD-containing NifA/NtrC-type transcription activators. In the latter proteins, phosphorylation of the receiver domain by a sensor kinase stimulates the ATPase function located in the central domain and, consequently, the formation of open complexes by RNA polymerase (943). As mentioned above, the central domain of LevR also contains a nucleotide binding site. It is therefore tempting to assume that phosphorylation of the EIIAMan-like domain by EI and HPr leads to increased LevR ATPase activity, while phosphorylation in PRD2 inhibits it. Nevertheless, how the inactivating/activating signals are transmitted from PRD2 and the EIIAMan-like domain, respectively, to the NifA/NtrC-like central domain remains an open question. The structure of LevR or one of its homologs has so far not been solved.
Distribution of LevR-like transcription activators.
For a long time, the only known homolog of LevR was CelR from G. stearothermophilus (445). CelR has the same regulatory domain organization as B. subtilis LevR (Fig. 7), to which it exhibits 47% sequence similarity. Histidyl residues equivalent to the two phosphorylation sites in B. subtilis LevR (His-585 and His-869) are also present in CelR. The celR gene precedes an operon encoding a cellobiose-specific PTS (lactose class) and a presumed 6-P-β-glucosidase (446). CelR was therefore assumed to control the expression of this operon by a mechanism similar to that used by LevR for the regulation of the B. subtilis lev operon.
The L. monocytogenes lmo1721 gene encodes a LevR-like regulator (called CsrA, but it is unrelated to E. coli CsrA), the inactivation of which prevents the repressive effect of cellobiose on the activity of PrfA, a transcription activator of numerous virulence genes in this organism, while the repressive effect of glucose on PrfA persisted in a csrA mutant (558a). The csrA gene precedes and probably regulates an operon containing a potential −12, −24 promoter and three genes, which code for an EIIA and an EIIB of a lactose/cellobiose class PTS and an outer surface protein. However, this operon is missing an EIIC, and it is therefore not clear whether the EIIA and EIIB are involved in cellobiose transport. Interestingly, L. monocytogenes contains an operon encoding a complete potential cellobiose-specific PTS (EIIA, EIIB, and EIIC, lmo2683 to lmo2685), but no regulator gene precedes or follows this operon. Nevertheless, this operon is preceded by a potential −12, −24 promoter identical to the one located in front of the csr operon [TGGCAC(N)5TTGCAT] (Deutscher, unpublished). It is possible that this operon is also regulated by CsrA and that csrA inactivation therefore prevents the expression of both potential cellobiose operons and, consequently, the synthesis of the cellobiose-specific PTS components and cellobiose transport. However, more specific effects of CsrA and/or the EIIA and EIIB encoded by the csr operon can presently not be excluded. It is noteworthy that cellobiose-specific inhibition of PrfA also disappeared in mutants affected in bvrAB, which code for a PRD-containing antiterminator and a β-glucoside-specific EIIBCA (81) (see the preceding section).
Homologs of B. subtilis LevR are present in many other low-G+C gram-positive organisms such as bacilli, clostridia, enterococci, lactobacilli, listeriae, Pediococcus pentosaceus, and Thermoanaerobacter tengcongensis, but they are also present in the actinobacterium S. thermophilum and in gram-negative bacteria such as S. enterica serovar Typhimurium, Salmonella enterica, K. pneumoniae, and Salmonella enteritidis, although they are much less frequent in the latter organisms. The highest number of LevR-like proteins (seven) was detected in C. difficile. The large number of LevR homologs in several bacteria suggests that these proteins are not used exclusively for the regulation of the expression of operons coding for fructose-, mannose-, or cellobiose-specific PTS. Indeed, the L. casei esu operon is regulated by a LevR-like protein and encodes a PTS that probably transports a sugar ester (976). Most of the approximately 100 LevR-like proteins detected have a size similar to that of B. subtilis LevR and exhibit between 40 and 60% sequence similarity to each other. They contain conserved equivalents of the two phosphorylatable histidyl residues, confirming the importance of these two amino acids in the regulation of the activity of LevR-like transcription activators. In contrast, an equivalent of His-506 of B. subtilis LevR, the first conserved histidine in PRD1, is absent from several LevR homologs, implying that this amino acid does not play an essential role in the regulation of LevR-like proteins. One E. faecalis and one C. difficile LevR homolog contain a complete second PRD, thus possessing an additional potentially phosphorylatable histidyl residue.
DeoR-type PTS-controlled transcription activators.
A protein containing two PRDs and an N-terminal domain resembling the DNA binding domain in transcription activators of the E. coli DeoR family was first detected in G. stearothermophilus (324). The corresponding gene is located in the mtlARFD operon, in which mtlA codes for a mannitol-specific EIICB, mtlR codes for the PRD-containing regulator of the operon, mtlF codes for an EIIAMtl, and mtlD codes for a mannitol-1-P dehydrogenase. Soon afterwards, another protein with a DeoR-like N-terminal DNA binding domain followed by two PRDs was found in B. subtilis (878). This protein, LicR, controls the expression of the licRBCAH operon, which encodes the transcription activator LicR; the EIIB, EIIC, and EIIA components of a β-glucoside-specific PTS (belonging to the lactose class); and a presumed 6-P-β-glucosidase, respectively. The genome sequence revealed that B. subtilis contains two other transcription activators possessing a DeoR-like N-terminal domain and PRDs: MtlR (YdaA) and ManR (YjdC). They were proposed to regulate the expression of a mannitol-specific and a mannose-specific PTS, respectively (844). Similar proteins in other bacteria have been described. They include MtlR (342) and SrlR (78) of S. mutans and MtlR of C. acetobutylicum (51). Extensive genetic and biochemical studies have been carried out only with MtlR of G. stearothermophilus and LicR from B. subtilis.
Domain organization in DeoR-type PRD-containing transcription activators.
The transcription activators MtlR of G. stearothermophilus and LicR of B. subtilis possess an N-terminal DNA binding domain (Fig. 7), which exhibits significant similarity (around 50%) to the first approximately 180 amino acids of transcription activators of the DeoR family such as E. coli FucR (125), B. anthracis AcpA (414), and S. pyogenes VirR (537). In most DeoR-type transcription activators, a traditional helix-turn-helix motif is located at the beginning of the N-terminal domain. However, in a few PTS-controlled regulators, the helix-turn-helix motif is degenerated and difficult to detect (51). The suggested presence of a second helix-turn-helix motif in LicR (879) has so far not been confirmed. The DNA binding domain is followed by two PRDs, with PRD1 beginning at about amino acid 185 (Fig. 7). Each PRD usually contains two conserved histidyl residues. The PRDs are followed by an EIIBGat domain containing a conserved and potentially phosphorylatable cysteyl residue (Cys-423 in G. stearothermophilus MtlR and Cys-413 in B. subtilis LicR) (295). The last domain of DeoR-type PRD-containing transcription activators is an EIIA domain belonging to the mannitol/fructose class PTS. In EIIAMtl of S. carnosus, His-62 has been identified as a PEP-dependent phosphorylation site (703). An equivalent histidyl residue is present in the EIIAMtl/Fru-like domains of LicR of B. subtilis (His-559) (879) and MtlR of G. stearothermophilus (His-598) (323). In total, these regulators contain six potential PTS phosphorylation sites (Fig. 7).
PTS-mediated control of DeoR-type PRD-containing transcription activators.
Phosphorylation of G. stearothermophilus MtlR was observed in the presence of PEP, EI, and HPr (323). The P∼His-HPr-mediated phosphorylation was completely prevented when either one of the two conserved histidyl residues in PRD2 was replaced with an alanine. However, His348Ala, His405Ala, and His348Ala/His405Ala mutant MtlRs were phosphorylated when, in addition to EI and HPr, EIIAMtl and EIICBMtl were also present in the phosphorylation assay (323). The phosphorylation of MtlR in the presence of EIIAMtl and EIICBMtl was strongly diminished when the conserved His-598 in the C-terminal EIIAMtl/Fru-like domain was replaced with an alanine. The residual weak phosphorylation of His598Ala mutant MtlR was probably due to phosphorylation in PRD2 by the low amounts of P∼His-HPr present in the reaction mixture. In conclusion, MtlR becomes phosphorylated by EI and HPr at one or both conserved histidyl residues in PRD2 and in an EI-, HPr-, EIIAMtl-, and EIICBMtl-requiring reaction at His-598 in the EIIAMtl/Fru-like domain. Phosphorylation of MtlR therefore clearly differs from B. subtilis LevR phosphorylation, where EI and HPr phosphorylate the EIIAMan-like domain and phosphorylation in the presence of EIIALev and EIIBLev occurs in PRD2 (520).
Similar to LevR, MtlR binds to a DNA region located upstream from the promoter, as was demonstrated in footprint experiments with G. stearothermophilus MtlR and a DNA fragment extending about 125 bp upstream and downstream from the transcription start site of the mtl operon. Apparently, MtlR does not interact with a continuous DNA stretch, as within a sequence of about 50 bp, five small regions were protected against DNase I digestion in the footprint experiments (325). The phosphorylation state of MtlR had a strong effect on its DNA binding activity. MtlR phosphorylated by EI and HPr in PRD2, but dephosphorylated in the EIIAMtl/Fru-like domain, exhibited an approximately 100-fold-higher affinity for its target DNA than did unphosphorylated MtlR (323). The DNA binding affinity of neither the His348Ala nor the His405Ala mutant MtlRs was enhanced by the presence of P∼His-HPr (as mentioned above, mutation of either His prevents phosphorylation in PRD2). In contrast, phosphorylation in the presence of EI, HPr, EIIAMtl, and EIICBMtl at His-598 in the EIIAMtl/Fru-like domain and in PRD2 lowered the affinity of MtlR for its DNA target about 10-fold compared to the unphosphorylated regulator. Surprisingly, not only phosphorylation of His-598 but also its replacement with an alanine had a negative effect on DNA binding, which could not be overcome by P∼His-HPr-mediated phosphorylation (323). It is possible that the mutation causes structural changes leading to the inactivation of the transcription regulator. In conclusion, MtlR is controlled by at least two PTS-mediated phosphorylation events that exert antagonistic effects. Similar to LicT and LevR, the absence of EI- and HPr-mediated phosphorylation in PRD2 of MtlR serves as an alternate CCR mechanism. In contrast, phosphorylation in the EIIAMtl/Fru-like domain, which requires the additional presence of EIIAMtl and EIICBMtl, exerts a negative effect on MtlR activity. This phosphorylation is probably prevented when mannitol is present in the growth medium, and it is therefore likely that dephosphorylation at His-598 serves as an induction mechanism for the mtl operon. The role of PRD1 in MtlR regulation is not clear. Although the replacement of either one of the conserved histidyl residues in PRD1 diminished the stimulatory effect of P∼His-HPr on MtlR activity, phosphorylation of these histidines could not be detected. Similar to what was proposed for LevR, PRD1 in MtlR might be important only for the signal transfer from PRD2 to the DNA binding domain.
Expression of the mtl operon in S. carnosus (232) and B. subtilis seems to be regulated by a mechanism resembling that in G. stearothermophilus, as the mtl operon of both organisms contains a 50-bp-long upstream activating sequence similar to that recognized by MtlR of G. stearothermophilus (325). In addition, the mtl operon of S. aureus exhibits a gene order (mtlARFD) identical to those in G. stearothermophilus and S. mutans (341, 342). In contrast, ydaA, which encodes the B. subtilis MtlR homolog, is a monocistronic transcription unit located about 15 kb downstream from the mtl operon (437). YdaA was renamed MtlR (844), as it exhibits significant sequence identity (39%) to MtlR from G. stearothermophilus. MtlR of S. mutans exhibits only 28% sequence identity and 48% similarity to MtlR of G. stearothermophilus. Surprisingly, only a polar insertion into mtlR was reported to prevent mannitol utilization by S. mutans, whereas a nonpolar insertion had no effect (342). Therefore, whether MtlR of S. mutans carries out functions similar to those of MtlR of G. stearothermophilus is questionable. Finally, an MtlR homolog of C. acetobutylicum was reported to control the expression of the mtlARFD operon of this organism (51).
The DNA binding site for LicR, the second PRD-containing DeoR-type transcription activator in B. subtilis, was determined by carrying out a deletion analysis with the promoter region of the lic operon. A sequence with a dyad symmetry precedes the lic promoter, which appears to be the target site for LicR (879). Similar to MtlR, the activity of LicR is negatively controlled by its corresponding sugar-specific PTS proteins, EIIACel (LicA) and EIIBCel (LicB). Inactivation of EIIACel causes constitutive expression of a licB′-lacZ fusion. In contrast, deletion of the ptsHI genes prevents expression of the licB′-lacZ fusion, suggesting a positive role for the general PTS proteins in LicR regulation (878). The four conserved histidyl residues in the two PRDs of LicR were proposed to be the targets of positive regulation by P∼His-HPr, as the replacement of either one of them with a nonphosphorylatable amino acid results in inactive LicR (879). In contrast, replacement of His-559 in the EIIA-like domain of LicR with a glycine causes constitutive expression of the licB′-lacZ fusion. His-559 therefore appears to be the site of negative control of LicR activity. Based on the genetic results, it was proposed that P∼EIIBCel transfers its phosphoryl group to His-559 in the EIIAMtl/Fru-like domain of LicR (879). Although EIIBCel and EIIAMtl/Fru belong to the same superfamily of PTS proteins (glucose-fructose-lactose), their phosphorylation sites do not exhibit significant sequence similarity (231, 703). In addition, the structures of EIIAs from these two PTS classes are completely different (730), and “cross”-phosphorylation between the EIIAs and EIIBs from the different PTS classes has not been reported. It therefore remains to be experimentally confirmed that the EIIAMtl/Fru-like domain in LicR can indeed be recognized and phosphorylated by P∼EIIBCel. Alternatively, His-559 in LicR could be phosphorylated by P∼His-HPr but only in the presence of P∼EIIBCel. This mechanism would resemble the regulation of the SacY antiterminator by P∼His-HPr-mediated phosphorylation at His-99 in the presence of P∼SacX (883).
Distribution of DeoR-type PRD-containing transcription activators.
A third gene, yjdC, encoding a protein containing a DeoR-like DNA binding domain followed by two PRDs and EIIBGat- and EIIAMtl/Fru-like domains is present in B. subtilis (437). The sequence identities between YjdC and the other two related transcription activators in B. subtilis were 26% to LicR and 21% to MtlR. The yjdC gene is monocistronic and is located in front of the yjdDEF operon encoding an EIIBCA of a fructose/mannitol class PTS (yjdD), an enzyme exhibiting similarity to mannose-6-P isomerase (yjdE) and a protein of unknown function (yjdF). YjdC was called ManR (844), although the sugar specificity of the encoded PTS remains to be determined. Another DeoR-type transcription regulator, SrlR, containing PRDs and EIIBGat- and EIIAMtl/Fru-like domains has been described for S. mutans. SrlR controls the expression of the srlDRMAEB operon (78). Besides the putative transcription activator, the operon encodes a glucitol (sorbitol)-specific PTS (srlAEB) and a glucitol-6-P dehydrogenase (srlD). Surprisingly, the operon also contains a homolog of E. coli SrlM, a small protein that has been reported to function as an activator for the srlR-less E. coli srl operon (961).
Homologs of G. stearothermophilus MtlR and B. subtilis LicR are found in the genomes of numerous gram-positive bacteria, including other bacilli, clostridia, enterococci, lactobacilli, listeriae, streptococci, staphylococci, Exiguobacterium spp., Geobacillus kaustophilus, Leuconostoc mesenteroides, L. lactis, Moorella thermoacetica, O. iheyensis, T. tengcongensis, Thermoanaerobacter ethanolicus, etc. In total, more than 100 proteins were found to exhibit significant sequence similarity to G. stearothermophilus MtlR or B. subtilis LicR. In many of these proteins, including S. mutans SrlR, one or both conserved histidines in PRD1 are missing, whereas the phosphorylatable histidines in PRD2 and the EIIAMtl/Fru-like domain were always present, confirming the importance of the EIIAMtl/Fru domain and PRD2 for the regulation of MtlR/LicR-type transcription activators. The organisms with the most DeoR-type PRD-containing transcription activators were C. difficile (13) and L. innocua (10). A few LicR/MtlR homologs are also present in gram-negative organisms such as E. coli, P. carotovorum, S. enterica serovar Typhimurium, S. enterica, S. enteritidis, S. paratyphi, Vibrio fischeri, and Yersinia enterocolitica.
The results obtained with the three best-characterized PRD-containing transcription activators LevR, MtlR, and LicR make it clear that the molecular mechanisms regulating their activity can vary largely. In LicR of B. subtilis, all four conserved histidyl residues in the two PRDs seem to be phosphorylated by P∼His-HPr, and their phosphorylation stimulates LicR activity. In MtlR of G. stearothermophilus, only the two histidyl residues present in PRD2 seem to have an equivalent function. Finally, B. subtilis LevR is activated by P∼His-HPr-mediated phosphorylation of a histidyl residue present in the EIIAMan-like domain. For each transcription activator, the absence of the stimulatory phosphorylation(s) during the uptake of a rapidly metabolizable PTS substrate initiates a CcpA-independent CCR mechanism. The three PRD-containing transcription activators are inhibited by P∼EIIB-requiring phosphorylation in their C-terminal domain. The presence of the appropriate inducer (PTS substrate) prevents this phosphorylation and leads to the induction of the corresponding operon. Inhibition of LicR of B. subtilis is mediated by phosphorylation at His-559 in the EIIAMtl/Fru-like domain. Although the detailed mechanism of this phosphorylation reaction is not known, it is probably mediated by P∼His-HPr in the presence of P∼EIIBCel. Similarly, MtlR of G. stearothermophilus is inactivated by P∼His-HPr- or P∼EIIBMtl-mediated phosphorylation at His-598 in the EIIAMtl/Fru-like domain. In contrast, inactivation of LevR apparently occurs by P∼EIIBLev-mediated phosphorylation of a conserved histidyl residue in the second, truncated PRD.
It is tempting to assume that the four regulatory domains present in PRD-containing transcription activators are organized in a row, similar to the PRDs in antiterminators (909). The inactivating signal elicited by phosphorylation in the C-terminal domain therefore has to be transmitted through the other three domains to affect DNA binding. Structure determination of a transcription activator will hopefully allow a better understanding of the signal transmission.
Regulation of EIIA-Containing Non-PTS Transporters by P∼His-HPr-Mediated Phosphorylation
Phosphorylation of an EIIAGlc-like domain in certain non-PTS transporters.
Lactose transport in S. thermophilus is catalyzed by LacS, an integral homodimeric membrane protein that exhibits similarities to the melibiose transporters of E. coli and other bacteria (673). Although LacS is an H+/lactose symporter (235), it contains a C-terminal EIIAGlc-like domain, which becomes phosphorylated by PEP, EI, and HPr (672). In E. coli and S. enterica serovar Typhimurium, the melibiose transporter is inhibited by an allosteric interaction with dephospho-EIIAGlc, whereas P∼EIIAGlc exhibits no affinity for the melibiose permease (439, 621) (also see the section on EIIAGlc-mediated inducer exclusion above). Regulation of LacS-mediated lactose transport in S. thermophilus was hypothesized to follow a similar mechanism, except that the transporter is already fused to its regulatory EIIAGlc domain. EI/HPr-catalyzed phosphorylation of the EIIAGlc domain (302) was thought to prevent the inhibition of LacS. However, deletion of the EIIAGlc domain of LacS or mutation of its phosphorylatable histidyl residue (His-552) did not affect the H+/lactose symport activity of LacS. In fact, when the lactose transport rate of LacS reconstituted into liposomes was measured, the presence of PEP, EI, and HPr even slightly inhibited proton motive force-driven lactose uptake.
Phosphorylation of LacS stimulates the lactose/galactose exchange reaction.
More detailed studies revealed that monomeric LacS of S. thermophilus (244) catalyzes lactose uptake via a lactose/galactose exchange reaction (915). Galactose produced by the β-galactosidase-catalyzed hydrolysis of intracellular lactose cannot be metabolized by S. thermophilus and therefore needs to be expelled. Galactose export is also catalyzed by LacS and is coupled to the import of lactose (counterflow). The lactose/galactose exchange reaction has a higher Vmax than LacS-catalyzed lactose/H+ symport and should therefore represent the major entry route for lactose. The Vmax of the counterflow reaction was two- to threefold higher when LacS was phosphorylated by PEP, EI, and HPr, and the Km was twofold lower (301). Thus, PEP-dependent phosphorylation of LacS specifically stimulates lactose uptake via the lactose/galactose exchange reaction (Fig. 6).
A lactose transporter exhibiting 95% sequence identity to LacS from S. thermophilus is present in S. salivarius. In vitro studies revealed that LacS of S. thermophilus and S. salivarius becomes efficiently phosphorylated at His-552 not only by P∼His-HPr but also by doubly phosphorylated (P∼His,P-Ser)-HPr (134, 468), which is present in large amounts in oral streptococci grown on glucose or lactose (660, 893) and in lactose-grown S. thermophilus cells (134). However, in contrast to S. thermophilus, S. salivarius did not expel galactose during growth on lactose. In addition, when grown in medium containing lactose or lactose plus galactose, an S. salivarius ptsI mutant showed the same growth characteristics as the wild-type strain. The role of LacS phosphorylation in S. salivarius is presently not known.
Transporters of the galactoside-pentose-hexuronide family, to which LacS belongs, are abundant in bacteria. It appears that only a few of them (those that transport lactose or raffinose in gram-positive organisms) gained a regulatory domain by fusing EIIAGlc to their C-terminal end, similar to the PTS-regulated NifA/NtrC- and DeoR-type transcription activators discussed in the previous section. In addition to S. thermophilus and S. salivarius, transporters exhibiting similarity to the E. coli melibiose permease and containing an EIIAGlc domain fused to the C terminus have been described for a few other gram-positive organisms. They include the lactose transport protein LacS of L. delbrueckii subsp. bulgaricus (467) and Leuconostoc lactis (914). L. plantarum contains not only a LacS homolog but also a raffinose transporter (RafP) with an EIIAGlc-like domain (815). Likewise, Pediococcus pentosaceus and Lactobacillus johnsonii possess both LacS and RafP homologs. S. thermophilus LacS-like proteins are also present in L. mesenteroides, Lactobacillus acidophilus, Lactobacillus reuteri, and Lactobacillus helveticus as well as in the proteobacterium Mannheimia succiniciproducens (MelB). The presence of an EIIAGlc-like domain in these proteins indicates that P∼His-HPr-mediated phosphorylation might represent a general mechanism for the stimulation of their lactose/galactose or raffinose/galactose exchange activities.
Regulation of Glycerol Kinase by P∼His-HPr-Mediated Phosphorylation
Glycerol metabolism in gram-positive bacteria requires functional EI and HPr.
Glycerol enters bacterial cells by facilitated diffusion, an energy-independent transport mechanism (321). The protein that catalyzes the equilibration of glycerol gradients across the bacterial cytoplasmic membrane is called the glycerol facilitator (GlpF) (481). GlpF, which has been studied extensively in E. coli (584, 849), is present in many other bacteria, archaea, and eukaryotes. It is a member of the major intrinsic protein family of transmembrane channels (39, 628). In addition to GlpF, the major intrinsic protein family includes other membrane-spanning bacterial proteins such as the water channel protein AqpZ from E. coli and several other bacteria (99) and PduF, a presumed propanediol diffusion facilitator of S. enterica serovar Typhimurium (119). A channel protein accepting glycerol and water as substrates was found in L. lactis (246). When the E. coli glpF gene was functionally expressed in Xenopus laevis oocytes, glycerol uptake by the oocytes exhibited the characteristics of diffusion by a pore-type mechanism (532). Because the glycerol facilitator catalyzes the reversible diffusion of glycerol across the cytoplasmic membrane, it is the subsequent metabolism of glycerol that allows a net (steady-state) flux of glycerol into the cell. The first metabolic step is catalyzed either by glycerol dehydrogenase, which converts glycerol into dihydroxyacetone, or by GlpK, which uses ATP to transform intracellular glycerol into glycerol-3-P (656). Glycerol-3-P is not a substrate for the glycerol facilitator and therefore remains entrapped in the cell. It functions as an inducer of the glp regulon by inactivating a repressor (GlpR) in E. coli (790) or by activating an antiterminator (GlpP) in B. subtilis (749). GlpP binds to the leader region in the mRNA of glpD but probably also of glpFK and glpTQ (274). The glpD gene encodes glycerol-3-P dehydrogenase (339), glpT encodes a glycerol-3-P transporter, and glpQ encodes a glycerophosphoryl diester phosphodiesterase.
Although glycerol is not transported by the PTS, mutants of gram-positive and gram-negative bacteria defective in one of the general PTS proteins EI or HPr had lost the ability to grow on glycerol as the sole carbon source (52, 265, 282, 600, 711, 741, 766). The inhibition of S. enterica serovar Typhimurium and E. coli glycerol kinase by unphosphorylated EIIAGlc and the resulting dependence of glycerol metabolism on a functional PTS are described above (see “REGULATION OF CARBON METABOLISM IN GRAM-NEGATIVE ENTERIC BACTERIA”) (166, 676, 899). A mechanism completely different from that operating in E. coli and S. enterica serovar Typhimurium seems to control glycerol metabolism in most low-G+C gram-positive bacteria.
EI- and HPr-catalyzed phosphorylation regulates GlpK activity in gram-positive bacteria.
In contrast to the Enterobacteriaceae E. coli and S. enterica serovar Typhimurium, most gram-positive organisms do not contain free soluble EIIAGlc (130, 350). In these bacteria, either EIIAGlc is fused to EIICBGlc, the membrane component of the glucose-specific PTS, or, when mannose and glucose are taken up by the same mannose class PTS, they possess no EIIAGlc at all. Even in Mycoplasma capricolum, which is one of the few gram-positive organisms possessing free EIIAGlc, the PTS protein did not inhibit GlpK activity (992). Moreover, while the inactivation of the EIIAGlc-encoding E. coli or S. enterica serovar Typhimurium crr gene enabled ptsHI mutants to grow on glycerol as the sole carbon source, deletion or disruption of the 3′ part of the B. subtilis ptsG gene encoding the EIIAGlc domain of EIICBAGlc had no such effect (282). Nevertheless, suppressor mutants that restored growth of a B. subtilis ptsHI mutant on glycerol have been obtained (52). They were not affected in ptsG but, rather, were affected in glpK (937), and glycerol uptake in gram-positive bacteria was therefore assumed to be regulated by an EIIAGlc-independent mechanism.
The observation that GlpK from E. faecalis is phosphorylated by PEP, EI, and HPr (174, 186) also suggested that glycerol metabolism in gram-positive bacteria is regulated by a mechanism different from that operating in gram-negative bacteria. PEP-dependent phosphorylation occurred at the N-3 position of a histidyl residue and stimulated GlpK activity about 10-fold (186). A similar PTS phosphorylation-mediated stimulation was observed for GlpK from Enterococcus casseliflavus (109) and B. subtilis (158). No specific protein phosphatase dephosphorylating P∼GlpK could be detected. Instead, dephosphorylation of purified P∼GlpK was observed when it was incubated together with an excess of HPr, indicating that P∼GlpK can transfer its phosphoryl group back to HPr (186). The site of PEP-dependent, EI- and HPr-catalyzed phosphorylation was determined to be His-232 in GlpK of E. casseliflavus (109) and His-230 in GlpK of B. subtilis (158). Interestingly, in one of the B. subtilis suppressor mutants that were able to grow on glycerol despite the absence of active EI and HPr, His-230 of GlpK was replaced with an arginine, and in another mutant, the neighboring Phe-232 was replaced with a serine (937). These mutations were thought to cause structural changes similar to those triggered by phosphorylation, and the ability of these glpK mutants to grow on glycerol was assumed to be due to PTS-independent elevated activity of the mutant GlpKs. Indeed, GlpK activity in the four B. subtilis ptsHI suppressor mutants was about 10-fold higher than the activity measured in a ptsHI strain producing wild-type GlpK (158). In addition, replacing the phosphorylatable His-232 in E. casseliflavus GlpK or His-230 in B. subtilis GlpK with an arginine led to an increase in activity similar to that caused by phosphorylation (109, 158). In contrast, E. casseliflavus (or B. subtilis) mutant GlpKs, in which His-232 (or His-230) had been replaced with an alanine or glutamine, exhibited low catalytic activity, which could not be stimulated by phosphorylation with PEP, EI, and HPr (109, 158). Compared to EIIAs, the usual phosphoryl acceptors of P∼His-HPr within the PTS phosphorylation cascade, phosphorylation of enterococcal GlpKs was about 100 times slower. Based on these in vitro results, it was concluded that PEP-dependent, EI- and HPr-catalyzed phosphorylation of GlpK serves to regulate glycerol metabolism in response to the presence or absence of a PTS substrate. In the absence of a PTS sugar, GlpK was assumed to be present primarily in the active, phosphorylated form, allowing the rapid uptake and metabolism of glycerol. However, when bacteria grow in a medium containing a PTS substrate, the phosphoryl transfer flux through the PTS will be high, and the concentration of the common phosphoryl donor P∼His-HPr will be low. As a result, GlpK will be present mainly in the less active dephospho form (Fig. 6).
P∼GlpK dephosphorylation leads to CCR via inducer exclusion.
E. faecalis GlpK and P∼GlpK migrate to slightly different positions on sodium dodecyl sulfate-polyacrylamide gels (175). By carrying out Western blot analyses with crude extracts from E. faecalis cells grown in the presence of various carbon sources, the influence of the growth conditions on the GlpK/P∼GlpK ratio could be determined. Growth in glycerol-containing medium induced the synthesis of GlpK, which was present primarily in the phosphorylated form under these conditions. If the growth medium contained glycerol plus glucose or mannitol, the synthesis of GlpK was strongly repressed, with the remaining GlpK being present mainly in the dephospho form. When cells were grown in glycerol-containing medium and glucose was added to one half of the culture 20 min before the cells were harvested, the amount of GlpK was similar to that in cells grown in the presence of glycerol only. However, while bacteria that continued to grow only on glycerol contained primarily P∼GlpK, the presence of glucose in the other half of the culture caused nearly complete dephosphorylation of P∼GlpK. These results support the above-described concept that the dephosphorylation of GlpK in response to the presence of a rapidly metabolizable PTS sugar inhibits glycerol uptake and metabolism. This regulatory mechanism can be considered a second form of “PTS-mediated inducer exclusion,” as the presence of a PTS substrate prevents the GlpK-catalyzed formation of the inducer glycerol-3-P.
The P∼GlpK dephosphorylation-based inducer exclusion mechanism represents a CcpA-independent CCR mechanism and has been studied in detail in B. subtilis, where expression of the glpFK operon is also submitted to P-Ser-HPr:CcpA-mediated CCR (158). In fact, in ptsH1, hprK (both cannot form P-Ser-HPr), and ccpA mutants, expression of glpK or a glpF′-lacZ fusion was still strongly repressed by glucose (158, 184), confirming that a second, P-Ser-HPr:CcpA-independent CCR mechanism is operative for the glp operon. Interestingly, the repressive effect of glucose on glpF′-lacZ expression was absent in a ccpA mutant producing His230Arg mutant GlpK (158), which is highly active even without being phosphorylated by PEP, EI, and HPr. These results provide additional evidence that the CcpA-independent CCR mechanism for the B. subtilis glpFK operon is based on poor phosphorylation of His-230 in GlpK. As discussed above, the prevalence of unphosphorylated, barely active GlpK during the rapid uptake of a PTS sugar is expected to lead to low concentrations of the inducer glycerol-3-P (Fig. 6). Growth of a ccpA mutant in the presence of glycerol-3-P, which is taken up by GlpT, should therefore also prevent CCR of glpF′-lacZ expression, and this has indeed been observed (158). In B. subtilis, the inducer glycerol-3-P activates the antiterminator GlpP, which prevents the formation of the ρ-independent terminator tglpFK located in the glpFK leader region and consequently leads to the induction of the glp operon. Deletion of tglpFK from the glpF′-lacZ fusion in a wild-type background therefore caused constitutive lacZ expression, which, however, was still strongly repressed by glucose (CcpA-mediated CCR). By contrast, when tglpFK was deleted in a ccpA or hprK mutant, the repressive effect of glucose on glpF′-lacZ expression completely disappeared (158).
The phosphorylation loop of enterococcal GlpK binds FBP in the E. coli enzyme.
The crystal structure of GlpK from E. casseliflavus (977) revealed that it forms dimers in which the phosphorylatable His-232 of each monomer is located about 25 Å from the active center on a loop that is part of the dimer interface. As a consequence, the two phosphorylatable histidyl residues of a GlpK dimer are located close to each other (about 5 Å apart). Because more than 80% of GlpK is present as P∼GlpK in cells grown in glycerol-containing medium (175), doubly phosphorylated GlpK dimers probably represent the activated form. Electrostatic repulsion due to the two neighboring negatively charged phosphoryl groups is likely to drive the structural changes leading to elevated activity of P∼GlpK. Owing to the instability of the P∼His bond, the structure of P∼GlpK could not be determined. Nevertheless, the E. casseliflavus His232Arg and Phe234Ser mutant GlpKs exhibit elevated activity even when they are not phosphorylated (109) and are therefore expected to possess a structure similar to that of P∼GlpK. It will therefore be interesting to determine the structure of one of the mutant proteins, work that is presently going on in the laboratories of J. Yeh, P. Briozzo, and J. Deutscher.
The overall structure of GlpK from E. casseliflavus is very similar to that of GlpK from E. coli. The loop containing the phosphorylatable histidine in E. casseliflavus GlpK protrudes less in E. coli GlpK. In addition, the corresponding loop in E. coli GlpK does not contain a histidine. While E. coli and E. casseliflavus GlpKs exhibit 60% overall sequence identity, the sequences corresponding to this loop are completely different in GlpKs from gram-positive and gram-negative bacteria. Interestingly, FBP, an allosteric inhibitor of GlpK from gram-negative bacteria, binds to this loop in E. coli GlpK (622), thereby connecting two GlpK dimers and leading to the formation of inactive GlpK tetramers. It therefore seems that GlpKs from gram-positive and gram-negative bacteria use the same regulatory loop but that the mechanisms controlling GlpK activity via this loop are completely different.
GlpKs from most other gram-positive bacteria with low G+C content, such as E. faecalis, S. pyogenes (109), L. lactis (67), E. sibiricum, L. monocytogenes, L. sakei, O. iheyensis, S. pneumoniae, S. aureus, etc., also contain a histidyl residue equivalent to His-232 of GlpK from E. casseliflavus, and the surrounding sequence, including the three aromatic amino acids, is well conserved. Interestingly, L. plantarum contains two GlpKs that exhibit 76% sequence identity. However, only GlpK1 possesses a regulatory loop with a phosphorylatable His (in boldface type) (T-K-D-Y-H-F-F-G-S), which is replaced with a Leu (in boldface type) in GlpK2 (T-K-D-Y-L-L-Y-G-S), implying that only GlpK1 is regulated by the PTS (Deutscher, unpublished). The gram-positive bacteria M. pneumoniae, Mycoplasma genitalium, and Carboxydothermus hydrogenoformans possess a GlpK that exhibits about 45% sequence identity to GlpK from E. casseliflavus and contains a histidine equivalent to His-232, but the surrounding amino acids are poorly conserved. Therefore, whether these three enzymes are phosphorylated and regulated by P∼His-HPr is questionable. Other gram-positive bacteria with low G+C content, such as clostridia, Desulfitobacterium hafniense, and T. tengcongensis, contain a GlpK exhibiting between 50% and 65% sequence identity to GlpK from E. casseliflavus, but they lack an equivalent of His-232, and the sequence corresponding to the regulatory loop is completely different. Surprisingly, the regulatory loop sequence of C. acetobutylicum and Clostridium perfringens GlpK is almost identical to that of E. coli GlpK, suggesting that these enzymes are regulated by FBP-stimulated tetramer formation and not by P∼His-HPr-mediated phosphorylation. In contrast, the site of interaction with EIIAGlc (Pro-472 to Tyr-481 in E. coli GlpK) (355) is not conserved in any known GlpK of gram-positive bacteria. These local sequence differences reflect the different modes of regulation of GlpK activity in gram-positive and gram-negative bacteria. Interestingly, amino acids Pro-472 to Tyr-481 are well conserved in GlpKs of only a few gram-negative bacteria including, in addition to E. coli and salmonellae, P. carotovorum, Photorhabdus luminescens, shigellae, and yersiniae, implying that the EIIAGlc-mediated regulation of GlpK is not very widespread.
At first glance, the mechanisms regulating GlpK activity in gram-positive bacteria (stimulation by P∼His-HPr-dependent phosphorylation of GlpK) and certain gram-negative bacteria (inhibition by EIIAGlc, which is prevented by P∼His-HPr-dependent phosphorylation at His-90 of EIIAGlc) seem to be completely different. Nevertheless, the two distinct mechanisms of GlpK regulation, which apparently developed in gram-positive and gram-negative organisms after their separation during evolution, serve identical physiological functions; i.e., they slow glycerol uptake and metabolism when a rapidly metabolizable carbohydrate is transported via the PTS.
GlpK phosphorylation in bacteria of the Thermus/Deinococcus group.
The thermostable GlpKs from Thermus flavus (347, 348) and Thermus aquaticus (346), which belong to the Thermus/Deinococcus group, exhibit more than 80% sequence identity to GlpK from B. subtilis. Other functionally related proteins from T. flavus or T. aquaticus and B. subtilis show a much lower degree of sequence identity (30 to 55%), implying that the GlpK-encoding gene present in T. flavus and T. aquaticus was gained by horizontal gene transfer from a gram-positive organism. Their GlpKs contain an equivalent of the phosphorylatable His-232, and the surrounding sequence is well conserved. It was therefore not surprising that EI and HPr from B. subtilis could phosphorylate T. flavus GlpK (157). In addition, T. flavus crude extracts were found to contain EI and HPr activity and were capable of phosphorylating T. flavus GlpK (157). However, phosphorylation of T. flavus GlpK did not increase its activity. A similar observation was made with G. stearothermophilus GlpK (714), and it was therefore hypothesized that the modification of GlpK from these two thermotolerant organisms affects their thermostability (157).
Is GlpK the only carbohydrate kinase regulated by P∼His-HPr?
Glycerol kinase is a member of a large family of carbohydrate kinases including xylulose kinase, gluconate kinase, l-fuculose kinase, and many others (954). A data bank search revealed that none of the presently known eubacterial members of this family contains a histidyl residue equivalent to His-232 of GlpK from E. casseliflavus. Nevertheless, some bacteria such as L. rhamnosus (17) and L. casei BL23 (570) possess a glpK-like gene in addition to glpK in the glpFK operon (up to 80% identity at the protein level), which was called gykA. GykA of both organisms contains a P∼His-HPr phosphorylation site in position 231 (His231, in boldface type; -Y-H-F-F-), which is identical to that in several other GlpKs. In L. casei, gykA is monocistronic, and its function is unknown (570). Because the L. rhamnosus gykA gene was not induced during growth on glycerol, it is possible that GykA phosphorylates a substrate other than glycerol. Nevertheless, the PTS-mediated activation mechanism based on P∼His-HPr-dependent phosphorylation at a regulatory loop in the target enzyme seems to have almost exclusively evolved for GlpK of low-G+C gram-positive bacteria and certain members of the Thermus/Deinococcus group.
SOME UNUSUAL PTS PATHWAYS AND PROTEINS
Genome sequencing has unveiled many genes that encode PTS-like proteins (see http://www.membranetransport.org/ [725] and http://www.tcdb.org/ [755]) (345, 720, 759, 867). Most gram-positive bacteria as well as chlamydiae and the spirochetes have a single EI and HPr, whereas bacilli, geobacilli, and oceanobacilli possess an additional HPr paralog, Crh (see “REGULATORY FUNCTIONS OF P-Ser-HPr”) (250). An HPr paralog is also present in U. urealyticum (308). In contrast, some proteobacteria carry several EI and HPr homologs. For instance, E. coli has at least five copies of each general PTS protein. Some of the paralogous genes encode multidomain proteins (e.g., diphosphoryl transfer proteins, triphosphoryl transfer proteins, or multiple-phosphoryl transfer proteins), which supposedly are the result of splicing and fusion as well as duplication of EI, HPr, EIIA, and EIIB domains (720, 867). EI, HPr, and EIIA homologs have even been identified in bacteria that lack any known EIIB and EIIC and therefore lack a PTS that is functional in sugar transport and phosphorylation (64). In the few cases studied in more detail, the paralogous proteins seem to constitute alternative phosphotransferase routes. We will discuss some of these alternative routes, which include (i) the unusual PTS-dependent utilization of dihydroxyacetone and (ii) the connection between carbon and nitrogen metabolism via the PTSNtr.
PEP-Dependent Dihydroxyacetone Phosphorylation
Dihydroxyacetone can be used by E. coli as the sole exogenous source of carbon. Although several bacteria metabolize dihydroxyacetone via ATP-dependent phosphorylation, a PEP-dependent pathway is operative in E. coli (376). Mutations in ptsI or the dha operon abolish phosphorylation of dihydroxyacetone and thereby growth on this carbon source. The E. coli dha operon comprises three cistrons (304, 640). dhaK (ycgT) and dhaL (ycgS) encode the dihydroxyacetone kinase subunits DhaK and DhaL (also called DhaK1 and DhaK2, respectively). Distinct DhaK and DhaL proteins are present in bacteria that phosphorylate dihydroxyacetone in a PEP-dependent reaction, whereas in bacteria that use ATP for dihydroxyacetone phosphorylation, such as Citrobacter freundii, DhaK and DhaL are fused to a single protein (153, 811). The third cistron encodes a tripartite PTS fusion protein, which was designated DhaH by Paulsen et al. (640) and DhaM by Gutknecht et al. (304). The corresponding gene is referred to as dhaM (ycgC) or ptsD. DhaM consists of an N-terminal EIIAMan-like domain followed by an HPr-like domain and a C-terminal domain resembling the N-terminal part of EI. Each domain contains a phosphorylation site characteristic of the corresponding PTS protein (304). For a better understanding, we will refer to the E. coli DhaM protein as EIIA-HPr-EIDha.
The PEP-dependent phosphorylation of dihydroxyacetone by E. coli involves seven phosphotransfer steps (depicted in Fig. 9). EIIA-HPr-EIDha was purified and shown to be phosphorylated by [32P]PEP in the presence of both EI and HPr but was also shown to be slightly phosphorylated in the presence of EI only (304, 640). This is consistent with the absolute requirement for EI in dihydroxyacetone phosphorylation that was determined previously (376). Furthermore, it was found that the addition of equimolar amounts of purified DhaK and DhaL to purified EIIA-HPr-EIDha allows PEP-dependent phosphorylation of dihydroxyacetone but that the phosphotransfer is blocked when either one of the phosphorylatable histidyl residues is replaced with an alanine (304). These observations imply that the phosphoryl group of PEP is sequentially transferred via EI and HPr to the EI domain of EIIA-HPr-EIDha and, from there, to the HPr and the EIIA domain of the protein. Finally, it was established in vitro that DhaK binds the substrate dihydroxyacetone with high specificity (255, 814), even in the presence of 2 M glycerol, while DhaL carries a tightly bound ADP molecule (36). The final transfer steps thus involve phosphotransfer from the EIIADha domain to the ADP cofactor in DhaL and from the presumably intermediately formed DhaL/ATP to the dihydroxyacetone molecule bound to DhaK. It should be noted that, in contrast to the sugar PTS, the dha PTS is not involved in membrane transport. Transport of dihydroxyacetone occurs probably via GlpF-catalyzed facilitated diffusion or direct diffusion through the membrane (848).
FIG. 9.
The Dha PTS of E. coli. Phosphotransfer from PEP to Dha is mediated by five distinct proteins (EI, HPr, DhaM, DhaL, and DhaK). DhaM itself is composed of three PTS domains: a truncated EI, HPr, and an EIIA of the mannose class PTS. From the P∼EIIA domain of DhaM, the phosphoryl group is transferred to an ADP molecule tightly bound to DhaL and from there is transferred to a Dha molecule bound to DhaK. Expression of the E. coli dha operon is regulated by the transcription activator DhaR. In addition, the gene encoding the activator DhaR is subject to negative autoregulation. When Dha is present in the cell, it is rapidly phosphorylated, and the ultimate phosphoryl donor, DhaL, therefore carries predominantly ADP. The DhaL:ADP complex binds to DhaR and stimulates its regulator functions. DhaK without Dha interacts with DhaR and down-regulates its activity, while the complex with its substrate, which is formed when Dha is present in the cell, does not interact with DhaR (37). It should be noted that many other organisms do not contain the three-domain DhaM but possess only EIIADha instead. In these bacteria, HPr probably transfers its phosphoryl group directly to EIIADha.
Proteins homologous to DhaK, DhaL, and DhaM of E. coli are found among many bacterial orders including the Enterobacteriales, Bacillales, Lactobacillales, and Clostridiales (41). K. pneumoniae and several members of the Clostridiales contain not only a PEP-dependent dha system but also a “normal” ATP-dependent dihydroxyacetone kinase (848). The dhaM gene encodes either a tripartite protein (E. coli), a bipartite protein (EIIA-HPrDha) (Corynebacterium diphtheriae and Leifsonia xyli), or, as in most species, a single EIIADha protein (41). It is likely that in the latter group of organisms, the phosphoryl group is transferred from PEP to dihydroxyacetone in five consecutive steps.
For E. coli, it was observed that the dha operon was inducible; i.e., PEP-requiring dihydroxyacetone phosphorylation activity increased when cells were grown on dihydroxyacetone (376, 640). It appeared that dha operon induction is mediated by the dephosphorylation of the phosphotransfer proteins, as the inactivation of ptsI or dhaM as well as the replacement of any one of the three phosphorylatable histidyl residues in EIIA-HPr-EIDha led to the constitutive expression of the dhaKLM operon (37). Transcription of the dhaKLM operon is subject to autoregulation by DhaK and DhaL, which interact with the N-terminal sensory domain of the transcriptional activator DhaR (Fig. 9). The protein is activated by DhaL/ADP and is inhibited by DhaK without bound dihydroxyacetone (37). This mode of autoregulation makes the expression of the dhaKLM operon sensitive to the availability of both substrate and sufficient phosphoryl donor molecules. In the presence of substrate, expression is expected to be high. The binding of dihydroxyacetone should prevent the inhibitory effect of DhaK on DhaR, and the formation of DhaL/ADP during dihydroxyacetone phosphorylation should stimulate DhaR (37). If, in addition to dihydroxyacetone, a rapidly metabolizable PTS sugar is present, PEP is probably used mainly for PTS-mediated sugar uptake and phosphorylation, and the metabolism of dihydroxyacetone should therefore be diminished. However, as a consequence of the expected decrease in phosphoryl group flux through DhaM, DhaL will primarily be present with bound ADP, and due to the presence of dihydroxyacetone, DhaK will primarily be present with bound substrate. Both signals favor dhaKLM expression, and this unusual regulation mode was therefore thought to ensure a constant dihydroxyacetone turnover rate and detoxification, which might be necessary if dihydroxyacetone accumulates owing to intracellular metabolic processes (567).
The PTSNtr
It has been known for quite some time that the extent of CCR depends not only on the nature of the carbon source but also on the available nitrogen source (137). Nevertheless, the underlying mechanism linking the regulation of carbon and nitrogen metabolism still remains obscure (107). Transcription of a number of genes involved in nitrogen assimilation, especially under nitrogen-limiting conditions, depends upon the sigma factor σ54, encoded by the rpoN gene (previously designated ntrA or glnF) (for reviews, see references 91, 704, 705, and 843). Transcription initiation at σ54-dependent promoters requires both the input of energy through nucleotide hydrolysis and the interaction with a specific EBP, such as NtrC or NifA, some of which can be activated by PTS-mediated phosphorylation (see “REGULATORY FUNCTIONS OF P-Ser-HPr”). Sequencing of the locus around the K. pneumoniae rpoN gene revealed two ORFs (ORF162 and ORF95) whose inactivation resulted in elevated transcription of σ54-dependent genes (553). ORF162 was shown to be homologous to EIIAs of the mannitol/fructose class PTS (718), which provided a putative link between nitrogen metabolism and the PTS for the first time. The gene was renamed ptsN, and the related protein was named EIIANtr. In K. pneumoniae, EIIANtr is phosphorylated in vitro in the presence of [32P]PEP, EI, and HPr (49). Overexpression of ptsN results in slightly slower growth on glucose minimal medium with NH4+ as the nitrogen source, which supports the assumption that EIIANtr negatively regulates transcription from σ54-dependent promoters.
Sequencing of the equivalent locus in E. coli revealed four ORFs downstream from rpoN, with the second ORF being similar to ptsN and the fourth ORF encoding a protein strongly resembling HPr (Fig. 10) (382, 680). The latter ORF was designated npr (ptsO), and its gene product was named NPr. Like the K. pneumoniae protein, purified E. coli EIIANtr was reversibly phosphorylated by EI and HPr in vitro (680). However, although EIIANtr exhibits strong similarity to EIIAFru and EIIAMtl, it does not complement mannitol or fructose phosphorylation in crude extracts prepared from mutants containing inactive EIIAFru or EIIAMtl. Likewise, although NPr proved to be phosphorylated by P∼EIIANtr and P∼EIIAFru of E. coli and EIIAGlc of B. subtilis, purified NPr does not replace HPr in the glucose or mannitol PTS assay (680), probably because phosphorylation of NPr by PEP/EI is a relatively slow process (689).
FIG. 10.
Gene organization downstream from rpoN in several bacteria. The genes encode the sigma factor σ54 (rpoN), the ribosome-associated protein Y (yhbH, in yellow), EIIANtr (ptsN), an ATP-binding protein (yhbJ, in red), and the HPr paralog NPr (npr), respectively.
The PTSNtr was completed by the discovery of a third “alternative” PTS gene (ptsP) in the E. coli genome, which encodes EINtr, a protein with considerable similarity to EI. Compared to EI, EINtr contains an N-terminal extension of 170 amino acids, which resembles the N-terminal regulatory GAF domain of NifA, a protein controlling nitrogen fixation. EINtr was therefore assumed to be involved in the regulation of nitrogen metabolism (716). PEP-dependent in vitro phosphorylation of NPr by EINtr was observed. However, EINtr does not replace EI in the overall PEP-dependent mannitol phosphorylation, which is at least 1,000-fold slower in the presence of EINtr compared to assays with EI (689). This finding was not surprising, as ptsI mutants (lacking EI) do not grow on PTS carbohydrates, and no phosphoryl group transfer from PEP to HPr was observed in crude extracts of such mutants.
The localization of ptsN and npr in the rpoN operon and, as a consequence, their cotranscription with rpoN are conserved in several gram-negative bacteria (382, 552, 867). In K. pneumoniae (553) and E. coli (680), expression of the rpoN operon is constitutive, whereas in Bradyrhizobium japonicum (433), Pseudomonas putida (415), and Rhizobium etli (555), it is negatively autoregulated. EINtr, NPr, and EIIANtr have so far not been detected in gram-positive bacteria. Attempts have been made to uncover the role of the PTSNtr in various species, mostly by studying growth and gene expression (via lacZ fusions) after inactivation of ptsN. The growth behavior was compared to those of a wild-type strain and mutants missing σ54 (RpoN). The results of these attempts are diverse and sometimes contradictory and will be described below.
Connection between carbon and nitrogen metabolism in E. coli.
In E. coli, the regulation of nitrogen metabolism appears to be coupled to specific “classical” PTS components as well as to the PTSNtr. The key enzyme and key regulators for the utilization of nitrogen by E. coli are located in the glnALG operon, which encodes glutamine synthethase and the two-component system NtrB and NtrC, respectively (607, 704). Under conditions of nitrogen deprivation, P∼NtrC blocks transcription from glnAp1 and induces transcription from the σ54-dependent glnAp2 promoter (607, 704). Although the weak σ70-dependent glnAp1 promoter requires activation by Crp/cAMP, glnA expression was found to be much higher during growth on glucose or glucose-6-phosphate than during growth on fructose or glycerol. It was assumed that Crp/cAMP stimulates glnALG expression from glnAp1 but represses expression from glnAp2 and that the stimulatory effect is relatively stronger than the effect of repression (875). In line with this assumption, it was found that the addition of cAMP to glucose-grown cells lowered GlnA activity by about sevenfold (506). However, further analysis showed that the effect of glucose is probably not mediated via Crp/cAMP but that it is mediated indirectly via the uridylylation state of GlnB. Furthermore, under nitrogen-limiting conditions, growth of a ptsN mutant on glucose and glutamine had a small but significant negative effect on expression from glnAp2 (680). The addition of cAMP under these conditions had no effect. E. coli ptsN mutants grow poorly on alanine, and this effect was accentuated by the presence of several sugars or TCA cycle intermediates (680). Repression is relieved by supplying the cells with ammonium salts or by expressing a ptsN gene in trans. Disruption of the gene preceding ptsN (probably also leading to the inactivation of ptsN) causes a slight reduction in transcription from glnAp2 and pnifL when K. pneumoniae cells are grown on glucose (plus formate) under nitrogen limitation (serine) (553). In contrast, when serine is replaced with ammonium as the nitrogen source, expression from the glnAp2, pnifL, and pnifH promoters is significantly higher than that in the wild type (553). A similarly elevated expression from glnAp2 can be observed in a genuine ptsN mutant, whereas the inactivation of npr leads to a decrease in transcription from glnAp2, pnifL, and pnifH (554). Overexpression of K. pneumoniae ptsN in an E. coli rpoN+ strain inhibits growth on glucose minimal medium with either glutamine or ammonium as the nitrogen source but had no effect on an rpoN mutant (49). In R. etli, the inactivation of ptsN slightly reduces growth on alanine or serine in the presence of mannitol or glucose, whereas growth is inhibited when the TCA cycle intermediates succinate and malate are present (555). In addition, a reduction in melanin production was observed. The activity of the pnifH promoter decreases, while the level of NifA, the activator controlling the expression of nifH, is not affected by the mutation. Mutation of ptsA, the gene that precedes the frw operon and codes for EIFrw in E. coli (345, 720), has effects similar to those of the inactivation of ptsN. A functional link between EIFrw and EIIANtr was therefore suggested (555).
A connection between EIIANtr and the regulation of branched-chain amino acid metabolism in E. coli was reported (456). The genes encoding the three heterodimeric E. coli acetohydroxy acid synthases, which catalyze the first step in leucine, isoleucine, and valine biosynthesis, are located in three different operons (891). One of the gene pairs (ilvGM) is normally not expressed due to a frameshift mutation. Among the other two gene pairs (ilvBN and ilvIH), the former is responsible for the majority of the enzyme activity (276, 277). The proteins encoded by ilvBN and ilvIH are sensitive to feedback inhibition by valine. As a consequence, wild-type cells exposed to valine as the sole amino acid or nitrogen source cannot make isoleucine and therefore cannot grow (452). Exposure to leucine-containing dipeptides also leads to an impairment of growth and a considerable increase in the intracellular leucine concentration (862). Besides abolishing the growth on leucine-containing dipeptides altogether, inactivation of ptsN completely prevents E. coli cells from growing on leucine and serine and reduces growth on isoleucine (456). Introduction of a plasmid encoding EIIANtr partly restores growth on these compounds, whereas transformants with a plasmid encoding His73Ala mutant EIIANtr (cannot be phosphorylated) grow even better than wild-type cells. Further experiments showed that unphosphorylated EIIANtr mediates derepression of the ilvBN operon and thereby enables the cells to synthesize the other branched-chain amino acids when grown on leucine, isoleucine, or serine.
Transcription regulation of the TOL plasmid of P. putida.
In P. putida, EIIANtr affects the regulation of gene expression from the TOL plasmid. The TOL plasmid harbors the genes necessary for the metabolism of toluene and xylenes and contains four transcription units: a so-called upper pathway and a meta pathway operon flanked by the promoters Pu and PM, respectively, and the regulator-encoding genes xylS and xylR connected to the promoters PS and PR, respectively (28, 388). Transcription from Pu and PS1 is σ54 dependent and is subject to CCR: it is low when cells grow exponentially on rich medium and is also down-regulated by glucose or gluconate but not by fructose (22, 101, 103). Repression of Pu (101, 103, 104) and PS1 (199, 281) transcription by rich medium or glucose is partially relieved (recovery of 50% of the activity) by the inactivation of ptsN or the catabolite repression control protein-encoding gene, crc (22). Relief of repression is achieved only in the presence of the inducer o-xylene (22), which increases the affinity of the activator XylR for Pu and PS1 (57, 514). Moreover, expression of xylR is repressed by rich medium, and this repression is absent in a ptsN mutant, whereas a crc mutation has no effect (22, 514). Thus, it seems that EIIANtr interferes with the activity of the transcription activator XylR, whereas repression via Crc should follow another mechanism (22). The involvement of Crc in the regulation of several sugar catabolic pathways (747) suggests a connection between the various metabolism-controlling regulatory networks (22). This complies with an observed down-regulation of Pu expression mediated via products of the Entner-Doudoroff pathway, where 6-phosphogluconate and/or 2-dehydro-3-deoxyphosphogluconate was identified as the intermediate that exerts repression (916).
Studies on active-site mutants suggest that the phosphorylated form of EIIANtr mediates repression of Pu and PS1. It was found that a mutant producing His68Asp EIIANtr is permanently repressed, the aspartyl residue is thought to mimic a phosphorylated histidine, whereas a mutant producing His68Ala EIIANtr is not (103). Surprisingly, inactivation of npr does not relieve inhibition by glucose but leads to repression in the absence of glucose, a phenotype resembling that of the His68Asp ptsN mutant (104). A ptsN npr double mutant has a ptsN-like phenotype, and a mutant in which the conserved histidine of NPr has been replaced with an alanine exhibits the same phenotype as the npr-disrupted mutant. Although a P. putida ptsP gene has been identified, its inactivation had no effect. Taking all of these findings into account, it appears that EIIANtr of P. putida is partly phosphorylated during growth on glucose, contrary to what is seen for EIIAGlc in E. coli. The presumed partial phosphorylation of the PTS components in the presence of glucose might be explained by the fact that glucose is not a PTS sugar in P. putida (103). Because the uptake of glucose by P. putida does not use but yields PEP, it is possible that the PEP-to-pyruvate ratio is raised by the glucose catabolism, which in turn might increase the phosphorylation state of PTS proteins, including EIIANtr. At the same time, the lack of repression by fructose can be explained by the fact that in P. putida, fructose uptake occurs via a PTS and thus lowers the concentration of P∼EIIANtr.
Other connections of the EIIANtr.
Inactivation of ptsN (and thereby also npr) in Pseudomonas aeruginosa impairs growth on minimal medium A (no carbon source mentioned) (377). Growth can be restored by the addition of glutamine, but not arginine, histidine, proline, or glutamate, and by trans-complementation (with only npr). There was no effect of ptsN overexpression or inactivation on the expression of the σ54-regulated pilin or flagellin genes. Likewise, in Caulobacter crescentus, expression from several σ54-dependent flagellar genes was unaffected by a ptsN mutation, although expression from the fljK promoter was slightly lower (371). Several bacteria, including numerous proteobacteria and the spirochetes Treponema pallidum, T. denticola, and Leptospira interrogans, the green photosynthetic bacterium Chlorobium tepidum, and the δ-proteobacterium Geobacter metallireducens, encode an EI, an HPr, an HPrK/P, and either one or two EIIANtr homologs within their genome but lack any known EIIB and EIIC (64, 345, 841). In T. pallidum, the EI-encoding gene seems to be an inactive pseudogene. Furthermore, it was shown in vitro that the T. denticola PTSNtr proteins are functional in phosphotransfer and that HPr is phosphorylated by HPrK/P requiring a much lower concentration of fructose-1,6-bisphosphate than the enzyme from B. subtilis for full activity (278).
Potential roles of EINtr.
EINtr, which can easily be detected in numerous proteobacteria due to its typical N-terminal extension, exerts diverse functions. Azotobacter vinelandii does not transport carbohydrates via the PTS (44, 740), and a ptsP mutation hardly affects growth on carbon sources like fructose, mannitol, sucrose, acetate, pyruvate, or succinate (793). However, growth of a ptsP mutant on glucose or glycerol is drastically slowed, although glucose uptake and phosphorylation are not affected. The impeded growth was traced to the inactivation of the enzyme nitrogenase, and the suggestion was made that respiratory protection of nitrogenase under carbon-limiting conditions is absent in the ptsP mutant (793). At the same time, it was observed that the inactivation of the ptsP gene in A. vinelandii reduces the capacity of the organism to accumulate poly-β-hydroxybutyrate (793), a carbohydrate storage molecule made from acetyl-CoA building blocks (397). The signaling pathway might also include NPr and EIIANtr, as genome sequencing has revealed the presence of both ptsN and npr in the A. vinelandii rpoN operon (551). Similarly, “leaky” Ralstonia eutropha phbI and phbH mutants show diminished accumulation of poly-β-hydroxybutyrate (687). A close relationship between phbI and phbH and the ptsP and npr genes of E. coli was established (680), although the PhbI protein of R. eutropha lacks the NifA-like extension typical of other EINtrs.
Mutants of P. aeruginosa (854) or Legionella pneumophila (333) affected in ptsP do not show an obvious phenotype when grown in rich medium. However, the ptsP mutants have almost completely lost their virulence due to strongly reduced growth after infection of nematodes, plants, mice, guinea pigs, or human epithelial cells. trans-Complementation with ptsP restores pathogenicity, which proves that the loss of virulence is due to the loss of functional EINtr. L. pneumophila stores poly-β-hydroxybutyrate in its cytoplasm as a nutrition source for long-term starvation survival (370). ptsP mutants showed normal accumulation of the carbon storage molecule when grown in rich medium, but unfortunately, poly-β-hydroxybutyrate levels were not monitored after infection of host cells (333).
In B. japonicum, ptsP is located next to lysC (encoding an aspartokinase), but the two genes do not form an operon (412). Nevertheless, inactivation of lysC or ptsP prevented the utilization of Pro-Gly-Gly and other oligopeptides. In vitro “pull-down” experiments revealed that EINtr interacts with the aspartokinase. Purified EINtr is phosphorylated by ATP in the presence of cell extracts, and the phosphorylation is prevented when aspartokinase is added (412).
What is the function of the PTSNtr?
The discovery of paralogous PTS proteins associated with the transcriptional regulation of genes involved in nitrogen assimilation has provided candidates for a regulatory connection between nitrogen and carbon metabolism. In fact, a potential PTSNtr phosphorylation cascade is present in many bacteria, but a coherent concept for a signaling pathway is lacking. Growth of ptsN null mutants on poor nitrogen sources is impaired, especially when a TCA cycle intermediate is used as the sole carbon source, whereas growth on rich nitrogen media (glutamine and NH4+) is not affected. The PTSNtr therefore seems to enable bacteria to grow (faster) on poor nitrogen sources and TCA cycle intermediates. A similar role in adaptation to changes in growth conditions can be attributed to EINtr of P. aeruginosa and L. pneumophila, considering that ptsP mutants of these pathogens grow normally in rich medium but grow very slowly after infection of a host cell. The effect of ptsN, npr, and ptsP mutations on gene expression is less clear. A connection to σ54-directed transcription is conceivable, because EIIANtr and NPr are encoded within the rpoN operon and EINtr has a putative regulatory domain present in other σ54-interacting proteins, but no data showing exactly how σ54-regulated genes are controlled by the PTSNtr are available. The amount of σ54 present in the cell does not seem to be controlled by PTSNtr, as it is always low and is affected neither by growth on glucose nor by ptsN or npr mutations (388). Many effects of the ptsN, npr, or ptsP mutations, such as those on melanin production, poly-β-hydroxybutyrate accumulation, Pu promoter expression, or era activity, seem to be unrelated to nitrogen metabolism. Two-dimensional gel electrophoresis with protein extracts prepared from P. putida wild-type or rpoN and ptsN mutant strains indicated that the role of EIIANtr in σ54-mediated transcription can be only minor. On the other hand, in a P. putida ptsN mutant, the level of about 9% of the proteins is altered (102).
Also, whether the PTSNtr indeed forms a functional phosphorylation cascade remains uncertain. Some organisms contain only part of the system. In R. etli, the rpoN operon contains ptsN but seems to lack npr, while ptsA (EIFrw) was identified based on sequence similarity (555). Inactivation of ptsA has effects similar to those of the inactivation of ptsN, suggesting a connection between EIFrw and EIIANtr. Moreover, genome analysis revealed that several proteobacteria, chlamydiae, and spirochetes lack any known EIIC permease but do contain EI, HPr, or both in combination with one or more components of the putative PTSNtr (41, 64). Although the PTSNtr is thought to require the phosphoryl group transfer P∼EINtr→P∼NPr→P∼EIIANtr in order to carry out its regulatory functions, the mixed appearance of the components in several bacteria suggests functional cross talk between the PTSNtr and “regular” PTS components. In fact, EIIANtr of the spirochete T. denticola (278) and EIIANtr and NPr of E. coli (680) can be phosphorylated in vitro by the regular PTS proteins. Interestingly, Powell et al. (680) found that in vitro NPr almost completely dephosphorylates P∼EIIANtr, implying that phosphoryl group transfer occurs in the opposite direction. In that case, EIIANtr, which is phosphorylated via EI and HPr, could communicate the phosphorylation state of the PTS proteins to NPr and EINtr. Nevertheless, as NPr was present in about an eightfold excess of both EI and EIIANtr in the phosphorylation assays, one might expect the dephosphorylation of P∼EI and P∼EIIANtr independent of which phosphoryl transfer direction is favored. Moreover, the finding that unphosphorylated EIIANtr mediates derepression of the ilvBN operon in E. coli and that the inactivation of ptsP or npr renders the derepression constitutive suggests a “normal” direction of the group transfer (456).
A comparison of the crystal structures of E. coli EIIANtr and the EIIAMtl domain shows that the fold of both proteins as well as the structure around the phosphorylation site, His-73 in EIIANtr and His-65 in EIIAMtl (703), are nearly identical (73, 905). However, the second conserved histidine, His-120 in EIIANtr and His-111 in EIIAMtl, respectively, is differently oriented. His-111 of EIIAMtl is part of the active site, whereas His-120 of EIIANtr is located in the vicinity but points away from the active site. Similar observations were made with the crystal structure of N. meningitidis EIIANtr (724). Interestingly, EIIANtr of H. influenzae lacks equivalents of the two histidines of the phosphorylation site altogether (717). These findings are consistent with a regulatory role of EIIANtr through binding rather than through phosphoryl group transfer. Perhaps phosphorylation of the PTSNtr proteins serves only to alter binding affinities. On the other hand, the solution structure of the NPr:EIIANtr complex suggests that the protein interface resembles that in the EIIAGlc:HPr complex (927).
CONCLUSIONS AND PERSPECTIVES
From an evolutionary point of view, it is likely that the PTS first served as a carbohydrate transport system before its numerous regulatory functions emerged. Although there is no direct evidence supporting this view, it is tempting to assume that during the course of evolution, the PTS grew in complexity by acquiring additional proteins/domains, which allowed additional regulatory functions. In this context, it is interesting that some PTS components resemble certain non-PTS enzymes, which, during catalysis, also become phosphorylated at histidyl or cysteyl residues. For example, EIIBs of the lactose/cellobiose class PTS exhibit structural similarity to low-molecular-weight P-tyrosine protein phosphatases (907) from either prokaryotes (such as YfkJ and YwlE of B. subtilis) (556) or eukaryotes. Proteins of both classes become phosphorylated at a cysteyl residue, which is located close to the N terminus and is surrounded by hydrophobic amino acids (132, 634). The sequence similarity between the lactose/cellobiose EIIBs and low-molecular-weight P-tyrosine protein phosphatases seems to be restricted to their N-terminal parts. Similarly, the active site of EIIAs of the mannose class PTS resembles the catalytic site in cofactor-dependent phosphoglycerate mutase and phosphatases dephosphorylating either low-molecular-weight substrates (such as eukaryotic fructose-2,6-bisphosphatase as well as E. coli glucose-1-P phosphatase and acid polyphosphatase AppA) or sensor kinases of two-component systems (such as E. coli SixA, which dephosphorylates P∼ArcB) (Deutscher, unpublished). Common to these phosphatases is the R-H-G signature (620), which is preceded by four to five hydrophobic residues. The histidine probably functions as a phosphoryl acceptor during catalysis. For E. coli phosphoglycerate mutase 1 (GpmA), a partially phosphorylated histidine, which is part of the R-H-G motif and is located in position 10, was observed in the crystal structure (69). The enzyme becomes phosphorylated during the interconversion of 1,3- and 2,3-bisphosphoglycerate. The phosphorylatable histidine of E. coli EIIAMan is also located in position 10 and is part of the T-H-G signature, which, similar to the R-H-G motif in the above-mentioned phosphatases, is preceded by several hydrophobic amino acids. It is not clear whether the similarities between PTS components and certain enzymes, mainly transiently phosphorylated phosphatases, result from divergent or convergent evolution.
It is also surprising that the two groups of bacteria that are mainly discussed in this review, i.e., Enterobacteriaceae and Firmicutes, both use PTS components for the complex regulation of carbohydrate metabolism but that the mechanisms of PTS-mediated regulation are often completely different. It has been pointed out that while EIIAGlc is the master regulator in Enterobacteriaceae, HPr carries out this function in low-G+C gram-positive bacteria. For CCR, P∼EIIAGlc stimulates the activity of adenylate cyclase and thereby activates Crp in Enterobacteriaceae, and P-Ser-HPr interacts with and stimulates CcpA in Firmicutes. For inducer exclusion, dephospo-EIIAGlc interacts with non-PTS permeases in Enterobacteriaceae, and P-Ser-HPr most likely interacts with non-PTS permeases in Firmicutes. For the regulation of GlpK, dephospo-EIIAGlc inhibits the catabolic enzyme by binding to its C-terminal part in Enterobacteriaceae, and P∼His-HPr phosphorylates a histidyl residue in the N-terminal half of GlpK and thereby stimulates the glycerol kinase activity in Firmicutes. According to today's commonly accepted branching order, the Firmicutes branched off first, and the γ-Proteobacteria, to which the Enterobacteriaceae belong, branched off last (303). P-Ser-HPr formation in firmicutes probably developed after branching off, and the occurrence of HprK/P in several proteobacteria might be the result of horizontal gene transfer. These proteobacteria usually contain EI, two EIIAs, and an HPr, in which the region around Ser-46 resembles the corresponding region in HPr of low-G+C gram-positive bacteria, but lack CcpA (41, 64). The role of P-Ser-HPr formation, which is also regulated by metabolites in most proteobacteria, remains obscure. Similarly, proteins with PRDs are much more abundant in firmicutes, and their rare appearance in proteobacteria probably also results from horizontal gene transfer. The early branching off of the firmicutes might partly explain why completely different mechanisms for the regulation of carbon metabolism evolved in the two phyla. At the same time, it underlines the importance of the PTS, as in the two groups of bacteria, evolution favored the development of mechanisms for the regulation of carbon metabolism and other cellular functions, in which PTS components play a major role, but apparently came up with quite different solutions for Enterobacteriaceae and Firmicutes.
Whatever the course of evolution may have been, the vast number of physiological processes controlled by the PTS makes it difficult to decide whether the main function of the PTS lies in carbohydrate transport or in regulation of cellular processes. The majority of PTS-mediated control mechanisms relate to the phosphorylation state of the PTS components and, therefore, to the energy state of the cell. Among others, the PTS senses the PEP-to-pyruvate ratio and the intracellular concentrations of ATP, glycolytic intermediates, PPi, etc., and its regulatory functions are therefore directly connected to the carbon and energy supply of the cell or, to use a more general term, to the fitness of the cell. The PTS-mediated regulatory functions can affect carbohydrate chemotaxis, the transport process, carbohydrate-specific metabolic steps, central metabolic pathways, and synthetic routes for secondary metabolites. In addition, specific mechanisms aimed at coordinating carbon metabolism and the metabolism of other essential elements, such as nitrogen and phosphorus, have developed. The scope of all these regulatory processes is to guarantee an optimal supply of carbon and energy without taking up too much of a carbon source. The latter might lead to futile cycles or might even have toxic effects. Although the general scope of the regulatory functions is very similar in gram-positive and gram-negative bacteria, the control mechanisms themselves can vary greatly.
Although a large number of PTS-mediated control mechanisms has been established, new PTS-related regulatory functions continue to emerge. An interesting new area is the relationship between the PTS and the pathogenicity of certain organisms. A connection between the PTS and the virulence of certain pathogens was suggested by the observation that some virulence genes underlie a kind of CCR. We extensively discussed this phenomenon for L. monocytogenes, where the diminished phosphorylation of PTS proteins during the uptake of glucose, fructose, cellobiose, etc., seems to be responsible for the inhibition of PrfA, a transcription activator of numerous virulence genes in this organism. In C. difficile, an important causative agent of nosocomial antibiotic-associated diarrhea and colitis, glucose and other rapidly metabolizable carbon sources inhibit the synthesis of toxin A and toxin B (202). It is not clear whether CCR of the toxA and toxB genes is mediated via P-Ser-HPr/CcpA or whether the repression of toxin synthesis occurs via another mechanism possibly involving the alternative σ factor TxeR, which is required for the expression of the tox genes (509). Synthesis of the catabolite-regulated transcription activator Mga, which is required for the expression of numerous surface-associated proteins involved in pathogenesis in S. pyogenes, seems to be controlled directly by CcpA. A connection between CcpA and virulence also exists in S. pneumoniae, where the inactivation of CcpA affects the synthesis of capsular polysaccharides and diminishes the virulence of this organism (273). Inactivation of the EI paralog PtsP in P. aeruginosa (854) or L. pneumophila (333) led to strongly reduced virulence, because the ptsP mutants exhibited reduced growth in their hosts. In addition, some EII components seem to play a role in the pathogenicity of certain bacteria. For example, inactivation of the Streptococcus suis manN gene, which codes for an EIID of the mannose class PTS, caused a hyperhemolytic phenotype due to the elevated synthesis of suilysin, the hemolysin produced by this organism. A mutant complementation test established that the presence of ManN lowers the synthesis of suilysin (496). Inactivation of an EIIABC of the glucose class PTS in S. mutans, the primary agent of dental caries in humans, led to the overproduction of the virulence factor fructan hydrolase. On the other hand, a mutant lacking the mannose-specific EIIAB exhibited an impaired capacity to form biofilms, which is probably due to the diminished synthesis of exopolysaccharide-forming glucosyltransferases (gtfBC) (5). EIIAs of the fructose and mannose class PTS were also proposed to play a role in the virulence of numerous gram-negative bacteria, which have in common that they possess an HprK/P and an incomplete PTS that is usually composed of an EI, an HPr, and one or two EIIAs. These bacteria therefore cannot transport sugars via the PTS, and P-Ser-HPr probably plays no role in CcpA-mediated CCR, as these organisms lack CcpA. Preliminary results suggesting a potential involvement of the incomplete PTS of these gram-negative bacteria in virulence regulation are discussed in this review.
A connection between the sensitivity/resistance of certain bacteria towards class IIa bacteriocins and PTS proteins has been reported. For example, the EIIAB component of a mannose class PTS (called Mpt) was found to be absent from several spontaneous leucocin A-resistant L. monocytogenes mutants (693), while overproduction of a β-glucoside-specific PTS in the same organism led to pediocin PA-1 resistance (294). The role of the Mpt PTS in class IIa bacteriocin resistance has been studied in more detail. The specificity of the Mpt PTS has not been determined, but it is likely to be the mannose/glucose-specific PTS, as its expression in L. monocytogenes is induced by these two sugars (151). In addition, the EIID domain of the Mpt PTS possesses a C-terminal extension characteristic of mannose/glucose-specific PTS, and the various sugar-specific EIIMpt components exhibit the strongest similarity to those of the recently characterized mannose/glucose PTS of L. casei (975). Homologs of the Mpt PTS are present in E. faecalis and Listeria innocua, and the absence of expression of the mpt operon in these organisms also leads to resistance towards class IIa bacteriocins such as mesentericin Y105 for E. faecalis (320) and pediocin AcH for L. innocua (959). The expression of the mpt operon containing the mptA, mptC, and mptD genes, which encode EIIAB, EIIC, and EIID, respectively, depends on σ54 (RpoN) and on a LevR-like protein, which was called ManR. In addition, a novel activator for the mpt genes, which distantly resembles proteins of the Crp/Fnr family, has been identified in L. innocua (Lin0142) and is also present in L. monocytogenes (Lmo0095) (959). It contains a winged-helix DNA binding motif, and its gene precedes the mpt operon. Its inactivation prevents mpt expression and leads to pediocin AcH resistance. It seems that both membrane components of the mannose/glucose PTS play a role in bacteriocin sensitivity. An L. monocytogenes strain carrying a 28-amino-acid in-frame deletion in the C-terminal part of EIIDMpt was fully resistant to mesentericin Y105 (151). On the other hand, expression of L. monocytogenes mptC in L. lactis rendered the latter organism sensitive towards class IIa bacteriocins (692). It was therefore proposed that the membrane components of the mannose/glucose PTS are necessary for the docking of the bacteriocin. Interestingly, the E. coli membrane-spanning mannose-specific PTS components are also important for the infection by bacteriophage lambda (218, 950), and they can be replaced in lambda infection by the B. subtilis PTS components of the lev operon (525).
PTS proteins also seem to play a role in certain stress responses. Some PTS components are either overproduced or barely synthesized in response to a specific stress. For example, HPr of L. lactis is strongly diminished or almost vanishes in cells that had been exposed to a pH of 5.5 or 4.5, respectively (243). In contrast, E. coli cells exposed to low pH contain elevated amounts of the PTS components HPr and EIIABMan (ManX) (63). Similarly, osmotic stress was found to increase the amount of CcpA and of an EIIA of a mannose class PTS in L. monocytogenes (200), while a cold shock led to the overproduction of HPr in B. subtilis (293) and L. lactis (956). Finally, the induction of the E. coli ompC gene in response to increasing temperatures was prevented by a specific mutation in the TD2 strain. A gene (hrsA, for heat response suppressor) that restored the thermoresponse in the TD2 mutant was identified (892). The hrsA gene codes for a fructose-like EIIABC, and it was renamed mngA when it was found to catalyze the transport of the osmolyte 2-O-α-mannosyl-d-glycerate (775). A connection between the cold shock response and the PTS was also inferred by the finding that an L. casei ptsH(Ile47Thr) mutant overproduced CspA (47), one of the small cold shock proteins found in most bacteria. Csps are normally among the first proteins overproduced in response to a cold shock. Interestingly, Csps exhibit sequence and structure similarities to the C-terminal part of EIIAs of the glucose class PTS. They contain a histidine homologous to the phosphorylatable His-91 in E. coli EIIAGlc (47), which, however, could not be phosphorylated by PEP, EI, and HPr. Nevertheless, this histidine is important for Csp function (659). In addition, various L. casei mutants affected in CCR exhibited reduced growth rates at low temperatures compared to that of the wild-type strain. Finally, the two mutants that were unable to form P-Ser-HPr (ptsH1 and hprK) showed an almost 100-fold-reduced survival rate after repeated freezing and thawing compared to the wild-type strain and other ptsH and ccpA mutants (47). It is not yet understood how the ability to form P-Ser-HPr increases the resistance to freezing and thawing.
PTS components not only interact with other proteins and enzymes to regulate the activity of their targets but they are sometimes found as domains in non-PTS proteins, suggesting that the activity of these hybrid proteins is controlled by the PTS domain, most likely in a phosphorylation-dependent manner. The examples of the lactose and raffinose transporters, which contain a regulatory EIIAGlc-like domain, and of transcription activators, which possess an EIIBGat and either an EIIAMan or EIIAMtl domain, have been discussed in detail. Another example of a non-PTS protein containing a domain of a PTS component is the C. acetobutylicum multidomain HprR protein (for HPr response regulator), parts of which exhibit significant sequence identity (about 40%) to several response regulators of two-component systems (B. subtilis BkdR, RocR, and AcoR and E. coli NtrC). Interestingly, instead of the usual N-terminal receiver module, HprR possesses an HPr-like domain, which contains a phosphorylatable His-15 (721), while Ser-46 is missing. It is therefore tempting to assume that HprR activity is regulated by phosphorylation at its His-15 or via interactions of the HPr domain with EI or EIIAs.
Pyruvate kinase of G. stearothermophilus contains a C-terminal domain that strongly resembles the phosphorylation domain of EI (599). From the numerous available genome sequences, it became clear that, in fact, most gram-positive bacteria possess a pyruvate kinase with an EI-resembling C-terminal extension. The phosphorylatable histidine is nicely conserved (His-539 in G. stearothermophilus and B. subtilis pyruvate kinase), and it was therefore proposed that pyruvate kinase of gram-positive bacteria might be regulated via PTS-catalyzed phosphorylation. However, attempts to phosphorylate the EI domain of B. subtilis pyruvate kinase with PEP or PEP plus EI and HPr have not been successful so far (G. Boël and J. Deutscher, unpublished results). In fact, the EI domain of pyruvate kinase from gram-positive bacteria shows more extended sequence similarity to PEP synthases.
The above-mentioned examples make it clear that we are far from understanding all the regulatory roles of the PTS. It seems almost certain that future research will lead to the discovery of as-yet-unimagined regulatory functions of this complex sugar transport and global cellular control system.
Acknowledgments
We are thankful to R. Brückner, A. Galinier, W. Hengstenberg, I. Martin-Verstraete, A. Mazé, W. Mitchell, C. Vadeboncoeur, and M. Zagorec for helpful suggestions and T. J. G. Wilson for critically reading the manuscript. C.F. thanks H. V. Westerhoff, K. J. Hellingwerf, and R. J. Siezen for their support.
We acknowledge the support of the University of Amsterdam, the Free University in Amsterdam, and the Wageningen Centre for Food Sciences as well as of the CNRS, the INRA, the INA-PG, and the Alexander von Humboldt Foundation.
Footnotes
Pieter W. Postma passed away during the preparation of this review. The loss that we experienced has been put into words by Gary R. Jacobson and Joseph W. Lengeler (368).
REFERENCES
- 1.Ab, E., G. Schuurman-Wolters, J. Reizer, M. H. Saier, Jr., K. Dijkstra, R. M. Scheek, and G. T. Robillard. 1997. The NMR side-chain assignments and solution structure of enzyme IIB(cellobiose) of the phosphoenolpyruvate-dependent phosphotransferase system of Escherichia coli. Protein Sci. 6:304-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ab, E., G. K. Schuurman-Wolters, D. Nijlant, K. Dijkstra, M. H. Saier, Jr., G. T. Robillard, and R. M. Scheek. 2001. NMR structure of cysteinyl-phosphorylated enzyme IIB of the N,N′-diacetylchitobiose-specific phosphoenolpyruvate-dependent phosphotransferase system of Escherichia coli. J. Mol. Biol. 308:993-1009. [DOI] [PubMed] [Google Scholar]
- 3.Abramson, J., I. Smirnova, V. Kasho, G. Verner, S. Iwata, and H. R. Kaback. 2003. The lactose permease of Escherichia coli: overall structure, the sugar-binding site and the alternating access model for transport. FEBS Lett. 555:96-101. [DOI] [PubMed] [Google Scholar]
- 4.Abramson, J., I. Smirnova, V. Kasho, G. Verner, H. R. Kaback, and S. Iwata. 2003. Structure and mechanism of the lactose permease of Escherichia coli. Science 301:610-615. [DOI] [PubMed] [Google Scholar]
- 5.Abranches, J., M. M. Candella, Z. T. Wen, H. V. Baker, and R. A. Burne. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J. Bacteriol. 188:3748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adhya, S. 1996. The lac and gal operons today, p. 181-200. In E. C. C. Lin and A. S. Lynch (ed.), Regulation of gene expression in Escherichia coli. R.G. Landes Company, Austin, Tex.
- 7.Adhya, S., and H. Echols. 1966. Glucose effect and the galactose enzymes of Escherichia coli: correlation between glucose inhibition of induction and inducer transport. J. Bacteriol. 92:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler, J., and W. Epstein. 1974. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc. Natl. Acad. Sci. USA 71:2895-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiba, H. 1983. Autoregulation of the Escherichia coli crp gene: CRP is a transcriptional repressor for its own gene. Cell 32:141-149. [DOI] [PubMed] [Google Scholar]
- 10.Aiba, H. 1985. Transcription of the Escherichia coli adenylate cyclase gene is negatively regulated by the cAMP-cAMP receptor protein. J. Biol. Chem. 260:3063-3070. [PubMed] [Google Scholar]
- 11.Alexandre, G., and I. B. Zhulin. 2001. More than one way to sense chemicals. J. Bacteriol. 183:4681-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali, N. O., J. Bignon, G. Rapoport, and M. Debarbouille. 2001. Regulation of the acetoin catabolic pathway is controlled by sigma L in Bacillus subtilis. J. Bacteriol. 183:2497-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen, G. S., K. Steinhauer, W. Hillen, J. Stülke, and R. G. Brennan. 2003. Crystal structure of HPr kinase/phosphatase from Mycoplasma pneumoniae. J. Mol. Biol. 326:1203-1217. [DOI] [PubMed] [Google Scholar]
- 14.Almengor, A. C., and K. S. McIver. 2004. Transcriptional activation of sclA by Mga requires a distal binding site in Streptococcus pyogenes. J. Bacteriol. 186:7847-7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almengor, A. C., M. S. Walters, and K. S. McIver. 2006. Mga is sufficient to activate transcription in vitro of sof-sfbX and other Mga-regulated virulence genes in the group A streptococcus. J. Bacteriol. 188:2038-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alpert, C.-A., R. Frank, K. Stüber, J. Deutscher, and W. Hengstenberg. 1985. Phosphoenolpyruvate-dependent protein kinase enzyme I of Streptococcus faecalis. Purification and properties of the enzyme and characterization of its active center. Biochemistry 24:959-964. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez, M. D. F., R. Medina, S. E. Pasteris, A. M. S. de Saad, and F. Sesma. 2004. Glycerol metabolism of Lactobacillus rhamnosus ATCC 7469: cloning and expression of two glycerol kinase genes. J. Mol. Microbiol. Biotechnol. 7:170-181. [DOI] [PubMed] [Google Scholar]
- 18.Amster-Choder, O., F. Houman, and A. Wright. 1989. Protein phosphorylation regulates transcription of the β-glucoside utilization operon in E. coli. Cell 58:847-855. [DOI] [PubMed] [Google Scholar]
- 19.An, C. L., W. J. Lim, S. Y. Hong, E. J. Kim, E. C. Shin, M. K. Kim, J. R. Lee, S. R. Park, J. G. Woo, Y. P. Lim, and H. D. Yun. 2004. Analysis of bgl operon structure and characterization of β-glucosidase from Pectobacterium carotovorum subsp. carotovorum LY34. Biosci. Biotechnol. Biochem. 68:2270-2278. [DOI] [PubMed] [Google Scholar]
- 20.Andersen, C., B. Rak, and R. Benz. 1999. The gene bglH present in the bgl operon of Escherichia coli, responsible for uptake and fermentation of β-glucosides encodes for a carbohydrate-specific outer membrane porin. Mol. Microbiol. 31:499-510. [DOI] [PubMed] [Google Scholar]
- 21.Angell, S., C. G. Lewis, M. J. Buttner, and M. J. Bibb. 1994. Glucose repression in Streptomyces coelicolor A3(2)—a likely regulatory role for glucose kinase. Mol. Gen. Genet. 244:135-143. [DOI] [PubMed] [Google Scholar]
- 22.Aranda-Olmedo, I., J. L. Ramos, and S. Marqués. 2005. Integration of signals through Crc and PtsN in catabolite repression of Pseudomonas putida TOL plasmid pWW0. Appl. Environ. Microbiol. 71:4191-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnaud, M., M. Débarbouillé, G. Rapoport, M. H. Saier, Jr., and J. Reizer. 1996. In vitro reconstitution of transcriptional antitermination by the SacT and SacY proteins of Bacillus subtilis. J. Biol. Chem. 271:18966-18972. [DOI] [PubMed] [Google Scholar]
- 24.Arnaud, M., P. Vary, M. Zagorec, A. Klier, M. Débarbouillé, P. Postma, and G. Rapoport. 1992. Regulation of the sacPA operon of Bacillus subtilis: identification of phosphotransferase components involved in SacT activity. J. Bacteriol. 174:3161-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arous, S., C. Buchrieser, P. Folio, P. Glaser, A. Namane, M. Hebraud, and Y. Hechard. 2004. Global analysis of gene expression in an rpoN mutant of Listeria monocytogenes. Microbiology 150:1581-1590. [DOI] [PubMed] [Google Scholar]
- 26.Asanuma, N., and T. Hino. 2003. Molecular characterization of HPr and related enzymes, and regulation of HPr phosphorylation in the ruminal bacterium Streptococcus bovis. Arch. Microbiol. 179:205-213. [DOI] [PubMed] [Google Scholar]
- 27.Asanuma, N., T. Yoshii, and T. Hino. 2004. Molecular characterization of CcpA and involvement of this protein in transcriptional regulation of lactate dehydrogenase and pyruvate formate-lyase in the ruminal bacterium Streptococcus bovis. Appl. Environ. Microbiol. 70:5244-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assinder, S. J., and P. A. Williams. 1990. The TOL plasmids: determinants of the catabolism of toluene and the xylenes. Adv. Microb. Physiol. 31:1-69. [DOI] [PubMed] [Google Scholar]
- 29.Audette, G. F., R. Engelmann, W. Hengstenberg, J. Deutscher, K. Hayakawa, J. W. Quail, and L. T. J. Delbaere. 2000. The 1.9 Å resolution structure of phospho-serine 46 HPr from Enterococcus faecalis. J. Mol. Biol. 303:545-553. [DOI] [PubMed] [Google Scholar]
- 30.Aung-Hilbrich, L. M., G. Seidel, A. Wagner, and W. Hillen. 2002. Quantification of the influence of HPrSer46P on CcpA-cre interaction. J. Mol. Biol. 319:77-85. [DOI] [PubMed] [Google Scholar]
- 31.Aymerich, S., G. Gonzy-Tréboul, and M. Steinmetz. 1986. 5′-noncoding region sacR is the target of all identified regulation affecting the levansucrase gene in Bacillus subtilis. J. Bacteriol. 166:993-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aymerich, S., and M. Steinmetz. 1992. Specificity determinants and structural features in the RNA target of the bacterial antiterminator proteins of the BglG/SacY family. Proc. Natl. Acad. Sci. USA 89:10410-10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azuaga, A. I., J. L. Neira, and N. A. van Nuland. 2005. HPr as a model protein in structure, interaction, folding and stability studies. Protein Pept. Lett. 12:123-137. [DOI] [PubMed] [Google Scholar]
- 34.Babu, M. M., and S. A. Teichmann. 2003. Evolution of transcription factors and the gene regulatory network in Escherichia coli. Nucleic Acids Res. 31:1234-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachem, S., and J. Stülke. 1998. Regulation of the Bacillus subtilis GlcT antiterminator protein by components of the phosphotransferase system. J. Bacteriol. 180:5319-5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bächler, C., K. Flükiger-Brühwiler, P. Schneider, P. Bähler, and B. Erni. 2005. From ATP as substrate to ADP as coenzyme: functional evolution of the nucleotide binding subunit of dihydroxyacetone kinase. J. Biol. Chem. 280:18321-18325. [DOI] [PubMed] [Google Scholar]
- 37.Bächler, C., P. Schneider, P. Bähler, A. Lustig, and B. Erni. 2005. Escherichia coli dihydroxyacetone kinase controls gene expression by binding to transcription factor DhaR. EMBO J. 24:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker, C. S., I. Morozov, K. Suzuki, T. Romeo, and P. Babitzke. 2002. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol. Microbiol. 44:1599-1610. [DOI] [PubMed] [Google Scholar]
- 39.Baker, M. E., and M. H. Saier, Jr. 1990. A common ancestor for bovine lens fiber major intrinsic protein, soybean nodulin-26 protein and E. coli glycerol facilitator. Cell 60:185-186. [DOI] [PubMed] [Google Scholar]
- 40.Balaeff, A., L. Mahadevan, and K. Schulten. 2004. Structural basis for cooperative DNA binding by CAP and lac repressor. Structure 12:123-132. [DOI] [PubMed] [Google Scholar]
- 41.Barabote, R. D., and M. H. Saier, Jr. 2005. Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol. Mol. Biol. Rev. 69:608-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bardowski, J., S. D. Ehrlich, and A. Chopin. 1994. BglR protein, which belongs to the BglG family of transcriptional antiterminators, is involved in β-glucoside utilization in Lactococcus lactis. J. Bacteriol. 176:5681-5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barford, D., A. K. Das, and M. P. Egloff. 1998. The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu. Rev. Biophys. Biomol. Struct. 27:133-164. [DOI] [PubMed] [Google Scholar]
- 44.Barnes, E. M., Jr. 1972. Respiration-coupled glucose transport in membrane vesicles from Azotobacter vinelandii. Arch. Biochem. Biophys. 152:795-799. [DOI] [PubMed] [Google Scholar]
- 45.Barrière, C., M. Veiga-da-Cunha, N. Pons, E. Guedon, S. A. van Hijum, J. Kok, O. P. Kuipers, D. S. Ehrlich, and P. Renault. 2005. Fructose utilization in Lactococcus lactis as a model for low-GC gram-positive bacteria: its regulator, signal, and DNA-binding site. J. Bacteriol. 187:3752-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bassias, J., and R. Brückner. 1998. Regulation of lactose utilization genes in Staphylococcus xylosus. J. Bacteriol. 180:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beaufils, S., N. Sauvageot, A. Mazé, J.-M. La Place, Y. Auffray, J. Deutscher, and A. Hartke. The cold shock response of Lactobacillus casei: relation between HPr phosphorylation and resistance to freeze/thaw cycles. J. Mol. Microbiol. Biotechnol., in press. [DOI] [PubMed]
- 48.Begley, G. S., D. E. Hansen, G. R. Jacobson, and J. R. Knowles. 1982. Stereochemical course of the reactions catalyzed by the bacterial phosphoenolpyruvate:glucose phosphotransferase system. Biochemistry 21:5552-5556. [DOI] [PubMed] [Google Scholar]
- 49.Begley, G. S., and G. R. Jacobson. 1994. Overexpression, phosphorylation, and growth effects of ORF162, a Klebsiella pneumoniae protein that is encoded by a gene linked to rpoN, the gene encoding σ54. FEMS Microbiol. Lett. 119:389-394. [DOI] [PubMed] [Google Scholar]
- 50.Behari, J., and P. Youngman. 1998. A homolog of CcpA mediates catabolite control in Listeria monocytogenes but not carbon source regulation of virulence genes. J. Bacteriol. 180:6316-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Behrens, S., W. J. Mitchell, and H. Bahl. 2001. Molecular analysis of the mannitol operon of Clostridium acetobutylicum encoding a phosphotransferase system and a putative PTS-modulated regulator. Microbiology 147:75-86. [DOI] [PubMed] [Google Scholar]
- 52.Beijer, L., and L. Rutberg. 1992. Utilisation of glycerol and glycerol 3-phosphate is differently affected by the phosphotransferase system in Bacillus subtilis. FEMS Microbiol. Lett. 100:217-220. [DOI] [PubMed] [Google Scholar]
- 53.Belitsky, B. R., H. J. Kim, and A. L. Sonenshein. 2004. CcpA-dependent regulation of Bacillus subtilis glutamate dehydrogenase gene expression. J. Bacteriol. 186:3392-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belitsky, B. R., and A. L. Sonenshein. 1998. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J. Bacteriol. 180:6298-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 56.Ben-Zeev, E., L. Fux, O. Amster-Choder, and M. Eisenstein. 2005. Experimental and computational characterization of the dimerization of the PTS-regulation domains of BglG from Escherichia coli. J. Mol. Biol. 347:693-706. [DOI] [PubMed] [Google Scholar]
- 57.Bertoni, G., S. Marqués, and V. de Lorenzo. 1998. Activation of the toluene-responsive regulator XylR causes a transcriptional switch between σ54 and σ70 promoters at the divergent Pr/Ps region of the TOL plasmid. Mol. Microbiol. 27:651-659. [DOI] [PubMed] [Google Scholar]
- 58.Bertram, R., M. Schlicht, K. Mahr, H. Nothaft, M. H. Saier, Jr., and F. Titgemeyer. 2004. In silico and transcriptional analysis of carbohydrate uptake systems of Streptomyces coelicolor A3(2). J. Bacteriol. 186:1362-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertram, R., A. Wünsche, M. Sprehe, and W. Hillen. 2006. Regulated expression of HPrK/P does not affect carbon catabolite repression of the xyn operon and of rocG in Bacillus subtilis. FEMS Microbiol. Lett. 259:147-152. [DOI] [PubMed] [Google Scholar]
- 60.Bertrand, K., C. Squires, and C. Yanofsky. 1976. Transcription termination in vivo in the leader region of the tryptophan operon of Escherichia coli. J. Mol. Biol. 103:319-337. [DOI] [PubMed] [Google Scholar]
- 61.Bischoff, D. S., and G. W. Ordal. 1992. Bacillus subtilis chemotaxis: a deviation from the Escherichia coli paradigm. Mol. Microbiol. 6:23-28. [DOI] [PubMed] [Google Scholar]
- 62.Bischoff, D. S., and G. W. Ordal. 1991. Sequence and characterization of Bacillus subtilis CheB, a homolog of Escherichia coli CheY, and its role in a different mechanism of chemotaxis. J. Biol. Chem. 266:12301-12305. [PubMed] [Google Scholar]
- 63.Blankenhorn, D., J. Phillips, and J. L. Slonczewski. 1999. Acid- and base-induced proteins during aerobic and anaerobic growth of Escherichia coli revealed by two-dimensional gel electrophoresis. J. Bacteriol. 181:2209-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boël, G., I. Mijakovic, A. Mazé, S. Poncet, M.-K. Taha, M. Larribe, E. Darbon, A. Khemiri, A. Galinier, and J. Deutscher. 2003. Transcription regulators potentially controlled by HPr kinase/phosphorylase in gram-negative bacteria. J. Mol. Microbiol. Biotechnol. 5:206-215. [DOI] [PubMed] [Google Scholar]
- 65.Böhm, A., and W. Boos. 2004. Gene regulation in prokaryotes by subcellular relocalization of transcription factors. Curr. Opin. Microbiol. 7:151-156. [DOI] [PubMed] [Google Scholar]
- 66.Böhm, A., J. Diez, K. Diederichs, W. Welte, and W. Boos. 2002. Structural model of MalK, the ABC subunit of the maltose transporter of Escherichia coli. Implications for mal gene regulation, inducer exclusion, and subunit assembly. J. Biol. Chem. 277:3708-3717. [DOI] [PubMed] [Google Scholar]
- 67.Bolotin, A., S. Mauger, K. Malarme, S. D. Ehrlich, and A. Sorokin. 1999. Low-redundancy sequencing of the entire Lactococcus lactis IL1403 genome. Antonie Leeuwenhoek 76:27-76. [PubMed] [Google Scholar]
- 68.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bond, C. S., M. F. White, and W. N. Hunter. 2001. High resolution structure of the phosphohistidine-activated form of Escherichia coli cofactor-dependent phosphoglycerate mutase. J. Biol. Chem. 276:3247-3253. [DOI] [PubMed] [Google Scholar]
- 70.Boos, W., and A. Böhm. 2000. Learning new tricks from an old dog: MalT of the Escherichia coli maltose system is part of a complex regulatory network. Trends Genet. 16:404-409. [DOI] [PubMed] [Google Scholar]
- 71.Boos, W., and J. M. Lucht. 1996. Periplasmic binding protein-dependent ABC transporters, p. 1175-1209. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 72.Boos, W., and H. Shuman. 1998. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 62:204-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bordo, D., R. L. M. van Monfort, T. Pijning, K. H. Kalk, J. Reizer, M. H. Saier, Jr., and B. W. Dijkstra. 1998. The three-dimensional structure of the nitrogen regulatory protein IIANtr from Escherichia coli. J. Mol. Biol. 279:245-255. [DOI] [PubMed] [Google Scholar]
- 74.Botfield, M. C., K. Naguchi, T. Tsuchiya, and T. H. Wilson. 1992. Membrane topology of the melibiose carrier of Escherichia coli. J. Biol. Chem. 267:1818-1822. [PubMed] [Google Scholar]
- 75.Botsford, J. L., and M. Drexler. 1978. The cAMP receptor protein and regulation of cAMP synthesis in Escherichia coli. Mol. Gen. Genet. 165:47-56. [DOI] [PubMed] [Google Scholar]
- 76.Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56:100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bourret, R. B., and A. M. Stock. 2002. Molecular information processing: lessons from bacterial chemotaxis. J. Biol. Chem. 277:9625-9628. [DOI] [PubMed] [Google Scholar]
- 78.Boyd, D. A., T. Thevenot, M. Gumbmann, A. L. Honeyman, and I. R. Hamilton. 2000. Identification of the operon for the sorbitol (glucitol) phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans. Infect. Immun. 68:925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bramley, H. F., and H. L. Kornberg. 1987. Nucleotide sequence of bglC, the gene specifying enzyme IIbgl of the PEP:sugar phosphotransferase system in Escherichia coli K12, and overexpression of the gene product. J. Gen. Microbiol. 133:563-573. [DOI] [PubMed] [Google Scholar]
- 80.Brana, H., and F. Chytil. 1966. Splitting of the cyclic 3′,5′-adenosine monophosphate in cell-free system of Escherichia coli. Folia Microbiol. 11:43-46. [DOI] [PubMed] [Google Scholar]
- 81.Brehm, K., M. T. Ripio, J. Kreft, and J. A. Vazquez-Boland. 1999. The bvr locus of Listeria monocytogenes mediates virulence gene repression by β-glucosides. J. Bacteriol. 181:5024-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bren, A., and M. Eisenbach. 2000. How signals are heard during bacterial chemotaxis: protein-protein interactions in sensory signal propagation. J. Bacteriol. 182:6865-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bringhurst, R. M., and D. J. Gage. 2002. Control of inducer accumulation plays a key role in succinate-mediated catabolite repression in Sinorhizobium meliloti. J. Bacteriol. 184:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brochu, D., and C. Vadeboncoeur. 1999. The HPr(Ser) kinase of Streptococcus salivarius: purification, properties, and cloning of the hprK gene. J. Bacteriol. 181:709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brokx, S. J., S. Napper, G. Wong, A. Mirza, F. Georges, L. T. J. Delbaere, and E. B. Waygood. 1999. Identification of the Escherichia coli enzyme I binding site in histidine-containing protein, HPr, by the effects of mutagenesis. Biochem. Cell Biol. 77:507-513. [PubMed] [Google Scholar]
- 86.Brokx, S. J., J. Talbot, F. Georges, and E. B. Waygood. 2000. Enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system. In vitro intragenic complementation: the roles of Arg126 in phosphoryl transfer and the C-terminal domain in dimerization. Biochemistry 39:3624-3635. [DOI] [PubMed] [Google Scholar]
- 87.Brown, G. D., and J. A. Thomson. 1998. Isolation and characterisation of an aryl-β-D-glucoside uptake and utilisation system (abg) from the gram-positive ruminal Clostridium species C. longisporum. Mol. Gen. Genet. 257:213-218. [DOI] [PubMed] [Google Scholar]
- 88.Browngardt, C. M., Z. T. Wen, and R. A. Burne. 2004. RegM is required for optimal fructosyltransferase and glucosyltransferase gene expression in Streptococcus mutans. FEMS Microbiol. Lett. 240:75-79. [DOI] [PubMed] [Google Scholar]
- 89.Browning, D. F., and S. J. W. Busby. 2004. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2:57-65. [DOI] [PubMed] [Google Scholar]
- 90.Brückner, R., and F. Titgemeyer. 2002. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209:141-148. [DOI] [PubMed] [Google Scholar]
- 91.Buck, M., M. T. Gallegos, D. J. Studholme, Y. Guo, and J. D. Gralla. 2000. The bacterial enhancer-dependent σ54 (σN) transcription factor. J. Bacteriol. 182:4129-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buhr, A., K. Flükiger, and B. Erni. 1994. The glucose transporter of Escherichia coli. Overexpression, purification, and characterization of functional domains. J. Biol. Chem. 269:23437-23443. [PubMed] [Google Scholar]
- 93.Burne, R. A., Z. T. Wen, Y.-Y. M. Chen, and J. E. C. Penders. 1999. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J. Bacteriol. 181:2863-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burstein, C., M. Cohn, A. Kepes, and J. Monod. 1965. Role of lactose and its metabolic products in the induction of the lactose operon in Escherichia coli. Biochim. Biophys. Acta 95:634-639. [PubMed] [Google Scholar]
- 95.Busby, S., and R. H. Ebright. 1999. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293:199-213. [DOI] [PubMed] [Google Scholar]
- 96.Busby, S., and A. Kolb. 1996. The CAP modulon, p. 255-279. In E. C. C. Lin and A. S. Lynch (ed.), Regulation of gene expression in Escherichia coli. R. G. Landes Company, Austin, Tex.
- 97.Butler, M. J., J. Deutscher, P. W. Postma, T. J. G. Wilson, A. Galinier, and M. J. Bibb. 1999. Analysis of a ptsH homologue from Streptomyces coelicolor A3(2). FEMS Microbiol. Lett. 177:279-288. [DOI] [PubMed] [Google Scholar]
- 98.Cai, M., D. C. Williams, Jr., G. Wang, B. R. Lee, A. Peterkofsky, and G. M. Clore. 2003. Solution structure of the phosphoryl transfer complex between the signal transducing protein IIAGlucose and the cytoplasmic domain of the glucose transporter IICBGlucose of the Escherichia coli glucose phosphotransferase system. J. Biol. Chem. 278:25191-25206. [DOI] [PubMed] [Google Scholar]
- 99.Calamita, G., W. R. Bishai, G. M. Preston, W. B. Guggino, and P. Agre. 1995. Molecular cloning and characterization of AqpZ, a water channel from Escherichia coli. J. Biol. Chem. 270:29063-29066. [DOI] [PubMed] [Google Scholar]
- 100.Cascales, E., K. Atmakuri, Z. Liu, A. N. Binns, and P. J. Christie. 2005. Agrobacterium tumefaciens oncogenic suppressors inhibit T-DNA and VirE2 protein substrate binding to the VirD4 coupling protein. Mol. Microbiol. 58:565-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cases, I., and V. de Lorenzo. 2000. Genetic evidence of distinct physiological regulation mechanisms in the σ54 Pu promoter of Pseudomonas putida. J. Bacteriol. 182:956-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cases, I., J.-A. Lopez, J.-P. Albar, and V. de Lorenzo. 2001. Evidence of multiple regulatory functions for the PtsN (IIANtr) protein of Pseudomonas putida. J. Bacteriol. 183:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cases, I., J. Perez-Martin, and V. de Lorenzo. 1999. The IIANtr (PtsN) protein of Pseudomonas putida mediates the C source inhibition of the σ54-dependent Pu promoter of the TOL plasmid. J. Biol. Chem. 274:15562-15568. [DOI] [PubMed] [Google Scholar]
- 104.Cases, I., F. Velazquez, and V. de Lorenzo. 2001. Role of ptsO in carbon-mediated inhibition of the Pu promoter belonging to the pWW0 Pseudomonas putida plasmid. J. Bacteriol. 183:5128-5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chandler, M. S. 1992. The gene encoding cAMP receptor protein is required for competence development in Haemophilus influenzae Rd. Proc. Natl. Acad. Sci. USA 89:1626-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chaptal, V., V. Gueguen-Chaignon, S. Poncet, C. Lecampion, P. Meyer, J. Deutscher, A. Galinier, S. Nessler, and S. Moréra. 2006. Structural analysis of B. subtilis CcpA effector binding site. Proteins 64:814-816. [DOI] [PubMed] [Google Scholar]
- 107.Charbit, A. 1996. Coordination of carbon and nitrogen metabolism. Res. Microbiol. 147:513-518. [DOI] [PubMed] [Google Scholar]
- 108.Charles, T. C., and E. W. Nester. 1993. A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 175:6614-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Charrier, V., E. Buckley, D. Parsonage, A. Galinier, E. Darbon, M. Jaquinod, E. Forest, J. Deutscher, and A. Claiborne. 1997. Cloning and sequencing of two enterococcal glpK genes and regulation of the encoded glycerol kinases by phosphoenolpyruvate-dependent, phosphotransferase system-catalyzed phosphorylation of a single histidyl residue. J. Biol. Chem. 272:14166-14174. [DOI] [PubMed] [Google Scholar]
- 110.Charrier, V., J. Deutscher, A. Galinier, and I. Martin-Verstraete. 1997. Protein phosphorylation chain of a Bacillus subtilis fructose-specific phosphotransferase system and its participation in regulation of the expression of the lev operon. Biochemistry 36:1163-1172. [DOI] [PubMed] [Google Scholar]
- 111.Chassy, B. M., and J. Thompson. 1983. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and β-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J. Bacteriol. 154:1195-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chatterjee, S., and L. C. Vining. 1982. Catabolite repression in Streptomyces venezuelae. Induction of beta-galactosidase, chloramphenicol production, and intracellular cyclic adenosine-3′,5′-monophosphate concentrations. Can. J. Microbiol. 28:311-317. [DOI] [PubMed] [Google Scholar]
- 113.Chauvaux, S., I. T. Paulsen, and M. H. Saier, Jr. 1998. CcpB, a novel transcription factor implicated in catabolite repression in Bacillus subtilis. J. Bacteriol. 180:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chauvin, F., L. Brand, and S. Roseman. 1996. Enzyme I: the first protein and potential regulator of the bacterial phosphoenolpyruvate:glycose phosphotransferase system. Res. Microbiol. 147:471-479. [DOI] [PubMed] [Google Scholar]
- 115.Chauvin, F., A. Fomenkov, C. R. Johnson, and S. Roseman. 1996. The N-terminal domain of Escherichia coli enzyme I of the phosphoenolpyruvate/glycose phosphotransferase system: molecular cloning and characterization. Proc. Natl. Acad. Sci. USA 93:7028-7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen, G. S., and I. H. Segel. 1968. Purification and properties of glycogen phosphorylase from Escherichia coli. Arch. Biochem. Biophys. 127:175-186. [DOI] [PubMed] [Google Scholar]
- 117.Chen, J., G. Lu, J. Lin, A. L. Davidson, and F. A. Quiocho. 2003. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell 12:651-661. [DOI] [PubMed] [Google Scholar]
- 118.Chen, J., S. Sharma, F. A. Quiocho, and A. L. Davidson. 2001. Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl. Acad. Sci. USA 98:1525-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen, P., D. I. Andersson, and J. R. Roth. 1994. The control region of the pdu/cob regulon in Salmonella typhimurium. J. Bacteriol. 176:5474-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen, Q., J. C. Arents, R. Bader, P. W. Postma, and O. Amster-Choder. 1997. BglF, the sensor of the E. coli bgl system, uses the same site to phosphorylate both a sugar and a regulatory protein. EMBO J. 16:4617-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen, Q., H. Engelberg-Kulka, and O. Amster-Choder. 1997. The localization of the phosphorylation site of BglG, the response regulator of the Escherichia coli bgl sensory system. J. Biol. Chem. 272:17263-17268. [DOI] [PubMed] [Google Scholar]
- 122.Chen, Y., D. A. Case, J. Reizer, M. H. Saier, Jr., and P. E. Wright. 1998. High-resolution solution structure of Bacillus subtilis IIAGlc. Proteins 31:258-270. [PubMed] [Google Scholar]
- 123.Chen, Y., W. J. Fairbrother, and P. E. Wright. 1993. Three-dimensional structures of the central regulatory proteins of the bacterial phosphotransferase system, HPr and IIAglc. J. Cell. Biochem. 51:75-82. [DOI] [PubMed] [Google Scholar]
- 124.Chen, Y., J. Reizer, M. H. Saier, Jr., W. J. Fairbrother, and P. E. Wright. 1993. Mapping of the binding interfaces of the proteins of the bacterial phosphotransferase system, HPr and IIAglc. Biochemistry 32:32-37. [DOI] [PubMed] [Google Scholar]
- 125.Chen, Y. M., Y. Zhu, and E. C. C. Lin. 1987. The organization of the fuc regulon specifying L-fucose dissimilation in Escherichia coli K12 as determined by gene cloning. Mol. Gen. Genet. 210:331-337. [DOI] [PubMed] [Google Scholar]
- 126.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J. Bacteriol. 180:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi, I.-D., K.-N. Kim, C.-W. Yun, and Y.-J. Choi. 2006. Cloning and characterization of the HPr kinase/phosphorylase gene from Bacillus stearothermophilus no. 236. Biosci. Biotechnol. Biochem. 70:1089-1101. [DOI] [PubMed] [Google Scholar]
- 128.Choi, S. K., and M. H. Saier, Jr. 2005. Regulation of sigL expression by the catabolite control protein CcpA involves a roadblock mechanism in Bacillus subtilis: potential connection between carbon and nitrogen metabolism. J. Bacteriol. 187:6856-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Christensen, D. P., A. K. Benson, and R. W. Hutkins. 1999. Mutational analysis of the role of HPr in Listeria monocytogenes. Appl. Environ. Microbiol. 65:2112-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Christiansen, I., and W. Hengstenberg. 1996. Cloning and sequencing of two genes from Staphylococcus carnosus coding for glucose-specific PTS and their expression in Escherichia coli K-12. Mol. Gen. Genet. 250:375-379. [DOI] [PubMed] [Google Scholar]
- 131.Christiansen, I., and W. Hengstenberg. 1999. Staphylococcal phosphoenolpyruvate-dependent phosphotransferase system—two highly similar glucose permeases in Staphylococcus carnosus with different glucoside specificity: protein engineering in vivo. Microbiology 145:2881-2889. [DOI] [PubMed] [Google Scholar]
- 132.Cirri, P., P. Chiarugi, G. Camici, G. Manao, G. Raugei, G. Cappugi, and G. Ramponi. 1993. The role of Cys12, Cys17 and Arg18 in the catalytic mechanism of low-M(r) cytosolic phosphotyrosine protein phosphatase. Eur. J. Biochem. 214:647-657. [DOI] [PubMed] [Google Scholar]
- 133.Cluzel, P., M. Surette, and S. Leibler. 2000. An ultrasensitive bacterial motor revealed by monitoring signaling proteins in single cells. Science 287:1652-1655. [DOI] [PubMed] [Google Scholar]
- 134.Cochu, A., D. Roy, K. Vaillancourt, J.-D. Lemay, I. Casabon, M. Frenette, S. Moineau, and C. Vadeboncoeur. 2005. The doubly phosphorylated form of HPr, HPr(Ser-P)(His∼P), is abundant in exponentially growing cells of Streptococcus thermophilus and phosphorylates the lactose transporter LacS as efficiently as HPr(His∼P). Appl. Environ. Microbiol. 71:1364-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Collier, D. N., P. W. Hager, and P. V. Phibbs. 1996. Catabolite repression control in the pseudomonads. Res. Microbiol. 147:551-561. [DOI] [PubMed] [Google Scholar]
- 136.Condon, C., M. Grunberg-Manago, and H. Putzer. 1996. Aminoacyl-tRNA synthetase gene regulation in Bacillus subtilis. Biochimie 78:381-389. [DOI] [PubMed] [Google Scholar]
- 137.Contesse, G., M. Crépin, F. Gros, A. Ullmann, and J. Monod. 1969. On the mechanism of catabolite repression, p. 401-415. In J. R. Beckwith and D. Zipser (ed.), The lactose operon. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 138.Cook, G. M., J.-J. Ye, J. B. Russell, and M. H. Saier, Jr. 1995. Properties of the two sugarphosphate phosphatases from Streptococcus bovis and their potential involvement in inducer expulsion. J. Bacteriol. 177:7007-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cornilescu, G., B. R. Lee, C. C. Cornilescu, G. Wang, A. Peterkofsky, and G. M. Clore. 2002. Solution structure of the phosphoryl transfer complex between the cytoplasmic A domain of the mannitol transporter IIMannitol and HPr of the Escherichia coli phosphotransferase system. J. Biol. Chem. 277:42289-42298. [DOI] [PubMed] [Google Scholar]
- 140.Cossart, P., and B. Gicquel-Sanzey. 1985. Regulation of expression of the crp gene of Escherichia coli K-12: in vivo study. J. Bacteriol. 161:454-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cote, C. K., and A. L. Honeyman. 2003. The LicT protein acts as both a positive and a negative regulator of loci within the bgl regulon of Streptococcus mutans. Microbiology 149:1333-1340. [DOI] [PubMed] [Google Scholar]
- 142.Cozzone, A. J. 1998. Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annu. Rev. Microbiol. 52:127-164. [DOI] [PubMed] [Google Scholar]
- 143.Crasnier, M., and A. Danchin. 1990. Characterization of Escherichia coli adenylate cyclase mutants with modified regulation. J. Gen. Microbiol. 136:1825-1831. [DOI] [PubMed] [Google Scholar]
- 144.Crasnier, M., V. Dumay, and A. Danchin. 1994. The catalytic domain of Escherichia coli K-12 adenylate cyclase as revealed by deletion analysis of the cya gene. Mol. Gen. Genet. 243:409-416. [DOI] [PubMed] [Google Scholar]
- 145.Crasnier-Mednansky, M., M. C. Park, W. K. Studley, and M. H. Saier, Jr. 1997. Cra-mediated regulation of Escherichia coli adenylate cyclase. Microbiology 143:785-792. [DOI] [PubMed] [Google Scholar]
- 146.Crutz, A.-M., and M. Steinmetz. 1992. Transcription of the Bacillus subtilis sacX and sacY genes, encoding regulators of sucrose metabolism, is both inducible by sucrose and controlled by the DegS-DegU signalling system. J. Bacteriol. 174:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Crutz, A.-M., M. Steinmetz, S. A. Aymerich, R. Richter, and D. Le Coq. 1990. Induction of levansucrase in Bacillus subtilis: an antitermination mechanism negatively controlled by the phosphotransferase system. J. Bacteriol. 172:1043-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dahl, M. K., and W. Hillen. 1995. Contributions of XylR, CcpA and HPr to catabolite repression of the xyl operon in Bacillus subtilis. FEMS Microbiol. Lett. 132:79-83. [Google Scholar]
- 149.Dai, J. Y., S. H. Lin, C. Kemmis, A. J. Chin, and J. C. Lee. 2004. Interplay between site-specific mutations and cyclic nucleotides in modulating DNA recognition by Escherichia coli cyclic AMP receptor protein. Biochemistry 43:8901-8910. [DOI] [PubMed] [Google Scholar]
- 150.Dalet, K., S. Arous, Y. Cenatiempo, and Y. Héchard. 2003. Characterization of a unique σ54-dependent PTS operon of the lactose family in Listeria monocytogenes. Biochimie 85:633-638. [DOI] [PubMed] [Google Scholar]
- 151.Dalet, K., Y. Cenatiempo, P. Cossart, the European Listeria Genome Consortium, and Y. Hechard. 2001. A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263-3269. [DOI] [PubMed] [Google Scholar]
- 152.Daniel, J., E. Joseph, and A. Danchin. 1984. Role of 2-ketobutyrate as an alarmone in E. coli K12: inhibition of adenylate cyclase activity mediated by the phosphoenolpyruvate:glycose phosphotransferase transport system. Mol. Gen. Genet. 193:467-472. [DOI] [PubMed] [Google Scholar]
- 153.Daniel, R., K. Stuertz, and G. Gottschalk. 1995. Biochemical and molecular characterization of the oxidative branch of glycerol utilization by Citrobacter freundii. J. Bacteriol. 177:4392-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dannelly, H. K., and S. Roseman. 1996. Active site phosphorylation of enzyme I of the bacterial phosphotransferase system by an ATP-dependent kinase. J. Biol. Chem. 271:15285-15291. [DOI] [PubMed] [Google Scholar]
- 155.Dannelly, H. K., and S. Roseman. 1992. NAD+ and NADH regulate an ATP-dependent kinase that phosphorylates enzyme I of the Escherichia coli phosphotransferase system. Proc. Natl. Acad. Sci. USA 89:11274-11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Darbon, E., A. Galinier, D. Le Coq, and J. Deutscher. 2001. Phosphotransfer functions of mutated Bacillus subtilis HPr-like protein Crh carrying a histidine in the active site. J. Mol. Microbiol. Biotechnol. 3:439-444. [PubMed] [Google Scholar]
- 157.Darbon, E., K. Ito, H.-S. Huang, T. Yoshimoto, S. Poncet, and J. Deutscher. 1999. Glycerol transport and phosphoenolpyruvate-dependent, enzyme I- and HPr-catalysed phosphorylation of glycerol kinase in Thermus flavus. Microbiology 145:3205-3212. [DOI] [PubMed] [Google Scholar]
- 158.Darbon, E., P. Servant, S. Poncet, and J. Deutscher. 2002. Antitermination by GlpP, catabolite repression via CcpA, and inducer exclusion elicited by P∼GlpK dephosphorylation control Bacillus subtilis glpFK expression. Mol. Microbiol. 43:1039-1052. [DOI] [PubMed] [Google Scholar]
- 159.Davidson, A. L., S. S. Laghaeian, and D. E. Mannering. 1996. The maltose transport system of Escherichia coli displays positive cooperativity in ATP hydrolysis. J. Biol. Chem. 271:4858-4863. [PubMed] [Google Scholar]
- 160.Davidson, A. L., and S. Sharma. 1997. Mutation of a single MalK subunit severely impairs maltose transport activity in Escherichia coli. J. Bacteriol. 179:5458-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Davidson, A. L., H. A. Shuman, and H. Nikaido. 1992. Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc. Natl. Acad. Sci. USA 89:2360-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davison, S. P., J. D. Santangelo, S. J. Reid, and D. R. Woods. 1995. A Clostridium acetobutylicum regulator gene (regA) affecting amylase production in Bacillus subtilis. Microbiology 141:989-996. [DOI] [PubMed] [Google Scholar]
- 163.Dean, D. A., J. Reizer, H. Nikaido, and M. H. Saier, Jr. 1990. Regulation of the maltose transport system of Escherichia coli by the glucose-specific enzyme III of the phosphoenolpyruvate-sugar phosphotransferase system. Characterization of inducer exclusion-resistant mutants and reconstitution of inducer exclusion in proteoliposomes. J. Biol. Chem. 265:21005-21010. [PubMed] [Google Scholar]
- 164.Débarbouillé, M., M. Arnaud, A. Fouet, A. Klier, and G. Rapoport. 1990. The sacT gene regulating the sacPA operon in Bacillus subtilis shares strong homology with transcriptional antiterminators. J. Bacteriol. 172:3966-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Débarbouillé, M., I. Martin-Verstraete, A. Klier, and G. Rapoport. 1991. The transcriptional regulator LevR of Bacillus subtilis has domains homologous to both σ54- and phosphotransferase system-dependent regulators. Proc. Natl. Acad. Sci. USA 88:2212-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.de Boer, M., C. P. Broekhuizen, and P. W. Postma. 1986. Regulation of glycerol kinase by enzyme IIIGlc of the phosphoenolpyruvate:carbohydrate phosphotransferase system. J. Bacteriol. 167:393-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Decker, K., J. Plumbridge, and W. Boos. 1998. Negative transcriptional regulation of a positive regulator: the expression of malT, encoding the transcriptional activator of the maltose regulon of Escherichia coli, is negatively controlled by Mlc. Mol. Microbiol. 27:381-390. [DOI] [PubMed] [Google Scholar]
- 168.Declerck, N., F. Vincent, F. Hoh, S. Aymerich, and H. van Tilbeurgh. 1999. RNA recognition by transcriptional antiterminators of the BglG/SacY family: functional and structural comparison of the CAT domain from SacY and LicT. J. Mol. Biol. 294:389-402. [DOI] [PubMed] [Google Scholar]
- 169.Delrue, R. M., P. Lestrate, A. Tibor, J. J. Letesson, and X. De Bolle. 2004. Brucella pathogenesis, genes identified from random large-scale screens. FEMS Microbiol. Lett. 231:1-12. [DOI] [PubMed] [Google Scholar]
- 170.den Blaauwen, J. L., and P. W. Postma. 1985. Regulation of cyclic AMP synthesis by enzyme IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system in crp strains of Salmonella typhimurium. J. Bacteriol. 164:477-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de Reuse, H., and A. Danchin. 1991. Positive regulation of the pts operon of Escherichia coli: genetic evidence for a signal transduction mechanism. J. Bacteriol. 173:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.de Reuse, H., and A. Danchin. 1988. The ptsH, ptsI, and crr genes of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J. Bacteriol. 170:3827-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.de Reuse, H., A. Kolb, and A. Danchin. 1992. Positive regulation of the expression of the Escherichia coli pts operon. Identification of the regulatory regions. J. Mol. Biol. 226:623-635. [DOI] [PubMed] [Google Scholar]
- 174.Deutscher, J. 1985. Phosphoenolpyruvate-dependent phosphorylation of a 55-kDa protein of Streptococcus faecalis catalyzed by the phosphotransferase system. FEMS Microbiol. Lett. 29:237-243. [Google Scholar]
- 175.Deutscher, J., B. Bauer, and H. Sauerwald. 1993. Regulation of glycerol metabolism in Enterococcus faecalis by phosphoenolpyruvate-dependent phosphorylation of glycerol kinase catalyzed by enzyme I and HPr of the phosphotransferase system. J. Bacteriol. 175:3730-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Deutscher, J., K. Beyreuther, M. H. Sobek, K. Stüber, and W. Hengstenberg. 1982. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: factor IIILac, a trimeric phospho-carrier protein that also acts as a phase transfer catalyst. Biochemistry 21:4867-4873. [DOI] [PubMed] [Google Scholar]
- 177.Deutscher, J., and R. Engelmann. 1984. Purification and characterization of an ATP-dependent protein kinase from Streptococcus faecalis. FEMS Microbiol. Lett. 23:157-162. [Google Scholar]
- 178.Deutscher, J., A. Galinier, and I. Martin-Verstraete. 2002. Carbohydrate uptake and metabolism, p. 129-150. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 179.Deutscher, J., R. Herro, A. Bourand, I. Mijakovic, and S. Poncet. 2005. P-Ser-HPr—a link between carbon metabolism and the virulence of some pathogenic bacteria. Biochim. Biophys. Acta 1754:118-125. [DOI] [PubMed] [Google Scholar]
- 180.Deutscher, J., U. Kessler, C. A. Alpert, and W. Hengstenberg. 1984. Bacterial phosphoenolpyruvate-dependent phosphotransferase system: P-Ser-HPr and its possible regulatory function. Biochemistry 23:4455-4460. [DOI] [PubMed] [Google Scholar]
- 181.Deutscher, J., U. Kessler, and W. Hengstenberg. 1985. Streptococcal phosphoenolpyruvate:sugar phosphotransferase system: purification and characterization of a phosphoprotein phosphatase which hydrolyzes the phosphoryl bond in seryl-phosphorylated histidine-containing protein. J. Bacteriol. 163:1203-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Deutscher, J., E. Küster, U. Bergstedt, V. Charrier, and W. Hillen. 1995. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol. Microbiol. 15:1049-1053. [DOI] [PubMed] [Google Scholar]
- 183.Deutscher, J., B. Pevec, K. Beyreuther, H.-H. Kiltz, and W. Hengstenberg. 1986. Streptococcal phosphoenolpyruvate-sugar phosphotransferase system: amino acid sequence and site of ATP-dependent phosphorylation of HPr. Biochemistry 25:6543-6551. [DOI] [PubMed] [Google Scholar]
- 184.Deutscher, J., J. Reizer, C. Fischer, A. Galinier, M. H. Saier, Jr., and M. Steinmetz. 1994. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J. Bacteriol. 176:3336-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Deutscher, J., and M. H. Saier, Jr. 1983. ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 80:6790-6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Deutscher, J., and H. Sauerwald. 1986. Stimulation of dihydroxyacetone and glycerol kinase activity in Streptococcus faecalis by phosphoenolpyruvate-dependent phosphorylation catalyzed by enzyme I and HPr of the phosphotransferase system. J. Bacteriol. 166:829-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Diederichs, K., J. Diez, G. Greller, C. Müller, J. Breed, C. Schnell, C. Vonrhein, W. Boos, and W. Welte. 2000. Crystal structure of MalK, the ATPase subunit of the trehalose/maltose ABC transporter of the archaeon Thermococcus litoralis. EMBO J. 19:5951-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dienert, F. 1900. Sur la fermentation du galactose et sur l'accoutumence des levures à ce sucre. Ann. Pasteur. 14:139-189. [Google Scholar]
- 189.Dills, S. S., M. R. Schmidt, and M. H. Saier, Jr. 1982. Regulation of lactose transport by the phosphoenolpyruvate-sugar phosphotransferase system in membrane vesicles of Escherichia coli. J. Cell. Biochem. 18:239-244. [DOI] [PubMed] [Google Scholar]
- 190.Dimitrova, M. N., A. Peterkofsky, and A. Ginsburg. 2003. Opposing effects of phosphoenolpyruvate and pyruvate with Mg2+ on the conformational stability and dimerization of phosphotransferase enzyme I from Escherichia coli. Protein Sci. 12:2047-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dimitrova, M. N., G. Piszcek, and A. Ginsburg. 2004. On the conformational stability and dimerization of phosphotransferase enzyme I from Escherichia coli. Thermochim. Acta 420:37-43. [Google Scholar]
- 192.Dimitrova, M. N., R. H. Szczepanowski, S. B. Ruvinov, A. Peterkofsky, and A. Ginsburg. 2002. Interdomain interaction and substrate coupling effects on dimerization and conformational stability of enzyme I of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system. Biochemistry 41:906-913. [DOI] [PubMed] [Google Scholar]
- 193.Djordjevic, G. M., J. H. Tchieu, and M. H. Saier, Jr. 2001. Genes involved in control of galactose uptake in Lactobacillus brevis and reconstitution of the regulatory system in Bacillus subtilis. J. Bacteriol. 183:3224-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Doan, T., and S. Aymerich. 2003. Regulation of the central glycolytic genes in Bacillus subtilis: binding of the repressor CggR to its single DNA target sequence is modulated by fructose-1,6-bisphosphate. Mol. Microbiol. 47:1709-1721. [DOI] [PubMed] [Google Scholar]
- 195.Dong, Y., Y.-Y. Chen, and R. A. Burne. 2004. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J. Bacteriol. 186:2511-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dorocicz, I., P. Williams, and R. J. Redfield. 1993. The Haemophilus influenzae adenylate cyclase gene: cloning, sequence, and essential role in competence. J. Bacteriol. 175:7142-7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dörschug, M., R. Frank, H. R. Kalbitzer, W. Hengstenberg, and J. Deutscher. 1984. Phosphoenolpyruvate-dependent phosphorylation site in enzyme IIIglc of the Escherichia coli phosphotransferase system. Eur. J. Biochem. 144:113-119. [DOI] [PubMed] [Google Scholar]
- 198.Dossonnet, V., V. Monedero, M. Zagorec, A. Galinier, G. Pérez-Martínez, and J. Deutscher. 2000. Phosphorylation of HPr by the bifunctional HPr kinase/P-Ser-HPr phosphatase from Lactobacillus casei controls catabolite repression and inducer exclusion but not inducer expulsion. J. Bacteriol. 182:2582-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Du, Y., A. Holtel, J. Reizer, and M. H. Saier, Jr. 1996. σ54-dependent transcription of the Pseudomonas putida xylS operon is influenced by the IIANtr protein of the phosphotransferase system in Escherichia coli. Res. Microbiol. 147:129-132. [DOI] [PubMed] [Google Scholar]
- 200.Duché, O., F. Trémoulet, P. Glaser, and J. Labadie. 2002. Salt stress proteins induced in Listeria monocytogenes. Appl. Environ. Microbiol. 68:1491-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dumay, V., A. Danchin, and M. Crasnier. 1996. Regulation of Escherichia coli adenylate cyclase activity during hexose phosphate transport. Microbiology 142:575-583. [DOI] [PubMed] [Google Scholar]
- 202.Dupuy, B., and A. L. Sonenshein. 1998. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 27:107-120. [DOI] [PubMed] [Google Scholar]
- 203.Eberstadt, M., S. G. Grdadolnik, G. Gemmecker, H. Kessler, A. Buhr, and B. Erni. 1996. Solution structure of the IIB domain of the glucose transporter of Escherichia coli. Biochemistry 35:11286-11292. [DOI] [PubMed] [Google Scholar]
- 204.Egeter, O., and R. Brückner. 1996. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol. Microbiol. 21:739-749. [DOI] [PubMed] [Google Scholar]
- 205.Egeter, O., and R. Brückner. 1995. Characterization of a genetic locus essential for maltose-maltotriose utilization in Staphylococcus xylosus. J. Bacteriol. 177:2408-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Eisermann, R., J. Deutscher, G. Gonzy-Tréboul, and W. Hengstenberg. 1988. Site-directed mutagenesis with the ptsH gene of Bacillus subtilis. Isolation and characterization of heat-stable proteins altered at the ATP-dependent regulatory phosphorylation site. J. Biol. Chem. 263:17050-17054. [PubMed] [Google Scholar]
- 207.Eiting, M., G. Hagelüken, W. D. Schubert, and D. W. Heinz. 2005. The mutation G145S in PrfA, a key virulence regulator of Listeria monocytogenes, increases DNA-binding affinity by stabilizing the HTH motif. Mol. Microbiol. 56:433-446. [DOI] [PubMed] [Google Scholar]
- 208.El Hassouni, M., B. Henrissat, M. Chippaux, and F. Barras. 1992. Nucleotide sequence of the arb genes, which control β-glucoside utilization in Erwinia chrysanthemi: comparison with the Escherichia coli bgl operon and evidence for a new β-glycohydrolase family including enzymes from eubacteria, archaebacteria, and humans. J. Bacteriol. 174:765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.El-Kazzaz, W., T. Morita, H. Tagami, T. Inada, and H. Aiba. 2004. Metabolic block at early stages of the glycolytic pathway activates the Rcs phosphorelay system via increased synthesis of dTDP-glucose in Escherichia coli. Mol. Microbiol. 51:1117-1128. [DOI] [PubMed] [Google Scholar]
- 210.Eppler, T., and W. Boos. 1999. Glycerol-3-phosphate-mediated repression of malT in Escherichia coli does not require metabolism, depends on enzyme IIAGlc and is mediated by cAMP levels. Mol. Microbiol. 33:1221-1231. [DOI] [PubMed] [Google Scholar]
- 211.Eppler, T., P. Postma, A. Schutz, U. Volker, and W. Boos. 2002. Glycerol-3-phosphate-induced catabolite repression in Escherichia coli. J. Bacteriol. 184:3044-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Epstein, W., L. B. Rothman-Denes, and J. Hesse. 1975. Adenosine 3′:5′-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc. Natl. Acad. Sci. USA 72:2300-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Erni, B. 1986. Glucose-specific permease of the bacterial phosphotransferase system: phosphorylation and oligomeric structure of the glucose-specific IIGlc-IIIGlc complex of Salmonella typhimurium. Biochemistry 25:305-312. [DOI] [PubMed] [Google Scholar]
- 214.Erni, B., H. Trachsel, P. W. Postma, and J. P. Rosenbusch. 1982. Bacterial phosphotransferase system. Solubilization and purification of the glucose-specific enzyme II from membranes of Salmonella typhimurium. J. Biol. Chem. 257:13726-13730. [PubMed] [Google Scholar]
- 215.Erni, B., and B. Zanolari. 1986. Glucose permease of the bacterial phosphotransferase system. Gene cloning, overproduction, and amino acid sequence of enzyme IIGlc. J. Biol. Chem. 261:16398-16403. [PubMed] [Google Scholar]
- 216.Erni, B., and B. Zanolari. 1985. The mannose-permease of the bacterial phosphotransferase system. Gene cloning and purification of the enzyme IIMan/IIIMan complex of Escherichia coli. J. Biol. Chem. 260:15495-15503. [PubMed] [Google Scholar]
- 217.Escalante, L., I. Ramos, I. Imriskova, E. Langley, and S. Sanchez. 1999. Glucose repression of anthracycline formation in Streptomyces peucetius var. caesius. Appl. Microbiol. Biotechnol. 52:572-578. [Google Scholar]
- 218.Esquinas-Rychen, M., and B. Erni. 2001. Facilitation of bacteriophage lambda DNA injection by inner membrane proteins of the bacterial phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS). J. Mol. Microbiol. Biotechnol. 3:361-370. [PubMed] [Google Scholar]
- 219.Fabret, C. 1996. Projet génome Bacillus subtilis: séquençage et analyse de la région chromosomique entre les loci cysB et hisA. Ph.D. thesis. Université de la Mediterranée Aix-Marseille, Marseille, France.
- 220.Faires, N., S. Tobisch, S. Bachem, I. Martin-Verstraete, M. Hecker, and J. Stülke. 1999. The catabolite control protein CcpA controls ammonium assimilation in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1:141-148. [PubMed] [Google Scholar]
- 221.Fandl, J. P., L. K. Thorner, and S. W. Artz. 1990. Mutations that affect transcription and cyclic AMP-CRP regulation of the adenylate cyclase gene (cya) of Salmonella typhimurium. Genetics 125:719-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Favier, A., B. Brutscher, M. Blackledge, A. Galinier, J. Deutscher, F. Penin, and D. Marion. 2002. Solution structure and dynamics of Crh, the Bacillus subtilis catabolite repression HPr. J. Mol. Biol. 317:131-144. [DOI] [PubMed] [Google Scholar]
- 223.Feese, M., D. W. Pettigrew, N. D. Meadow, S. Roseman, and S. J. Remington. 1994. Cation-promoted association of a regulatory and target protein is controlled by protein phosphorylation. Proc. Natl. Acad. Sci. USA 91:3544-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Feese, M. D., L. Comolli, N. D. Meadow, S. Roseman, and S. J. Remington. 1997. Structural studies of the Escherichia coli signal transducing protein IIAGlc: implications for target recognition. Biochemistry 36:16087-16096. [DOI] [PubMed] [Google Scholar]
- 225.Feldheim, D. A., A. M. Chin, C. T. Nierva, B. U. Feucht, Y. W. Cao, Y. F. Xu, S. L. Sutrina, and M. H. Saier, Jr. 1990. Physiological consequences of the complete loss of phosphoryl-transfer proteins HPr and FPr of the phosphoenolpyruvate:sugar phosphotransferase system and analysis of fructose (fru) operon expression in Salmonella typhimurium. J. Bacteriol. 172:5459-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fetsch, E. E., and A. L. Davidson. 2003. Maltose transport through the inner membrane of E. coli. Front. Biosci. 8:D652-D660. [DOI] [PubMed] [Google Scholar]
- 227.Feucht, B. U., and M. H. Saier, Jr. 1980. Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 141:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fieulaine, S., S. Morera, S. Poncet, I. Mijakovic, A. Galinier, J. Janin, J. Deutscher, and S. Nessler. 2002. X-ray structure of a bifunctional protein kinase in complex with its protein substrate HPr. Proc. Natl. Acad. Sci. USA 99:13437-13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fieulaine, S., S. Morera, S. Poncet, V. Monedero, V. Gueguen-Chaignon, A. Galinier, J. Janin, J. Deutscher, and S. Nessler. 2001. X-ray structure of HPr kinase: a bacterial protein kinase with a P-loop nucleotide binding domain. EMBO J. 20:3917-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fillinger, S., S. Boschi-Muller, S. Azza, E. Dervyn, G. Branlant, and S. Aymerich. 2000. Two glyceraldehyde-3-phosphate dehydrogenases with opposite physiological roles in a nonphotosynthetic bacterium. J. Biol. Chem. 275:14031-14037. [DOI] [PubMed] [Google Scholar]
- 231.Finkeldei, U., H. R. Kalbitzer, R. Eisermann, G. C. Stewart, and W. Hengstenberg. 1991. Enzyme IIILac of the staphylococcal phosphoenolpyruvate-dependent phosphotransferase system: site-specific mutagenesis of histidine residues, biochemical characterization and 1H-NMR studies. Protein Eng. 4:469-473. [DOI] [PubMed] [Google Scholar]
- 232.Fischer, R., and W. Hengstenberg. 1992. Mannitol-specific enzyme II of the phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus carnosus. Sequence and expression in Escherichia coli and structural comparison with the enzyme IIMannitol of Escherichia coli. Eur. J. Biochem. 204:963-969. [DOI] [PubMed] [Google Scholar]
- 233.Fisher, S. H., and B. Magasanik. 1984. Isolation of Bacillus subtilis mutants pleiotropically insensitive to glucose catabolite repression. J. Bacteriol. 157:942-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fomenkov, A., A. Valiakhmetov, L. Brand, and S. Roseman. 1998. In vivo and in vitro complementation of the N-terminal domain of enzyme I of the Escherichia coli phosphotransferase system by the cloned C-terminal domain. Proc. Natl. Acad. Sci. USA 95:8491-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Foucaud, C., and B. Poolman. 1992. Lactose transport system of Streptococcus thermophilus. Functional reconstitution of the protein and characterization of the kinetic mechanism of transport. J. Biol. Chem. 267:22087-22094. [PubMed] [Google Scholar]
- 236.Fox, D. K., N. D. Meadow, and S. Roseman. 1986. Phosphate transfer between acetate kinase and enzyme I of the bacterial phosphotransferase system. J. Biol. Chem. 261:13498-13503. [PubMed] [Google Scholar]
- 237.Fox, D. K., K. A. Presper, S. Adhya, S. Roseman, and S. Garges. 1992. Evidence for two promoters upstream of the pts operon: regulation by the cAMP receptor protein regulatory complex. Proc. Natl. Acad. Sci. USA 89:7056-7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fox, D. K., and S. Roseman. 1986. Isolation and characterization of homogeneous acetate kinase from Salmonella typhimurium and Escherichia coli. J. Biol. Chem. 261:13487-13497. [PubMed] [Google Scholar]
- 239.Francke, C., P. W. Postma, H. V. Westerhoff, J. G. Blom, and M. A. Peletier. 2003. Why the phosphotransferase system of Escherichia coli escapes diffusion limitation. Biophys. J. 85:612-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Francke, C., H. V. Westerhoff, J. G. Blom, and M. A. Peletier. 2002. Flux control of the bacterial phosphoenolpyruvate:glucose phosphotransferase system and the effect of diffusion. Mol. Biol. Rep. 29:21-26. [DOI] [PubMed] [Google Scholar]
- 241.Fraser, A. D. E., and H. Yamazaki. 1978. Determination of the rates of synthesis of cAMP in Escherichia coli CRP− and CRP+ strains. Can. J. Biochem. 56:849-852. [DOI] [PubMed] [Google Scholar]
- 242.Fraser, A. D. E., and H. Yamazaki. 1982. Significance of β-galactosidase repression in glucose inhibition of lactose utilization in Escherichia coli. Curr. Microbiol. 7:241-244. [Google Scholar]
- 243.Frees, D., F. K. Vogensen, and H. Ingmer. 2003. Identification of proteins induced at low pH in Lactococcus lactis. Int. J. Food Microbiol. 87:293-300. [DOI] [PubMed] [Google Scholar]
- 244.Friesen, R. H., J. Knol, and B. Poolman. 2000. Quaternary structure of the lactose transport protein of Streptococcus thermophilus in the detergent-solubilized and membrane-reconstituted state. J. Biol. Chem. 275:33527-33535. [DOI] [PubMed] [Google Scholar]
- 245.Frillingos, S., and H. R. Kaback. 1996. Probing the conformation of the lactose permease of Escherichia coli by in situ site-directed sulfhydryl modification. Biochemistry 35:3950-3956. [DOI] [PubMed] [Google Scholar]
- 246.Froger, A., J. P. Rolland, P. Bron, V. Lagree, F. L. Caherec, S. Deschamps, J. F. Hubert, I. Pellerin, D. Thomas, and C. Delamarche. 2001. Functional characterization of a microbial aquaglyceroporin. Microbiology 147:1129-1135. [DOI] [PubMed] [Google Scholar]
- 247.Fujita, Y., Y. Miwa, A. Galinier, and J. Deutscher. 1995. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol. Microbiol. 17:953-960. [DOI] [PubMed] [Google Scholar]
- 248.Gaidenko, T. A., T.-J. Kim, and C. W. Price. 2002. The PrpC serine-threonine phosphatase and PrkC kinase have opposing physiological roles in stationary-phase Bacillus subtilis cells. J. Bacteriol. 184:6109-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Galinier, A., J. Deutscher, and I. Martin-Verstraete. 1999. Phosphorylation of either Crh or HPr mediates catabolite repression and binding of CcpA to the cre of the Bacillus subtilis xyn operon. J. Mol. Biol. 286:307-314. [DOI] [PubMed] [Google Scholar]
- 250.Galinier, A., J. Haiech, M.-C. Kilhoffer, M. Jaquinod, J. Stülke, J. Deutscher, and I. Martin-Verstraete. 1997. The Bacillus subtilis crh gene encodes a HPr-like protein involved in carbon catabolite repression. Proc. Natl. Acad. Sci. USA 94:8439-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Galinier, A., M. Kravanja, R. Engelmann, W. Hengstenberg, M.-C. Kilhoffer, J. Deutscher, and J. Haiech. 1998. New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression. Proc. Natl. Acad. Sci. USA 95:1823-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Galinier, A., J. P. Lavergne, C. Geourjon, S. Fieulaine, S. Nessler, and J. M. Jault. 2002. A new family of phosphotransferases with a P-loop motif. J. Biol. Chem. 277:11362-11367. [DOI] [PubMed] [Google Scholar]
- 253.Gao, B., R. Paramanathan, and R. S. Gupta. 2006. Signature proteins that are distinctive characteristics of Actinobacteria and their subgroups. Antonie Leeuwenhoek 90:69-91. [DOI] [PubMed] [Google Scholar]
- 254.Garcia-Alles, L. F., K. Flükiger, J. Hewel, R. Gutknecht, C. Siebold, S. Schurch, and B. Erni. 2002. Mechanism-based inhibition of enzyme I of the Escherichia coli phosphotransferase system. Cysteine 502 is an essential residue. J. Biol. Chem. 277:6934-6942. [DOI] [PubMed] [Google Scholar]
- 255.Garcia-Alles, L. F., C. Siebold, T. L. Nyffeler, K. Flükiger-Brühwiler, P. Schneider, H. B. Bürgi, U. Baumann, and B. Erni. 2004. Phosphoenolpyruvate- and ATP-dependent dihydroxyacetone kinases: covalent substrate-binding and kinetic mechanism. Biochemistry 43:13037-13045. [DOI] [PubMed] [Google Scholar]
- 256.Garcia-Alles, L. F., A. Zahn, and B. Erni. 2002. Sugar recognition by the glucose and mannose permeases of Escherichia coli. Steady-state kinetics and inhibition studies. Biochemistry 41:10077-10086. [DOI] [PubMed] [Google Scholar]
- 257.Garnak, M., and H. C. Reeves. 1979. Phosphorylation of isocitrate dehydrogenase of Escherichia coli. Science 203:1111-1112. [DOI] [PubMed] [Google Scholar]
- 258.Garrett, D. S., Y.-J. Seok, D.-I. Liao, A. Peterkofsky, A. M. Gronenborn, and G. M. Clore. 1997. Solution structure of the 30 kDa N-terminal domain of enzyme I of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system by multidimensional NMR. Biochemistry 36:2517-2530. [DOI] [PubMed] [Google Scholar]
- 259.Garrett, D. S., Y.-J. Seok, A. Peterkofsky, G. M. Clore, and A. M. Gronenborn. 1997. Identification by NMR of the binding surface for the histidine-containing phosphocarrier protein HPr on the N-terminal domain of enzyme I of the Escherichia coli phosphotransferase system. Biochemistry 36:4393-4398. [DOI] [PubMed] [Google Scholar]
- 260.Garrett, D. S., Y.-J. Seok, A. Peterkofsky, A. M. Gronenborn, and G. M. Clore. 1999. Solution structure of the 40,000 Mr phosphoryl transfer complex between the N-terminal domain of enzyme I and HPr. Nat. Struct. Biol. 6:166-173. [DOI] [PubMed] [Google Scholar]
- 261.Garrity, L. F., S. L. Schiel, R. Merrill, J. Reizer, M. H. Saier, Jr., and G. W. Ordal. 1998. Unique regulation of carbohydrate chemotaxis in Bacillus subtilis by the phosphoenolpyruvate-dependent phosphotransferase system and the methyl-accepting chemotaxis protein McpC. J. Bacteriol. 180:4475-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gassner, M., D. Stehlik, O. Schrecker, W. Hengstenberg, W. Maurer, and H. Rüterjans. 1977. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 2. 1H and 31P nuclear-magnetic-resonance studies on the phosphocarrier protein HPr, phosphohistidines and phosphorylated HPr. Eur. J. Biochem. 75:287-296. [DOI] [PubMed] [Google Scholar]
- 263.Gauthier, M., D. Brochu, L. D. Eltis, S. Thomas, and C. Vadeboncoeur. 1997. Replacement of isoleucine-47 by threonine in the HPr protein of Streptococcus salivarius abrogates the preferential metabolism of glucose and fructose over lactose and melibiose but does not prevent the phosphorylation of HPr on serine-46. Mol. Microbiol. 25:695-705. [DOI] [PubMed] [Google Scholar]
- 264.Gay, P. 1979. The vectorial metabolism of carbohydrates and the catabolism of fructose in Bacillus subtilis Marburg: genetic and biochemical studies. Ph.D. thesis. University of Paris VI, Paris, France.
- 265.Gay, P., P. Cordier, M. Marquet, and A. Delobbe. 1973. Carbohydrate metabolism and transport in Bacillus subtilis. A study of ctr mutations. Mol. Gen. Genet. 121:355-368. [DOI] [PubMed] [Google Scholar]
- 266.Geerse, R. H., F. Izzo, and P. W. Postma. 1989. The PEP:fructose phosphotransferase system in Salmonella typhimurium: FPr combines enzyme IIIFru and pseudo-HPr activities. Mol. Gen. Genet. 216:517-525. [DOI] [PubMed] [Google Scholar]
- 267.Geerse, R. H., C. R. Ruig, A. R. J. Schuitema, and P. W. Postma. 1986. Relationship between pseudo-HPr and the PEP:fructose phosphotransferase system in Salmonella typhimurium and Escherichia coli. Mol. Gen. Genet. 203:435-444. [DOI] [PubMed] [Google Scholar]
- 268.Geerse, R. H., J. van der Pluijm, and P. W. Postma. 1989. The repressor of the PEP:fructose phosphotransferase system is required for the transcription of the pps gene of Escherichia coli. Mol. Gen. Genet. 218:348-352. [DOI] [PubMed] [Google Scholar]
- 269.Geissmann, T. A., and D. Touati. 2004. Hfq, a new chaperoning role: binding to messenger RNA determines access for small RNA regulator. EMBO J. 23:396-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gemmecker, G., M. Eberstadt, A. Buhr, R. Lanz, S. G. Grdadolnik, H. Kessler, and B. Erni. 1997. Glucose transporter of Escherichia coli: NMR characterization of the phosphocysteine form of the IIBGlc domain and its binding interface with the IIAGlc subunit. Biochemistry 36:7408-7417. [DOI] [PubMed] [Google Scholar]
- 271.Georgellis, D., O. Kwon, and E. C. C. Lin. 2001. Quinones as the redox signal for the Arc two-component system of bacteria. Science 292:2314-2316. [DOI] [PubMed] [Google Scholar]
- 272.Gershanovitch, V. N., T. N. Bolshakova, M. L. Molchanova, A. M. Umyarov, O. Y. Dobrynina, Y. A. Grigorenko, and R. S. Erlagaeva. 1989. Fructose-specific phosphoenolpyruvate dependent phosphotransferase system of Escherichia coli: its alterations and adenylate cyclase activity. FEMS Microbiol. Rev. 63:125-134. [DOI] [PubMed] [Google Scholar]
- 273.Giammarinaro, P., and J. C. Paton. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 70:5454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Glatz, E., M. Persson, and B. Rutberg. 1998. Antiterminator protein GlpP of Bacillus subtilis binds to glpD leader mRNA. Microbiology 144:449-456. [DOI] [PubMed] [Google Scholar]
- 275.Goldenbaum, P. E., and G. A. Hale. 1979. Transport of cAMP across Escherichia coli vesicle membrane. J. Bacteriol. 140:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gollop, N., D. M. Chipman, and Z. Barak. 1983. Inhibition of acetohydroxy acid synthase by leucine. Biochim. Biophys. Acta 748:34-39. [DOI] [PubMed] [Google Scholar]
- 277.Gollop, N., H. Tavori, and Z. Barak. 1982. Acetohydroxy acid synthase is a target for leucine containing peptide toxicity in Escherichia coli. J. Bacteriol. 149:387-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gonzalez, C. F., A. J. Stonestrom, G. L. Lorca, and M. H. Saier, Jr. 2005. Biochemical characterization of phosphoryl transfer involving HPr of the phosphoenolpyruvate-dependent phosphotransferase system in Treponema denticola, an organism that lacks PTS permeases. Biochemistry 44:598-608. [DOI] [PubMed] [Google Scholar]
- 279.González-Gil, G., P. Bringmann, and R. Kahmann. 1996. FIS is a regulator of metabolism in Escherichia coli. Mol. Microbiol. 22:21-29. [DOI] [PubMed] [Google Scholar]
- 280.González-Gil, G., R. Kahmann, and G. Muskhelishvili. 1998. Regulation of crp transcription by oscillation between distinct nucleoprotein complexes. EMBO J. 17:2877-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.González-Pérez, M. M., J. L. Ramos, and S. Marqués. 2004. Cellular XylS levels are a function of transcription of xylS from two independent promoters and the differential efficiency of translation of the two mRNAs. J. Bacteriol. 186:1898-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gonzy-Tréboul, G., J. H. de Waard, M. Zagorec, and P. W. Postma. 1991. The glucose permease of the phosphotransferase system of Bacillus subtilis: evidence for IIGlc and IIIGlc domains. Mol. Microbiol. 5:1241-1249. [DOI] [PubMed] [Google Scholar]
- 283.Gonzy-Tréboul, G., and M. Steinmetz. 1987. Phosphoenolpyruvate:sugar phosphotransferase system of Bacillus subtilis: cloning of the region containing the ptsH and ptsI genes and evidence for a crr-like gene. J. Bacteriol. 169:2287-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gordon, E., B. Flouret, L. Chantalat, J. van Heijenoort, D. Mengin-Lecreulx, and O. Dideberg. 2001. Crystal structure of UDP-N-acetylmuramoyl-l-alanyl-d-glutamate:meso-diaminopimelate ligase from Escherichia coli. J. Biol. Chem. 276:10999-11006. [DOI] [PubMed] [Google Scholar]
- 285.Görke, B. 2003. Regulation of the Escherichia coli antiterminator protein BglG by phosphorylation at multiple sites and evidence for transfer of phosphoryl groups between monomers. J. Biol. Chem. 278:46219-46229. [DOI] [PubMed] [Google Scholar]
- 286.Görke, B., L. Fraysse, and A. Galinier. 2004. Drastic differences in Crh and HPr synthesis levels reflect their different impact on catabolite repression in Bacillus subtilis. J. Bacteriol. 186:2992-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Görke, B., and B. Rak. 1999. Catabolite control of Escherichia coli regulatory protein BglG activity by antagonistically acting phosphorylations. EMBO J. 18:3370-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gosalbes, M. J., C. D. Esteban, and G. Pérez-Martínez. 2002. In vivo effect of mutations in the antiterminator LacT in Lactobacillus casei. Microbiology 148:695-702. [DOI] [PubMed] [Google Scholar]
- 289.Gosalbes, M. J., V. Monedero, and G. Pérez-Martínez. 1999. Elements involved in catabolite repression and substrate induction of the lactose operon in Lactobacillus casei. J. Bacteriol. 181:3928-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gösseringer, R., E. Küster, A. Galinier, J. Deutscher, and W. Hillen. 1997. Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J. Mol. Biol. 266:665-676. [DOI] [PubMed] [Google Scholar]
- 291.Gosset, G., Z. G. Zhang, S. N. Nayyar, W. A. Cuevas, and M. H. Saier, Jr. 2004. Transcriptome analysis of Crp-dependent catabolite control of gene expression in Escherichia coli. J. Bacteriol. 186:3516-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Graille, M., C. Z. Zhou, V. Receveur-Brechot, B. Collinet, N. Declerck, and H. van Tilbeurgh. 2005. Activation of the LicT transcriptional antiterminator involves a domain swing/lock mechanism provoking massive structural changes. J. Biol. Chem. 280:14780-14789. [DOI] [PubMed] [Google Scholar]
- 293.Graumann, P., and M. A. Marahiel. 1996. Some like it cold: response of microorganisms to cold shock. Arch. Microbiol. 166:293-300. [DOI] [PubMed] [Google Scholar]
- 294.Gravesen, A., P. Warthoe, S. Knochel, and K. Thirstrup. 2000. Restriction fragment differential display of pediocin-resistant Listeria monocytogenes 412 mutants shows consistent overexpression of a putative β-glucoside-specific PTS system. Microbiology 146:1381-1389. [DOI] [PubMed] [Google Scholar]
- 295.Greenberg, D. B., J. Stülke, and M. H. Saier, Jr. 2002. Domain analysis of transcriptional regulators bearing PTS regulatory domains. Res. Microbiol. 153:519-526. [DOI] [PubMed] [Google Scholar]
- 296.Greer-Phillips, S. E., G. Alexandre, B. L. Taylor, and I. B. Zhulin. 2003. Aer and Tsr guide Escherichia coli in spatial gradients of oxidizable substrates. Microbiology 149:2661-2667. [DOI] [PubMed] [Google Scholar]
- 297.Grisafi, P. L., A. Scholle, J. Sugiyama, C. Briggs, G. R. Jacobson, and J. W. Lengeler. 1989. Deletion mutants of the Escherichia coli K-12 mannitol permease: dissection of transport-phosphorylation, phospho-exchange, and mannitol-binding activities. J. Bacteriol. 171:2719-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Griswold, A., Y.-Y. M. Chen, J. A. Snyder, and R. A. Burne. 2004. Characterization of the arginine deiminase operon of Streptococcus rattus FA-1. Appl. Environ. Microbiol. 70:1321-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Grundy, F. J., A. J. Turinsky, and T. Henkin. 1994. Catabolite regulation of Bacillus subtilis acetate and acetoin utilization genes by CcpA. J. Bacteriol. 176:4527-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Guan, L., Y. Hu, and H. R. Kaback. 2003. Aromatic stacking in the sugar binding site of the lactose permease. Biochemistry 42:1377-1382. [DOI] [PubMed] [Google Scholar]
- 301.Gunnewijk, M. G. W., and B. Poolman. 2000. HPr(His∼P)-mediated phosphorylation differently affects counterflow and proton motive force-driven uptake via the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem. 275:34080-34085. [DOI] [PubMed] [Google Scholar]
- 302.Gunnewijk, M. G. W., P. W. Postma, and B. Poolman. 1999. Phosphorylation and functional properties of the IIA domain of the lactose transport protein of Streptococcus thermophilus. J. Bacteriol. 181:632-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Gupta, R. S. 2001. The branching order and phylogenetic placement of species from completed bacterial genomes, based on conserved indels found in various proteins. Int. Microbiol. 4:187-202. [DOI] [PubMed] [Google Scholar]
- 304.Gutknecht, R., R. Beutler, L. F. Garcia-Alles, U. Baumann, and B. Erni. 2001. The dihydroxyacetone kinase of Escherichia coli utilizes a phosphoprotein instead of ATP as phosphoryl donor. EMBO J. 15:2480-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gwinn, M. L., D. Yi, O. Smith, and J.-F. Tomb. 1996. Role of the two-component signal transduction and the phosphoenolpyruvate:carbohydrate phosphotransferase systems in competence development of Haemophilus influenzae Rd. J. Bacteriol. 178:6366-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Halbedel, S., J. Busse, S. R. Schmidl, and J. Stülke. 2006. Regulatory protein phosphorylation in Mycoplasma pneumoniae: a PP2C-type phosphatase serves to dephosphorylate HPr(Ser-P). J. Biol. Chem. 281:26253-26259. [DOI] [PubMed] [Google Scholar]
- 307.Halbedel, S., C. Hames, and J. Stülke. 2004. In vivo activity of enzymatic and regulatory components of the phosphoenolpyruvate:sugar phosphotransferase system in Mycoplasma pneumoniae. J. Bacteriol. 186:7936-7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Halbedel, S., C. Hames, and J. Stülke. 2007. Regulation of carbon metabolism in the mollicutes and its relation to virulence. J. Mol. Microbiol. Biotechnol. 12:145-152. [DOI] [PubMed] [Google Scholar]
- 309.Halpern, Y. S., and M. Lupo. 1966. Effect of glucose and other carbon sources on the transport of α-methylglucoside in Escherichia coli K12. Biochim. Biophys. Acta 126:163-167. [DOI] [PubMed] [Google Scholar]
- 310.Hanamura, A., and H. Aiba. 1992. A new aspect of transcriptional control of the Escherichia coli crp gene: positive autoregulation. Mol. Microbiol. 6:2489-2497. [DOI] [PubMed] [Google Scholar]
- 311.Hanamura, A., and H. Aiba. 1991. Molecular mechanism of negative autoregulation of Escherichia coli crp gene. Nucleic Acids Res. 19:4413-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Hanks, S. K., and T. Hunter. 1995. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 313.Hanlon, D. W., and G. W. Ordal. 1994. Cloning and characterization of genes encoding methyl-accepting chemotaxis proteins in Bacillus subtilis. J. Biol. Chem. 269:14038-14046. [PubMed] [Google Scholar]
- 314.Hansen, T., B. Reichstein, R. Schmid, and P. Schönheit. 2002. The first archaeal ATP-dependent glucokinase, from the hyperthermophilic crenarchaeon Aeropyrum pernix, represents a monomeric, extremely thermophilic ROK glucokinase with broad hexose specificity. J. Bacteriol. 184:5955-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hanson, K. G., K. Steinhauer, J. Reizer, W. Hillen, and J. Stülke. 2002. HPr kinase/phosphatase of Bacillus subtilis: expression of the gene and effects of mutations on enzyme activity, growth and carbon catabolite repression. Microbiology 148:1805-1811. [DOI] [PubMed] [Google Scholar]
- 316.Harman, J. G. 2001. Allosteric regulation of the cAMP receptor protein. Biochim. Biophys. Acta 1547:1-17. [DOI] [PubMed] [Google Scholar]
- 317.Harwood, J. P., C. Gazdar, C. Prasad, A. Peterkofsky, S. J. Curtis, and W. Epstein. 1976. Involvement of the glucose enzymes II of the sugar phosphotransferase system in the regulation of adenylate cyclase by glucose in Escherichia coli. J. Biol. Chem. 251:2462-2468. [PubMed] [Google Scholar]
- 318.Harwood, J. P., and A. Peterkofsky. 1975. Glucose-sensitive adenylate cyclase in toluene-treated cells of Escherichia coli B. J. Biol. Chem. 250:4656-4662. [PubMed] [Google Scholar]
- 319.Hays, J. B., R. D. Simoni, and S. Roseman. 1973. Sugar transport. V. A trimeric lactose-specific phosphocarrier protein of the Staphylococcus aureus phosphotransferase system. J. Biol. Chem. 248:941-956. [PubMed] [Google Scholar]
- 320.Héchard, Y., C. Pelletier, Y. Cenatiempo, and J. Frère. 2001. Analysis of σ54-dependent genes in Enterococcus faecalis: a mannose PTS permease (EIIMan) is involved in sensitivity to a bacteriocin, mesentericin Y105. Microbiology 147:1575-1580. [DOI] [PubMed] [Google Scholar]
- 321.Heller, K. B., E. C. C. Lin, and T. H. Wilson. 1980. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J. Bacteriol. 144:274-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Henkin, T. M., F. J. Grundy, W. L. Nicholson, and G. H. Chambliss. 1991. Catabolite repression of α-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacI and galR repressors. Mol. Microbiol. 5:575-584. [DOI] [PubMed] [Google Scholar]
- 323.Henstra, S. A., R. H. Duurkens, and G. T. Robillard. 2000. Multiple phosphorylation events regulate the activity of the mannitol transcriptional regulator MtlR of the Bacillus stearothermophilus phosphoenolpyruvate-dependent mannitol phosphotransferase system. J. Biol. Chem. 275:7037-7044. [DOI] [PubMed] [Google Scholar]
- 324.Henstra, S. A., B. Tolner, R. H. ten Hoeve Duurkens, W. N. Konings, and G. T. Robillard. 1996. Cloning, expression, and isolation of the mannitol transport protein from the thermophilic bacterium Bacillus stearothermophilus. J. Bacteriol. 178:5586-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Henstra, S. A., M. Tuinhof, R. H. Duurkens, and G. T. Robillard. 1999. The Bacillus stearothermophilus mannitol regulator, MtlR, of the phosphotransferase system. A DNA-binding protein, regulated by HPr and IICBmtl-dependent phosphorylation. J. Biol. Chem. 274:4754-4763. [DOI] [PubMed] [Google Scholar]
- 326.Herro, R., S. Poncet, P. Cossart, C. Buchrieser, E. Gouin, P. Glaser, and J. Deutscher. 2005. How seryl-phosphorylated HPr inhibits PrfA, a transcription activator of Listeria monocytogenes virulence genes. J. Mol. Microbiol. Biotechnol. 9:224-234. [DOI] [PubMed] [Google Scholar]
- 327.Herzberg, O. 1992. An atomic model for protein-protein phosphoryl group transfer. J. Biol. Chem. 267:24819-24823. [PubMed] [Google Scholar]
- 328.Herzberg, O., C. C. H. Chen, G. Kapadia, M. McGuire, L. J. Carroll, S. J. Noh, and D. Dunaway-Mariano. 1996. Swiveling-domain mechanism for enzymatic phosphotransfer between remote reaction sites. Proc. Natl. Acad. Sci. USA 93:2652-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Herzberg, O., and R. Klevit. 1994. Unraveling a bacterial hexose transport pathway. Curr. Opin. Struct. Biol. 4:814-822. [DOI] [PubMed] [Google Scholar]
- 330.Herzberg, O., P. Reddy, S. Sutrina, M. H. Saier, Jr., J. Reizer, and G. Kapadia. 1992. Structure of the histidine-containing phosphocarrier protein HPr from Bacillus subtilis at 2.0-Å resolution. Proc. Natl. Acad. Sci. USA 89:2499-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hester, K. L., J. Lehman, F. Najar, L. Song, B. A. Roe, C. H. MacGregor, P. W. Hager, P. V. Phibbs, and J. R. Sokatch. 2000. Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa. J. Bacteriol. 182:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Hester, K. L., K. T. Madhusudhan, and J. R. Sokatch. 2000. Catabolite repression control by Crc in 2xYT medium is mediated by posttranscriptional regulation of bkdR expression in Pseudomonas putida. J. Bacteriol. 182:1150-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Higa, F., and P. H. Edelstein. 2001. Potential virulence role of the Legionella pneumophila ptsP ortholog. Infect. Immun. 69:4782-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hogema, B. M., J. C. Arents, R. Bader, K. Eijkemans, T. Inada, H. Aiba, and P. W. Postma. 1998. Inducer exclusion by glucose 6-phosphate in Escherichia coli. Mol. Microbiol. 28:755-765. [DOI] [PubMed] [Google Scholar]
- 335.Hogema, B. M., J. C. Arents, R. Bader, K. Eijkemans, H. Yoshida, H. Takahashi, H. Aiba, and P. W. Postma. 1998. Inducer exclusion in Escherichia coli by non-PTS substrates: the role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol. Microbiol. 30:487-498. [DOI] [PubMed] [Google Scholar]
- 336.Hogema, B. M., J. C. Arents, R. Bader, and P. W. Postma. 1999. Autoregulation of lactose uptake through the LacY permease by enzyme IIAGlc of the PTS in Escherichia coli K-12. Mol. Microbiol. 31:1825-1833. [DOI] [PubMed] [Google Scholar]
- 337.Hogema, B. M., J. C. Arents, T. Inada, H. Aiba, K. van Dam, and P. W. Postma. 1997. Catabolite repression by glucose 6-phosphate, gluconate and lactose in Escherichia coli. Mol. Microbiol. 24:857-867. [DOI] [PubMed] [Google Scholar]
- 338.Hoischen, C., J. Levin, S. Pitaknarongphorn, J. Reizer, and M. H. Saier, Jr. 1996. Involvement of the central loop of the lactose permease of Escherichia coli in its allosteric regulation by the glucose-specific enzyme IIA of the phosphoenolpyruvate-dependent phosphotransferase system. J. Bacteriol. 178:6082-6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Holmberg, C., and B. Rutberg. 1991. Expression of the gene encoding glycerol-3-phosphate dehydrogenase (glpD) in Bacillus subtilis is controlled by antiterminatation. Mol. Microbiol. 5:2891-2900. [DOI] [PubMed] [Google Scholar]
- 340.Holtman, C. K., A. C. Pawlyk, N. D. Meadow, and D. W. Pettigrew. 2001. Reverse genetics of Escherichia coli glycerol kinase allosteric regulation and glucose control of glycerol utilization in vivo. J. Bacteriol. 183:3336-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Honeyman, A. L., and R. Curtiss III. 1992. Isolation, characterization, and nucleotide sequence of the Streptococcus mutans mannitol-phosphate dehydrogenase gene and the mannitol-specific factor III gene of the phosphoenolpyruvate phosphotransferase system. Infect. Immun. 60:3369-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Honeyman, A. L., and R. Curtiss III. 2000. The mannitol-specific enzyme II (mtlA) gene and the mtlR gene of the PTS of Streptococcus mutans. Microbiology 146:1565-1572. [DOI] [PubMed] [Google Scholar]
- 343.Hosono, K., H. Kakuda, and S. Ichihara. 1995. Decreasing accumulation of acetate in a rich medium by Escherichia coli on introduction of genes on a multicopy plasmid. Biosci. Biotechnol. Biochem. 59:256-261. [DOI] [PubMed] [Google Scholar]
- 344.Houman, F., M. R. Diaz-Torres, and A. Wright. 1990. Transcriptional antitermination in the bgl operon of E. coli is modulated by a specific RNA binding protein. Cell 62:1153-1163. [DOI] [PubMed] [Google Scholar]
- 345.Hu, K. Y., and M. H. Saier, Jr. 2002. Phylogeny of phosphoryl transfer proteins of the phosphoenolpyruvate-dependent sugar-transporting phosphotransferase system. Res. Microbiol. 153:405-415. [DOI] [PubMed] [Google Scholar]
- 346.Huang, H. S., K. Ito, C. H. Yin, T. Kabashima, and T. Yoshimoto. 1998. Cloning, sequencing, high expression, and crystallization of the thermophile Thermus aquaticus glycerol kinase. Biosci. Biotechnol. Biochem. 62:2375-2381. [DOI] [PubMed] [Google Scholar]
- 347.Huang, H. S., T. Kabashima, K. Ito, C. H. Yin, Y. Nishiya, Y. Kawamura, and T. Yoshimoto. 1998. Thermostable glycerol kinase from Thermus flavus: cloning, sequencing, and expression of the enzyme gene. Biochim. Biophys. Acta 1382:186-190. [DOI] [PubMed] [Google Scholar]
- 348.Huang, H. S., T. Yoshida, Y. Meng, T. Kabashima, K. Ito, Y. Nishiya, Y. Kawamura, and T. Yoshimoto. 1997. Purification and characterization of thermostable glycerol kinase from Thermus flavus. J. Ferment. Bioeng. 83:328-332. [Google Scholar]
- 349.Huang, K., G. Kapadia, P. P. Zhu, A. Peterkofsky, and O. Herzberg. 1998. A promiscuous binding surface: crystal structure of the IIA domain of the glucose-specific permease from Mycoplasma capricolum. Structure 6:697-710. [DOI] [PubMed] [Google Scholar]
- 350.Hüdig, H., and W. Hengstenberg. 1980. The bacterial phosphoenolpyruvate dependent phosphotransferase system: solubilization and kinetic parameters of the glucose-specific membrane-bound enzyme II component of Streptococcus faecalis. FEBS Lett. 114:103-106. [DOI] [PubMed] [Google Scholar]
- 351.Hudson, J. M., and M. G. Fried. 1990. Cooperative interactions between the catabolite gene activator protein and the lac repressor at the lactose promoter. J. Mol. Biol. 214:381-396. [DOI] [PubMed] [Google Scholar]
- 352.Hudson, J. W., G. B. Golding, and M. M. Crerar. 1993. Evolution of allosteric control in glycogen phosphorylase. J. Mol. Biol. 234:700-721. [DOI] [PubMed] [Google Scholar]
- 353.Hueck, C. J., W. Hillen, and M. H. Saier, Jr. 1994. Analysis of a cis-active sequence mediating catabolite repression in gram-positive bacteria. Res. Microbiol. 145:503-518. [DOI] [PubMed] [Google Scholar]
- 354.Hunke, S., M. Mourez, M. Jehanno, E. Dassa, and E. Schneider. 2000. ATP modulates subunit-subunit interactions in an ATP-binding cassette transporter (MalFGK2) determined by site-directed chemical cross-linking. J. Biol. Chem. 275:15526-15534. [DOI] [PubMed] [Google Scholar]
- 355.Hurley, J. H., H. R. Faber, D. Worthylake, N. D. Meadow, S. Roseman, D. W. Pettigrew, and S. J. Remington. 1993. Structure of the regulatory complex of Escherichia coli IIIGlc with glycerol kinase. Science 259:673-677. [PubMed] [Google Scholar]
- 356.Hurtado-Gomez, E., G. Fernandez-Ballester, H. Nothaft, J. Gomez, F. Titgemeyer, and J. L. Neira. 2006. Biophysical characterization of the enzyme I of the Streptomyces coelicolor phosphoenolpyruvate:sugar phosphotransferase system. Biophys. J. 90:4592-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Huynh, P. L., I. Jankovic, N. F. Schnell, and R. Brückner. 2000. Characterization of an HPr kinase mutant of Staphylococcus xylosus. J. Bacteriol. 182:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hvorup, R., A. B. Chang, and M. H. Saier, Jr. 2003. Bioinformatic analyses of the bacterial L-ascorbate phosphotransferase system permease family. J. Mol. Microbiol. Biotechnol. 6:191-205. [DOI] [PubMed] [Google Scholar]
- 359.Idelson, M., and O. Amster-Choder. 1998. SacY, a transcriptional antiterminator from Bacillus subtilis, is regulated by phosphorylation in vivo. J. Bacteriol. 180:660-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ihssen, J., and T. Egli. 2005. Global physiological analysis of carbon- and energy-limited growing Escherichia coli confirms a high degree of catabolic flexibility and preparedness for mixed substrate utilization. Environ. Microbiol. 7:1568-1581. [DOI] [PubMed] [Google Scholar]
- 361.Inacio, J. M., C. Costa, and I. de Sa-Nogueira. 2003. Distinct molecular mechanisms involved in carbon catabolite repression of the arabinose regulon in Bacillus subtilis. Microbiology 149:2345-2355. [DOI] [PubMed] [Google Scholar]
- 362.Inada, T., K. Kimata, and H. Aiba. 1996. Mechanism responsible for glucose-lactose diauxie in Escherichia coli: challenge to the cAMP model. Genes Cells 1:293-301. [DOI] [PubMed] [Google Scholar]
- 363.Inada, T., H. Takahashi, T. Mizuno, and H. Aiba. 1996. Down regulation of cAMP production by cAMP receptor protein in Escherichia coli: an assessment of the contributions of transcriptional and posttranscriptional control of adenylate cyclase. Mol. Gen. Genet. 253:198-204. [DOI] [PubMed] [Google Scholar]
- 364.Ishizuka, H., A. Hanamura, T. Inada, and H. Aiba. 1994. Mechanism of the down-regulation of cAMP receptor protein by glucose in Escherichia coli: role of autoregulation of the crp gene. EMBO J. 13:3077-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ishizuka, H., A. Hanamura, T. Kunimura, and H. Aiba. 1993. A lowered concentration of cAMP receptor protein caused by glucose is an important determinant for catabolite repression in Escherichia coli. Mol. Microbiol. 10:341-350. [DOI] [PubMed] [Google Scholar]
- 366.Iyer, R., N. S. Baliga, and A. Camilli. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 187:8340-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Jacob, F., and J. Monod. 1961. Genetic regulatory mechnaisms in the synthesis of proteins. J. Mol. Biol. 3:318-356. [DOI] [PubMed] [Google Scholar]
- 368.Jacobson, G. R., and J. W. Lengeler. 2002. Pieter W. Postma 1942-2002. Mol. Microbiol. 45:1461-1462. [DOI] [PubMed] [Google Scholar]
- 369.Jacobson, G. R., and C. Saraceni-Richards. 1993. The Escherichia coli mannitol permease as a model for transport via the bacterial phosphotransferase system. J. Bioenerg. Biomembr. 25:621-626. [DOI] [PubMed] [Google Scholar]
- 370.James, B. W., W. S. Mauchline, P. J. Dennis, C. W. Keevil, and R. Wait. 1999. Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Appl. Environ. Microbiol. 65:822-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Janakiraman, R. S., and Y. V. Brun. 1997. Transcriptional and mutational analyses of the rpoN operon in Caulobacter crescentus. J. Bacteriol. 179:5138-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Jault, J.-M., S. Fieulaine, S. Nessler, P. Gonzalo, A. Di Pietro, J. Deutscher, and A. Galinier. 2000. The HPr kinase from Bacillus subtilis is a homo-oligomeric enzyme which exhibits strong positive cooperativity for nucleotide and fructose 1,6-bisphosphate binding. J. Biol. Chem. 275:1773-1780. [DOI] [PubMed] [Google Scholar]
- 373.Jeong, J. Y., Y. J. Kim, N. W. Cho, D. W. Shin, T. W. Nam, S. Ryu, and Y.-J. Seok. 2004. Expression of ptsG encoding the major glucose transporter is regulated by ArcA in Escherichia coli. J. Biol. Chem. 279:38513-38518. [DOI] [PubMed] [Google Scholar]
- 374.Jia, Z., J. W. Quail, L. T. J. Delbaere, and E. B. Waygood. 1994. Structural comparison of the histidine-containing phosphocarrier protein HPr. Biochem. Cell Biol. 72:202-217. [DOI] [PubMed] [Google Scholar]
- 375.Jia, Z., M. Vandonselaar, W. Hengstenberg, J. W. Quail, and L. T. J. Delbaere. 1994. The 1.6 Å structure of histidine-containing phosphotransfer protein HPr from Streptococcus faecalis. J. Mol. Biol. 236:1341-1355. [DOI] [PubMed] [Google Scholar]
- 376.Jin, R. Z., and E. C. C. Lin. 1984. An inducible phosphoenolpyruvate:dihydroxyacetone phosphotransferase system in Escherichia coli. J. Gen. Microbiol. 130:83-88. [DOI] [PubMed] [Google Scholar]
- 377.Jin, S., K. Ishimito, and S. Lory. 1994. Nucleotide sequence of the rpoN gene and characterization of two downstream open reading frames in Pseudomonas aeruginosa. J. Bacteriol. 176:1316-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Johansson, J., C. Balsalobre, S. Y. Wang, J. Urbonaviciene, D. J. Jin, B. Sonden, and B. E. Uhlin. 2000. Nucleoid proteins stimulate stringently controlled bacterial promoters: a link between the cAMP-CRP and the (p)ppGpp regulons in Escherichia coli. Cell 102:475-485. [DOI] [PubMed] [Google Scholar]
- 379.Johnson, M. S., E. H. Rowsell, and B. L. Taylor. 1995. Investigation of transphosphorylation between chemotaxis proteins and the phosphoenolpyruvate:sugar phosphotransferase system. FEBS Lett. 374:161-164. [DOI] [PubMed] [Google Scholar]
- 380.Joly, N., A. Böhm, W. Boos, and E. Richet. 2004. MalK, the ATP-binding cassette component of the Escherichia coli maltodextrin transporter, inhibits the transcriptional activator MalT by antagonizing inducer binding. J. Biol. Chem. 279:33123-33130. [DOI] [PubMed] [Google Scholar]
- 381.Jones, B. E., V. Dossonnet, E. Küster, W. Hillen, J. Deutscher, and R. E. Klevit. 1997. Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorylation of its corepressor HPr. J. Biol. Chem. 272:26530-26535. [DOI] [PubMed] [Google Scholar]
- 382.Jones, D. H. A., F. C. H. Franklin, and C. M. Thomas. 1994. Molecular analysis of the operon which encodes the RNA polymerase sigma factor σ54 of Escherichia coli. Microbiology 140:1035-1043. [DOI] [PubMed] [Google Scholar]
- 383.Jones-Mortimer, M. C., and H. L. Kornberg. 1980. Amino-sugar transport systems of Escherichia coli K12. J. Gen. Microbiol. 117:369-376. [DOI] [PubMed] [Google Scholar]
- 384.Joseph, E., C. Bernsley, N. Guiso, and A. Ullmann. 1982. Multiple regulation of the activity of adenylate cyclase in Escherichia coli. Mol. Gen. Genet. 185:262-268. [DOI] [PubMed] [Google Scholar]
- 385.Joseph, E., A. Danchin, and A. Ullmann. 1981. Regulation of galactose operon expression: glucose effects and role of cyclic adenosine 3′,5′-monophosphate. J. Bacteriol. 146:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Jourlin-Castelli, C., N. Mani, M. M. Nakano, and A. L. Sonenshein. 2000. CcpC, a novel regulator of the LysR family required for glucose repression of the citB gene in Bacillus subtilis. J. Mol. Biol. 295:865-878. [DOI] [PubMed] [Google Scholar]
- 387.Jovanovitch, S. B. 1985. Regulation of a cya-lac fusion by cyclic AMP in Salmonella typhimurium. J. Bacteriol. 161:641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Jurado, P., L. A. Fernández, and V. de Lorenzo. 2003. σ54 levels and physiological control of the Pseudomonas putida Pu promoter. J. Bacteriol. 185:3379-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Juy, M., F. Penin, A. Favier, A. Galinier, R. Montserret, R. Haser, J. Deutscher, and A. Böckmann. 2003. Dimerization of Crh by reversible 3D domain swapping induces structural adjustments to its monomeric homologue HPr. J. Mol. Biol. 332:767-776. [DOI] [PubMed] [Google Scholar]
- 390.Kaback, H. R. 1997. A molecular mechanism for energy coupling in a membrane transport protein, the lactose permease of Escherichia coli. Proc. Natl. Acad. Sci. USA 94:5539-5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Kaback, H. R., M. Sahin-Tóth, and A. B. Weinglass. 2001. The kamikaze approach to membrane transport. Nat. Rev. Mol. Cell Biol. 2:610-620. [DOI] [PubMed] [Google Scholar]
- 392.Kamionka, A., S. Parche, H. Nothaft, J. Siepelmeyer, K. Jahreis, and F. Titgemeyer. 2002. The phosphotransferase system of Streptomyces coelicolor. Eur. J. Biochem. 269:2143-2150. [DOI] [PubMed] [Google Scholar]
- 393.Kao, J. S., D. M. Stucker, J. W. Warren, and H. L. Mobley. 1997. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect. Immun. 65:2812-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Kawamoto, H., T. Morita, A. Shimizu, T. Inada, and H. Aiba. 2005. Implication of membrane localization of target mRNA in the action of a small RNA: mechanism of post-transcriptional regulation of glucose transporter in Escherichia coli. Genes Dev. 19:328-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Kawamukai, M., J. Kishimoto, R. Utsumi, M. Himeno, T. Komano, and H. Aiba. 1985. Negative regulation of adenylate cyclase gene (cya) expression by cyclic AMP-cyclic AMP receptor protein in Escherichia coli: studies with cya-lac protein and operon fusion plasmids. J. Bacteriol. 164:872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Keener, J., and S. Kustu. 1988. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc. Natl. Acad. Sci. USA 85:4976-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Kessler, B., and B. Witholt. 2001. Factors involved in the regulatory network of polyhydroxyalkanoate metabolism. J. Biotechnol. 86:97-104. [DOI] [PubMed] [Google Scholar]
- 398.Keyhani, N., M. E. Rodgers, B. Demeler, J. C. Hansen, and S. Roseman. 2000. Analytical sedimentation of the IIAChb and IIBChb proteins of the Escherichia coli N,N′-diacetylchitobiose phosphotransferase system. Demonstration of a model phosphotransfer transition state complex. J. Biol. Chem. 275:33110-33115. [DOI] [PubMed] [Google Scholar]
- 399.Khan, S. R., and N. Banerjee-Bhatnagar. 2002. Loss of catabolite repression function of HPr, the phosphocarrier protein of the bacterial phosphotransferase system, affects expression of the cry4A toxin gene in Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 184:5410-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Khan, S. R., J. Deutscher, R. Vishwakarma, V. Monedero, and N. B. Bhatnagar. 2001. The ptsH gene from Bacillus thuringiensis israelensis. Characterization of a new phosphorylation site on the protein HPr. Eur. J. Biochem. 268:521-530. [DOI] [PubMed] [Google Scholar]
- 401.Kim, H. J., C. Jourlin-Castelli, S. I. Kim, and A. L. Sonenshein. 2002. Regulation of the Bacillus subtilis ccpC gene by CcpA and CcpC. Mol. Microbiol. 43:399-410. [DOI] [PubMed] [Google Scholar]
- 402.Kim, H. J., M. Mittal, and A. L. Sonenshein. 2006. CcpC-dependent regulation of citB and lmo0847 in Listeria monocytogenes. J. Bacteriol. 188:179-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Kim, H. J., A. Roux, and A. L. Sonenshein. 2002. Direct and indirect roles of CcpA in regulation of Bacillus subtilis Krebs cycle genes. Mol. Microbiol. 45:179-190. [DOI] [PubMed] [Google Scholar]
- 404.Kim, J.-H., M. I. Voskuil, and G. H. Chambliss. 1998. NADP, corepressor for the Bacillus catabolite control protein CcpA. Proc. Natl. Acad. Sci. USA 95:9590-9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Kim, J. H., Z. T. Guvener, J. Y. Cho, K.-C. Chung, and G. H. Chambliss. 1995. Specificity of DNA binding activity of the Bacillus subtilis catabolite control protein CcpA. J. Bacteriol. 177:5129-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Kim, J. H., Y. K. Yang, and G. H. Chambliss. 2005. Evidence that Bacillus catabolite control protein CcpA interacts with RNA polymerase to inhibit transcription. Mol. Microbiol. 56:155-162. [DOI] [PubMed] [Google Scholar]
- 407.Kim, S.-Y., T.-W. Nam, D. Shin, B.-M. Koo, Y.-J. Seok, and S. Ryu. 1999. Purification of Mlc and analysis of its effects on the pts expression in Escherichia coli. J. Biol. Chem. 274:25398-25402. [DOI] [PubMed] [Google Scholar]
- 408.Kimata, K., T. Inada, H. Tagami, and H. Aiba. 1998. A global repressor (Mlc) is involved in glucose induction of the ptsG gene encoding major glucose transporter in Escherichia coli. Mol. Microbiol. 29:1509-1519. [DOI] [PubMed] [Google Scholar]
- 409.Kimata, K., H. Takahashi, T. Inada, P. Postma, and H. Aiba. 1997. cAMP receptor protein—cAMP plays a crucial role in glucose-lactose diauxie by activating the glucose transporter gene in Escherichia coli. Proc. Natl. Acad. Sci. USA 94:12914-12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Kimata, K., Y. Tanaka, T. Inada, and H. Aiba. 2001. Expression of the glucose transporter gene, ptsG, is regulated at the mRNA degradation step in response to glycolytic flux in Escherichia coli. EMBO J. 20:3587-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Kinch, L. N., S. Cheek, and N. V. Grishin. 2005. EDD, a novel phosphotransferase domain common to mannose transporter EIIA, dihydroxy acetone kinase, and DegV. Protein Sci. 14:360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.King, N. D., and M. R. O'Brian. 2001. Evidence for direct interaction between enzyme INtr and aspartokinase to regulate bacterial oligopeptide transport. J. Biol. Chem. 276:21311-21316. [DOI] [PubMed] [Google Scholar]
- 413.Knezevic, I., S. Bachem, A. Sickmann, H. E. Meyer, J. Stülke, and W. Hengstenberg. 2000. Regulation of the glucose-specific phosphotransferase system (PTS) of Staphylococcus carnosus by the antiterminator protein GlcT. Microbiology 146:2333-2342. [DOI] [PubMed] [Google Scholar]
- 414.Koehler, T. M. 2002. Bacillus anthracis genetics and virulence gene regulation. Curr. Top. Microbiol. Immunol. 271:143-164. [DOI] [PubMed] [Google Scholar]
- 415.Köhler, T., J. F. Alvarez, and S. Harayama. 1994. Regulation of the rpoN, ORF102 and ORF154 genes in Pseudomonas putida. FEMS Microbiol. Lett. 115:177-184. [DOI] [PubMed] [Google Scholar]
- 416.Kolb, A., S. Busby, H. Buc, S. Garges, and S. Adhya. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62:749-795. [DOI] [PubMed] [Google Scholar]
- 417.Kolb, A., A. Spassky, C. Chapon, B. Blazy, and H. Buc. 1983. On the different binding affinities of CRP at the lac, gal, and malT promoter regions. Nucleic Acids Res. 11:7833-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Koo, B. M., and Y.-J. Seok. 2001. Regulation of glycogen concentration by the histidine-containing phosphocarrier protein HPr in Escherichia coli. J. Microbiol. 39:24-30. [Google Scholar]
- 419.Koo, B. M., M. J. Yoon, C. R. Lee, T. W. Nam, Y. J. Choe, H. Jaffe, A. Peterkofsky, and Y.-J. Seok. 2004. A novel fermentation/respiration switch protein regulated by enzyme IIAGlc in Escherichia coli. J. Biol. Chem. 279:31613-31621. [DOI] [PubMed] [Google Scholar]
- 420.Kornberg, H. L., and R. E. Reeves. 1972. Inducible phosphoenolpyruvate-dependent hexose phosphotransferase activities in Escherichia coli. Biochem. J. 128:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Kraus, A., C. Hueck, D. Gärtner, and W. Hillen. 1994. Catabolite repression of the Bacillus subtilis xyl operon involves a cis element functional in the context of an unrelated sequence, and glucose exerts additional xylR-dependent repression. J. Bacteriol. 176:1738-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Kraus, A., E. Küster, A. Wagner, K. Hoffmann, and W. Hillen. 1998. Identification of a co-repressor binding site in catabolite control protein CcpA. Mol. Microbiol. 30:955-963. [DOI] [PubMed] [Google Scholar]
- 423.Kravanja, M., R. Engelmann, V. Dossonnet, M. Blüggel, H. E. Meyer, R. Frank, A. Galinier, J. Deutscher, N. Schnell, and W. Hengstenberg. 1999. The hprK gene of Enterococcus faecalis encodes a novel bifunctional enzyme: the HPr kinase/phosphatase. Mol. Microbiol. 31:59-66. [DOI] [PubMed] [Google Scholar]
- 424.Kreikemeyer, B., K. S. McIver, and A. Podbielski. 2003. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol. 11:224-232. [DOI] [PubMed] [Google Scholar]
- 425.Kremling, A., K. Bettenbrock, B. Laube, K. Jahreis, J. W. Lengeler, and E. D. Gilles. 2001. The organization of metabolic reaction networks. III. Application for diauxic growth on glucose and lactose. Metab. Eng. 3:362-379. [DOI] [PubMed] [Google Scholar]
- 426.Kremling, A., S. Fischer, T. Sauter, K. Bettenbrock, and E. D. Gilles. 2004. Time hierarchies in the Escherichia coli carbohydrate uptake and metabolism. Biosystems 73:57-71. [DOI] [PubMed] [Google Scholar]
- 427.Krin, E., O. Sismeiro, A. Danchin, and P. N. Bertin. 2002. The regulation of enzyme IIAGlc expression controls adenylate cyclase activity in Escherichia coli. Microbiology 148:1553-1559. [DOI] [PubMed] [Google Scholar]
- 428.Kristich, C. J., G. D. Glekas, and G. W. Ordal. 2003. The conserved cytoplasmic module of the transmembrane chemoreceptor McpC mediates carbohydrate chemotaxis in Bacillus subtilis. Mol. Microbiol. 47:1353-1366. [DOI] [PubMed] [Google Scholar]
- 429.Krüger, S., S. Gertz, and M. Hecker. 1996. Transcriptional analysis of bglPH expression in Bacillus subtilis: evidence for two distinct pathways mediating carbon catabolite repression. J. Bacteriol. 178:2637-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Krüger, S., and M. Hecker. 1995. Regulation of the putative bglPH operon for aryl-β-glucoside utilization in Bacillus subtilis. J. Bacteriol. 177:5590-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Kühnau, S., M. Reyes, A. Sievertsen, H. A. Shuman, and W. Boos. 1991. The activities of the Escherichia coli MalK protein in maltose transport, regulation, and inducer exclusion can be separated by mutations. J. Bacteriol. 173:2180-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Kukuruzinska, M. A., W. F. Harrington, and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system. Studies on the molecular weight and association of enzyme I. J. Biol. Chem. 257:14470-14476. [PubMed] [Google Scholar]
- 433.Kullik, I., S. Fritsche, H. Knobel, J. Sanjuan, H. Hennecke, and H. M. Fischer. 1991. Bradyrhizobium japonicum has two differentially regulated, functional homologs of the σ54 gene (rpoN). J. Bacteriol. 173:1125-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Kundig, W., S. Ghosh, and S. Roseman. 1964. Phosphate bound to histidine in a protein as an intermediate in a novel phospho-transferase system. Proc. Natl. Acad. Sci. USA 52:1067-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Kundig, W., and S. Roseman. 1971. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J. Biol. Chem. 246:1393-1406. [PubMed] [Google Scholar]
- 436.Kundig, W., and S. Roseman. 1971. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J. Biol. Chem. 246:1407-1418. [PubMed] [Google Scholar]
- 437.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, N. J. Cummings, R. A. Daniel, F. Denizot, K. M. Devine, A. Dusterhoft, S. D. Ehrlich, P. T. Emmerson, K. D. Entian, J. Errington, C. Fabret, E. Ferrari, D. Foulger, C. Fritz, M. Fujita, Y. Fujita, S. Fuma, A. Galizzi, N. Galleron, S. Y. Ghim, P. Glaser, A. Goffeau, E. J. Golightly, G. Grandi, G. Guiseppi, B. J. Guy, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 438.Kuo, J. T., Y. J. Chang, and C. P. Tseng. 2003. Growth rate regulation of lac operon expression in Escherichia coli is cyclic AMP dependent. FEBS Lett. 553:397-402. [DOI] [PubMed] [Google Scholar]
- 439.Kuroda, M., S. De Waard, K. Mizushima, M. Tsuda, P. Postma, and T. Tsuchiya. 1992. Resistance of the melibiose carrier to inhibition by the phosphotransferase system due to substitutions of amino acid residues in the carrier of Salmonella typhimurium. J. Biol. Chem. 267:18336-18341. [PubMed] [Google Scholar]
- 440.Kuroda, M., T. H. Wilson, and T. Tsuchiya. 2001. Regulation of galactoside transport by the PTS. J. Mol. Microbiol. Biotechnol. 3:381-384. [PubMed] [Google Scholar]
- 441.Küster, E., E. J. Luesink, W. M. de Vos, and W. Hillen. 1996. Immunological crossreactivity to the catabolite control protein CcpA from Bacillus megaterium is found in many gram-positive bacteria. FEMS Microbiol. Lett. 139:109-115. [DOI] [PubMed] [Google Scholar]
- 442.Küster-Schöck, E., A. Wagner, U. Völker, and W. Hillen. 1999. Mutations in catabolite control protein CcpA showing glucose-independent regulation in Bacillus megaterium. J. Bacteriol. 181:7634-7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Kwakman, J. H. J. M., and P. W. Postma. 1994. Glucose kinase has a regulatory role in carbon catabolite repression in Streptomyces coelicolor. J. Bacteriol. 176:2694-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Kwaw, I., K. C. Zen, Y. Hu, and H. R. Kaback. 2001. Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: helices IV and V that contain the major determinants for substrate binding. Biochemistry 40:10491-10499. [DOI] [PubMed] [Google Scholar]
- 445.Lai, X., and L. O. Ingram. 1993. Cloning and sequencing of a cellobiose phosphotransferase system operon from Bacillus stearothermophilus XL-65-6 and functional expression in Escherichia coli. J. Bacteriol. 175:6441-6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Lai, X. K., F. C. Davis, R. B. Hespell, and L. O. Ingram. 1997. Cloning of cellobiose phosphoenolpyruvate-dependent phosphotransferase genes: functional expression in recombinant Escherichia coli and identification of a putative binding region for disaccharides. Appl. Environ. Microbiol. 63:355-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Landmesser, H., A. Stein, B. Bluschke, M. Brinkmann, S. Hunke, and E. Schneider. 2002. Large-scale purification, dissociation and functional reassembly of the maltose ATP-binding cassette transporter (MalFGK2) of Salmonella typhimurium. Biochim. Biophys. Acta 1565:64-72. [DOI] [PubMed] [Google Scholar]
- 448.Langbein, I., S. Bachem, and J. Stülke. 1999. Specific interaction of the RNA-binding domain of the Bacillus subtilis transcriptional antiterminator GlcT with its RNA target, RAT. J. Mol. Biol. 293:795-805. [DOI] [PubMed] [Google Scholar]
- 449.Lanz, R., and B. Erni. 1998. The glucose transporter of the Escherichia coli phosphotransferase system. Mutant analysis of the invariant arginines, histidines, and domain linker. J. Biol. Chem. 273:12239-12243. [DOI] [PubMed] [Google Scholar]
- 450.LaPorte, D. L., and D. E. Koshland. 1982. A protein with kinase and phosphatase activities involved in regulation of tricarboxylic acid cycle. Nature 300:458-460. [DOI] [PubMed] [Google Scholar]
- 451.Lawhon, S. D., J. G. Frye, M. Suyemoto, S. Porwollik, M. McClelland, and C. Altier. 2003. Global regulation by CsrA in Salmonella typhimurium. Mol. Microbiol. 48:1633-1645. [DOI] [PubMed] [Google Scholar]
- 452.Lawther, R. P., D. H. Calhoun, C. W. Adams, C. A. Hauser, J. Gray, and G. W. Hatfield. 1981. Molecular basis of valine resistance in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 78:922-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Leboeuf, C., L. Leblanc, Y. Auffray, and A. Hartke. 2000. Characterization of the ccpA gene of Enterococcus faecalis: identification of starvation-inducible proteins regulated by CcpA. J. Bacteriol. 182:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Le Coq, D., C. Lindner, S. Krüger, M. Steinmetz, and J. Stülke. 1995. New β-glucoside (bgl) genes in Bacillus subtilis: the bglP gene product has both transport and regulatory functions similar to those of BglF, its Escherichia coli homolog. J. Bacteriol. 177:1527-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Lee, B. R., P. Lecchi, L. Pannell, H. Jaffe, and A. Peterkofsky. 1994. Identification of the N-terminal domain of enzyme I of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system produced by proteolytic digestion. Arch. Biochem. Biophys. 312:121-124. [DOI] [PubMed] [Google Scholar]
- 456.Lee, C. R., B. M. Koo, S. H. Cho, Y. J. Kim, M. J. Yoon, A. Peterkofsky, and Y.-J. Seok. 2005. Requirement of the dephospho-form of enzyme IIANtr for derepression of Escherichia coli K-12 ilvBN expression. Mol. Microbiol. 58:334-344. [DOI] [PubMed] [Google Scholar]
- 457.Lee, J. S., K. D. Wittchen, C. Stahl, J. Strey, and F. Meinhardt. 2001. Cloning, expression, and carbon catabolite repression of the bamM gene encoding β-amylase of Bacillus megaterium DSM319. Appl. Microbiol. Biotechnol. 56:205-211. [DOI] [PubMed] [Google Scholar]
- 458.Lee, S. J., W. Boos, J. P. Bouche, and J. Plumbridge. 2000. Signal transduction between a membrane-bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J. 19:5353-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Legler, P. M., M. L. Cai, A. Peterkofsky, and G. M. Clore. 2004. Three-dimensional solution structure of the cytoplasmic B domain of the mannitol transporter IIMannitol of the Escherichia coli phosphotransferase system. J. Biol. Chem. 279:39115-39121. [DOI] [PubMed] [Google Scholar]
- 460.Lengeler, J. 1975. Mutations affecting transport of the hexitols d-mannitol, d-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J. Bacteriol. 124:26-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Lengeler, J. 1975. Nature and properties of hexitol transport systems in Escherichia coli. J. Bacteriol. 124:39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Lengeler, J., A.-M. Auburger, R. Mayer, and A. Pecher. 1981. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K12. Mol. Gen. Genet. 183:163-170. [DOI] [PubMed] [Google Scholar]
- 463.Lengeler, J. W. 2000. Metabolic networks: a signal-oriented approach to cellular models. Biol. Chem. 381:911-920. [DOI] [PubMed] [Google Scholar]
- 464.Lengeler, J. W., K. Jahreis, and U. F. Wehmeier. 1994. Enzymes II of the phosphoenolpyruvate-dependent phosphotransferase systems: their structure and function in carbohydrate transport. Biochim. Biophys. Acta 1188:1-28. [DOI] [PubMed] [Google Scholar]
- 465.Lengeler, J. W., and H. Steinberger. 1978. Analysis of regulatory mechanisms controlling the activity of the hexitol transport systems in Escherichia coli K12. Mol. Gen. Genet. 167:75-82. [DOI] [PubMed] [Google Scholar]
- 466.Lengeler, J. W., and H. Steinberger. 1978. Analysis of the regulatory mechanisms controlling the synthesis of the hexitol transport systems in Escherichia coli K12. Mol. Gen. Genet. 164:163-169. [DOI] [PubMed] [Google Scholar]
- 467.Leong-Morgenthaler, P., M. C. Zwahlen, and H. Hottinger. 1991. Lactose metabolism in Lactobacillus bulgaricus: analysis of the primary structure and expression of the genes involved. J. Bacteriol. 173:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Lessard, C., A. Cochu, J. D. Lemay, D. Roy, K. Vaillancourt, M. Frenette, S. Moineau, and C. Vadeboncoeur. 2003. Phosphorylation of Streptococcus salivarius lactose permease (LacS) by HPr(His∼P) and HPr(Ser-P)(His∼P) and effects on growth. J. Bacteriol. 185:6764-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Lévy, S., G.-Q. Zeng, and A. Danchin. 1990. Cyclic AMP synthesis in Escherichia coli strains bearing known deletions in the pts phosphotransferase operon. Gene 86:27-33. [DOI] [PubMed] [Google Scholar]
- 470.Lewis, M., G. Chang, N. C. Horton, M. A. Kercher, H. C. Pace, M. A. Schumacher, R. G. Brennan, and P. Z. Lu. 1996. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science 271:1247-1254. [DOI] [PubMed] [Google Scholar]
- 471.Li, J., X. Cheng, and J. C. Lee. 2002. Structure and dynamics of the modular halves of Escherichia coli cyclic AMP receptor protein. Biochemistry 41:14771-14778. [DOI] [PubMed] [Google Scholar]
- 472.Li, L., Y. Jia, Q. Hou, T. C. Charles, E. W. Nester, and S. Q. Q. Pan. 2002. A global pH sensor: Agrobacterium sensor protein ChvG regulates acid-inducible genes on its two chromosomes and Ti plasmid. Proc. Natl. Acad. Sci. USA 99:12369-12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Li, Y., and T. Ferenci. 1997. Gene organisation and regulatory sequences in the sucrose utilisation cluster of Bacillus stearothermophilus NUB36. Gene 195:195-200. [DOI] [PubMed] [Google Scholar]
- 474.Liao, D.-I., G. Kapadia, P. Reddy, M. H. Saier, Jr., J. Reizer, and O. Herzberg. 1991. Structure of the IIA domain of the glucose permease of Bacillus subtilis at 2.2-Å resolution. Biochemistry 30:9583-9594. [DOI] [PubMed] [Google Scholar]
- 475.Liao, D.-I., E. Silverton, Y.-J. Seok, B. R. Lee, A. Peterkofsky, and D. R. Davies. 1996. The first step in sugar transport: crystal structure of the amino terminal domain of enzyme I of the E. coli PEP:sugar phosphotransferase system and a model of the phosphotransfer complex with HPr. Structure 4:861-872. [DOI] [PubMed] [Google Scholar]
- 476.Liberman, E., P. Reddy, C. Gazdar, and A. Peterkofsky. 1985. The Escherichia coli adenylate cyclase complex. Stimulation by potassium and phosphate. J. Biol. Chem. 260:4075-4081. [PubMed] [Google Scholar]
- 477.Liberman, E., D. Saffen, S. Roseman, and A. Peterkofsky. 1986. Inhibition of E. coli adenylate cyclase activity by inorganic orthophosphate is dependent on IIIglc of the phosphoenolpyruvate:glycose phosphotransferase system. Biochem. Biophys. Res. Commun. 141:1138-1144. [DOI] [PubMed] [Google Scholar]
- 478.LiCalsi, C., T. S. Crocenzi, E. Freire, and S. Roseman. 1991. Sugar transport by the bacterial phosphotransferase system. Structural and thermodynamic domains of enzyme I of Salmonella typhimurium. J. Biol. Chem. 266:19519-19527. [PubMed] [Google Scholar]
- 479.Lin, E. C. C. 1996. Dissimilatory pathways for sugars, polyols, and carboxylates, p. 307-342. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 480.Lin, E. C. C. 1976. Glycerol dissimilation and its regulation in bacteria. Annu. Rev. Microbiol. 30:535-578. [DOI] [PubMed] [Google Scholar]
- 481.Lin, E. C. C. 1986. Glycerol facilitator in Escherichia coli. Methods Enzymol. 125:467-473. [DOI] [PubMed] [Google Scholar]
- 482.Lindner, C., A. Galinier, M. Hecker, and J. Deutscher. 1999. Regulation of the activity of the Bacillus subtilis antiterminator LicT by multiple PEP-dependent, enzyme I- and HPr-catalysed phosphorylation. Mol. Microbiol. 31:995-1006. [DOI] [PubMed] [Google Scholar]
- 483.Lindner, C., M. Hecker, D. Le Coq, and J. Deutscher. 2002. Bacillus subtilis mutant LicT antiterminators exhibiting enzyme I- and HPr-independent antitermination affect catabolite repression of the bglPH operon. J. Bacteriol. 184:4819-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Lis, J. T., and R. Schleif. 1973. Different cyclic AMP requirements for induction of the arabinose and lactose operons of Escherichia coli. J. Mol. Biol. 79:149-162. [DOI] [PubMed] [Google Scholar]
- 485.Liu, M., T. Durfee, J. E. Cabrera, K. Zhao, D. J. Jin, and F. R. Blattner. 2005. Global transcriptional programs reveal a carbon source foraging strategy by Escherichia coli. J. Biol. Chem. 280:15921-15927. [DOI] [PubMed] [Google Scholar]
- 486.Liu, M. Y., H. H. Yang, and T. Romeo. 1995. The product of the pleiotropic Escherichia coli gene csrA modulates glycogen biosynthesis via effects on messenger RNA stability. J. Bacteriol. 177:2663-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Liu, W. Z., R. Faber, M. Feese, S. J. Remington, and D. W. Pettigrew. 1994. Escherichia coli glycerol kinase: role of a tetramer interface in regulation by fructose 1,6-bisphosphate and phosphotransferase system regulatory protein IIIGlc. Biochemistry 33:10120-10126. [DOI] [PubMed] [Google Scholar]
- 488.Liu, X. M., and H. W. Taber. 1998. Catabolite regulation of the Bacillus subtilis ctaBCDEF gene cluster. J. Bacteriol. 180:6154-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Lokman, B. C., M. Heerikhuisen, R. J. Leer, A. van den Broek, Y. Borsboom, S. Chaillou, P. W. Postma, and P. H. Pouwels. 1997. Regulation of expression of the Lactobacillus pentosus xylAB operon. J. Bacteriol. 179:5391-5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Lopez, J. M., and B. Thoms. 1977. Role of sugar uptake and metabolic intermediates on catabolite repression in Bacillus subtilis. J. Bacteriol. 129:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Lorca, G. L., Y. J. Chung, R. D. Barabote, W. Weyler, C. H. Schilling, and M. H. Saier, Jr. 2005. Catabolite repression and activation in Bacillus subtilis: dependency on CcpA, HPr, and HprK. J. Bacteriol. 187:7826-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Ludwig, H., N. Rebhan, H.-M. Blencke, M. Merzbacher, and J. Stülke. 2002. Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol. Microbiol. 45:543-553. [DOI] [PubMed] [Google Scholar]
- 493.Luesink, E. J., C. M. A. Beumer, O. P. Kuipers, and W. M. de Vos. 1999. Molecular characterization of the Lactococcus lactis ptsHI operon and analysis of the regulatory role of HPr. J. Bacteriol. 181:764-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Luesink, E. J., R. E. M. A. van Harpen, B. P. Grossiord, O. P. Kuipers, and W. M. de Vos. 1998. Transcriptional activation of the glycolytic las operon and catabolite repression of the gal operon in Lactococcus lactis are mediated by the catabolite control protein CcpA. Mol. Microbiol. 30:789-798. [DOI] [PubMed] [Google Scholar]
- 495.Reference deleted.
- 496.Lun, S., and P. J. Willson. 2005. Putative mannose-specific phosphotransferase system component IID represses expression of suilysin in serotype 2 Streptococcus suis. Vet. Microbiol. 105:169-180. [DOI] [PubMed] [Google Scholar]
- 497.Lux, R., K. Jahreis, K. Bettenbrock, J. S. Parkinson, and J. W. Lengeler. 1995. Coupling of the phosphotransferase system and the methyl-accepting chemotaxis protein-dependent chemotaxis signaling pathways of Escherichia coli. Proc. Natl. Acad. Sci. USA 92:11583-11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Lux, R., V. R. N. Munasinghe, F. Castellano, J. W. Lengeler, J. E. T. Corrie, and S. Khan. 1999. Elucidation of a PTS-carbohydrate chemotactic signal pathway in Escherichia coli using a time-resolved behavioral assay. Mol. Biol. Cell 10:1133-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Macfadyen, L. P., I. R. Dorocicz, J. Reizer, M. H. Saier, Jr., and R. J. Redfield. 1996. Regulation of competence development and sugar utilization in Haemophilus influenzae Rd by a phosphoenolpyruvate:fructose phosphotransferase system. Mol. Microbiol. 21:941-952. [DOI] [PubMed] [Google Scholar]
- 500.Macfadyen, L. P., and R. J. Redfield. 1996. Life in mucus: sugar metabolism in Haemophilus influenzae. Res. Microbiol. 147:541-551. [DOI] [PubMed] [Google Scholar]
- 501.MacNab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 502.Magasanik, B. 1970. Glucose effects: inducer exclusion and repression, p. 189-219. In J. R. Beckwith and D. Zipser (ed.), The lactose operon. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 503.Magill, N. G., A. E. Cowan, D. E. Koppel, and P. Setlow. 1994. The internal pH of the forespore compartment of Bacillus megaterium decreases by about 1 pH unit during sporulation. J. Bacteriol. 176:2252-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Mahadevan, S., A. E. Reynolds, and A. Wright. 1987. Positive and negative regulation of the bgl operon in Escherichia coli. J. Bacteriol. 169:2570-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Mahadevan, S., and A. Wright. 1987. A bacterial gene involved in transcription antitermination: regulation at a rho-independent terminator in the bgl operon of E. coli. Cell 50:485-494. [DOI] [PubMed] [Google Scholar]
- 506.Maheswaran, M., and K. Forchhammer. 2003. Carbon-source-dependent nitrogen regulation in Escherichia coli is mediated through glutamine-dependent GlnB signalling. Microbiology 149:2163-2172. [DOI] [PubMed] [Google Scholar]
- 507.Mahr, K., W. Hillen, and F. Titgemeyer. 2000. Carbon catabolite repression in Lactobacillus pentosus: analysis of the ccpA region. Appl. Environ. Microbiol. 66:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Makman, R. S., and E. W. Sutherland. 1965. Adenosine 3′,5′-phosphate in Escherichia coli. J. Biol. Chem. 240:1309-1314. [PubMed] [Google Scholar]
- 509.Mani, N., and B. Dupuy. 2001. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc. Natl. Acad. Sci. USA 98:5844-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Manival, X., Y. S. Yang, M. P. Strub, M. Kochoyan, M. Steinmetz, and S. Aymerich. 1997. From genetic to structural characterization of a new class of RNA-binding domain within the SacY/BglG family of antiterminator proteins. EMBO J. 16:5019-5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Marasco, R., L. Muscariello, M. Rigano, and M. Sacco. 2002. Mutational analysis of the bglH catabolite-responsive element (cre) in Lactobacillus plantarum. FEMS Microbiol. Lett. 208:143-146. [DOI] [PubMed] [Google Scholar]
- 512.Marasco, R., L. Muscariello, M. Varcamonti, M. De Felice, and M. Sacco. 1998. Expression of the bglH gene of Lactobacillus plantarum is controlled by carbon catabolite repression. J. Bacteriol. 180:3400-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Marasco, R., I. Salatiello, M. De Felice, and M. Sacco. 2000. A physical and functional analysis of the newly-identified bglGPT operon of Lactobacillus plantarum. FEMS Microbiol. Lett. 186:269-273. [DOI] [PubMed] [Google Scholar]
- 514.Marqués, S., M. T. Gallegos, M. Manzanera, A. Holtel, K. N. Timmis, and J. L. Ramos. 1998. Activation and repression of transcription at the double tandem divergent promoters for the xylR and xylS genes of the TOL plasmid of Pseudomonas putida. J. Bacteriol. 180:2889-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Marquez, J. A., S. Hasenbein, B. Koch, S. Fieulaine, S. Nessler, R. B. Russell, W. Hengstenberg, and K. Scheffzek. 2002. Structure of the full-length HPr kinase/phosphatase from Staphylococcus xylosus at 1.95 Å resolution: mimicking the product/substrate of the phospho transfer reactions. Proc. Natl. Acad. Sci. USA 99:3458-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Marquez, J. A., S. Reinelt, B. Koch, R. Engelmann, W. Hengstenberg, and K. Scheffzek. 2006. Structure of the full-length enzyme I of the phosphoenopyruvate dependent sugar phosphotransferase system. J. Biol. Chem. 281:32508-32515. [DOI] [PubMed] [Google Scholar]
- 517.Marr, A. K., B. Joseph, S. Mertins, R. Ecke, S. Müller-Altrock, and W. Goebel. 2006. Overexpression of PrfA leads to growth inhibition of Listeria monocytogenes in glucose-containing culture media by interfering with glucose uptake. J. Bacteriol. 188:3887-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Martin, I., M. Débarbouillé, A. Klier, and G. Rapoport. 1989. Induction and metabolite regulation of levanase synthesis in Bacillus subtilis. J. Bacteriol. 171:1885-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Martin, S. A., and J. B. Russell. 1987. Transport and phosphorylation of disaccharides by the ruminal bacterium Streptococcus bovis. Appl. Environ. Microbiol. 53:2388-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Martin-Verstraete, I., V. Charrier, J. Stülke, A. Galinier, B. Erni, G. Rapoport, and J. Deutscher. 1998. Antagonistic effects of dual PTS-catalysed phosphorylation on the Bacillus subtilis transcriptional activator LevR. Mol. Microbiol. 28:293-303. [DOI] [PubMed] [Google Scholar]
- 521.Martin-Verstraete, I., M. Débarbouillé, A. Klier, and G. Rapoport. 1994. Interaction of wild-type and truncated LevR of Bacillus subtilis with the upstream activating sequence of the levanase operon. J. Mol. Biol. 241:178-192. [DOI] [PubMed] [Google Scholar]
- 522.Martin-Verstraete, I., M. Débarbouillé, A. Klier, and G. Rapoport. 1990. Levanase operon of Bacillus subtilis includes a fructose-specific phosphotransferase system regulating the expression of the operon. J. Mol. Biol. 214:657-671. [DOI] [PubMed] [Google Scholar]
- 523.Martin-Verstraete, I., J. Deutscher, and A. Galinier. 1999. Phosphorylation of HPr and Crh by HprK, early steps in the catabolite repression signalling pathway for the Bacillus subtilis levanase operon. J. Bacteriol. 181:2966-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Martin-Verstraete, I., A. Galinier, E. Darbon, Y. Quentin, V. Charrier, M.-C. Kilhoffer, J. Haiech, G. Rapoport, and J. Deutscher. 1999. The Q15H mutation enables Crh, a Bacillus subtilis HPr-like protein, to carry out some regulatory HPr functions, but does not make it an effective phosphocarrier for sugar transport. Microbiology 145:3195-3204. [DOI] [PubMed] [Google Scholar]
- 525.Martin-Verstraete, I., V. Michel, and A. Charbit. 1996. The levanase operon of Bacillus subtilis expressed in Escherichia coli can substitute for the mannose permease in mannose uptake and bacteriophage lambda infection. J. Bacteriol. 178:7112-7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Martin-Verstraete, I., J. Stülke, A. Klier, and G. Rapoport. 1995. Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J. Bacteriol. 177:6919-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Martínez-Antonio, A., and J. Collado-Vides. 2003. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 6:482-489. [DOI] [PubMed] [Google Scholar]
- 528.Mason, P. W., D. P. Carbone, R. A. Cushman, and A. S. Waggoner. 1981. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J. Biol. Chem. 256:1861-1866. [PubMed] [Google Scholar]
- 529.Matin, A., and M. K. Matin. 1982. Cellular levels, excretion, and synthesis rates of cyclic AMP in Escherichia coli grown in continuous culture. J. Bacteriol. 149:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Mattern, S. G., M. E. Brawner, and J. Westpheling. 1993. Identification of a complex operator for galp1, the glucose-sensitive, galactose-dependent promoter of the Streptomyces galactose operon. J. Bacteriol. 175:1213-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Mattoo, R. L., and E. B. Waygood. 1983. Determination of the levels of HPr and enzyme I of the phosphoenolpyruvate-sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Can. J. Biochem. 61:29-37. [DOI] [PubMed] [Google Scholar]
- 532.Maurel, C., J. Reizer, J. I. Schroeder, M. J. Chrispeels, and M. H. Saier, Jr. 1994. Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J. Biol. Chem. 269:11869-11872. [PubMed] [Google Scholar]
- 533.Maurer, T., S. Meier, N. Kachel, C. E. Munte, S. Hasenbein, B. Koch, W. Hengstenberg, and H. R. Kalbitzer. 2004. High-resolution structure of the histidine-containing phosphocarrier protein (HPr) from Staphylococcus aureus and characterization of its interaction with the bifunctional HPr kinase/phosphorylase. J. Bacteriol. 186:5906-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Mazé, A., G. Boël, S. Poncet, I. Mijakovic, Y. Le Breton, A. Benachour, V. Monedero, J. Deutscher, and A. Hartke. 2004. The Lactobacillus casei ptsHI47T mutation causes overexpression of a LevR-regulated but RpoN-independent operon encoding a mannose class phosphotransferase system. J. Bacteriol. 186:4543-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534a.Mazé, A., M. O'Connell-Motherway, G. F. Fitzgerald, J. Deutscher, and D. van Sinderen. Identification and characterization of a fructose phosphotransferase system in Bifidobacterium breve UCC2003. Appl. Environ. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 535.McIver, K. S., and J. R. Scott. 1997. Role of mga in growth phase regulation of virulence genes of the group A streptococcus. J. Bacteriol. 179:5178-5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.McIver, K. S., A. S. Thurman, and J. R. Scott. 1999. Regulation of mga transcription in the group A streptococcus: specific binding of Mga within its own promoter and evidence for a negative regulator. J. Bacteriol. 181:5373-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.McLandsborough, L. A., and P. P. Cleary. 1995. Insertional inactivation of virR in Streptococcus pyogenes M49 demonstrates that VirR functions as a positive regulator of ScpA, FcRA, OF, and M protein. FEMS Microbiol. Lett. 128:45-51. [DOI] [PubMed] [Google Scholar]
- 538.Meadow, N. D., P. Coyle, A. Komoryia, C. B. Anfinsen, and S. Roseman. 1986. Limited proteolysis of IIIGlc, a regulatory protein of the phosphoenolpyruvate:glycose phosphotransferase system, by membrane-associated enzymes from Salmonella typhimurium and Escherichia coli. J. Biol. Chem. 261:13504-13509. [PubMed] [Google Scholar]
- 539.Meadow, N. D., R. L. Mattoo, R. S. Savtchenko, and S. Roseman. 2005. Transient state kinetics of enzyme I of the phosphoenolpyruvate:glycose phosphotransferase system of Escherichia coli: equilibrium and second-order rate constants for the phosphotransfer reactions with phosphoenolpyruvate and HPr. Biochemistry 44:12790-12796. [DOI] [PubMed] [Google Scholar]
- 540.Meadow, N. D., and S. Roseman. 1996. Rate and equilibrium constants for phosphoryltransfer between active site histidines of Escherichia coli HPr and the signal transducing protein IIIGlc. J. Biol. Chem. 271:33440-33445. [DOI] [PubMed] [Google Scholar]
- 541.Meadow, N. D., and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J. Biol. Chem. 257:14526-14537. [PubMed] [Google Scholar]
- 542.Meadow, N. D., J. M. Rosenberg, H. M. Pinkert, and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system. Evidence that crr is the structural gene for the Salmonella typhimurium glucose-specific phosphocarrier protein IIIGlc. J. Biol. Chem. 257:14538-14542. [PubMed] [Google Scholar]
- 543.Meadow, N. D., D. W. Saffen, R. P. Dottin, and S. Roseman. 1982. Molecular cloning of the crr gene and evidence that it is the structural gene for IIIGlc, a phosphocarrier protein of the bacterial phosphotransferase system. Proc. Natl. Acad. Sci. USA 79:2528-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Meadow, N. D., R. S. Savtchenko, A. Nezami, and S. Roseman. 2005. Transient state kinetics of enzyme IICBGlc, a glucose transporter of the phosphoenolpyruvate phosphotransferase system of Escherichia coli: equilibrium and second order rate constants for the glucose binding and phosphotransfer reactions. J. Biol. Chem. 280:41872-41880. [DOI] [PubMed] [Google Scholar]
- 545.Meadow, N. D., R. S. Savtchenko, S. J. Remington, and S. Roseman. 2006. Effects of mutations and truncations on the kinetic behavior of IIAGlc, a phosphocarrier and regulatory protein of the phosphoenolpyruvate phosphotransferase system of Escherichia coli. J. Biol. Chem. 281:11450-11455. [DOI] [PubMed] [Google Scholar]
- 546.Meijberg, W., G. K. Schuurman-Wolters, and G. T. Robillard. 1998. Thermodynamic evidence for conformational coupling between the B and C domains of the mannitol transporter of Escherichia coli, enzyme IImtl. J. Biol. Chem. 273:7949-7956. [DOI] [PubMed] [Google Scholar]
- 547.Meins, M., P. Jenö, D. Müller, W. J. Richter, J. R. Rosenbusch, and B. Erni. 1993. Cysteine phosphorylation of the glucose transporter of Escherichia coli. J. Biol. Chem. 268:11604-11609. [PubMed] [Google Scholar]
- 548.Meins, M., B. Zanolari, J. P. Rosenbusch, and B. Erni. 1988. Glucose permease of Escherichia coli. Purification of the IIGlc subunit and functional characterization of its oligomeric forms. J. Biol. Chem. 263:12986-12993. [PubMed] [Google Scholar]
- 549.Mekjian, K. R., E. M. Bryan, B. W. Beall, and C. P. Moran, Jr. 1999. Regulation of hexuronate utilization in Bacillus subtilis. J. Bacteriol. 181:426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Melton, T., P. E. Hartman, J. P. Stratis, T. L. Lee, and A. T. Davis. 1978. Chemotaxis of Salmonella typhimurium to amino acids and some sugars. J. Bacteriol. 133:708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Merrick, M., J. Gibbins, and A. Toukdarian. 1987. The nucleotide sequence of the sigma factor gene ntrA (rpoN) of Azotobacter vinelandii: analysis of conserved sequences in NtrA proteins. Mol. Gen. Genet. 210:323-330. [DOI] [PubMed] [Google Scholar]
- 552.Merrick, M. J. 1993. In a class of its own—the RNA polymerase sigma factor σ54 (σN). Mol. Microbiol. 10:903-909. [DOI] [PubMed] [Google Scholar]
- 553.Merrick, M. J., and J. R. Coppard. 1989. Mutations in genes downstream of the rpoN gene (encoding σ54) of Klebsiella pneumoniae affect expression from σ54-dependent promoters. Mol. Microbiol. 3:1765-1775. [DOI] [PubMed] [Google Scholar]
- 554.Merrick, M. J., M. Taylor, M. H. Saier, Jr., and J. Reizer. 1995. The role of genes downstream of the σN structural gene rpoN in Klebsiella pneumoniae, p. 189-194. In I. A. Tikhonovitch, N. A. Provorov, V. I. Romanov, and W. E. Newton (ed.), Nitrogen fixation: fundamentals and applications. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 555.Michiels, J., T. van Soom, I. D'Hooghe, B. Dombrecht, T. Benhassine, P. de Wilde, and J. Vanderleyden. 1998. The Rhizobium etli rpoN locus: DNA sequence analysis and phenotypical characterization of rpoN, ptsN, and ptsA mutants. J. Bacteriol. 180:1729-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Mijakovic, I., D. Petranovic, N. Bottini, J. Deutscher, and, P. R. Jensen. 2005. Protein-tyrosine phosphorylation in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 9:189-197. [DOI] [PubMed] [Google Scholar]
- 557.Mijakovic, I., S. Poncet, A. Galinier, V. Monedero, S. Fieulaine, J. Janin, S. Nessler, J. A. Marquez, K. Scheffzek, S. Hasenbein, W. Hengstenberg, and J. Deutscher. 2002. Pyrophosphate-producing protein dephosphorylation by HPr kinase/phosphorylase: a relic of early life? Proc. Natl. Acad. Sci. USA 99:13442-13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Milenbachs, A. A., D. P. Brown, M. Moors, and P. Youngman. 1997. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 23:1075-1085. [DOI] [PubMed] [Google Scholar]
- 558a.Milenbachs Lukowiak, A., K. J. Mueller, N. E. Freitag, and P. Youngman. 2004. Deregulation of Listeria monocytogenes virulence gene expression by two distinct and semi-independent pathways. Microbiology 150:321-333. [DOI] [PubMed] [Google Scholar]
- 559.Misko, T. P., W. J. Mitchell, N. D. Meadow, and S. Roseman. 1987. Sugar transport by the bacterial phosphotransferase system. Reconstitution of inducer exclusion in Salmonella typhimurium membrane vesicles. J. Biol. Chem. 262:16261-16266. [PubMed] [Google Scholar]
- 560.Misset, O., M. Brouwer, and G. T. Robillard. 1980. Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system. Evidence that the dimer is the active form of enzyme I. Biochemistry 19:883-890. [DOI] [PubMed] [Google Scholar]
- 561.Mitchell, W. J., T. P. Misko, and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system. Regulation of other transport systems (lactose and melibiose). J. Biol. Chem. 257:14553-14564. [PubMed] [Google Scholar]
- 562.Mitchell, W. J., D. W. Saffen, and S. Roseman. 1987. Sugar transport by the bacterial phosphotransferase system. In vivo regulation of lactose transport in Escherichia coli by IIIGlc, a protein of the phosphoenolpyruvate:glycose phosphotransferase system J. Biol. Chem. 262:16254-16260. [PubMed] [Google Scholar]
- 563.Miwa, Y., and Y. Fujita. 2001. Involvement of two distinct catabolite-responsive elements in catabolite repression of the Bacillus subtilis myo-inositol (iol) operon. J. Bacteriol. 183:5877-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Miwa, Y., K. Nagura, S. Eguchi, H. Fukuda, J. Deutscher, and Y. Fujita. 1997. Catabolite repression of the Bacillus subtilis gnt operon exerted by two catabolite-responsive elements. Mol. Microbiol. 23:1203-1213. [DOI] [PubMed] [Google Scholar]
- 565.Miwa, Y., A. Nakata, A. Ogiwara, M. Yamamoto, and Y. Fujita. 2000. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 28:1206-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Miwa, Y., M. Saikawa, and Y. Fujita. 1994. Possible function and some properties of the CcpA protein of Bacillus subtilis. Microbiology 140:2567-2575. [DOI] [PubMed] [Google Scholar]
- 567.Molin, M., J. Norbeck, and A. Blomberg. 2003. Dihydroxyacetone kinases in Saccharomyces cerevisiae are involved in detoxification of dihydroxyacetone. J. Biol. Chem. 278:1415-1423. [DOI] [PubMed] [Google Scholar]
- 568.Monedero, V., M. J. Gosalbes, and G. Pérez-Martínez. 1997. Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J. Bacteriol. 179:6657-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Monedero, V., O. P. Kuipers, E. Jamet, and J. Deutscher. 2001. Regulatory functions of serine-46-phosphorylated HPr in Lactococcus lactis. J. Bacteriol. 183:3391-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Monedero, V., A. Mazé, G. Boël, M. Zúñiga, S. Beaufils, A. Hartke, and J. Deutscher. 2007. The phosphotransferase system of Lactobacillus casei: regulation of carbon metabolism and connection to cold shock response. J. Mol. Microbiol. Biotechnol. 12:18-30. [DOI] [PubMed] [Google Scholar]
- 571.Monedero, V., S. Poncet, I. Mijakovic, S. Fieulaine, V. Dossonnet, I. Martin-Verstraete, S. Nessler, and J. Deutscher. 2001. Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism. EMBO J. 20:3928-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Monod, J. 1942. Recherches sur la croissance des cultures bactériennes. Hermann et Cie, Paris, France.
- 573.Morales, G., J. F. Linares, A. Beloso, J. P. Albar, J. L. Martínez, and F. Rojo. 2004. The Pseudomonas putida Crc global regulator controls the expression of genes from several chromosomal catabolic pathways for aromatic compounds. J. Bacteriol. 186:1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Morel, F., M. Lamarque, I. Bissardon, D. Atlan, and A. Galinier. 2001. Autoregulation of the biosynthesis of the CcpA-like protein, PepR1, in Lactobacillus delbrueckii subsp. bulgaricus. J. Mol. Microbiol. Biotechnol. 3:63-66. [PubMed] [Google Scholar]
- 575.Moreno, M. S., B. L. Schneider, R. R. Maile, W. Weyler, and M. H. Saier, Jr. 2001. Catabolite repression mediated by CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses. Mol. Microbiol. 39:1366-1381. [DOI] [PubMed] [Google Scholar]
- 576.Mori, K., and H. Aiba. 1985. Evidence for negative control of cya transcription by cAMP and cAMP receptor protein in intact Escherichia coli cells. J. Biol. Chem. 260:14838-14843. [PubMed] [Google Scholar]
- 577.Morita, T., W. El-Kazzaz, Y. Tanaka, T. Inada, and H. Aiba. 2003. Accumulation of glucose 6-phosphate or fructose 6-phosphate is responsible for destabilization of glucose transporter mRNA in Escherichia coli. J. Biol. Chem. 278:15608-15614. [DOI] [PubMed] [Google Scholar]
- 578.Morita, T., H. Kawamoto, T. Mizota, T. Inada, and H. Aiba. 2004. Enolase in the RNA degradosome plays a crucial role in the rapid decay of glucose transporter mRNA in the response to phosphosugar stress in Escherichia coli. Mol. Microbiol. 54:1063-1075. [DOI] [PubMed] [Google Scholar]
- 579.Morris, P. W., J. P. Binkley, J. M. Henson, and P. L. Kuempel. 1985. Cloning and location of the dgsA gene of Escherichia coli. J. Bacteriol. 163:785-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Mourez, M., N. Hofnung, and E. Dassa. 1997. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 16:3066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Mourez, M., M. Jéhanno, E. Schneider, and E. Dassa. 1998. In vitro interaction between components of the inner membrane complex of the maltose ABC transporter of Escherichia coli: modulation by ATP. Mol. Microbiol. 30:353-363. [DOI] [PubMed] [Google Scholar]
- 582.Müller, J., S. Schiel, G. W. Ordal, and H. H. Saxild. 1997. Functional and genetic characterization of mcpC, which encodes a third methyl-accepting chemotaxis protein in Bacillus subtilis. Microbiology 143:3231-3240. [DOI] [PubMed] [Google Scholar]
- 583.Müller, W., N. Horstmann, W. Hillen, and H. Sticht. 2006. The transcription regulator RbsR represents a novel interaction partner of the phosphoprotein HPr-Ser46-P in Bacillus subtilis. FEBS J. 273:1251-1261. [DOI] [PubMed] [Google Scholar]
- 584.Muramatsu, S., and T. Mizuno. 1989. Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 17:4378. [PMC free article] [PubMed] [Google Scholar]
- 585.Murphy, N., D. J. McConnell, and B. A. Cantwell. 1984. The DNA sequence of the gene and genetic control sites for the excreted B. subtilis enzyme β-glucanase. Nucleic Acids Res. 12:5355-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Mus-Veteau, I., and G. Leblanc. 1996. Melibiose permease of Escherichia coli: structural organization of cosubstrate binding sites as deduced from tryptophan fluorescence analyses. Biochemistry 35:12053-12060. [DOI] [PubMed] [Google Scholar]
- 587.Nakanishi, T., T. Nakatsu, M. Matsuoka, K. Sakata, and H. Kato. 2005. Crystal structures of pyruvate phosphate dikinase from maize revealed an alternative conformation in the swiveling-domain motion. Biochemistry 44:1136-1144. [DOI] [PubMed] [Google Scholar]
- 588.Nam, T. W., S. H. Cho, D. Shin, J. H. Kim, J. Y. Jeong, J. H. Lee, J. H. Roe, A. Peterkofsky, S. O. Kang, S. Ryu, and Y.-J. Seok. 2001. The Escherichia coli glucose transporter enzyme IICBGlc recruits the global repressor Mlc. EMBO J. 20:491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Napper, S., J. W. Anderson, F. Georges, J. W. Quail, L. T. J. Delbaere, and E. B. Waygood. 1996. Mutation of serine-46 to aspartate in the histidine-containing protein of Escherichia coli mimics the inactivation by phosphorylation of serine-46 in HPrs from gram-positive bacteria. Biochemistry 35:11260-11267. [DOI] [PubMed] [Google Scholar]
- 590.Narindrasorasak, S., and W. A. Bridger. 1977. Phosphoenolpyruvate synthetase of Escherichia coli. Molecular weight, subunit composition, and identification of phosphohistidine in phosphoenzyme intermediate. J. Biol. Chem. 252:3121-3127. [PubMed] [Google Scholar]
- 591.Nasser, W., R. Schneider, A. Travers, and G. Muskhelishvili. 2001. CRP modulates fis transcription by alternate formation of activating and repressing nucleoprotein complexes. J. Biol. Chem. 276:17878-17886. [DOI] [PubMed] [Google Scholar]
- 592.Neidhardt, F. C., and M. A. Savageau. 1996. Regulation beyond the operon, p. 1310-1324. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 593.Nelson, S. O., J. Lengeler, and P. W. Postma. 1984. Role of IIIGlc of the phosphoenolpyruvate-glucose phosphotransferase system in inducer exclusion in Escherichia coli. J. Bacteriol. 160:360-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Nelson, S. O., and P. W. Postma. 1984. Interactions in vivo between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and the glycerol and maltose uptake systems of Salmonella typhimurium. Eur. J. Biochem. 139:29-34. [DOI] [PubMed] [Google Scholar]
- 595.Nelson, S. O., B. J. Scholte, and P. W. Postma. 1982. Phosphoenolpyruvate:sugar phosphotransferase system-mediated regulation of carbohydrate metabolism in Salmonella typhimurium. J. Bacteriol. 150:604-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Nelson, S. O., A. R. J. Schuitema, and P. W. Postma. 1986. The phosphoenolpyruvate:glucose phosphotransferase system of Salmonella typhimurium. The phosphorylated form of IIIGlc. Eur. J. Biochem. 154:337-341. [DOI] [PubMed] [Google Scholar]
- 597.Nelson, S. O., J. K. Wright, and P. W. Postma. 1983. The mechanism of inducer exclusion. Direct interaction between purified IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and the lactose carrier of Escherichia coli. EMBO J. 2:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Neves, A. R., A. Ramos, M. C. Nunes, M. Kleerebezem, J. Hugenholtz, W. M. de Vos, J. Almeida, and H. Santos. 1999. In vivo nuclear magnetic resonance studies of glycolytic kinetics in Lactococcus lactis. Biotechnol. Bioeng. 64:200-212. [DOI] [PubMed] [Google Scholar]
- 599.Nguyen, C. C., and M. H. Saier, Jr. 1995. Phylogenetic analysis of the putative phosphorylation domain in the pyruvate kinase of Bacillus stearothermophilus. Res. Microbiol. 146:713-719. [DOI] [PubMed] [Google Scholar]
- 600.Niaudet, B., P. Gay, and R. Dedonder. 1975. Identification of the structural gene of the PEP-phosphotransferase enzyme I in Bacillus subtilis Marburg. Mol. Gen. Genet. 136:337-349. [DOI] [PubMed] [Google Scholar]
- 601.Nicholson, W. L., and G. H. Chambliss. 1985. Isolation and characterization of a cis-acting mutation conferring catabolite repression resistance to α-amylase synthesis in Bacillus subtilis. J. Bacteriol. 161:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Nicholson, W. L., and G. H. Chambliss. 1986. Molecular cloning of cis-acting regulatory alleles of the Bacillus subtilis amyR region by using gene conversion transformation. J. Bacteriol. 165:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Nicholson, W. L., Y.-K. Park, T. M. Henkin, M. Won, M. J. Weickert, J. A. Gaskell, and G. H. Chambliss. 1987. Catabolite repression-resistant mutations of the Bacillus subtilis alpha-amylase promoter affect transcription levels and are in an operator-like sequence. J. Mol. Biol. 198:609-618. [DOI] [PubMed] [Google Scholar]
- 604.Nielsen, L. D., D. Monard, and H. V. Rickenberg. 1973. Cyclic 3′,5′-adenosine monophosphate phosphodiesterase of Escherichia coli. J. Bacteriol. 116:857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Niersbach, M., F. Kreuzaler, R. H. Geerse, P. W. Postma, and H. J. Hirsch. 1992. Cloning and nucleotide sequence of the Escherichia coli K-12 ppsA gene, encoding PEP synthase. Mol. Gen. Genet. 231:332-336. [DOI] [PubMed] [Google Scholar]
- 606.Nihashi, J.-I., and Y. Fujita. 1984. Catabolite repression of inositol dehydrogenase and gluconate kinase in Bacillus subtilis. Biochim. Biophys. Acta 798:88-95. [DOI] [PubMed] [Google Scholar]
- 607.Ninfa, A. J., P. Jiang, M. R. Atkinson, and J. A. Peliska. 2000. Integration of antagonistic signals in the regulation of nitrogen assimilation in Escherichia coli. Curr. Top. Cell. Regul. 36:31-75. [DOI] [PubMed] [Google Scholar]
- 608.Ninfa, A. J., and B. Magasanik. 1986. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc. Natl. Acad. Sci. USA 83:5909-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Niwano, M., and B. L. Taylor. 1982. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc. Natl. Acad. Sci. USA 79:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Nobelmann, B., and J. W. Lengeler. 1996. Molecular analysis of the gat genes from Escherichia coli and of their roles in galactitol transport and metabolism. J. Bacteriol. 178:6790-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Norris, V., P. Gascuel, J. Guespin-Michel, C. Ripoli, and M. H. Saier, Jr. 1999. Metabolite-induced metabolons: the activation of transporter-enzyme complexes by substrate binding. Mol. Microbiol. 31:1592-1595. [DOI] [PubMed] [Google Scholar]
- 612.Nothaft, H., S. Parche, A. Kamionka, and F. Titgemeyer. 2003. In vivo analysis of HPr reveals a fructose-specific phosphotransferase system that confers high-affinity uptake in Streptomyces coelicolor. J. Bacteriol. 185:929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Notley, L., and T. Ferenci. 1995. Differential expression of mal genes under cAMP and endogenous inducer control in nutrient-stressed Escherichia coli. Mol. Microbiol. 16:121-129. [DOI] [PubMed] [Google Scholar]
- 614.Notley-McRobb, L., A. Death, and T. Ferenci. 1997. The relationship between external glucose concentration and cAMP levels inside Escherichia coli: implications for models of phosphotransferase-mediated regulation of adenylate cyclase. Microbiology 143:1909-1918. [DOI] [PubMed] [Google Scholar]
- 615.Notley-McRobb, L., and T. Ferenci. 2000. Substrate specificity and signal transduction pathways in the glucose-specific enzyme II (EIIGlc) component of the Escherichia coli phosphotransferase system. J. Bacteriol. 182:4437-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Novotny, M. J., W. L. Frederickson, E. B. Waygood, and M. H. Saier, Jr. 1985. Allosteric regulation of glycerol kinase by enzyme IIIglc of the phosphotransferase system in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 162:810-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Nunn, R. S., Z. Markovic-Housley, J.-C. Genovesio-Taverne, K. Flükiger, P. J. Rizkallah, J. N. Jansonius, T. Schirmer, and B. Erni. 1996. Structure of the IIA domain of the mannose transporter from Escherichia coli at 1.7 Å resolution. J. Mol. Biol. 259:502-511. [DOI] [PubMed] [Google Scholar]
- 618.Nuoffer, C., B. Zanolari, and B. Erni. 1988. Glucose permease of Escherichia coli. The effect of cysteine to serine mutations on the function, stability, and regulation of transport and phosphorylation. J. Biol. Chem. 263:6647-6655. [PubMed] [Google Scholar]
- 619.Oberholzer, A. E., M. Bumann, P. Schneider, C. Bächler, C. Siebold, U. Baumann, and B. Erni. 2005. Crystal structure of the phosphoenolpyruvate-binding enzyme I-domain from the Thermoanaerobacter tengcongensis PEP:sugar phosphotransferase system (PTS). J. Mol. Biol. 346:521-532. [DOI] [PubMed] [Google Scholar]
- 620.Ogino, T., M. Matsubara, N. Kato, Y. Nakamura, and T. Mizuno. 1998. An Escherichia coli protein that exhibits phosphohistidine phosphatase activity towards the HPt domain of the ArcB sensor involved in the multistep His-Asp phosphorelay. Mol. Microbiol. 27:573-585. [DOI] [PubMed] [Google Scholar]
- 621.Okada, T., K. Ueyama, S. Niiya, H. Kanazawa, M. Futai, and T. Tsuchiya. 1981. Role of inducer exclusion in preferential utilization of glucose over melibiose in diauxic growth of Escherichia coli. J. Bacteriol. 146:1030-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Ormö, M., C. E. Bystrom, and S. J. Remington. 1998. Crystal structure of a complex of Escherichia coli glycerol kinase and an allosteric effector fructose 1,6-bisphosphate. Biochemistry 37:16565-16572. [DOI] [PubMed] [Google Scholar]
- 623.Orriss, G. L., B. Erni, and T. Schirmer. 2003. Crystal structure of the IIBSor domain of the sorbose permease from Klebsiella pneumoniae solved to 1.75 Å resolution. J. Mol. Biol. 327:1111-1119. [DOI] [PubMed] [Google Scholar]
- 624.Osumi, T., and M. H. Saier, Jr. 1982. Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc. Natl. Acad. Sci. USA 79:1457-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Overath, P., U. Weigel, J.-M. Neuhaus, J. Soppa, R. Seckler, I. Riede, H. Bocklage, B. Müller-Hill, G. Aichele, and J. K. Wright. 1987. Lactose permease of Escherichia coli: properties of mutants defective in substrate translocation. Proc. Natl. Acad. Sci. USA 84:5535-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Ozbudak, E. M., M. Thattai, H. N. Lim, B. I. Shraiman, and A. van Oudenaarden. 2004. Multistability in the lactose utilization network of Escherichia coli. Nature 427:737-740. [DOI] [PubMed] [Google Scholar]
- 627.Panagiotidis, C. H., W. Boos, and H. A. Shuman. 1998. The ATP-binding cassette subunit of the maltose transporter MalK antagonizes MalT, the activator of the Escherichia coli mal regulon. Mol. Microbiol. 30:535-546. [DOI] [PubMed] [Google Scholar]
- 628.Pao, G. M., L.-F. Wu, K. D. Johnson, H. Höfte, M. J. Chrispeels, G. Sweet, N. N. Sandal, and M. H. Saier, Jr. 1991. Evolution of the MIP family of integral membrane transport proteins. Mol. Microbiol. 5:33-37. [DOI] [PubMed] [Google Scholar]
- 629.Parche, S., H. Nothaft, A. Kamionka, and F. Titgemeyer. 2000. Sugar uptake and utilisation in Streptomyces coelicolor: a PTS view to the genome. Antonie Leeuwenhoek 78:243-251. [DOI] [PubMed] [Google Scholar]
- 630.Parche, S., R. Schmid, and F. Titgemeyer. 1999. The phosphotransferase system (PTS) of Streptomyces coelicolor. Identification and biochemical analysis of a histidine phosphocarrier protein HPr encoded by the gene ptsH. Eur. J. Biochem. 265:308-317. [DOI] [PubMed] [Google Scholar]
- 631.Parche, S., A. W. Thomae, M. Schlicht, and F. Titgemeyer. 2001. Corynebacterium diphtheriae: a PTS view to the genome. J. Mol. Microbiol. Biotechnol. 3:415-422. [PubMed] [Google Scholar]
- 632.Park, Y.-H., B. R. Lee, Y.-J. Seok, and A. Peterkofsky. 2006. In vitro rerconstitution of catabolite repression in Escherichia coli. J. Biol. Chem. 281:6448-6454. [DOI] [PubMed] [Google Scholar]
- 633.Pas, H. H., G. H. Meyer, W. H. Kruizinga, K. S. Tamminga, R. P. van Weeghel, and G. T. Robillard. 1991. 31Phospho-NMR demonstration of phosphocysteine as a catalytic intermediate on the Escherichia coli phosphotransferase system EIIMtl. J. Biol. Chem. 266:6690-6692. [PubMed] [Google Scholar]
- 634.Pas, H. H., and G. T. Robillard. 1988. S-Phosphocysteine and phosphohistidine are intermediates in the phosphoenolpyruvate-dependent mannitol transport catalyzed by Escherichia coli EIIMtl. Biochemistry 27:5835-5839. [DOI] [PubMed] [Google Scholar]
- 635.Pascal, M. J. F. 1976. The enzymes of sucrose metabolism and the regulation of their synthesis in Bacillus subtilis Marburg. Ph.D. thesis. University of Paris VII, Paris, France.
- 636.Pastan, I., and S. Adhya. 1976. Cyclic adenosine 5′-monophosphate in Escherichia coli. Bacteriol. Rev. 40:527-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Patel, H. V., K. A. Vyas, X. Li, R. Savtchenko, and S. Roseman. 2004. Subcellular distribution of enzyme I of the Escherichia coli phosphoenolpyruvate:glycose phosphotransferase system depends on growth conditions. Proc. Natl. Acad. Sci. USA 101:17486-17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Patel, H. V., K. A. Vyas, R. L. Mattoo, M. Southworth, F. B. Perler, D. Comb, and S. Roseman. 2006. Properties of the C-terminal domain of enzyme I of the Escherichia coli phosphotransferase system. J. Biol. Chem. 281:17579-17587. [DOI] [PubMed] [Google Scholar]
- 639.Patel, H. V., K. A. Vyas, R. Savtchenko, and S. Roseman. 2006. The monomer/dimer transition of enzyme I of the Escherichia coli phosphotransferase system. J. Biol. Chem. 281:17570-17578. [DOI] [PubMed] [Google Scholar]
- 640.Paulsen, I. T., J. Reizer, R. Z. Jin, E. C. C. Lin, and M. H. Saier, Jr. 2000. Functional genomic studies of dihydroxyacetone utilization in Escherichia coli. Microbiology 146:2343-2344. [DOI] [PubMed] [Google Scholar]
- 641.Pecher, A., I. Rennner, and J. W. Lengeler. 1983. The phosphoenolpyruvate-dependent carbohydrate:phosphotransferase system enzyme II. A new class of chemosensors in bacterial chemotaxis, p. 517-531. In H. Sund and C. Veegher (ed.), Mobility and recognition in cell biology. Walter de Gruyter & Co., Berlin, Germany.
- 642.Pelton, J. G., D. A. Torchia, N. D. Meadow, and S. Roseman. 1992. Structural comparison of phosphorylated and unphosphorylated forms of IIIGlc, a signal-transducing protein from Escherichia coli, using three-dimensional NMR techniques. Biochemistry 31:5215-5224. [DOI] [PubMed] [Google Scholar]
- 643.Pelton, J. G., D. A. Torchia, N. D. Meadow, C.-Y. Wong, and S. Roseman. 1991. Secondary structure of the phosphocarrier protein IIIGlc, a signal-transducing protein from Escherichia coli, determined by heteronuclear three-dimensional NMR spectroscopy. Proc. Natl. Acad. Sci. USA 88:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Penin, F., A. Favier, R. Montserret, B. Brutscher, J. Deutscher, D. Marion, and A. Galinier. 2001. Characterisation of the oligomerisation state of the Bacillus subtilis catabolite repression HPr-like protein, Crh. J. Mol. Microbiol. Biotechnol. 3:429-432. [PubMed] [Google Scholar]
- 645.Perez-Martin, J., and V. de Lorenzo. 1997. Clues and consequences of DNA bending in transcription. Annu. Rev. Microbiol. 51:593-628. [DOI] [PubMed] [Google Scholar]
- 646.Peri, K. G., H. Goldie, and E. B. Waygood. 1990. Cloning and characterization of the N-acetylglucosamine operon of Escherichia coli. Biochem. Cell Biol. 68:123-137. [DOI] [PubMed] [Google Scholar]
- 647.Perlman, R. L., B. de Crombrugghe, and I. Pastan. 1969. cAMP regulates catabolite and transient repression in Escherichia coli. Nature 223:810-812. [DOI] [PubMed] [Google Scholar]
- 648.Peterkofsky, A. 1981. Escherichia coli adenylate cyclase as a sensor of sugar transport function. Adv. Cyclic Nucleotide Res. 14:215-228. [PubMed] [Google Scholar]
- 649.Peterkofsky, A., and C. Gazdar. 1979. Escherichia coli adenylate cyclase complex: regulation by the proton electrochemical gradient. Proc. Natl. Acad. Sci. USA 76:1099-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Peterkofsky, A., and C. Gazdar. 1974. Glucose inhibition of adenylate cyclase in intact cells of Escherichia coli B. Proc. Natl. Acad. Sci. USA 71:2324-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Peterkofsky, A., and C. Gazdar. 1975. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc. Natl. Acad. Sci. USA 72:2920-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Peterkofsky, A., and C. Gazdar. 1973. Measurements of rates of adenosine 3′:5′-cyclic monophosphate synthesis in intact Escherichia coli B. Proc. Natl. Acad. Sci. USA 70:2149-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Peterkofsky, A., A. Reizer, J. Reizer, N. Gollop, P.-P. Zhu, and N. Amin. 1993. Bacterial adenylyl cyclases. Prog. Nucleic Acid Res. Mol. Biol. 44:31-65. [DOI] [PubMed] [Google Scholar]
- 654.Pettigrew, D. W. 1986. Inactivation of Escherichia coli glycerol kinase by 5,5′-dithiobis(2-nitrobenzoic acid) and N-ethylmaleimide: evidence for nucleotide regulatory binding sites. Biochemistry 25:4711-4718. [DOI] [PubMed] [Google Scholar]
- 655.Pettigrew, D. W., W. Z. Liu, C. Holmes, N. D. Meadow, and S. Roseman. 1996. A single amino acid change in Escherichia coli glycerol kinase abolishes glucose control of glycerol utilization in vivo. J. Bacteriol. 178:2846-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Pettigrew, D. W., D.-P. Ma, C. A. Conrad, and J. R. Johnson. 1988. Escherichia coli glycerol kinase. Cloning and sequencing of the glpK gene and the primary structure of the enzyme. J. Biol. Chem. 263:135-139. [PubMed] [Google Scholar]
- 657.Pettigrew, D. W., N. D. Meadow, S. Roseman, and S. J. Remington. 1998. Cation promoted association of Escherichia coli phosphocarrier protein IIAGlc with regulatory target protein glycerol kinase: substitutions of a Zn(II) ligand and implications for inducer exclusion. Biochemistry 37:4875-4883. [DOI] [PubMed] [Google Scholar]
- 658.Pettigrew, D. W., G. B. Smith, K. P. Thomas, and D. C. Dodds. 1998. Conserved active site aspartates and domain-domain interactions in regulatory properties of the sugar kinase superfamily. Arch. Biochem. Biophys. 349:236-245. [DOI] [PubMed] [Google Scholar]
- 659.Phadtare, S., S. Tyagi, M. Inouye, and K. Severinov. 2002. Three amino acids in Escherichia coli CspE surface-exposed aromatic patch are critical for nucleic acid melting activity leading to transcription antitermination and cold acclimation of cells. J. Biol. Chem. 277:46706-46711. [DOI] [PubMed] [Google Scholar]
- 660.Plamondon, P., D. Brochu, S. Thomas, J. Fradette, L. Gauthier, K. Vaillancourt, N. Buckley, M. Frenette, and C. Vadeboncoeur. 1999. Phenotypic consequences resulting from a methionine-to-valine substitution at position 48 in the HPr protein of Streptococcus salivarius. J. Bacteriol. 181:6914-6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Plumbridge, J. 2000. A mutation which affects both the specificity of PtsG sugar transport and the regulation of ptsG expression by Mlc in Escherichia coli. Microbiology 146:2655-2663. [DOI] [PubMed] [Google Scholar]
- 662.Plumbridge, J. 1998. Control of the expression of the manXYZ operon in Escherichia coli: Mlc is a negative regulator of the mannose PTS. Mol. Microbiol. 27:369-380. [DOI] [PubMed] [Google Scholar]
- 663.Plumbridge, J. 2001. DNA binding sites for the Mlc and NagC proteins: regulation of nagE, encoding the N-acetylglucosamine-specific transporter in Escherichia coli. Nucleic Acids Res. 29:506-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Plumbridge, J. 1998. Expression of ptsG, the gene for the major glucose PTS transporter in Escherichia coli, is repressed by Mlc and induced by growth on glucose. Mol. Microbiol. 29:1053-1063. [DOI] [PubMed] [Google Scholar]
- 665.Plumbridge, J. 1999. Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol. Microbiol. 33:260-273. [DOI] [PubMed] [Google Scholar]
- 666.Plumbridge, J. 2002. Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr. Opin. Microbiol. 5:187-193. [DOI] [PubMed] [Google Scholar]
- 667.Plumbridge, J., and A. Kolb. 1993. DNA loop formation between Nag repressor molecules bound to its two operator sites is necessary for repression of the nag regulon of Escherichia coli in vivo. Mol. Microbiol. 10:973-981. [DOI] [PubMed] [Google Scholar]
- 668.Plumbridge, J. A. 1991. Repression and induction of the nag regulon of Escherichia coli K-12: the roles of nagC and nagA in maintenance of the uninduced state. Mol. Microbiol. 5:2053-2062. [DOI] [PubMed] [Google Scholar]
- 669.Plumbridge, J. A. 1989. Sequence of the nagBACD operon in Escherichia coli K12 and pattern of transcription within the nag regulon. Mol. Microbiol. 3:505-515. [DOI] [PubMed] [Google Scholar]
- 670.Pocalyko, D. J., L. J. Carroll, B. M. Martin, P. C. Babbitt, and D. Dunaway-Mariano. 1990. Analysis of sequence homologies in plant and bacterial pyruvate phosphate dikinase, enzyme I of the bacterial phosphoenolpyruvate:sugar phosphotransferase system and other PEP-utilizing enzymes. Identification of potential catalytic and regulatory motifs. Biochemistry 29:10757-10765. [DOI] [PubMed] [Google Scholar]
- 671.Polit, A., U. Blaszczyk, and Z. Wasylewski. 2003. Steady-state and time-resolved fluorescence studies of conformational changes induced by cyclic AMP and DNA binding to cyclic AMP receptor protein from Escherichia coli. Eur. J. Biochem. 270:1413-1423. [DOI] [PubMed] [Google Scholar]
- 672.Poolman, B., R. Modderman, and J. Reizer. 1992. Lactose transport system in Streptococcus thermophilus. The role of histidine residues. J. Biol. Chem. 267:9150-9157. [PubMed] [Google Scholar]
- 673.Poolman, B., T. J. Royer, S. E. Mainzer, and B. F. Schmidt. 1989. Lactose transport system of Streptococcus thermophilus: a hybrid protein with homology to the melibiose carrier and enzyme III of phosphoenolpyruvate-dependent phosphotransferase systems. J. Bacteriol. 171:244-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Posthuma, C. C., R. Bader, R. Engelmann, P. W. Postma, W. Hengstenberg, and P. H. Pouwels. 2002. Expression of the xylulose 5-phosphate phosphoketolase gene, xpkA, from Lactobacillus pentosus MD363 is induced by sugars that are fermented via the phosphoketolase pathway and is repressed by glucose mediated by CcpA and the mannose phosphoenolpyruvate phosphotransferase system. Appl. Environ. Microbiol. 68:831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Postma, P. W., C. P. Broekhuizen, A. R. J. Schuitema, A. P. Vogler, and J. W. Lengeler. 1988. Carbohydrate transport and metabolism in Escherichia coli and Salmonella typhimurium: regulation by the PEP:carbohydrate phosphotransferase system, p. 43-52. In F. Palmieri and E. Quagliariello (ed.), Molecular basis of biomembrane transport. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 676.Postma, P. W., W. Epstein, A. R. J. Schuitema, and S. O. Nelson. 1984. Interaction between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and glycerol kinase of Salmonella typhimurium. J. Bacteriol. 158:351-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Postma, P. W., and J. W. Lengeler. 1985. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol. Rev. 49:232-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Potter, K., G. Chaloner-Larsson, and H. Yamazaki. 1974. Abnormally high rate of cyclic AMP excretion from an Escherichia coli mutant deficient in cyclic AMP receptor protein. Biochem. Biophys. Res. Commun. 57:379-385. [DOI] [PubMed] [Google Scholar]
- 680.Powell, B. S., D. L. Court, T. Inada, Y. Nakamura, V. Michotey, X. Cui, A. Reizer, M. H. Saier, Jr., and J. Reizer. 1995. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. Enzyme IIANtr affects growth on organic nitrogen and the conditional lethality of an erats mutant. J. Biol. Chem. 270:4822-4839. [DOI] [PubMed] [Google Scholar]
- 681.Powers, D. A., and S. Roseman. 1984. The primary structure of Salmonella typhimurium HPr, a phosphocarrier protein of the phosphoenolpyruvate:glycose phosphotransferase system. A correction. J. Biol. Chem. 259:15212-15214. [PubMed] [Google Scholar]
- 682.Prasad, I., and S. Schaefler. 1974. Regulation of the β-glucoside system in Escherichia coli K-12. J. Bacteriol. 120:638-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Preiss, J., and T. Romeo. 1994. Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 47:299-329. [DOI] [PubMed] [Google Scholar]
- 684.Preiss, J., and T. Romeo. 1989. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv. Microb. Physiol. 30:183-238. [DOI] [PubMed] [Google Scholar]
- 685.Presecan-Siedel, E., A. Galinier, R. Longin, J. Deutscher, A. Danchin, P. Glaser, and I. Martin-Verstraete. 1999. Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J. Bacteriol. 181:6889-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Presper, K. A., C.-Y. Wong, L. Liu, N. M. Meadow, and S. Roseman. 1989. Site-directed mutagenesis of the phosphocarrier protein, IIIGlc, a major signal-transducing protein in Escherichia coli. Proc. Natl. Acad. Sci. USA 86:4052-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Pries, A., H. Priefert, N. Krüger, and A. Steinbüchel. 1991. Identification and characterization of two Alcaligenes eutrophus gene loci relevant to the poly(β-hydroxybutyric acid)-leaky phenotype which exhibit homology to ptsH and ptsI of Escherichia coli. J. Bacteriol. 173:5843-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Puchalka, J., and A. M. Kierzek. 2004. Bridging the gap between stochastic and deterministic regimes in the kinetic simulations of the biochemical reaction networks. Biophys. J. 86:1357-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Rabus, R., J. Reizer, I. Paulsen, and M. H. Saier, Jr. 1999. Enzyme INtr from Escherichia coli. A novel enzyme of the phosphoenolpyruvate-dependent phosphotransferase system exhibiting strict specificity for its phosphoryl acceptor, NPr. J. Biol. Chem. 274:26185-26191. [DOI] [PubMed] [Google Scholar]
- 690.Raghunand, T. R., and S. Mahadevan. 2003. The beta-glucoside genes of Klebsiella aerogenes: conservation and divergence in relation to the cryptic bgl genes of Escherichia coli. FEMS Microbiol. Lett. 223:267-274. [DOI] [PubMed] [Google Scholar]
- 691.Rajagopal, P., E. B. Waygood, J. Reizer, M. H. Saier, Jr., and R. E. Klevit. 1997. Demonstration of protein-protein interaction specificity by NMR chemical shift mapping. Protein Sci. 6:2624-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Ramnath, M., S. Arous, A. Gravesen, J. W. Hastings, and Y. Hechard. 2004. Expression of mptC of Listeria monocytogenes induces sensitivity to class IIa bacteriocins in Lactococcus lactis. Microbiology 150:2663-2668. [DOI] [PubMed] [Google Scholar]
- 693.Ramnath, M., M. Beukes, K. Tamura, and J. W. Hastings. 2000. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl. Environ. Microbiol. 66:3098-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Ramos, I., S. Guzmán, L. Escalante, I. Imriskova, R. Rodríguez-Sanoja, S. Sanchez, and E. Langley. 2004. Glucose kinase alone cannot be responsible for carbon source regulation in Streptomyces peucetius var. caesius. Res. Microbiol. 155:267-274. [DOI] [PubMed] [Google Scholar]
- 695.Ramseier, T. M., J. Reizer, E. Küster, W. Hillen, and M. H. Saier, Jr. 1995. In vitro binding of the CcpA protein of Bacillus megaterium to cis-acting catabolite responsive elements (CREs) of gram-positive bacteria. FEMS Microbiol. Lett. 129:207-214. [DOI] [PubMed] [Google Scholar]
- 696.Ramström, H., M. Bourotte, C. Philippe, M. Schmitt, J. Haiech, and J. J. Bourguignon. 2004. Heterocyclic bis-cations as starting hits for design of inhibitors of the bifunctional enzyme histidine-containing protein kinase/phosphatase from Bacillus subtilis. J. Med. Chem. 47:2264-2275. [DOI] [PubMed] [Google Scholar]
- 697.Ramström, H., S. Sanglier, E. Leize-Wagner, C. Philippe, A. van Dorsselaer, and J. Haiech. 2003. Properties and regulation of the bifunctional HPr kinase/phosphatase in Bacillus subtilis. J. Biol. Chem. 278:1174-1185. [DOI] [PubMed] [Google Scholar]
- 698.Ray, P., K. J. Smith, R. A. Parslow, R. Dixon, and E. I. Hyde. 2002. Secondary structure and DNA binding by the C-terminal domain of the transcriptional activator NifA from Klebsiella pneumoniae. Nucleic Acids Res. 30:3972-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Reddy, P., J. Hoskins, and K. McKenney. 1995. Mapping domains in proteins: dissection and expression of Escherichia coli adenylyl cyclase. Anal. Biochem. 231:282-286. [DOI] [PubMed] [Google Scholar]
- 700.Reddy, P., and M. Kamireddi. 1998. Modulation of Escherichia coli adenylyl cyclase activity by catalytic-site mutants of protein IIAGlc of the phosphoenolpyruvate:sugar phosphotransferase system. J. Bacteriol. 180:732-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Reddy, P., N. Meadow, S. Roseman, and A. Peterkofsky. 1985. Reconstitution of regulatory properties of adenylate cyclase in Escherichia coli extracts. Proc. Natl. Acad. Sci. USA 82:8300-8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Reddy, P., D. Miller, and A. Peterkofsky. 1986. Stimulation of Escherichia coli adenylate cyclase activity by elongation factor Tu, a GTP-binding protein essential for protein synthesis. J. Biol. Chem. 261:11448-11451. [PubMed] [Google Scholar]
- 703.Reiche, B., R. Frank, J. Deutscher, N. Meyer, and W. Hengstenberg. 1988. Staphylococcal phosphoenolpyruvate-dependent phosphotransferase system: purification and characterization of the mannitol-specific enzyme III mtl of Staphylococcus aureus and Staphylococcus carnosus and homology with the enzyme II mtl of Escherichia coli. Biochemistry 27:6512-6516. [DOI] [PubMed] [Google Scholar]
- 704.Reitzer, L. 2003. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 57:155-176. [DOI] [PubMed] [Google Scholar]
- 705.Reitzer, L., and B. L. Schneider. 2001. Metabolic context and possible physiological themes of σ54-dependent genes in Escherichia coli. Microbiol. Mol. Biol. Rev. 65:422-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Reizer, J., S. Bachem, A. Reizer, M. Arnaud, M. H. Saier, Jr., and J. Stülke. 1999. Novel phosphotransferase system genes revealed by genome analysis—the complete complement of PTS proteins encoded within the genome of Bacillus subtilis. Microbiology 145:3419-3429. [DOI] [PubMed] [Google Scholar]
- 707.Reizer, J., U. Bergstedt, A. Galinier, E. Küster, M. H. Saier, Jr., W. Hillen, M. Steinmetz, and J. Deutscher. 1996. Catabolite repression resistance of gnt operon expression in Bacillus subtilis conferred by mutation of His-15, the site of phosphoenolpyruvate-dependent phosphorylation of the phosphocarrier protein HPr. J. Bacteriol. 178:5480-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Reizer, J., J. Deutscher, S. Sutrina, J. Thompson, and M. H. Saier, Jr. 1985. Sugar accumulation in gram-positive bacteria: exclusion and expulsion mechanisms. Trends Biochem. Sci. 10:32-35. [Google Scholar]
- 709.Reizer, J., C. Hoischen, F. Titgemeyer, C. Rivolta, R. Rabus, J. Stülke, D. Karamata, M. H. Saier, Jr., and W. Hillen. 1998. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol. Microbiol. 27:1157-1169. [DOI] [PubMed] [Google Scholar]
- 710.Reizer, J., M. J. Novotny, C. Panos, and M. H. Saier, Jr. 1983. Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J. Bacteriol. 156:354-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Reizer, J., M. J. Novotny, I. Stuiver, and M. H. Saier, Jr. 1984. Regulation of glycerol uptake by the phosphoenolpyruvate-sugar phosphotransferase system in Bacillus subtilis. J. Bacteriol. 159:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Reizer, J., and C. Panos. 1980. Regulation of β-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc. Natl. Acad. Sci. USA 77:5497-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Reizer, J., I. T. Paulsen, A. Reizer, F. Titgemeyer, and M. H. Saier, Jr. 1996. Novel phosphotransferase system genes revealed by bacterial genome analysis: the complete complement of pts genes in Mycoplasma genitalium. Microb. Comp. Genomics 1:151-163. [DOI] [PubMed] [Google Scholar]
- 714.Reizer, J., and A. Peterkofsky. 1987. Regulatory mechanisms for sugar transport in gram-positive bacteria, p. 333-364. In J. Reizer and A. Peterkofsky (ed.), Sugar transport and metabolism in gram-positive bacteria. Ellis Horwood, Chichester, United Kingdom.
- 715.Reizer, J., A. Peterkofsky, and A. H. Romano. 1988. Evidence for the presence of heat-stable protein (HPr) and ATP-dependent HPr kinase in heterofermentative lactobacilli lacking a phosphoenolpyruvate:glycose phosphotransferase activity. Proc. Natl. Acad. Sci. USA 85:2041-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Reizer, J., A. Reizer, M. J. Merrick, G. Plunkett III, D. J. Rose, and M. H. Saier, Jr. 1996. Novel phosphotransferase-encoding genes revealed by analysis of the Escherichia coli genome: a chimeric gene encoding an enzyme I homologue that possesses a putative sensory transduction domain. Gene 181:103-108. [DOI] [PubMed] [Google Scholar]
- 717.Reizer, J., A. Reizer, and M. H. Saier, Jr. 1996. Novel PTS proteins revealed by bacterial genome sequencing: a unique fructose-specific phosphoryl transfer protein with two HPr-like domains in Haemophilus influenzae. Res. Microbiol. 147:209-215. [DOI] [PubMed] [Google Scholar]
- 718.Reizer, J., A. Reizer, M. H. Saier, Jr., and G. R. Jacobson. 1992. A proposed link between nitrogen and carbon metabolism involving protein phosphorylation in bacteria. Protein Sci. 1:722-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Reizer, J., and M. H. Saier, Jr. 1983. Involvement of lactose enzyme II of the phosphotransferase system in rapid expulsion of free galactosides from Streptococcus pyogenes. J. Bacteriol. 156:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Reizer, J., and M. H. Saier, Jr. 1997. Modular multidomain phosphoryl transfer proteins of bacteria. Curr. Opin. Struct. Biol. 7:407-415. [DOI] [PubMed] [Google Scholar]
- 721.Reizer, J., B. Schneider, A. Reizer, and M. H. Saier, Jr. 1999. A hybrid response regulator possessing a PEP-dependent phosphorylation domain. Microbiology 145:987-989. [DOI] [PubMed] [Google Scholar]
- 722.Reizer, J., S. L. Sutrina, M. H. Saier, Jr., G. C. Stewart, A. Peterkofsky, and P. Reddy. 1989. Mechanistic and physiological consequences of HPr (ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J. 8:2111-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Reizer, J., S. L. Sutrina, L.-F. Wu, J. Deutscher, P. Reddy, and M. H. Saier, Jr. 1992. Functional interactions between proteins of the phosphoenolpyruvate:sugar phosphotransferase systems of Bacillus subtilis and Escherichia coli. J. Biol. Chem. 267:9158-9169. [PubMed] [Google Scholar]
- 724.Ren, J., S. Sainsbury, N. S. Berrow, D. Alderton, J. E. Nettleship, D. K. Stammers, N. J. Saunders, and R. J. Owens. 2005. Crystal structure of nitrogen regulatory protein IIANtr from Neisseria meningitidis. BMC Struct. Biol. 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Ren, Q. H., K. H. Kang, and I. T. Paulsen. 2004. TransportDB: a relational database of cellular membrane transport systems. Nucleic Acids Res. 32:D284-D288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Renna, M. C., N. Najimudin, L. R. Winik, and S. A. Zahler. 1993. Regulation of the Bacillus subtilis alsS, alsD, and alsR genes involved in post-exponential-phase production of acetoin. J. Bacteriol. 175:3863-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Rephaeli, A. W., and M. H. Saier, Jr. 1980. Regulation of genes coding for enzyme constituents of the bacterial phosphotransferase system. J. Bacteriol. 141:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Ripio, M.-T., K. Brehm, M. Lara, M. Suarez, and J.-A. Vazquez-Boland. 1997. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J. Bacteriol. 179:7174-7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Rivolta, C., B. Soldo, V. Lazarevic, B. Joris, C. Mauël, and D. Karamata. 1998. A 35.7 kb DNA fragment from the Bacillus subtilis chromosome containing a putative 12.3 kb operon involved in hexuronate catabolism and a perfectly symmetrical hypothetical catabolite responsive element. Microbiology 144:877-884. [DOI] [PubMed] [Google Scholar]
- 730.Robillard, G. T., and J. Broos. 1999. Structure/function studies on the bacterial carbohydrate transporters, enzymes II, of the phosphoenolpyruvate-dependent phosphotransferase system. Biochim. Biophys. Acta 1422:73-104. [DOI] [PubMed] [Google Scholar]
- 731.Rodionov, D. A., A. A. Mironov, and M. S. Gelfand. 2001. Transcriptional regulation of pentose utilisation systems in the Bacillus/Clostridium group of bacteria. FEMS Microbiol. Lett. 205:305-314. [DOI] [PubMed] [Google Scholar]
- 732.Roehl, R. A., and R. T. Vinopal. 1980. Genetic locus, distant from ptsM, affecting enzyme IIA/IIB function in Escherichia coli K-12. J. Bacteriol. 142:120-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Rogers, J. D., and F. A. Scannapieco. 2001. RegG, a CcpA homolog, participates in regulation of amylase-binding protein A gene (abpA) expression in Streptococcus gordonii. J. Bacteriol. 183:3521-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Rohwer, J. M., R. Bader, H. V. Westerhoff, and P. W. Postma. 1998. Limits to inducer exclusion: inhibition of the bacterial phosphotransferase system by glycerol kinase. Mol. Microbiol. 29:641-652. [DOI] [PubMed] [Google Scholar]
- 735.Rohwer, J. M., J.-H. S. Hofmeyr, and P. W. Postma. 1998. Retro-regulation of the bacterial phosphotransferase system: a kinetic model, p. 340-344. In C. Larsson, I. L. Pählman, and L. Gustafsson (ed.), BioThermoKinetics in the post genomic era. Chalmers University of Technology, Göteborg, Sweden.
- 736.Rohwer, J. M., P. R. Jensen, Y. Shinohara, P. W. Postma, and H. V. Westerhoff. 1996. Changes in the cellular energy state affect the activity of the bacterial phosphotransferase system. Eur. J. Biochem. 235:225-230. [DOI] [PubMed] [Google Scholar]
- 737.Rohwer, J. M., N. D. Meadow, S. Roseman, H. V. Westerhoff, and P. W. Postma. 2000. Understanding glucose transport by the bacterial phosphoenolpyruvate:glycose phosphotransferase system on the basis of kinetic measurements in vitro. J. Biol. Chem. 275:34909-34921. [DOI] [PubMed] [Google Scholar]
- 738.Rohwer, J. M., P. W. Postma, B. N. Kholodenko, and H. V. Westerhoff. 1998. Implications of macromolecular crowding for signal transduction and metabolite channeling. Proc. Natl. Acad. Sci. USA 95:10547-10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Romano, A. H., G. Brino, A. Peterkofsky, and J. Reizer. 1987. Regulation of β-galactoside transport and accumulation in heterofermentative lactic acid bacteria. J. Bacteriol. 169:5589-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Romano, A. H., and M. H. Saier, Jr. 1992. Evolution of the bacterial phosphoenolpyruvate:sugar phosphotransferase system. Section I. Physiological and organismic considerations, p. 143-170. In R. P. Mortlock (ed.), Evolution of metabolic function. CRC Press, Boca Raton, Fla.
- 741.Romano, A. H., M. H. Saier, Jr., O. T. Harriott, and J. Reizer. 1990. Physiological studies on regulation of glycerol utilization by the phosphoenolpyruvate:sugar phosphotransferase system in Enterococcus faecalis. J. Bacteriol. 172:6741-6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Romeo, T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321-1330. [DOI] [PubMed] [Google Scholar]
- 743.Romeo, T., M. Gong, M. Y. Liu, and A.-M. Brun-Zinkernagel. 1993. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J. Bacteriol. 175:4744-4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Roseman, S., and N. D. Meadow. 1990. Signal transduction by the bacterial phosphotransferase system. Diauxie and the crr gene (J. Monod revisited). J. Biol. Chem. 265:2993-2996. [PubMed] [Google Scholar]
- 745.Rowsell, E. R., J. M. Smith, A. Wolfe, and B. L. Taylor. 1995. CheA, CheW, and CheY are required for chemotaxis to oxygen and sugars of the phosphotransferase system in Escherichia coli. J. Bacteriol. 177:6011-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Roy, A., A. Danchin, E. Joseph, and A. Ullmann. 1983. Two functional domains in adenylate cyclase of Escherichia coli. J. Mol. Biol. 165:197-202. [DOI] [PubMed] [Google Scholar]
- 747.Ruiz-Manzano, A., L. Yuste, and F. Rojo. 2005. Levels and activity of the Pseudomonas putida global regulatory protein Crc vary according to growth conditions. J. Bacteriol. 187:3678-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Russell, R. B., J. A. Marquez, W. Hengstenberg, and K. Scheffzek. 2002. Evolutionary relationship between the bacterial HPr kinase and the ubiquitous PEP-carboxykinase: expanding the P-loop nucleotidyl transferase superfamily. FEBS Lett. 517:1-6. [DOI] [PubMed] [Google Scholar]
- 749.Rutberg, B. 1997. Antitermination of transcription of catabolic operons. Mol. Microbiol. 23:413-421. [DOI] [PubMed] [Google Scholar]
- 750.Ryu, S., and S. Garges. 1994. Promoter switch in the Escherichia coli pts operon. J. Biol. Chem. 269:4767-4772. [PubMed] [Google Scholar]
- 751.Ryu, S., T. Ramseier, V. Michotey, M. H. Saier, Jr., and S. Garges. 1995. Effects of the FruR regulator on transcription of the pts operon of Escherichia coli. J. Biol. Chem. 270:2489-2496. [DOI] [PubMed] [Google Scholar]
- 752.Sabnis, N. A., H. Yang, and T. Romeo. 1995. Pleiotropic regulation of central carbohydrate metabolism in Escherichia coli via the gene csrA. J. Biol. Chem. 270:29096-29104. [DOI] [PubMed] [Google Scholar]
- 753.Saffen, D. W., K. A. Presper, T. L. Doering, and S. Roseman. 1987. Sugar transport by the bacterial phosphotransferase system. Molecular cloning and structural analysis of the Escherichia coli ptsH, ptsI, and crr genes. J. Biol. Chem. 262:16241-16253. [PubMed] [Google Scholar]
- 754.Sahin-Tóth, M., K. M. Akhoon, J. Runner, and H. R. Kaback. 2000. Ligand recognition by the lactose permease of Escherichia coli: specificity and affinity are defined by distinct structural elements of galactopyranosides. Biochemistry 39:5097-5103. [DOI] [PubMed] [Google Scholar]
- 755.Saier, M. H., Jr. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64:354-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Saier, M. H., Jr., S. Chauvaux, J. Deutscher, J. Reizer, and J.-J. Ye. 1995. Protein phosphorylation and regulation of carbon metabolism in gram-negative versus gram-positive bacteria. Trends Biochem. Sci. 20:267-271. [DOI] [PubMed] [Google Scholar]
- 757.Saier, M. H., Jr., D. F. Cox, B. U. Feucht, and M. J. Novotny. 1982. Evidence for the functional association of enzyme I and HPr of the phosphoenolpyruvate-sugar phosphotransferase system with the membrane in sealed vesicles of Escherichia coli. J. Cell. Biochem. 18:231-238. [DOI] [PubMed] [Google Scholar]
- 758.Saier, M. H., Jr., B. U. Feucht, and M. T. McCaman. 1975. Regulation of intracellular adenosine cyclic 3′:5′-monophosphate levels in Escherichia coli and Salmonella typhimurium. Evidence for energy-dependent excretion of the cyclic nucleotide. J. Biol. Chem. 250:7593-7601. [PubMed] [Google Scholar]
- 759.Saier, M. H., Jr., R. N. Hvorup, and R. D. Barabote. 2005. Evolution of the bacterial phosphotransferase system: from carriers and enzymes to group translocators. Biochem. Soc. Trans. 33:220-224. [DOI] [PubMed] [Google Scholar]
- 760.Saier, M. H., Jr., D. K. Keeler, and B. U. Feucht. 1982. Physiological desensitization of carbohydrate permeases and adenylate cyclase to regulation by the phosphoenolpyruvate:sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Involvement of adenosine cyclic 3′,5′-phosphate and inducer. J. Biol. Chem. 257:2509-2517. [PubMed] [Google Scholar]
- 761.Saier, M. H., Jr., M. J. Novotny, D. Comeau-Fuhrman, T. Osumi, and J. D. Desai. 1983. Cooperative binding of the sugar substrates and allosteric regulatory protein (enzyme IIIGlc of the phosphotransferase system) to the lactose and melibiose permeases in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 155:1351-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Saier, M. H., Jr., and T. M. Ramseier. 1996. The catabolite repressor/activator (Cra) protein of enteric bacteria. J. Bacteriol. 178:3411-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Saier, M. H., Jr., T. M. Ramseier, and J. Reizer. 1996. Regulation of carbon utilization, p. 1325-1343. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 764.Saier, M. H., Jr., and J. Reizer. 1992. Proposed uniform nomenclature for the proteins and protein domains of the bacterial phosphoenolpyruvate:sugar phosphotransferase system. J. Bacteriol. 174:1433-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Saier, M. H., Jr., and S. Roseman. 1972. Inducer exclusion and repression of enzyme synthesis in mutants of Salmonella typhimurium defective in enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system. J. Biol. Chem. 247:972-975. [PubMed] [Google Scholar]
- 766.Saier, M. H., Jr., and S. Roseman. 1976. Sugar transport. Inducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J. Biol. Chem. 251:6606-6615. [PubMed] [Google Scholar]
- 767.Saier, M. H., Jr., and S. Roseman. 1976. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J. Biol. Chem. 251:6598-6605. [PubMed] [Google Scholar]
- 768.Saier, M. H., Jr., M. R. Schmidt, and P. Lin. 1980. Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate:sugar phosphotransferase system. Kinetic characterization. J. Biol. Chem. 255:8579-8584. [PubMed] [Google Scholar]
- 769.Saier, M. H., Jr., and R. D. Simoni. 1976. Regulation of carbohydrate uptake in gram-positive bacteria. J. Biol. Chem. 251:893-894. [PubMed] [Google Scholar]
- 770.Saier, M. H., Jr., R. D. Simoni, and S. Roseman. 1976. Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate:sugar phosphotransferase system. J. Biol. Chem. 251:6584-6597. [PubMed] [Google Scholar]
- 771.Saier, M. H., Jr., R. D. Simoni, and S. Roseman. 1970. The physiological behavior of enzyme I and heat-stable protein mutants of a bacterial phosphotransferase system. J. Biol. Chem. 245:5870-5873. [PubMed] [Google Scholar]
- 772.Saier, M. H., Jr., J.-J. Ye, S. Klinke, and E. Nino. 1996. Identification of an anaerobically induced phosphoenolpyruvate-dependent fructose-specific phosphotransferase system and evidence for the Embden-Meyerhof glycolytic pathway in the heterofermentative bacterium Lactobacillus brevis. J. Bacteriol. 178:314-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Saier, M. H., Jr., W. S. Young, and S. Roseman. 1971. Utilization and transport of hexoses by mutant strains of Salmonella typhimurium lacking enzyme I of the phosphoenolpyruvate-dependent phosphotransferase system. J. Biol. Chem. 246:5838-5840. [PubMed] [Google Scholar]
- 774.Samanta, S., T. Ayvaz, M. Reyes, H. A. Shuman, J. Chen, and A. L. Davidson. 2003. Disulfide cross-linking reveals a site of stable interaction between C-terminal regulatory domains of the two MalK subunits in the maltose transport complex. J. Biol. Chem. 278:35265-35271. [DOI] [PubMed] [Google Scholar]
- 775.Sampaio, M. M., F. Chevance, R. Dippel, T. Eppler, A. Schlegel, W. Boos, Y. J. Lu, and C. O. Rock. 2004. Phosphotransferase-mediated transport of the osmolyte 2-O-alpha-mannosyl-D-glycerate in Escherichia coli occurs by the product of the mngA (hrsA) gene and is regulated by the mngR (farR) gene product acting as repressor. J. Biol. Chem. 279:5537-5548. [DOI] [PubMed] [Google Scholar]
- 776.Santillán, M., and M. C. Mackey. 2004. Influence of catabolite repression and inducer exclusion on the bistable behavior of the lac operon. Biophys. J. 86:1282-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Sauter, T., and E. D. Gilles. 2004. Modeling and experimental validation of the signal transduction via the Escherichia coli sucrose phospho transferase system. J. Biotechnol. 110:181-199. [DOI] [PubMed] [Google Scholar]
- 778.Schauder, S., R. S. Nunn, R. Lanz, B. Erni, and T. Schirmer. 1998. Crystal structure of the IIB subunit of a fructose permease (IIBLev) from Bacillus subtilis. J. Mol. Biol. 276:591-602. [DOI] [PubMed] [Google Scholar]
- 779.Schick, J., B. Weber, J. R. Klein, and B. Henrich. 1999. PepR1, a CcpA-like transcription regulator of Lactobacillus delbrueckii subsp. lactis. Microbiology 145:3147-3154. [DOI] [PubMed] [Google Scholar]
- 780.Schiefner, A., K. Gerber, S. Seitz, W. Welte, K. Diederichs, and W. Boos. 2005. The crystal structure of Mlc, a global regulator of sugar metabolism in Escherichia coli. J. Biol. Chem. 280:29073-29079. [DOI] [PubMed] [Google Scholar]
- 781.Schmalisch, M. H., S. Bachem, and J. Stülke. 2003. Control of the Bacillus subtilis antiterminator protein GlcT by phosphorylation: elucidation of the phosphorylation chain leading to inactivation of GlcT. J. Biol. Chem. 278:51108-51115. [DOI] [PubMed] [Google Scholar]
- 782.Schneider, E. 2001. ABC transporters catalyzing carbohydrate uptake. Res. Microbiol. 152:303-310. [DOI] [PubMed] [Google Scholar]
- 783.Schnetz, K., and B. Rak. 1990. β-Glucoside permease represses the bgl operon of Escherichia coli by phosphorylation of the antiterminator protein and also interacts with glucose-specific enzyme IIIGlc, the key element in catabolite control. Proc. Natl. Acad. Sci. USA 87:5074-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Schnetz, K., S. L. Sutrina, M. H. Saier, Jr., and B. Rak. 1990. Identification of catalytic residues in the β-glucoside permease of Escherichia coli by site-specific mutagenesis and demonstration of interdomain cross-reactivity between the β-glucoside and glucose systems. J. Biol. Chem. 265:13464-13471. [PubMed] [Google Scholar]
- 785.Schnetz, K., C. Toloczyki, and B. Rak. 1987. β-Glucoside (bgl) operon of Escherichia coli K-12: nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J. Bacteriol. 169:2579-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Scholte, B. J., A. R. J. Schuitema, and P. W. Postma. 1982. Characterization of factor IIIGlc in catabolite repression-resistant (crr) mutants of Salmonella typhimurium. J. Bacteriol. 149:576-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Schumacher, M. A., G. S. Allen, M. Diel, G. Seidel, W. Hillen, and R. G. Brennan. 2004. Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P. Cell 118:731-741. [DOI] [PubMed] [Google Scholar]
- 788.Schumacher, M. A., K. Y. Choi, H. Zalkin, and R. G. Brennan. 1994. Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by α helices. Science 266:763-770. [DOI] [PubMed] [Google Scholar]
- 789.Schumacher, M. A., G. Seidel, W. Hillen, and R. G. Brennan. 2006. Phosphoprotein Crh-Ser46-P displays altered binding to CcpA to effect carbon catabolite regulation. J. Biol. Chem. 281:6793-6800. [DOI] [PubMed] [Google Scholar]
- 790.Schweizer, H., W. Boos, and T. J. Larson. 1985. Repressor for the sn-glycerol-3-phosphate regulon of Escherichia coli K-12: cloning of the glpR gene and identification of its product. J. Bacteriol. 161:563-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791.Scovill, W. H., H. J. Schreier, and K. W. Bayles. 1996. Identification and characterization of the pckA gene from Staphylococcus aureus. J. Bacteriol. 178:3362-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Seeto, S., L. Notley-McRobb, and T. Ferenci. 2004. The multifactorial influences of RpoS, Mlc and cAMP on ptsG expression under glucose-limited and anaerobic conditions. Res. Microbiol. 155:211-215. [DOI] [PubMed] [Google Scholar]
- 793.Segura, D., and G. Espin. 1998. Mutational inactivation of a gene homologous to Escherichia coli ptsP affects poly-β-hydroxybutyrate accumulation and nitrogen fixation in Azotobacter vinelandii. J. Bacteriol. 180:4790-4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Seidel, G., M. Diel, N. Fuchsbauer, and W. Hillen. 2005. Quantitative interdependence of coeffectors, CcpA and cre in carbon catabolite regulation of Bacillus subtilis. FEBS J. 272:2566-2577. [DOI] [PubMed] [Google Scholar]
- 795.Seitz, S., S. J. Lee, C. Pennetier, W. Boos, and J. Plumbridge. 2003. Analysis of the interaction between the global regulator Mlc and EIIBGlc of the glucose-specific phosphotransferase system in Escherichia coli. J. Biol. Chem. 278:10744-10751. [DOI] [PubMed] [Google Scholar]
- 796.Senba, M., N. Kashige, Y. Nakashima, F. Miake, and K. Watanabe. 2000. Cloning of the gene of beta-N-acetylglucosaminidase from Lactobacillus casei ATCC 27092 and characterization of the enzyme expressed in Escherichia coli. Biol. Pharm. Bull. 23:527-531. [DOI] [PubMed] [Google Scholar]
- 797.Seok, Y.-J., B. M. Koo, M. Sondej, and A. Peterkofsky. 2001. Regulation of E. coli glycogen phosphorylase activity by HPr. J. Mol. Microbiol. Biotechnol. 3:385-393. [PubMed] [Google Scholar]
- 798.Seok, Y.-J., B. R. Lee, C. Gazdar, I. Svenson, N. Yadla, and A. Peterkofsky. 1996. Importance of the region around glycine-338 for the activity of enzyme I of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system. Biochemistry 35:236-242. [DOI] [PubMed] [Google Scholar]
- 799.Seok, Y.-J., M. Sondej, P. Badawi, M. S. Lewis, M. C. Briggs, H. Jaffe, and A. Peterkofsky. 1997. High affinity binding and allosteric regulation of Escherichia coli glycogen phosphorylase by the histidine phosphocarrier protein, HPr. J. Biol. Chem. 272:26511-26521. [DOI] [PubMed] [Google Scholar]
- 800.Seok, Y.-J., J. Sun, H. R. Kaback, and A. Peterkofsky. 1997. Topology of allosteric regulation of lactose permease. Proc. Natl. Acad. Sci. USA 94:13515-13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Setlow, B., and P. Setlow. 1977. Levels of oxidized and reduced pyridine nucleotides in dormant spores and during growth, sporulation, and spore germination of Bacillus megaterium. J. Bacteriol. 129:857-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Setty, Y., A. E. Mayo, M. G. Surette, and U. Alon. 2003. Detailed map of a cis-regulatory input function. Proc. Natl. Acad. Sci. USA 100:7702-7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803.Shaw, G. C., H. S. Kao, and C. Y. Chiou. 1998. Cloning, expression, and catabolite repression of a gene encoding β-galactosidase of Bacillus megaterium ATCC 14581. J. Bacteriol. 180:4734-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Sheehan, B., A. Klarsfeld, T. Msadek, and P. Cossart. 1995. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J. Bacteriol. 177:6469-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Shenolikar, S. 1994. Protein serine/threonine phosphatases—new avenues for cell regulation. Annu. Rev. Cell Biol. 10:55-86. [DOI] [PubMed] [Google Scholar]
- 806.Shimotsu, H., and D. J. Henner. 1986. Modulation of Bacillus subtilis levansucrase gene expression by sucrose and regulation of the steady-state mRNA level by sacU and sacQ genes. J. Bacteriol. 168:380-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Shin, D., N. Cho, S. Heu, and S. Ryu. 2003. Selective regulation of ptsG expression by Fis. Formation of either activating or repressing nucleoprotein complex in response to glucose. J. Biol. Chem. 278:14776-14781. [DOI] [PubMed] [Google Scholar]
- 808.Shin, D., S. Lim, Y.-J. Seok, and S. Ryu. 2001. Heat shock RNA polymerase (Eσ32) is involved in the transcription of mlc and crucial for induction of the Mlc regulon by glucose in Escherichia coli. J. Biol. Chem. 276:25871-25875. [DOI] [PubMed] [Google Scholar]
- 809.Shingler, V. 1996. Signal sensing by σ54-dependent regulators: derepression as a control mechanism. Mol. Microbiol. 19:409-416. [DOI] [PubMed] [Google Scholar]
- 810.Shivers, R. P., and A. L. Sonenshein. 2005. Bacillus subtilis ilvB operon: an intersection of global regulons. Mol. Microbiol. 56:1549-1559. [DOI] [PubMed] [Google Scholar]
- 811.Siebold, C., I. Arnold, L. F. Garcia-Alles, U. Baumann, and B. Erni. 2003. Crystal structure of the Citrobacter freundii dihydroxyacetone kinase reveals an eight-stranded α-helical barrel ATP-binding domain. J. Biol. Chem. 278:48236-48244. [DOI] [PubMed] [Google Scholar]
- 812.Siebold, C., and B. Erni. 2002. Intein-mediated cyclization of a soluble and a membrane protein in vivo: function and stability. Biophys. Chem. 96:163-171. [DOI] [PubMed] [Google Scholar]
- 813.Siebold, C., K. Flukiger, R. Beutler, and B. Erni. 2001. Carbohydrate transporters of the bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS). FEBS Lett. 504:104-111. [DOI] [PubMed] [Google Scholar]
- 814.Siebold, C., L. F. Garcia-Alles, B. Erni, and U. Baumann. 2003. A mechanism of covalent substrate binding in the X-ray structure of subunit K of the Escherichia coli dihydroxyacetone kinase. Proc. Natl. Acad. Sci. USA 100:8188-8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Silvestroni, A., C. Connes, F. Sesma, G. S. De Giori, and J. C. Piard. 2002. Characterization of the melA locus for α-galactosidase in Lactobacillus plantarum. Appl. Environ. Microbiol. 68:5464-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.Simpson, C. L., and R. R. Russell. 1998. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect. Immun. 66:2085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 817.Singh-Wissmann, K., C. Ingram-Smith, R. D. Miles, and J. G. Ferry. 1998. Identification of essential glutamates in the acetate kinase from Methanosarcina thermophila. J. Bacteriol. 180:1129-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Sliz, P., R. Engelmann, W. Hengstenberg, and E. F. Pai. 1997. The structure of enzyme IIAlactose from Lactococcus lactis reveals a new fold and points to possible interactions of a multicomponent system. Structure 5:775-788. [DOI] [PubMed] [Google Scholar]
- 819.Smirnova, I. N., and H. R. Kaback. 2003. A mutation in the lactose permease of Escherichia coli that decreases conformational flexibility and increases protein stability. Biochemistry 42:3025-3031. [DOI] [PubMed] [Google Scholar]
- 820.Söhngen, N. L., and C. Coolhaas. 1924. The fermentation of galactose by Saccharomyces cerevisiae. J. Bacteriol. 9:131-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821.Sola-Landa, A., J. Pizarro-Cerda, M. J. Grillo, E. Moreno, I. Moriyon, J. M. Blasco, J. P. Gorvel, and I. Lopez-Goni. 1998. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29:125-138. [DOI] [PubMed] [Google Scholar]
- 822.Sondej, M., Y.-J. Seok, P. Badawi, B.-M. Koo, T.-W. Nam, and A. Peterkofsky. 2000. Topography of the surface of the Escherichia coli phosphotransferase system protein enzyme IIAGlc that interacts with lactose permease. Biochemistry 39:2931-2939. [DOI] [PubMed] [Google Scholar]
- 823.Sondej, M., J. Z. Sun, Y.-J. Seok, H. R. Kaback, and A. Peterkofsky. 1999. Deduction of consensus binding sequences on proteins that bind IIAGlc of the phosphoenolpyruvate:sugar phosphotransferase system by cysteine scanning mutagenesis of Escherichia coli lactose permease. Proc. Natl. Acad. Sci. USA 96:3525-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Sondej, M., J. L. Vazquez-Ibar, A. Farshidi, A. Peterkofsky, and H. R. Kaback. 2003. Characterization of a lactose permease mutant that binds IIAGlc in the absence of ligand. Biochemistry 42:9153-9159. [DOI] [PubMed] [Google Scholar]
- 825.Sondej, M., A. B. Weinglass, A. Peterkofsky, and H. R. Kaback. 2002. Binding of enzyme IIAGlc, a component of the phosphoenolpyruvate:sugar phosphotransferase system, to the Escherichia coli lactose permease. Biochemistry 41:5556-5565. [DOI] [PubMed] [Google Scholar]
- 826.Spelbrink, R. E., A. Kolkman, M. Slijper, J. A. Killian, and B. de Kruijff. 2005. Detection and identification of stable oligomeric protein complexes in Escherichi coli inner membranes: a proteomics approach. J. Biol. Chem. 280:28742-28748. [DOI] [PubMed] [Google Scholar]
- 827.Sridharan, S., A. Razvi, J. M. Scholtz, and J. C. Sacchettini. 2005. The HPr proteins from the thermophile Bacillus stearothermophilus can form domain-swapped dimers. J. Mol. Biol. 346:919-931. [DOI] [PubMed] [Google Scholar]
- 828.Stein, A., M. Seifert, R. Volkmer-Engert, J. Siepelmeyer, K. Jahreis, and E. Schneider. 2002. Functional characterization of the maltose ATP-binding-cassette transporter of Salmonella typhimurium by means of monoclonal antibodies directed against the MalK subunit. Eur. J. Biochem. 269:4074-4085. [DOI] [PubMed] [Google Scholar]
- 829.Stein, J. M., H. L. Kornberg, and B. R. Martin. 1985. Effects of GTP, GDP[βS] and glucose on adenylate cyclase activity of E. coli B. FEBS Lett. 182:429-434. [DOI] [PubMed] [Google Scholar]
- 830.Steinhauer, K., G. S. Allen, W. Hillen, J. Stülke, and R. G. Brennan. 2002. Crystallization, preliminary X-ray analysis and biophysical characterization of HPr kinase/phosphatase of Mycoplasma pneumoniae. Acta Crystallogr. 58:515-518. [DOI] [PubMed] [Google Scholar]
- 831.Steinhauer, K., T. Jepp, W. Hillen, and J. Stülke. 2002. A novel mode of control of Mycoplasma pneumoniae HPr kinase/phosphatase activity reflects its parasitic lifestyle. Microbiology 148:3277-3284. [DOI] [PubMed] [Google Scholar]
- 832.Steinmetz, M., D. Le Coq, and S. Aymerich. 1989. Induction of saccharolytic enzymes by sucrose in Bacillus subtilis: evidence for two partially interchangeable regulatory pathways. J. Bacteriol. 171:1519-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Steinmetz, M., D. Le Coq, S. Aymerich, G. Gonzy-Tréboul, and P. Gay. 1985. The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol. Gen. Genet. 200:220-228. [DOI] [PubMed] [Google Scholar]
- 834.Stentz, R., R. Lauret, S. D. Ehrlich, F. Morel-Deville, and M. Zagorec. 1997. Molecular cloning and analysis of the ptsHI operon in Lactobacillus sake. Appl. Environ. Microbiol. 63:2111-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Stentz, R., and M. Zagorec. 1999. Ribose utilization in Lactobacillus sakei: analysis of the regulation of the rbs operon and putative involvement of a new transporter. J. Mol. Microbiol. Biotechnol. 1:165-173. [PubMed] [Google Scholar]
- 836.Stephenson, M., and E. F. Gale. 1937. The adaptability of glucozymase and galactozymase in Bacterium coli. Biochem. J. 31:1311-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Stock, J. B., A. J. Ninfa, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53:450-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Stock, J. B., and M. G. Surette. 1996. Chemotaxis, p. 1103-1129. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 839.Stock, J. B., E. B. Waygood, N. D. Meadow, P. W. Postma, and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system. The glucose receptors of the Salmonella typhimurium phosphotransferase system. J. Biol. Chem. 257:14543-14552. [PubMed] [Google Scholar]
- 840.Stolz, B., M. Huber, Z. Markovic-Housley, and B. Erni. 1993. The mannose transporter of Escherichia coli. Structure and function of the IIABMan subunit. J. Biol. Chem. 268:27094-27099. [PubMed] [Google Scholar]
- 841.Stonestrom, A., R. D. Barabote, C. F. Gonzalez, and M. H. Saier, Jr. 2005. Bioinformatic analyses of bacterial HPr kinase/phosphorylase homologues. Res. Microbiol. 156:443-451. [DOI] [PubMed] [Google Scholar]
- 842.Storz, G., J. A. Opdyke, and A. X. Zhang. 2004. Controlling mRNA stability and translation with small, noncoding RNAs. Curr. Opin. Microbiol. 7:140-144. [DOI] [PubMed] [Google Scholar]
- 843.Studholme, D. J., and R. Dixon. 2003. Domain architectures of σ54-dependent transcriptional activators. J. Bacteriol. 185:1757-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Stülke, J., M. Arnaud, G. Rapoport, and I. Martin-Verstraete. 1998. PRD—a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol. Microbiol. 28:865-874. [DOI] [PubMed] [Google Scholar]
- 845.Stülke, J., and W. Hillen. 1999. Carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 846.Stülke, J., I. Martin-Verstraete, V. Charrier, A. Klier, J. Deutscher, and G. Rapoport. 1995. The HPr protein of the phosphotransferase system links induction and catabolite repression of the Bacillus subtilis levanase operon. J. Bacteriol. 177:6928-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Stülke, J., I. Martin-Verstraete, M. Zagorec, M. Rose, A. Klier, and G. Rapoport. 1997. Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol. Microbiol. 25:65-78. [DOI] [PubMed] [Google Scholar]
- 848.Sun, J. B., J. van den Heuvel, P. Soucaille, Y. B. Qu, and A. P. Zeng. 2003. Comparative genomic analysis of dha regulon and related genes for anaerobic glycerol metabolism in bacteria. Biotechnol. Prog. 19:263-272. [DOI] [PubMed] [Google Scholar]
- 849.Sweet, G., C. Gandor, R. Voegele, N. Wittekindt, J. Beuerle, V. Truniger, E. C. C. Lin, and W. Boos. 1990. Glycerol facilitator of Escherichia coli: cloning of glpF and identification of the glpF product. J. Bacteriol. 172:424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850.Szurmant, H., and G. W. Ordal. 2004. Diversity in chemotaxis mechanisms among the bacteria and archaea. Microbiol. Mol. Biol. Rev. 68:301-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.Tagami, H., T. Inada, T. Kunimura, and H. Aiba. 1995. Glucose lowers CRP* levels resulting in repression of the lac operon in cells lacking cAMP. Mol. Microbiol. 17:251-258. [DOI] [PubMed] [Google Scholar]
- 852.Takahashi, H., T. Inada, P. Postma, and H. Aiba. 1998. CRP down-regulates adenylate cyclase activity by reducing the level of phosphorylated IIAGlc, the glucose-specific phosphotransferase protein, in Escherichia coli. Mol. Gen. Genet. 259:317-326. [DOI] [PubMed] [Google Scholar]
- 853.Takahashi, M., B. Blazy, A. Baudras, and W. Hillen. 1989. Ligand-modulated binding of a gene regulatory protein to DNA. Quantitative analysis of cyclic-AMP induced binding of CRP from Escherichia coli to non-specific and specific DNA targets. J. Mol. Biol. 207:783-796. [DOI] [PubMed] [Google Scholar]
- 854.Tan, M.-W., L. G. Rahme, J. A. Sternberg, R. G. Tompkins, and F. M. Ausubel. 1999. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 96:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855.Tanaka, Y., F. Itoh, K. Kimata, and H. Aiba. 2004. Membrane localization itself but not binding to IICBGlc is directly responsible for the inactivation of the global repressor Mlc in Escherichia coli. Mol. Microbiol. 53:941-951. [DOI] [PubMed] [Google Scholar]
- 856.Tanaka, Y., K. Kimata, and H. Aiba. 2000. A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J. 19:5344-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 857.Tanaka, Y., K. Kimata, T. Inada, H. Tagami, and H. Aiba. 1999. Negative regulation of the pts operon by Mlc: mechanism underlying glucose induction in Escherichia coli. Genes Cells. 4:391-399. [DOI] [PubMed] [Google Scholar]
- 858.Tang, C., D. C. Williams, Jr., R. Ghirlando, and G. M. Clore. 2005. Solution structure of enzyme IIAChitobiose from the N,N′-diacetylchitobiose branch of the Escherichia coli phosphotransferase system. J. Biol. Chem. 280:11770-11780. [DOI] [PubMed] [Google Scholar]
- 859.Tangney, M., A. Galinier, J. Deutscher, and W. J. Mitchell. 2003. Analysis of the elements of catabolite repression in Clostridium acetobutylicum ATCC 824. J. Mol. Microbiol. Biotechnol. 6:6-11. [DOI] [PubMed] [Google Scholar]
- 860.Tangney, M., and W. J. Mitchell. 2000. Analysis of a catabolic operon for sucrose transport and metabolism in Clostridium acetobutylicum ATCC 824. J. Mol. Microbiol. Biotechnol. 2:71-80. [PubMed] [Google Scholar]
- 861.Tatarko, M., and T. Romeo. 2001. Disruption of a global regulatory gene to enhance central carbon flux into phenylalanine biosynthesis in Escherichia coli. Curr. Microbiol. 43:26-32. [DOI] [PubMed] [Google Scholar]
- 862.Tavori, H., Y. Kimmel, and Z. Barak. 1981. Toxicity of leucine-containing peptides in Escherichia coli caused by circumvention of leucine transport regulation. J. Bacteriol. 146:676-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Taylor, B. L., M. S. Johnson, and J. M. Smith. 1988. Signaling pathways in bacterial chemotaxis. Bot. Acta 101:101-104. [Google Scholar]
- 864.Taylor, B. L., and J. W. Lengeler. 1990. Transductive coupling by methylated transducing proteins and permeases of the phosphotransferase system in bacterial chemotaxis, p. 69-90. In R. C. Aloia, C. C. Curtain, and L. M. Gordon (ed.), Membrane transport and information storage. Wiley Liss, New York, N.Y.
- 865.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63:479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Taylor, S. S., D. R. Knighton, J. Zheng, J. M. Sowadski, C. S. Gibbs, and M. J. Zoller. 1993. A template for the protein kinase family. Trends Biochem. Sci. 18:84-89. [DOI] [PubMed] [Google Scholar]
- 867.Tchieu, J. H., V. Norris, J. S. Edwards, and M. H. Saier, Jr. 2001. The complete phosphotranferase system in Escherichia coli. J. Mol. Microbiol. Biotechnol. 3:329-346. [PubMed] [Google Scholar]
- 868.Thattai, M., and B. I. Shraiman. 2003. Metabolic switching in the sugar phosphotransferase system of Escherichia coli. Biophys. J. 85:744-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869.Thevenot, T., D. Brochu, C. Vadeboncoeur, and I. R. Hamilton. 1995. Regulation of ATP-dependent P-(Ser)-HPr formation in Streptococcus mutans and Streptococcus salivarius. J. Bacteriol. 177:2751-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870.Thomas, S., D. Brochu, and C. Vadeboncoeur. 2001. Diversity of Streptococcus salivarius ptsH mutants that can be isolated in the presence of 2-deoxyglucose and galactose and characterization of two mutants synthesizing reduced levels of HPr, a phosphocarrier of the phosphoenolpyruvate:sugar phosphotransferase system. J. Bacteriol. 183:5145-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871.Thompson, J., and B. M. Chassy. 1983. Regulation of glycolysis and sugar phosphotransferase activities in Streptococcus lactis: growth in the presence of 2-deoxy-d-glucose. J. Bacteriol. 154:819-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872.Thompson, J., and M. H. Saier, Jr. 1981. Regulation of thiomethylgalactoside 6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J. Bacteriol. 146:885-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Thompson, J., and D. A. Torchia. 1984. Use of 31P nuclear magnetic resonance spectroscopy and 14C fluorography in studies of glycolysis and regulation of pyruvate kinase in Streptococcus lactis. J. Bacteriol. 158:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 874.Thorner, J. W., and H. Paulus. 1973. Catalytic and allosteric properties of glycerol kinase from Escherichia coli. J. Biol. Chem. 248:3922-3932. [PubMed] [Google Scholar]
- 875.Tian, Z. X., Q. S. Li, M. Buck, A. Kolb, and Y. P. Wang. 2001. The CRP-cAMP complex and downregulation of the glnAp2 promoter provides a novel regulatory linkage between carbon metabolism and nitrogen assimilation in Escherichia coli. Mol. Microbiol. 41:911-924. [DOI] [PubMed] [Google Scholar]
- 876.Titgemeyer, F., R. E. Mason, and M. H. Saier, Jr. 1994. Regulation of the raffinose permease of Escherichia coli by the glucose-specific enzyme IIA of the phosphoenolpyruvate:sugar phosphotransferase system. J. Bacteriol. 176:543-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 877.Titgemeyer, F., J. Reizer, A. Reizer, and M. H. Saier, Jr. 1994. Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology 140:2349-2354. [DOI] [PubMed] [Google Scholar]
- 878.Tobisch, S., P. Glaser, S. Krüger, and M. Hecker. 1997. Identification and characterization of a new β-glucoside utilization system in Bacillus subtilis. J. Bacteriol. 179:496-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 879.Tobisch, S., J. Stülke, and M. Hecker. 1999. Regulation of the lic operon of Bacillus subtilis and characterization of potential phosphorylation sites of the LicR regulator protein by site-directed mutagenesis. J. Bacteriol. 181:4995-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 880.Tobisch, S., D. Zühlke, J. Bernhardt, J. Stülke, and M. Hecker. 1999. Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis. J. Bacteriol. 181:6996-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 881.Todhunter, J. A., and D. L. Purich. 1974. Evidence for the formation of a γ-phosphorylated glutamyl residue in the Escherichia coli acetate kinase reaction. Biochem. Biophys. Res. Commun. 60:273-280. [DOI] [PubMed] [Google Scholar]
- 882.Tojo, S., T. Satomura, K. Morisaki, J. Deutscher, K. Hirooka, and Y. Fujita. 2005. Elaborate expression regulation of the Bacillus subtilis ilv-leu operon involved in the biosynthesis of the branched-chain amino acids through global regulators of CcpA, CodY and TnrA. Mol. Microbiol. 56:1560-1573. [DOI] [PubMed] [Google Scholar]
- 883.Tortosa, P., S. Aymerich, C. Lindner, M. H. Saier, Jr., J. Reizer, and D. Le Coq. 1997. Multiple phosphorylation of SacY, a Bacillus subtilis transcriptional antiterminator negatively controlled by the phosphotransferase system. J. Biol. Chem. 272:17230-17237. [DOI] [PubMed] [Google Scholar]
- 884.Tortosa, P., N. Declerck, H. Dutartre, C. Lindner, J. Deutscher, and D. Le Coq. 2001. Sites of positive and negative regulation in the Bacillus subtilis antiterminators LicT and SacY. Mol. Microbiol. 41:1381-1393. [DOI] [PubMed] [Google Scholar]
- 885.Tortosa, P., and D. Le Coq. 1995. A ribonucleic antiterminator sequence (RAT) and a distant palindrome are both involved in sucrose induction of the Bacillus subtilis sacXY regulatory operon. Microbiology 141:2921-2927. [DOI] [PubMed] [Google Scholar]
- 886.Turinsky, A. J., F. J. Grundy, J.-H. Kim, G. H. Chambliss, and T. M. Henkin. 1998. Transcriptional activation of the Bacillus subtilis ackA gene requires sequences upstream of the promoter. J. Bacteriol. 180:5961-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887.Turinsky, A. J., T. R. Moir-Blais, F. J. Grundy, and T. M. Henkin. 2000. Bacillus subtilis ccpA gene mutants specifically defective in activation of acetoin biosynthesis. J. Bacteriol. 182:5611-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Tworzyd[strok]lo, M., A. Polit, J. Mikolajczak, and Z. Wasylewski. 2005. Fluorescence quenching and kinetic studies of conformational changes induced by DNA and cAMP binding to cAMP receptor protein from Escherichia coli. FEBS J. 272:1103-1116. [DOI] [PubMed] [Google Scholar]
- 889.Ucker, D. S., and E. R. Signer. 1978. Catabolite-repression-like phenomenon in Rhizobium meliloti. J. Bacteriol. 136:1197-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Ullmann, A., and A. Danchin. 1983. Role of cyclic AMP in bacteria. Adv. Cyclic Nucleotide Res. 15:32-53. [Google Scholar]
- 891.Umbarger, H. E. 1996. Biosynthesis of the branched-chain amino acids, p. 442-457. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 892.Utsumi, R., T. Horie, A. Katoh, Y. Kaino, H. Tanabe, and M. Noda. 1996. Isolation and characterization of the heat-responsive genes in Escherichia coli. Biosci. Biotechnol. Biochem. 60:309-315. [DOI] [PubMed] [Google Scholar]
- 893.Vadeboncoeur, C., D. Brochu, and J. Reizer. 1991. Quantitative determination of the intracellular concentration of the various forms of HPr, a phosphocarrier protein of the phosphoenolpyruvate:sugar phosphotransferase system in growing cells of oral streptococci. Anal. Biochem. 196:24-30. [DOI] [PubMed] [Google Scholar]
- 894.Vadeboncoeur, C., M. Frenette, and L. A. Lortie. 2000. Regulation of the pts operon in low G+C gram-positive bacteria. J. Mol. Microbiol. Biotechnol. 2:483-490. [PubMed] [Google Scholar]
- 895.Valentin-Hansen, P., M. Eriksen, and C. Udesen. 2004. The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol. Microbiol. 51:1525-1533. [DOI] [PubMed] [Google Scholar]
- 896.van Dam, K., J. van der Vlag, B. Kholodenko, and H. Westerhoff. 1993. The sum of the control coefficients of all enzymes on the flux through a group transfer pathway can be as high as two. Eur. J. Biochem. 212:791-799. [DOI] [PubMed] [Google Scholar]
- 897.van den Bogaard, P. T. C., M. Kleerebezem, O. P. Kuipers, and W. M. de Vos. 2000. Control of lactose transport, β-galactosidase activity, and glycolysis by CcpA in Streptococcus thermophilus: evidence for carbon catabolite repression by a non-phosphoenolpyruvate-dependent phosphotransferase system sugar. J. Bacteriol. 182:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898.Vanderpool, C. K., and S. Gottesman. 2004. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol. Microbiol. 54:1076-1089. [DOI] [PubMed] [Google Scholar]
- 899.van der Vlag, J., and P. W. Postma. 1995. Regulation of glycerol and maltose uptake by the IIAGlc-like domain of IINag of the phosphotransferase system in Salmonella typhimurium. Mol. Gen. Genet. 248:236-241. [DOI] [PubMed] [Google Scholar]
- 900.van der Vlag, J., K. van Dam, and P. W. Postma. 1994. Quantification of the regulation of glycerol and maltose metabolism by IIAGlc of the phosphoenolpyruvate-dependent glucose phoshotransferase system in Salmonella typhimurium. J. Bacteriol. 176:3518-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.van der Vlag, J., R. van't Hof, K. van Dam, and P. W. Postma. 1995. Control of glucose metabolism by the enzymes of the glucose phosphotransferase system in Salmonella typhimurium. Eur. J. Biochem. 230:170-182. [DOI] [PubMed] [Google Scholar]
- 902.van Iwaarden, P. R., A. J. Driessen, J. S. Lolkema, H. R. Kaback, and W. N. Konings. 1993. Exchange, efflux, and substrate binding by cysteine mutants of the lactose permease of Escherichia coli. Biochemistry 32:5419-5424. [DOI] [PubMed] [Google Scholar]
- 903.van Montfort, B. A., G. K. Schuurman-Wolters, R. H. Duurkens, R. Mensen, B. Pollman, and G. T. Robillard. 2001. Cysteine cross-linking defines part of the dimer and B/C domain interface of the Escherichia coli mannitol permease. J. Biol. Chem. 276:12756-12763. [DOI] [PubMed] [Google Scholar]
- 904.van Montfort, B. A., G. K. Schuurman-Wolters, J. Wind, J. Broos, G. T. Robillard, and B. Pollman. 2002. Mapping of the dimer interface of the Escherichia coli mannitol permease by cysteine cross-linking. J. Biol. Chem. 277:14717-14723. [DOI] [PubMed] [Google Scholar]
- 905.van Montfort, R. L. M., and B. W. Dijkstra. 1998. The functional importance of structural differences between the mannitol-specific IIAmannitol and the regulatory IIAnitrogen. Protein Sci. 7:2210-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.van Montfort, R. L. M., T. Pijning, K. H. Kalk, I. Hangyi, M. L. C. E. Kouwijzer, G. T. Robillard, and B. W. Dijkstra. 1998. The structure of the Escherichia coli phosphotransferase IIAmannitol reveals a novel fold with two conformations of the active site. Structure 6:377-388. [DOI] [PubMed] [Google Scholar]
- 907.van Montfort, R. L. M., T. Pijning, K. H. Kalk, J. Reizer, M. H. Saier, Jr., M. M. G. M. Thunnissen, G. T. Robillard, and B. W. Dijkstra. 1997. The structure of an energy-coupling protein from bacteria, IIBcellobiose, reveals similarity to eukaryotic protein tyrosine phosphatases. Structure 5:217-225. [DOI] [PubMed] [Google Scholar]
- 908.van Nuland, N. A. J., G. J. A. Kroon, K. Dijkstra, G. K. Wolters, R. M. Scheek, and G. T. Robillard. 1993. The NMR determination of the IIAmtl binding site on HPr of the Escherichia coli phosphoenol pyruvate-dependent phosphotransferase system. FEBS Lett. 315:11-15. [DOI] [PubMed] [Google Scholar]
- 909.van Tilbeurgh, H., D. Le Coq, and N. Declerck. 2001. Crystal structure of an activated form of the PTS regulation domain from the LicT transcriptional antiterminator. EMBO J. 20:3789-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 910.van Tilbeurgh, H., X. Manival, S. Aymerich, J. M. Lhoste, C. Dumas, and M. Kochoyan. 1997. Crystal structure of a new RNA-binding domain from the antiterminator protein SacY of Bacillus subtilis. EMBO J. 16:5030-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911.van Wezel, G. P., K. Mahr, M. König, B. A. Traag, E. F. Pimentel-Schmitt, A. Willimek, and F. Titgemeyer. 2005. GlcP constitutes the major glucose uptake system of Streptomyces coelicolor A3(2). Mol. Microbiol. 55:624-636. [DOI] [PubMed] [Google Scholar]
- 912.van Wezel, G. P., J. White, P. Young, P. W. Postma, and M. J. Bibb. 1997. Substrate induction and glucose repression of maltose utilization by Streptomyces coelicolor A3(2) is controlled by malR, a member of the lacI-galR family of regulatory genes. Mol. Microbiol. 23:537-549. [DOI] [PubMed] [Google Scholar]
- 913.Varga, J., V. L. Stirewalt, and S. B. Melville. 2004. The CcpA protein is necessary for efficient sporulation and enterotoxin gene (cpe) regulation in Clostridium perfringens. J. Bacteriol. 186:5221-5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 914.Vaughan, E. E., S. David, and W. de Vos. 1996. The lactose transporter in Leuconostoc lactis is a new member of the LacS subfamily of galactoside-pentose-hexuronide translocators. Appl. Environ. Microbiol. 62:1574-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Veenhoff, L. M., and B. Poolman. 1999. Substrate recognition at the cytoplasmic and extracellular binding site of the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem. 274:33244-33250. [DOI] [PubMed] [Google Scholar]
- 916.Velázquez, F., I. di Bartolo, and V. de Lorenzo. 2004. Genetic evidence that catabolites of the Entner-Doudoroff pathway signal C source repression of the σ54 Pu promoter of Pseudomonas putida. J. Bacteriol. 186:8267-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917.Vemuri, G. N., and A. A. Aristidou. 2005. Metabolic engineering in the -omics era: elucidating and modulating regulatory networks. Microbiol. Mol. Biol. Rev. 69:197-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918.Vervoort, E. B., J. B. Bultema, G. K. Schuurman-Wolters, E. R. Geertsma, J. Broos, and B. Poolman. 2005. The first cytoplasmic loop of the mannitol permease from Escherichia coli is accessible for sulfhydryl reagents from the periplasmic side of the membrane. J. Mol. Biol. 346:733-743. [DOI] [PubMed] [Google Scholar]
- 919.Viana, R., V. Monedero, V. Dossonnet, C. Vadeboncoeur, G. Pérez-Martínez, and J. Deutscher. 2000. Enzyme I and HPr from Lactobacillus casei: their role in sugar transport, carbon catabolite repression and inducer exclusion. Mol. Microbiol. 36:570-584. [DOI] [PubMed] [Google Scholar]
- 920.Vögele, R. T., G. D. Sweet, and W. Boos. 1993. Glycerol kinase of Escherichia coli is activated by interaction with the glycerol facilitator. J. Bacteriol. 175:1087-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 921.Vogler, A. P., C. P. Broekhuizen, A. Schuitema, J. W. Lengeler, and P. W. Postma. 1988. Suppression of IIIGlc-defects by enzymes IINag and IIBgl of the PEP:carbohydrate phosphotransferase system. Mol. Microbiol. 2:719-726. [DOI] [PubMed] [Google Scholar]
- 922.Vogler, A. P., and J. W. Lengeler. 1989. Analysis of the nag regulon from Escherichia coli K12 and Klebsiella pneumoniae and of its regulation. Mol. Gen. Genet. 219:97-105. [DOI] [PubMed] [Google Scholar]
- 923.Vogler, A. P., and J. W. Lengeler. 1988. Complementation of a truncated membrane-bound enzyme IINag from Klebsiella pneumoniae with a soluble enzyme III in Escherichia coli K12. Mol. Gen. Genet. 213:175-178. [DOI] [PubMed] [Google Scholar]
- 924.Voskuil, M. I., and G. H. Chambliss. 1996. Significance of HPr in catabolite repression of α-amylase. J. Bacteriol. 178:7014-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 925.Wacker, I., H. Ludwig, I. Reif, H.-M. Blencke, C. Detsch, and J. Stülke. 2003. The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA. Microbiology 149:3001-3009. [DOI] [PubMed] [Google Scholar]
- 926.Wang, G., A. Peterkofsky, and G. M. Clore. 2000. A novel membrane anchor function for the N-terminal amphipathic sequence of the signal-transducing protein IIAGlucose of the Escherichia coli phosphotransferase system. J. Biol. Chem. 275:39811-39814. [DOI] [PubMed] [Google Scholar]
- 927.Wang, G., A. Peterkofsky, P. A. Keifer, and X. Li. 2005. NMR characterization of the Escherichia coli nitrogen regulatory protein IIANtr in solution and interaction with its partner protein, NPr. Protein Sci. 14:1082-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.Wang, G. S., P. A. Keifer, and A. Peterkofsky. 2003. Solution structure of the N-terminal amphitropic domain of Escherichia coli glucose-specific enzyme IIA in membrane-mimetic micelles. Protein Sci. 12:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929.Wang, G. S., J. M. Louis, M. Sondej, Y.-J. Seok, A. Peterkofsky, and G. M. Clore. 2000. Solution structure of the phosphoryl transfer complex between the signal transducing proteins HPr and IIAGlucose of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system. EMBO J. 19:5635-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 930.Wang, G. S., M. Sondej, D. S. Garrett, A. Peterkofsky, and G. M. Clore. 2000. A common interface on histidine-containing phosphocarrier protein for interaction with its partner proteins. J. Biol. Chem. 275:16401-16403. [DOI] [PubMed] [Google Scholar]
- 931.Wang, J., E. D. Gilles, J. W. Lengeler, and K. Jahreis. 2001. Modeling of inducer exclusion and catabolite repression based on a PTS-dependent sucrose and non-PTS-dependent glycerol transport systems in Escherichia coli K-12 and its experimental verification. J. Biotechnol. 92:133-158. [DOI] [PubMed] [Google Scholar]
- 932.Wanner, B. L., R. Kodaira, and F. C. Neidhardt. 1978. Regulation of the lac operon expression: reappraisel of the theory of catabolite repression. J. Bacteriol. 136:947-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 933.Ward, D. E., C. C. van Der Weijden, M. J. van Der Merwe, H. V. Westerhoff, A. Claiborne, and J. L. Snoep. 2000. Branched-chain α-keto acid catabolism via the gene products of the bkd operon in Enterococcus faecalis: a new, secreted metabolite serving as a temporary redox sink. J. Bacteriol. 182:3239-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 934.Warner, J. B., and J. S. Lolkema. 2003. A Crh-specific function in carbon catabolite repression in Bacillus subtilis. FEMS Microbiol. Lett. 220:277-280. [DOI] [PubMed] [Google Scholar]
- 935.Warner, J. B., and J. S. Lolkema. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67:475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 936.Waygood, E. B. 1998. The structure and function of HPr. Biochem. Cell Biol. 76:359-367. [DOI] [PubMed] [Google Scholar]
- 937.Wehtje, C., L. Beijer, R.-P. Nilsson, and B. Rutberg. 1995. Mutations in the glycerol kinase gene restore the ability of a ptsGHI mutant of Bacillus subtilis to grow on glycerol. Microbiology 141:1193-1198. [DOI] [PubMed] [Google Scholar]
- 938.Weickert, M. J., and S. Adhya. 1992. A family of bacterial regulators homologous to Gal and Lac repressor. J. Biol. Chem. 267:15869-15874. [PubMed] [Google Scholar]
- 939.Weickert, M. J., and G. H. Chambliss. 1990. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 87:6238-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 940.Weigel, N., M. A. Kukuruzinska, A. Nakazawa, E. B. Waygood, and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J. Biol. Chem. 257:14477-14491. [PubMed] [Google Scholar]
- 941.Weigel, N., D. A. Powers, and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system. Primary structure and active site of a general phosphocarrier protein (HPr) from Salmonella typhimurium. J. Biol. Chem. 257:14499-14509. [PubMed] [Google Scholar]
- 942.Weinglass, A. B., M. Sondej, and H. R. Kaback. 2002. Manipulating conformational equilibria in the lactose permease of Escherichia coli. J. Mol. Biol. 315:561-571. [DOI] [PubMed] [Google Scholar]
- 943.Weiss, D. S., J. Batut, K. E. Klose, J. Keener, and S. Kustu. 1991. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell 67:155-167. [DOI] [PubMed] [Google Scholar]
- 944.Weissenborn, D. L., N. Wittekindt, and T. J. Larson. 1992. Structure and regulation of the glpFK operon encoding glycerol diffusion facilitator and glycerol kinase of Escherichia coli K-12. J. Biol. Chem. 267:6122-6131. [PubMed] [Google Scholar]
- 945.Welch, M., K. Oosawa, S. Aizawa, and M. Eisenbach. 1993. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc. Natl. Acad. Sci. USA 90:8787-8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946.Wen, Z. T., and R. A. Burne. 2002. Analysis of cis- and trans-acting factors involved in regulation of the Streptococcus mutans fructanase gene (fruA). J. Bacteriol. 184:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947.Wen, Z. T., and R. A. Burne. 2002. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl. Environ. Microbiol. 68:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 948.Weng, Q.-P., J. Elder, and G. R. Jacobson. 1992. Site-specific mutagenesis of residues in the Escherichia coli mannitol permease that have been suggested to be important for its phosphorylation and chemoreception functions. J. Biol. Chem. 267:19529-19535. [PubMed] [Google Scholar]
- 949.Williams, D. C., Jr., M. Cai, J. Y. Suh, A. Peterkofsky, and G. M. Clore. 2005. Solution NMR structure of the 48-kDa IIAMannose-HPr complex of the Escherichia coli mannose phosphotransferase system. J. Biol. Chem. 280:20775-20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 950.Williams, N., D. K. Fox, C. Shea, and S. Roseman. 1986. Pel, the protein that permits λ DNA penetration of Escherichia coli, is encoded by a gene in ptsM and is required for mannose utilization by the phosphotransferase system. Proc. Natl. Acad. Sci. USA 83:8934-8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 951.Wilson, T. H., P. L. Yunker, and C. L. Hansen. 1990. Lactose transport mutants of Escherichia coli resistant to inhibition by the phosphotransferase system. Biochim. Biophys. Acta 1029:113-116. [DOI] [PubMed] [Google Scholar]
- 952.Wittekind, M., J. Reizer, J. Deutscher, M. H. Saier, Jr., and R. E. Klevit. 1989. Common structural changes accompagny the functional inactivation of HPr by seryl phosphorylation or by serine to aspartate substitution. Biochemistry 28:9908-9912. [DOI] [PubMed] [Google Scholar]
- 953.Wong, P., S. Gladney, and J. D. Keasling. 1997. Mathematical model of the lac operon: inducer exclusion, catabolite repression, and diauxic growth on glucose and lactose. Biotechnol. Prog. 13:132-143. [DOI] [PubMed] [Google Scholar]
- 954.Worley, K. C., K. Y. King, S. Chua, E. R. McCabe, and R. F. Smith. 1995. Identification of new members of a carbohydrate kinase-encoding gene family. J. Comput. Biol. 2:451-458. [DOI] [PubMed] [Google Scholar]
- 955.Worthylake, D., N. D. Meadow, S. Roseman, D.-I. Liao, O. Herzberg, and S. J. Remington. 1991. Three-dimensional structure of the Escherichia coli phosphocarrier protein IIIGlc. Proc. Natl. Acad. Sci. USA 88:10382-10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 956.Wouters, J. A., H. H. Kamphuis, J. Hugenholtz, O. P. Kuipers, W. M. de Vos, and T. Abee. 2000. Changes in glycolytic activity of Lactococcus lactis induced by low temperature. Appl. Environ. Microbiol. 66:3686-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 957.Wright, L. F., D. P. Milne, and C. J. Knowles. 1979. The regulatory effects of growth rate and cyclic AMP levels on carbon catabolism and respiration in Escherichia coli K-12. Biochim. Biophys. Acta 583:73-80. [DOI] [PubMed] [Google Scholar]
- 958.Wu, L.-F., J. M. Tomich, and M. H. Saier, Jr. 1990. Structure and evolution of a multidomain multiphosphoryl transfer protein. Nucleotide sequence of the fruB(HI) gene in Rhodobacter capsulatus and comparisons with homologous genes from other organisms. J. Mol. Biol. 213:687-703. [DOI] [PubMed] [Google Scholar]
- 959.Xue, J., I. Hunter, T. Steinmetz, A. Peters, B. Ray, and K. W. Miller. 2005. Novel activator of mannose-specific phosphotransferase system permease expression in Listeria innocua, identified by screening for pediocin AcH resistance. Appl. Environ. Microbiol. 71:1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 960.Yagur-Kroll, S., and O. Amster-Choder. 2005. Dynamic membrane topology of the Escherichia coli β-glucoside transporter BglF. J. Biol. Chem. 280:19306-19318. [DOI] [PubMed] [Google Scholar]
- 961.Yamada, M., and M. H. Saier, Jr. 1988. Positive and negative regulators for glucitol (gut) operon expression in Escherichia coli. J. Mol. Biol. 203:569-583. [DOI] [PubMed] [Google Scholar]
- 962.Yang, H., M. Y. Liu, and T. Romeo. 1996. Coordinate genetic regulation of glycogen catabolism and biosynthesis in Escherichia coli via the CsrA gene product. J. Bacteriol. 178:1012-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963.Yang, J. K., and W. Epstein. 1983. Purification and characterization of adenylate cyclase from Escherichia coli K12. J. Biol. Chem. 258:3750-3758. [PubMed] [Google Scholar]
- 964.Yang, Y., N. Declerck, X. Manival, S. Aymerich, and M. Kochoyan. 2002. Solution structure of the LicT-RNA antitermination complex: CAT clamping RAT. EMBO J. 21:1987-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 965.Yanofsky, C. 2000. Transcription attenuation: once viewed as a novel regulatory strategy. J. Bacteriol. 182:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 966.Yazyu, H., S. Shiota-Niiya, T. Shimamoto, H. Kanazawa, M. Futai, and T. Tsuchiya. 1984. Nucleotide sequence of the melB gene and characteristics of deduced amino acid sequence of the melibiose carrier in Escherichia coli. J. Biol. Chem. 259:4320-4326. [PubMed] [Google Scholar]
- 967.Ye, J.-J., J. Minarcik, and M. H. Saier, Jr. 1996. Inducer expulsion and the occurrence of an HPr(Ser-P)-activated sugar phosphate phosphatase in Enterococcus faecalis and Streptococcus pyogenes. Microbiology 142:585-592. [DOI] [PubMed] [Google Scholar]
- 968.Ye, J.-J., J. W. Neal, X. Cui, J. Reizer, and M. H. Saier, Jr. 1994. Regulation of the glucose:H+ symporter by metabolite-activated ATP-dependent phosphorylation of HPr in Lactobacillus brevis. J. Bacteriol. 176:3484-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969.Ye, J.-J., J. Reizer, X. Cui, and M. H. Saier, Jr. 1994. ATP-dependent phosphorylation of serine-46 in the phosphocarrier protein HPr regulates lactose/H+ symport in Lactobacillus brevis. Proc. Natl. Acad. Sci. USA 91:3102-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 970.Ye, J.-J., J. Reizer, X. Cui, and M. H. Saier, Jr. 1994. Inhibition of the phosphoenolpyruvate:lactose phosphotransferase system and activation of a cytoplasmic sugar-phosphate phosphatase in Lactococcus lactis by ATP-dependent metabolite-activated phosphorylation of serine 46 in the phosphocarrier protein HPr. J. Biol. Chem. 269:11837-11844. [PubMed] [Google Scholar]
- 971.Ye, J.-J., J. Reizer, and M. H. Saier, Jr. 1994. Regulation of 2-deoxyglucose phosphate accumulation in Lactococcus lactis vesicles by metabolite-activated, ATP-dependent phosphorylation of serine-46 in HPr of the phosphotransferase system. Microbiology 140:3421-3429. [DOI] [PubMed] [Google Scholar]
- 972.Ye, J.-J., and M. H. Saier, Jr. 1995. Cooperative binding of lactose and the phosphorylated phosphocarrier HPr(Ser-P) to the lactose/H+ symport permease of Lactobacillus brevis. Proc. Natl. Acad. Sci. USA 92:417-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973.Ye, J.-J., and M. H. Saier, Jr. 1995. Purification and characterization of a small membrane-associated sugar phosphate phosphatase that is allosterically activated by HPr(Ser(P)) of the phosphotransferase system in Lactococcus lactis. J. Biol. Chem. 270:16740-16744. [DOI] [PubMed] [Google Scholar]
- 974.Ye, J.-J., and M. H. Saier, Jr. 1996. Regulation of sugar uptake via the phosphoenolpyruvate-dependent phosphotransferase system in Bacillus subtilis and Lactococcus lactis is mediated by ATP-dependent phosphorylation of seryl residue 46 in HPr. J. Bacteriol. 178:3557-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 975.Yebra, M. J., V. Monedero, M. Zúñiga, J. Deutscher, and G. Pérez-Martínez. 2006. Molecular analysis of the glucose-specific phosphoenolpyruvate:sugar phosphotransferase system from Lactobacillus casei and its links with the control of sugar metabolism. Microbiology 152:95-104. [DOI] [PubMed] [Google Scholar]
- 976.Yebra, M. J., R. Viana, V. Monedero, J. Deutscher, and G. Perez-Martinez. 2004. An esterase gene from Lactobacillus casei cotranscribed with genes encoding a phosphoenolpyruvate:sugar phosphotransferase system and regulated by a LevR-like activator and σ54 factor. J. Mol. Microbiol. Biotechnol. 8:117-128. [DOI] [PubMed] [Google Scholar]
- 977.Yeh, J. I., V. Charrier, J. Paulo, L. Hou, E. Darbon, A. Claiborne, W. G. J. Hol, and J. Deutscher. 2004. Structures of enterococcal glycerol kinase in the absence and presence of glycerol: correlation of conformation to substrate binding and a mechanism of activation by phosphorylation. Biochemistry 43:362-373. [DOI] [PubMed] [Google Scholar]
- 978.Yildirim, N., and M. C. Mackey. 2003. Feedback regulation in the lactose operon: a mathematical modeling study and comparison with experimental data. Biophys. J. 84:2841-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Yildirim, N., M. Santillán, D. Horike, and M. C. Mackey. 2004. Dynamics and bistability in a reduced model of the lac operon. Chaos 14:279-292. [DOI] [PubMed] [Google Scholar]
- 980.Yoshida, K.-I., K. Kobayashi, Y. Miwa, C. M. Kang, M. Matsunaga, H. Yamaguchi, S. Tojo, M. Yamamoto, R. Nishi, N. Ogasawara, T. Nakayama, and Y. Fujita. 2001. Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucleic Acids Res. 29:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Yoshida, K. I., T. Shibayama, D. Aoyama, and Y. Fujita. 1999. Interaction of a repressor and its binding sites for regulation of the Bacillus subtilis iol divergon. J. Mol. Biol. 285:917-929. [DOI] [PubMed] [Google Scholar]
- 982.Yuste, L., and F. Rojo. 2001. Role of the crc gene in catabolic repression of the Pseudomonas putida GPo1 alkane degradation pathway. J. Bacteriol. 183:6197-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983.Zahler, S. A., L. G. Benjamin, B. S. Glatz, P. F. Winter, and B. J. Goldstein. 1976. Genetic mapping of the alsA, alsR, thyA, kauA, and citD markers in Bacillus subtilis, p. 35-43. In D. Schlessinger (ed.), Microbiology—1976. American Society for Microbiology, Washington, D.C.
- 984.Zalieckas, J. M., L. V. Wray, Jr., and S. H. Fisher. 1998. Expression of the Bacillus subtilis acsA gene: position and sequence context affect cre-mediated carbon catabolite repression. J. Bacteriol. 180:6649-6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 985.Zalieckas, J. M., L. V. Wray, Jr., and S. H. Fisher. 1999. trans-Acting factors affecting carbon catabolite repression of the hut operon in Bacillus subtilis. J. Bacteriol. 181:2883-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Zeng, G., S. Ye, and T. J. Larson. 1996. Repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K-12: primary structure and identification of the DNA-binding domain. J. Bacteriol. 178:7080-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987.Zeng, G. Q., H. de Reuse, and A. Danchin. 1992. Mutational analysis of the enzyme IIIGlc of the phosphoenolpyruvate phosphotransferase system in Escherichia coli. Res. Microbiol. 143:251-261. [DOI] [PubMed] [Google Scholar]
- 988.Zeppenfeld, T., C. Larisch, J. W. Lengeler, and K. Jahreis. 2000. Glucose transporter mutants of Escherichia coli K-12 with changes in substrate recognition of IICBGlc and induction behavior of the ptsG gene. J. Bacteriol. 182:4443-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 989.Zhang, Z. G., M. Aboulwafa, M. H. Smith, and M. H. Saier, Jr. 2003. The ascorbate transporter of Escherichia coli. J. Bacteriol. 185:2243-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 990.Zheng, D. L., C. Constantinidou, J. L. Hobman, and S. D. Minchin. 2004. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 32:5874-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 991.Zhu, P.-P., O. Herzberg, and A. Peterkofsky. 1998. Topography of the interaction of HPr(Ser) kinase with HPr. Biochemistry 37:11762-11770. [DOI] [PubMed] [Google Scholar]
- 992.Zhu, P. P., N. Nosworthy, A. Ginsburg, M. Miyata, Y.-J. Seok, and A. Peterkofsky. 1997. Expression, purification, and characterization of enzyme IIAglc of the phosphoenolpyruvate:sugar phosphotransferase system of Mycoplasma capricolum. Biochemistry 36:6947-6953. [DOI] [PubMed] [Google Scholar]
- 993.Zukowski, M. M., L. Miller, P. Cogswell, K. Chen, S. Aymerich, and M. Steinmetz. 1990. Nucleotide sequence of the sacS locus of Bacillus subtilis reveals the presence of two regulatory genes. Gene 90:153-155. [DOI] [PubMed] [Google Scholar]
- 994.Zwaig, N., and E. C. C. Lin. 1966. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science 153:755-757. [DOI] [PubMed] [Google Scholar]