Abstract
The double-stranded RNA-dependent protein kinase PKR is a critical mediator of the antiproliferative and antiviral effects exerted by interferons. Not only is PKR an effector molecule on the cellular response to double-stranded RNA, but it also integrates signals in response to Toll-like receptor activation, growth factors, and diverse cellular stresses. In this review, we provide a detailed picture on how signaling downstream of PKR unfolds and what are the ultimate consequences for the cell fate. PKR activation affects both transcription and translation. PKR phosphorylation of the alpha subunit of eukaryotic initiation factor 2 results in a blockade on translation initiation. However, PKR cannot avoid the translation of some cellular and viral mRNAs bearing special features in their 5′ untranslated regions. In addition, PKR affects diverse transcriptional factors such as interferon regulatory factor 1, STATs, p53, activating transcription factor 3, and NF-κB. In particular, how PKR triggers a cascade of events involving IKK phosphorylation of IκB and NF-κB nuclear translocation has been intensively studied. At the cellular and organism levels PKR exerts antiproliferative effects, and it is a key antiviral agent. A point of convergence in both effects is that PKR activation results in apoptosis induction. The extent and strength of the antiviral action of PKR are clearly understood by the findings that unrelated viral proteins of animal viruses have evolved to inhibit PKR action by using diverse strategies. The case for the pathological consequences of the antiproliferative action of PKR is less understood, but therapeutic strategies aimed at targeting PKR are beginning to offer promising results.
INTRODUCTION
The interferons (IFN) were discovered by Isaacs and Lindenmann in 1957 as substances that protect cells from viral infection (162). Since then, the antiviral activities of IFN have been studied intensively. It was soon recognized that IFN have a wide range of biological functions, including antiviral, antiproliferative, and immunomodulatory properties (304, 316). Pioneering work leading to the cloning of the interferon genes, the determination of the structures of the ligand and their receptors, and the determination of the signaling pathways and transcription of IFN-induced genes has been instrumental in the understanding of how these molecules exert their function in the cell (281, 333a). This knowledge opened the way to the discovery of similar signaling pathways in the cytokine family. Among the molecules with important biological functions induced by IFN is the double-stranded RNA (dsRNA)-dependent protein kinase (PKR), an enzyme with multiple effects in cells, which plays a critical role in the antiviral defense mechanism of the host (42, 171, 305, 376, 377).
PKR was discovered after it was observed at the National Institute for Medical Research, London, United Kingdom, by the groups of Metz and Kerr that cell extracts prepared from IFN-treated vaccinia virus-infected cells, which are known to have restricted translation of viral and cellular mRNAs in cultured cells (244), were exquisitely sensitive to a translational block after addition to a cell-free system of the exogenous mRNA (99) and of the synthetic form of dsRNA, poly(rI) · poly(rC) (pIC) (190). These seminal studies led to the identification of a protein with dsRNA-dependent kinase activity (296, 317), now known as PKR (43), which was later cloned (245) after the laboratory of Hovanessian prepared specific antibodies for PKR purification and partial sequencing (211). The research attempting to define the mechanism by which dsRNA inhibits protein synthesis led to the discovery of another important enzyme, the 2′,5′-oligoadenylate synthetase (158). PKR is the most-studied member of the alpha subunit of eukaryotic initiation factor 2 (eIF-2α)-specific kinase subfamily (74). It is a serine/threonine kinase, characterized by two distinct kinase activities: autophosphorylation, which represents the activation reaction, and the phosphorylation of eIF-2α (101, 156), which impairs eIF-2 activity, resulting in inhibition of protein synthesis (293). In addition to its translational regulatory function, PKR has a role in signal transduction and transcriptional control through the IκB/NF-κB pathway (201). PKR, which is expressed constitutively in mammalian cells, has also been implicated in the control of cell growth and proliferation with tumor suppressor function (198, 224, 246).
PKR is encoded from a single gene located on human chromosome 2p21-22 and mouse chromosome 17E2 (12, 200, 331). The human Pkr gene consists of 17 exons, whereas the mouse gene has 16 (200, 350). In mice, three PKR transcripts with tissue-specific differential expression have been described, but their molecular basis and functional significance remain unresolved (160, 188). Expression of PKR varies in a time- and tissue-specific manner during human fetal development; levels are imperceptible in blastema and immature mesenchymal cells but high in a variety of more differentiated tissues such as epithelial cells (141). In adult tissues, PKR levels are low in the proliferating immature zone of squamous mucosa and increase progressively in the nonproliferating mature keratinocytes (141).
Due to its intrinsic properties, PKR has been studied extensively to document its relevance as a first-line defense mechanism against infection and as a cell growth regulator. Numerous reviews on PKR action have been published over the years (10, 11, 118, 125, 170, 171, 180, 183, 184, 280, 305, 324, 368, 376-378). In this review, we provide an in-depth analysis of current knowledge of this important serine/threonine protein kinase. We summarize PKR structure and function, with particular emphasis on the impact of PKR activation on translation and the signal transduction pathways it mediates. We review several effectors regulated by PKR, with special attention to the NF-κB-PKR activation pathway and approaches to the identification of new effectors, regulators, and targets of PKR activation. We also examine its numerous cellular and viral regulators, highlighting the importance of PKR regulation under normal and stress conditions. We discuss the mechanisms and pathways involved in PKR-induced apoptosis and review the experimental findings that support or do not support PKR involvement as a tumor suppressor, and we discuss how it might be used as a target in anticancer therapies.
TRANSLATION REGULATION BY PKR
PKR is one of the four mammalian kinases (the others are GCN2, PERK, and HRI) that phosphorylate eIF-2α in response to stress signals, mainly as a result of viral infections (11, 78, 146, 376). Phosphorylation of eIF-2α at residue S51 prevents the recycling of this factor that is required for ongoing translation, leading to general inhibition of translation. In addition to the kinase domain (KD) shared by the other eIF-2α kinases, PKR also has a dsRNA-binding domain (dsRBD) that regulates its activity. As a consequence of dsRNA accumulation in infected cells, PKR-triggered eIF-2α phosphorylation also inhibits translation of viral mRNA (7, 214, 216, 335); this constitutes the basic mechanism by which PKR exerts its antiviral activity on a wide spectrum of DNA and RNA viruses. A number of recent reports have provided insights into the mechanism of PKR activation and eIF-2α phosphorylation, consisting of a three-step pathway in which KD dimerization triggers autophosphorylation, in turn promoting specific recognition of eIF-2α.
eIF-2α Recognition and Phosphorylation
PKR activation segment phosphorylation on Thr446 promotes substrate recognition and phosphorylation. Recent structural data from KD-eIF-2α crystals revealed the determinants for the exquisite specificity of PKR for its natural substrate. eIF-2α recognition involves bipartite interactions with the αG helix and the PKR phospho-acceptor-binding site located in the catalytic pocket between lobes (Fig. 1). The αG helix of PKR in the surface of the C lobe of KD interacts initially with eIF-2α to promote a conformational change in this factor that brings the phosphorylatable S51 residue close to the PKR phospho-acceptor site for catalysis. This change in eIF-2α involves local unfolding of S51, which affords this residue full accessibility to the catalytic cleft of PKR (61, 80, 355). Other eIF-2α kinases also have the αG helix in a similar orientation, suggesting a fully conserved mechanism for eIF-2α recognition. This allosteric mechanism of catalysis explains earlier observations that short peptides derived from the eIF-2α sequence containing unfold S51 were much poorer substrates than complete eIF-2α. Given this high-order substrate recognition mechanism, the existence of another PKR substrate(s) apart from eIF-2α should be considered with caution. Recently, phosphorylation at tyrosine residues in PKR upon activation has been reported (337). Phosphorylation at these tyrosine residues (Y101, Y162, and Y293) appears to influence the binding to dsRNA, autophosphorylation, and eIF-2 phosphorylation. These data support the idea that PKR is a kinase of dual specificity that also requires tyrosine phosphorylation for full-scale activation.
FIG. 1.
Top panel, schematic drawing of PKR activation by dsRNA-mediated dimerization, autophosphorylation, and phosphorylation of eIF-2α. R, dsRNA-binding domain., N and C, N-terminal and C-terminal lobes of kinase domain. Middle panel, crystallographic structure of PKR dimer bound to eIF-2α. Bottom panel, conformational change in eIF-2α induced by binding to PKR. Isolated eIF-2α and eIF-2α bound to PKR are in blue and red, respectively. RMSD, root mean square deviation. (The middle and bottom panels were adapted from reference 61 with permission from Elsevier.)
Impact of PKR Activation on Translation
In mammals, eIF-2 promotes Met-tRNAi delivery to the 40S ribosome to initiate polypeptide chain synthesis. eIF-2 is composed of three subunits (α, β, and γ); it binds the Met-tRNAi in a GTP-dependent manner to form the ternary complex, which joins the 40S subunit (153, 236). Once Met-tRNAi is delivered, eIF-2 is released from the 48S initiation complex after eIF-5-promoted GTP hydrolysis (153, 236). Inactive eIF-2-GDP complexes are continuously regenerated by GDP-to-GTP exchange in a process catalyzed by the GTP exchange factor eIF-2B. eIF-2 activity is regulated by phosphorylation at S51 of its α subunit. As a consequence of eIF-2α phosphorylation, eIF-2 affinity for eIF-2B increases up to 100-fold, leading to competitive inhibition of eIF-2B and the resulting inhibition of translation initiation (338). Since eIF-2B is present in limited amounts with respect to eIF-2, small increases in eIF-2α phosphorylation can thus lead to an exacerbated effect on protein synthesis (153).
eIF-2α phosphorylation has emerged not only as the main regulation point in protein synthesis but also as a critical trigger of the stress response. This is illustrated by the existence of four eIF-2α kinases (PKR, GCN2, PERK, and HRI) that sensitize mammalian cells to different stress signals and allow them to respond to adverse situations (78). Although translation of most cell and viral mRNAs is inhibited by eIF-2α phosphorylation, translation of few mRNAs involved in the stress response is enhanced by limited eIF-2α phosphorylation. This is the case for yeast GCN4 and mammalian activating transcription factor 4 (ATF-4), ATF-3, and CAT-1 mRNAs (79, 134, 232, 386). Under nonstress conditions, translation of these mRNAs is inhibited by the presence of upstream short open reading frames (ORFs) that attract ribosomes to translate short peptides, restricting the flow of scanning ribosomes to the bona fide GCN4 and ATF-4 ORFs (78). Phosphorylation of eIF-2α limits the number of active 43S complexes, promoting reinitiation from GCN4 and ATF-4 ORFs. Although for most viruses, translation of their mRNAs also requires the participation of eIF-2, translation in some insect viruses (cricket paralysis virus) and alphaviruses (Sindbis virus [SV] and Semliki Forest virus) can proceed in the absence of functional eIF-2 (363, 375). Alphaviruses are a case apart from the rest of the viruses, since complete eIF-2α phosphorylation is detected in infected cells (363). Translation of SV and Semliki Forest virus subgenomic mRNAs resists eIF-2α phosphorylation by the existence of a stable stem-loop structure in the RNA downstream of the AUG codon that stalls the ribosomes on the correct site to initiate translation (Fig. 2). To promote this, alphaviruses can alternatively use eIF-2A to deliver the Met-tRNAi and initiate translation in the presence of high levels of phosphorylated eIF-2α (363).
FIG. 2.
(A) Impact of PKR activation and eIF-2α phosphorylation on translation. See the text for details. (B) Two examples of mRNAs whose translation is resistant to eIF-2α phosphorylation. Translation of the bona fide ATF-4 ORF is induced by low levels of functional eIF-2 (eIF-2α phosphorylation), whereas under normal conditions (high availability of eIF-2), ribosomes initiate at upstream ORFs that lead to a premature translation halt. For SV capsid mRNA, a very stable stem-loop structure downstream of initiation codon stalls the ribosome on the correct site to initiate translation. When eIF-2α is phosphorylated, SV mRNA can alternatively use the translation initiation factor 2A for delivering the Met-tRNA.
As predicted for a translation regulator, PKR is associated to ribosomes, mainly to 40S subunits (203, 401). Ribosomal association of PKR appears to be mediated by the dsRBDs, strengthening the role of these domains in the correct regulation of PKR activity (383, 401). Several ribosomal proteins are reported to interact with PKR, although it is still unclear whether these interactions simply anchor PKR to ribosomes or are involved in a more complex functional regulation of the kinase (203). PKR localization in ribosomes offers a satisfactory explanation for its local activation in response to a limited stimulus, as reported by many groups (7, 18, 186, 401). This is illustrated by the finding that PKR upregulation by IFN treatment leads to discrete eIF-2α phosphorylation in response to viral infection, preventing translation of viral mRNA without affecting the overall translation of the cell mRNAs. Restricted activation of PKR to sites of viral RNA synthesis and translation could thus specifically prevent accumulation of viral proteins. Although direct experimental evidence for such spatial regulation of PKR activity is still lacking, the availability of specific antibodies to PKR will allow the localization of active PKR during viral infections or other stress. In addition, PKR has been also detected in the nuclei of human and murine cells, specially when the kinase is overexpressed from transfected plasmids (172, 269). The biological significance of PKR translocation to the nucleus is unknown, but recent data suggest that it could be involved in stress-induced apoptosis, since accumulation of phosphorylated PKR has been detected in tunicamycin-treated cultured cells and in neurons from patients with Alzheimer's disease (269).
PKR ENGAGEMENT BY DIFFERENT SIGNAL TRANSDUCTION PATHWAYS
In addition to its well-established role as a translational regulator as discussed above, PKR is involved in signal transduction. The first indication came from the observation that chemical inhibition of PKR by using the nucleoside analogue 2-aminopurine interfered with the gene induction normally triggered by IFN (314). The fact that dsRNA signals to activate the NF-κB pathway (365) was a step towards the identification of a role for PKR as a signal transducer in that pathway (201), although its participation was found to be more complex than initially thought, as we discuss below. In addition to mediating a critical role in response to dsRNA, thus acting as a sensor of viral infections, PKR is switched on by a set of other activators, such as proinflammatory stimuli, growth factors, cytokines, and oxidative stress. In addition, PKR integrates and transmits these signals not only to eIF-2α and the translational machinery but also to various factors such as STAT, interferon regulatory factor 1 (IRF-1), p53, Jun N-terminal protein kinase (JNK), and p38, as well as engaging the NF-κB pathway (364, 376, 378).
PKR Activation by dsRNA
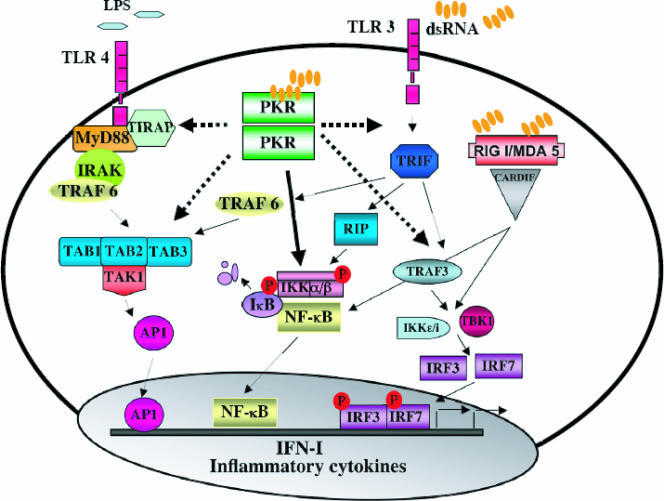
Immunorecognition of dsRNA include Toll-like receptor 3 (TLR3) and cytosolic RNA-binding proteins such as PKR and the helicases RIGI and MDA5 (3, 181, 393) (Fig. 3).
FIG. 3.
PKR is an intermediary component in TLR signaling. PKR is implicated in the LPS/TLR4-mediated pathway probably recruited by the TIRAP complex. PKR is also involved during the dsRNA/TLR3 pathway, recruited by a TAK1-containing complex. Several proteins act downstream from PKR, such as TRAF6, which has been identified downstream of PKR in the signaling cascades triggered by TLR3 and TLR4. Moreover, TRAF3 is also involved in PKR downstream events during the activation of TLR3. The activation of these pathways in turn triggers the induction of proinflammatory cytokines. RIGI/MDA5 in response to dsRNA signaling is indicated.
In nonstressed cells, PKR is in a monomeric latent state due to the autoinhibitory effect of its dsRBDs, which occlude the KD and regulate activation of the kinase (Fig. 1). As mentioned in the introduction, PKR is activated in response to dsRNA of cellular, viral, or synthetic (such as pIC) origin. The PKR can also be activated by polyanions such as heparin, dextran sulfate, chondroitin sulfate, and poly-l-glutamine (157). The different dsRNA molecules are recognized and bound by PKR through the two N-terminal dsRNA-binding motifs, resulting in PKR activation and autophosphorylation (42). The PKR dsRBDs consist of two motifs of 70 amino acids each, connected by a short, 20-amino-acid linker. These motifs are also found in other dsRNA-binding proteins, such as Staufen or RNase III, and appear to constitute an universal motif for dsRNA recognition (309). The structure of the PKR dsRNA-binding domain was determined by nuclear magnetic resonance; it consists of two identical α-β-β-α folds, whereas the 20-amino-acid linker is entirely in a random-coil conformation (258, 259). This allows the dsRBD to wrap around the dsRNA molecule for optimal protein-RNA interactions, and it offers a satisfactory explanation for length requirements for dsRNA molecules to be effective PKR activators. dsRNA molecules shorter than 30 base pairs thus fail to bind or activate PKR (238). This explains why generally PKR is not activated by short interfering RNAs (siRNAs) of 19 to 29 nucleotides in length. However, experiments have to be carried out with caution, as PKR activation upon siRNA treatment has been reported as a potential nonspecific effect under certain circumstances (312, 329). PKR is activated by dsRNA of greater than 30 bp, but optimal PKR activation is achieved with dsRNA of 80 bp or longer, with no sequence requirements. This indicates that dsRBDs have intrinsic affinity for the A conformation of dsRNA and explains why PKR can be activated by dsRNAs of diverse origins.
Most natural dsRNA activators of PKR are synthesized in virus-infected cells as by-products of viral replication or transcription. For RNA viruses, dsRNA replicative forms are obligatory intermediates for the synthesis of new genomic RNA copies. Complex DNA viruses such as vaccinia virus (VV), adenovirus, or herpes simplex virus (HSV) have ORFs in opposite orientation; they produce overlapping mRNA transcripts that can fold to form dsRNA stretches responsible for PKR activation in infected cells (169, 210, 240). One of the most striking enzymatic properties of PKR is that high dsRNA concentrations inhibit enzyme activity (238, 365). Some endogenous molecules reported to be PKR inhibitors, such as Alu RNA, can thus efficiently activate the kinase in vitro at lower concentrations (57). More-specific structures, such as a pseudoknot present at the 5′ end of IFN-γ mRNA, are reported to activate PKR, although the role of this interaction in the control of IFN-γ synthesis remains to be analyzed (18).
After binding dsRNA, PKR undergoes a number of conformational changes that relieve the autoinhibitory interactions of the enzyme and allow subsequent substrate recognition. Biochemical and genetic data have underscored the importance of homodimerization in PKR activation (80). Thus, replacement of the dsRBD with an unrelated domain that is able to dimerize, such as glutathione S-transferase, constitutively activates PKR, both in vitro and in vivo (359). Recent crystallographic data show a dimer interphase on the N-terminal lobe of the KD, so that PKR monomers associate in a back-to-back conformation in which the R262-D266 salt bridge and the Y293-D289-Y323H bond triad are involved in interlobe contacts (80). After homodimerization, PKR undergoes rapid autophosphorylation in a stretch of amino acids termed the activation segment. Among others, residues Thr446 and Thr451 in this segment are consistently phosphorylated during activation (80, 353, 399). This further stabilizes PKR dimerization, which in turn increases the catalytic activity of the kinase. In contrast to the case for receptor tyrosine kinases, the paradigm archetype of kinase activation, autophosphorylation of PKR monomers appears to occur in cis or through the action of one PKR dimer on another PKR dimer or monomer (61, 80).
Whether of viral origin or pIC, dsRNA thus not only induces effects on translation but also influences various signal transduction pathways that affect different transcriptional activities. As such, PKR mediates the dsRNA-induced transcription of many genes (84, 134, 190).
PKR Activation by TLRs
The TLR family consists of more than 10 members, which have key roles in activating the innate immune response (343). TLR family members recognize different microbial products including lipopolysaccharide (LPS), CpG motifs characteristic of bacterial DNA, dsRNA, and peptidoglycans. The TLRs function through four TIR domain adapters (MyD88, TIRAP, TRIF, and TRAM) (24a). Subsequently, tumor necrosis factor (TNF) receptor-associated factor (TRAF) family proteins (TRAF3 or TRAF6) are engaged (128, 138, 266), and eventually signal transduction cascades that turn on JNK, p38, IRF-3, and/or NF-κB are activated. As a result, cytokines such as type I IFN or interleukin-10 (IL-10) are secreted (343), leading to boosting of antiviral activities.
Studies of PKR-deficient mice and cells derived from these animals showed impaired responses to different TLR ligands and reduced production of proinflammatory cytokines in response to LPS, suggesting that PKR is an intermediary in TLR signaling (127). PKR interacts with TIRAP and is phosphorylated in LPS-stimulated wild-type (wt) macrophages, suggesting that PKR is a component of the TLR4 signaling pathway (155).
In addition, PKR is phosphorylated and activated in response to CpG (155). CpG engages a different TLR family member, TLR9, which uses a different adapter molecule, MyD88 (155). PKR is also engaged in dsRNA-activated TLR3 signaling, although it does not interact directly with TLR3; it is recruited by a TAK1-containing complex in response to dsRNA binding to the TLR3 receptor (174). PKR is therefore a common component, integrating at least three TLR family members (Fig. 4).
FIG. 4.
PKR acts as an activator on the signaling cascades involved during stress-activated protein kinases (MAPK) action. PKR is located upstream of MKK3 or MKK6 and MKK4 during the activation of JNK and p38 in response to several cytokines, such as IL-1 and TNF-α, and other components, such as LPS and dsRNA. Inflammatory transcription factors such as NF-κB, ATF-2, and STAT1 are finally activated.
PKR Activation by Growth Receptors and Cytokines
PKR signals downstream of various growth factors and cytokines, such as IFN, platelet-derived growth factor (PDGF), TNF-α, and IL-1. The interferons are known transcriptional inducers of PKR, and type I IFN (IFN-α and IFN-β) are better PKR inducers than IFN-γ (212). As mentioned above, however, transcription of human IFN-γ mRNA is in turn self-regulated through a pseudoknot that results in local PKR activation (18). In addition to its direct transcriptional activation by IFN-γ, PKR mediates the IFN-γ-triggered NF-κB activation and c-myc expression that is STAT1 independent (72, 290). Another context in which PKR has a role downstream of IFN-γ signaling is in preneural cells, where it synergizes with TNF-α to activate NF-κB by affecting IκBβ stability (54). Overall, PKR function as a downstream mediator of IFN-γ signaling is revealed clearly by the reduced antiviral response observed in PKR−/− mice treated with IFN-γ (390).
Analysis of TNF-α signaling in cells with altered PKR levels (using antisense strategies to suppress PKR expression or in PKR−/− mouse embryo fibroblasts [MEF]) showed a slight decrease in NF-κB activation in the absence of PKR (202, 239). It is noteworthy that, in contrast to the profound defects observed in response to pIC, ablation of PKR results in slight although sustained and reproducible defects in NF-κB activation by TNF-α.
PKR has also been implicated in the signal cascades initiated by several growth factors. PKR is phosphorylated in response to PDGF, activating expression of immediate-early genes such as c-fos (73, 254). Moreover, transcriptional induction of c-fos is impaired in PDGF-treated PKR-null MEF compared to wt MEF (73). Thus, it seems that once activated by PDGF, PKR plays a critical role in mediating phosphorylation of STAT3 on its Ser727 residue (73). This phosphorylation is extracellular signal-regulated kinase-1/2 (ERK1/2) dependent, as it can be inhibited by treatment with a specific chemical inhibitor. Overall, PKR is a downstream mediator of PDGF action, integrating both growth-promoting and growth-inhibitory signals.
PKR also mediates IL-1 signaling, although the evidence suggests that it does so in a cell type-specific manner. For example, PKR−/− MEF show a defect in p38 activation in response to IL-1 (127). In contrast, IL-1 treatment does not activate PKR in endothelial cells (265). PKR also regulates the transcription of cytokines such as IL-1α, IL-1β, and TNF-α. Therefore, PKR is an important mediator for regulating the coordinated induction of cytokine responses. Further investigation is needed to assign roles to different cytokines in PKR activation.
PKR Activation in Response to Cell Stress
A range of cellular stresses, such as arsenite, thapsigargin, and H2O2, can activate PKR, and the second messenger ceramide can promote PKR activation (165, 302). Activation of PKR by all of these stimuli, as well as by others such as IL-3 deprivation, is PKR-associated activator (PACT)/RAX dependent. PACT and its mouse orthologue RAX are cell proteins that bind to and activate PKR independently of dsRNA or other molecules (165, 279). RAX levels do not vary during cell stress, but RAX is phosphorylated, after which it binds to and activates PKR (20). PACT/RAX therefore acts a physiological mediator that links a wide range of different cell stresses to PKR.
REGULATION OF SIGNAL TRANSDUCTION BY PKR
PKR was initially identified because of its ability to regulate translation in response to dsRNA. As mentioned above, however, several signal transduction pathways are affected by PKR. Here we outline different pathways that PKR regulates to modulate transcription.
IRF-1
The IRF family of transcription factors is composed of nine proteins that are key regulators of the innate immune response (352). IRF-1 is a tumor suppressor, as suggested by gene deletions observed in leukemia patients (379) and from data derived from mouse models (352). In addition, ectopic expression of IRF-1 inhibits cell growth. It was suggested that PKR can mediate the growth-inhibitory activities of IRF-1 (197). The relationship between PKR and IRF-1 is probably more complex. IRF-1 is proposed to mediate PKR-triggered apoptosis induction (77), as the activation of IRF-1 in response to IFN-γ or pIC treatment is defective in PKR−/− mice (202).
STAT
The STAT (signal transducers and activators of transcription) proteins mediate a number of biological functions and form part of the signaling cascades triggered by IFN and other cytokines (62). One PKR mechanism for controlling IFN and dsRNA signaling pathways is the modulation of STAT function. PKR associates with STAT1 in mouse and human cells; this association is not a kinase-substrate interaction, since the STAT1 phosphorylation status is unaffected by PKR binding (381). In addition, PKR-STAT1 complex formation is not dependent on PKR catalytic activity but requires the PKR dsRNA-binding domain. It has been suggested that PKR-STAT1 interaction results in inhibition of STAT1 DNA binding activity. PKR−/− cells are defective in STAT1 phosphorylation on Ser727, however, resulting in a fourfold decrease in STAT1-dependent transactivation (290). Consistent with the observation that PKR does not phosphorylate STAT1 directly, the role of PKR seems to be to control a kinase cascade in which ERK2 is the kinase phosphorylating STAT1 (290). STAT1 is also a target for PKR-mediated activation in response to LPS in glial cells (213). PKR also associates with STAT3, and it is required for full STAT3 activation in response to PDGF (73). STAT3 phosphorylation on Tyr and Ser residues, which is necessary for full activation, is PKR dependent. As proposed for STAT1, PKR regulates the ERK activation ultimately involved in STAT3 phosphorylation (73).
Tumor Suppressor p53
The tumor suppressor p53 is central for sensing genotoxic stress; once activated, it mounts a transcriptional response that results in cell cycle arrest or apoptosis, depending on the cellular context. PKR can influence apoptosis in U937 cells in response to TNF-α, correlating with the ability of PKR to induce p53 (392). In U937 cells overexpressing PKR, inhibition of p53 expression by antisense techniques prevents TNF-α-induced apoptosis, and p53 overexpression confers susceptibility to apoptosis. Another study showed that PKR could interact directly with the C-terminal part of p53 and phosphorylate p53 on the Ser392 residue (48). The ability of p53 to cause cell cycle arrest and regulate transcription of target genes is impaired in PKR−/− MEF. In these cells, a minor induction of mdm2 and p21 transcripts correlates with defective phosphorylation of Ser18 in p53 (the equivalent to Ser15 in human p53). Experiments using PKR−/− MEF also hint at a role for PKR in modulating p53 function in response to adriamycin or gamma irradiation. A role for phosphatidylinositol 3-kinase in this process is suggested by studies with chemical inhibitors that diminished Ser18 phosphorylation on p53 (48). Further studies are needed to clarify the link between PKR and p53.
MAPK Activation by PKR
Mitogen-activated protein kinases (MAPK) are evolutionarily conserved serine/threonine kinases that regulate many cell events. Mammalian MAPK are classified in several families, including ERK, p38, and JNK. These MAPK are activated by specific MAPK kinases (MAPKK): ERK by MEK1 and MEK2, p38 by MKK3 and MKK6, and SAPK/JNK by MKK4 and MKK7. These MAPKK are in turn activated by various MAPKK kinases (MAPKKK), including Raf, MLK, MEKK1, TAK1, and ASK1 (100).
JNK is expressed ubiquitously and can be activated by many types of stress, such as UV and gamma irradiation, protein synthesis inhibitors (anisomycin), hyperosmolarity, toxins, ischemia/reperfusion injury, heat shock, chemotherapeutic drugs, ceramide, T-cell receptor stimulation, peroxide, and inflammatory cytokines such as TNF. p38, on the other hand, is activated in response to cytokines such as IFN-γ, IL-1, or TNF-α or cell stress such as UV irradiation, osmotic shock, heat shock, LPS, and others (207).
PKR is an activator for signaling cascades involving stress-activated protein kinases and is described as mediating JNK and p38 activation in response to specific stimuli (58, 127). For full activation in response to LPS or cytokines such as IFN-γ, IL-1, or TNF-α, both p38 and JNK are dependent on PKR (127) (Fig. 4). A study using a chemical inhibitor of p38 MAPK (SB203580) showed that p38 MAPK has a critical role in IFN signaling. p38 MAPK is needed for activation of phospholipase A2 and for phosphorylation of Ser727 in STAT1 (98) (Fig. 4). Using MEF derived from PKR-null mice, it was shown that PKR is required for p38 MAPK activation in response to dsRNA, LPS, and proinflammatory cytokines but not in response to other forms of stress. p38 MAPK is integrated in a signaling cascade and is activated directly by phosphorylation by MKK3 or MKK6 (289), whereas JNK is downstream of MKK4. Experiments using PKR−/− cells showed that PKR is upstream of MKK3 or MKK6 and MKK4 (Fig. 4). The requirement for PKR in p38 activation is maintained in immortalized cell lines, in contrast to the case for JNK, which can be activated independently of PKR in immortalized cells (58, 127).
An interesting insight into the role of PKR in p38 activation came from analysis of the specific requirements in response to different stimuli. The catalytic function of PKR is required in response to LPS and dsRNA, but in response to TNF-α, PKR seems to have a merely structural role, as a noncatalytic mutant (K296R) mimics the effect of PKR in p38 signaling. As p38 MAPK is a master regulator that controls several transcription factors, such as NF-κB, ATF-2, and STAT1, these transcriptional pathways are also influenced by PKR. At least to a certain extent, p38 MAPK is thus central in PKR control of these factors and in response to certain specific stimuli. Finally, although solid evidence has yet to be produced, PKR might also activate ERK, as suggested by its role in mediating STAT1 and STAT3 phosphorylation in residues located in ERK consensus sequences (73).
ATF-3
ATF-3, a 181-amino-acid protein, is a member of the ATF/CREB family of transcription factors, which are expressed at low levels in quiescent cells (140). Although ATF-3 induction is associated with cell damage (139, 140), the physiological relevance of ATF-3 induction by stress signals is not understood. The role of ATF-3 in p53-dependent apoptosis (189, 398), and cell fate (147, 260, 346) remains controversial. ATF-3 induces apoptosis following curcumin treatment and participates in stress-induced β cell apoptosis (147, 388). ATF-3 acts as a sensor that interacts with and activates p53 under various types of stress by blocking its ubiquitination (389). Furthermore, ATF-3 overexpression enhances caspase 3 activity (340). Overexpression of full-length ATF-3 protein in colorectal cancer has antitumorigenic properties, whereas an antisense RNA targeting ATF-3 has the opposite effect (27). Using microarray analysis of human cells infected with a VV recombinant expressing wt PKR or the catalytically inactive form of PKR-K296R under inducible conditions, ATF-3 was recently identified as a gene that is selectively upregulated by the active PKR enzyme (134). Activation of endogenous PKR with a VV mutant lacking the viral protein E3L (VVΔE3L) triggered an increase in ATF-3 expression that was not observed in PKR−/− cells. Since protein synthesis was severely reduced at 16 h after VV-PKR infection, the increase in ATF-3 protein levels is possible because its translation is not affected by eIF-2α phosphorylation due to the special 5′ untranslated region of the ATF-3 mRNA (134). ATF-3 can also be induced by PERK and GCN4 (173), and is involved in PKR-induced apoptosis (134).
NF-κB
The NF-κB family of transcription factors controls the expression of genes involved in immune and inflammatory responses, cell differentiation, and apoptosis, among others (115). The human family includes NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB, c-Rel, and the proteins p100 and p105. The key mechanism that regulates NF-κB activation is its cytoplasmic retention mediated by interaction with inhibitory molecules of the IκB family (364). IκB proteins are phosphorylated by the IKK complex at two close serine residues in response to a variety of stimuli, which tags them for ubiquitin-proteasome-mediated degradation (297). This event allows NF-κB translocation to the nucleus, where it regulates transcription (115). The IKK complex contains a structural protein termed IKKγ or NEMO and two kinase subunits, IKKα and IKKβ (114). Another pathway that regulates NF-κB activation involves NIK phosphorylation of a complex containing IKKα, which phosphorylates p100, which once processed can activate p52/RelB target genes (286).
The first clues suggesting a role for PKR in NF-κB activation arose from observations that dsRNA could induce NF-κB activity in different cell lines (365). Subsequent experiments using the kinase inhibitor 2-aminopurine suggested a role for PKR in this process. Additional evidence came from analysis of NF-κB activation following dsRNA treatment in cells lacking PKR expression. When PKR expression was downregulated using 2-5A antisense oligonucleotides, diminished NF-κB activation was observed in response to dsRNA, with no significant change in the response to TNF-α (239). Similarly, experiments performed with PKR−/− MEF showed that NF-κB activation was impaired in response to pIC treatment (390). As a consequence of the NF-κB activation impairment, PKR−/− MEF show defects in IFN production compared with wt MEF (390). Finally, experiments performed with human cells and VV recombinants expressing PKR in an inducible manner showed the ability of PKR to activate NF-κB in the context of viral infection (116, 117). Because the NF-κB pathway plays a pivotal role in the regulation of cell growth, next we discuss mechanistic aspects of NF-κB when is activated by PKR.
MECHANISM OF NF-κΒ ACTIVATION BY PKR
Initial reports indicated that PKR was the protein kinase that phosphorylated IκBα directly in response to dsRNA, based on in vivo and in vitro evidence (201). Although PKR appears to be necessary for transducing this signal (202, 239, 390), later evidence pointed to an indirect role for PKR in IκB phosphorylation. Mutant cells lacking IKKγ were unable to induce NF-κB in response to pIC treatment (387). Leaman et al. isolated a mutant cell line defective in dsRNA-induced NF-κB activation, in which PKR activation was normal and NF-κB could be activated in response to other stimuli (212).
PKR Activates the IKK Complex
Analysis of NF-κB activation in response to PKR showed that IκBα phosphorylation on serines 32 and 36 precedes its degradation and translocation of NF-κB to the nucleus (116, 117). As phosphorylation on these residues is the hallmark of IKK kinase activity, a role for the IKK complex in the process seemed obvious. The effect of IKK on PKR activation of NF-κB, induced either by dsRNA or by infection with vesicular stomatitis virus (VSV), was confirmed independently by several studies using MEF lacking expression of different IKK subunits or dominant negative versions of the IKK proteins (26, 58, 116, 397). Although NIK was initially proposed to be downstream of PKR in the IKK activation process (397), the participation of NIK in this pathway seems dubious by virtue of current knowledge of IKK signaling.
Although the evidence is consistent with a role for PKR in activating IKK in response to dsRNA, the nature of the PKR effect is unclear. PKR interacts physically with the IKK complex in a way similar to that observed for other kinases upstream of IKK (26, 116, 397). Association with the IKK complex seems to involve the PKR catalytic domain, as suggested by mutational analysis (124). Whether this interaction is direct or indirect nonetheless remains to be adequately defined.
It needs to be determined whether PKR catalytic activity is required for dsRNA activation of the IKK complex or whether PKR is merely a structural component in this process. Catalytically inactive PKR mutants (K296R) can be coimmunoprecipitated with IKK (124). Experiments with NIH 3T3 cells suggested that a catalytically inactive PKR mutant is a poor IKK activator, but when PKR is expressed at high levels it can activate IKK efficiently (58). Purified PKR, either wt or mutant K296R, activates the recombinant IKKβ protein, suggesting that PKR catalytic activity is not needed in the process. These results support the hypothesis that protein-protein interaction, but not PKR catalytic activity, is needed to activate IKK.
In contrast, experiments using PKR−/− cells suggested that PKR catalytic activity is needed for IKK activation. Complementation of PKR−/− MEF with wt PKR, but not with a catalytically inactive mutant, restored appropriate NF-κB (and IRF-1) activation (202). Similar results were obtained upon expression of a battery of PKR mutants in PKR−/− cells by using VV recombinants (124). In this setting, a direct relationship was clearly drawn between the catalytic ability of the PKR mutants and NF-κB activation (124). Based on the experiments described above, it is reasonable to consider that under normal circumstances, PKR catalytic activity is necessary to signal dsRNA-dependent activation of IKK. It is noteworthy that results that indicate only a protein-protein interaction between PKR and IKK to be necessary for activating the IKK complex were obtained under experimental conditions in which endogenous wt PKR was still present; this could confuse interpretation of the findings, as both endogenous wt PKR and the overexpressed exogenous mutants would form heterotypic complexes in association with IKK. In any event, the question as to whether PKR catalytic activity is needed for activating IKK clearly remains to be settled.
TRAFs Link PKR to IKK Activation
Several pathway-specific adapter proteins, such as members of the TRAF family, MyD88, TIRAP, and TRIF, act as mediators that link different pathways with IKK activation (4, 155, 159, 266, 374). TRAF proteins have emerged as key signal transducers, not only downstream of TNF receptors but also in other pathways (59). There are two putative TRAF-interacting motifs in the PKR sequence, and the viability of the PKR/TRAF interaction was suggested by bioinformatic analysis and confirmed in vivo (122). The interaction between PKR and TRAF2 or TRAF5 was shown to be dependent on PKR dimerization and is functionally relevant, as demonstrated in cells genetically deficient in TRAF2 and TRAF5 or after expression of TRAF dominant negative molecules. TRAF family proteins are suggested to act downstream of PKR and signal towards the activation of NF-κB (122).
The PKR/TRAF relationship does not end here, and it probably has a role in linking PKR with other signaling pathways, such as some TLR-related pathways. A complex containing TRAF6 has been identified downstream of PKR in the signaling cascades triggered by TLR3 and TLR4 (155, 174) (Fig. 3). It was recently reported that TRAF3 is required to activate NF-κB and to produce type I IFN downstream of TLR3, -4, -7, and -9 (138, 266). In addition, TRAF3 is involved in TLR-independent antiviral responses and associates physically with PKR (266). TRAF3 is also involved in PKR-dependent type I IFN induction, a process that requires NF-κB activation as shown by the fact that TRAF3−/− MEF do not produce type I IFN in response to VSV infection (266) (Fig. 3). A putative complex involving TRAF3 downstream of PKR would therefore be a convergence point in the regulation of NF-κB activation and type I IFN production and is consistent with the proposed model of a functional relationship between TRAF and PKR.
PKR-Independent NF-κB Activation by dsRNA
At least two distinct pathways link dsRNA with NF-κB activation. dsRNA is generated as a step or a by-product during viral replication and hence can be used as a perfect reporter for cells to detect viral infections. Different cell signaling pathways are activated in response to dsRNA, which eventually result in the triggering of a robust antiviral response through the production of type I IFN. At the molecular level, IFN production is boosted through the activation of several transcription factors, such as IRF-3, IRF-7, or NF-κB (167).
We have already discussed the prominent role of PKR in dsRNA-dependent NF-κB activation. The impaired NF-κB activation in response to viral infection or pIC observed in PKR−/− MEF exemplifies the effect of PKR (202). Although NF-κB activation was largely suppressed, some NF-κB activity can still be triggered by dsRNA in the absence of PKR. In addition, work with PKR−/− mice showed that they remain susceptible to VSV infection, depending on the route of virus inoculation. These observations suggest that there could be cell-specific, PKR-independent mechanisms of NF-κB activation and of IFN production (7, 58).
The search for a dsRNA receptor that senses viral infection independently of PKR resulted in the identification of one member of the TLR family, TLR3 (3). TLR3−/− mice show impaired responses to dsRNA and pIC, whereas TLR3 expression conferred dsRNA responsiveness in dsRNA-insensitive 293 cells. The activation of NF-κB triggered by dsRNA and mediated by TLR3 is PKR independent (3). Given the tissue distribution of TLR3 (179), the presence of additional pathways to activate NF-κB and produce IFN in response to dsRNA, like the recently discovered RIGI and MDA5 (181, 393), cannot be excluded.
IDENTIFICATION OF NOVEL GENES INDUCED IN RESPONSE TO PKR
As described in the preceding section, PKR is involved in signaling various pathways that activate and engage a number of transcription factors. Since these transcription factors regulate the expression of many cellular genes, it is anticipated that PKR controls the expression of multiple genes. In the following section, we describe the as-yet-limited efforts to uncover the gene expression profile associated with PKR activation in uninfected and virus-infected cell systems by using microarrays. Similar approaches have led to identification of hundreds of genes whose expression is regulated in response to IFN (76, 329), such as genes regulated by the 2′-5′ oligoadenylate synthetase, the enzyme that activates the RNase L protein through the production of 2′-5′-linked oligoadenylates in response to dsRNA (237).
Genes Induced by PKR in the Absence of Viral Infection
A global approach to identifying cellular genes induced by PKR expression was first taken by Donze et al., using murine cells expressing PKR in a tetracycline-inducible manner (84). This effort identified NF-κB-dependent genes induced by PKR. Interestingly, they also showed distinct PKR-mediated signaling. Upregulation of survival genes such as those for c-IAP1, c-IAP2, and A20, which are NF-κB dependent, occurs soon after PKR activation, but cells ultimately die by PKR-triggered apoptosis, concomitant with upregulation of other mRNAs such as those of GADD34/MYD116 and GADD153/CHOP, whose induction is dependent on eIF-2α phosphorylation. Similar studies by Ung et al., who used human cells expressing a Gyrb-PKR fusion that dimerizes and is activated following coumermycin addition (359), identified 22 induced genes, some of which encode proteins involved in apoptosis, such as GADD45A, GADD45B, BIK, and IRF-1, highlighting the importance of transcriptional regulation in PKR-induced apoptosis.
Genes Induced by PKR during Viral Infection
The studies described above investigated the transcriptional profile associated with PKR activation in the absence of viral infection. To define the cell transcriptional response after PKR expression in virus-infected cells, IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible VV recombinants expressing wild-type PKR (VV-PKR) or the Lys296Arg mutant PKR (VV-PKR-K296R) were used (134). Of the 15,000 human genes analyzed, 111 showed changes specifically dependent on PKR catalytic activity; of these, 97 genes were upregulated and 14 downregulated. Nine of the genes activated encode cytoskeletal proteins involved in cell adhesion, 3 have a role in immune modulation, and 19 have metabolic and signaling functions. In addition, 21 of the upregulated genes are implicated in transcription and 11 in translation functions. PKR upregulates the expression of two proteasome subunits, PSMD8 and PSMA7, both of which are involved in antigen peptide production. PKR also induces major histocompatibility complex class I (human leukocyte antigen subtypes) and class II molecules, such as the coactivator of the immune complex, β2-macroglobulin. This protein coordinates the activation of immune effector cells and is important for a robust, long-lasting immune response against infectious agents (221).
Although Hsp70 is known to be induced following VV infection (175, 313), after VV-PKR infection the microarray data showed downregulation of several heat shock protein family genes, including those for Hsp10, Hsp40, and Hsp70. Hsp70 is an antiapoptotic chaperone protein (251) that inhibits mitochondrial release of cytochrome c and blocks procaspase 9 recruitment to the apoptosome complex (16). These results suggest that PKR might induce apoptosis by upregulating apoptotic genes and downregulating antiapoptotic genes (133). The identification of nine upregulated genes with roles in apoptosis concurs with a role for PKR in the apoptotic response to viral infection. One of these genes is that for ATF-3, as we described above. These findings provide new insights into specific mechanisms used by PKR and indicate that during infection PKR produces transcriptional alteration in genes of various pathways, coordinating PKR-regulated apoptotic functions.
MODULATION OF PKR ACTION BY CELL AND VIRAL PROTEINS
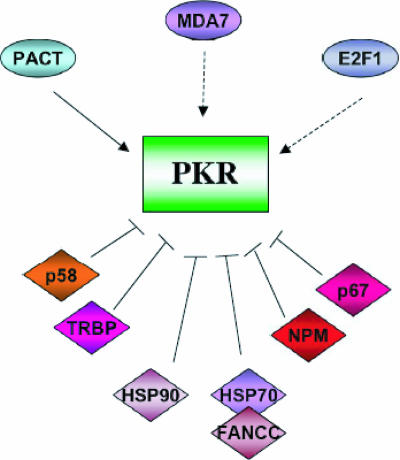
The biological importance of PKR function is further indicated by the existence of a multitude of cellular and viral regulators of PKR action (Fig. 5; Table 1).
FIG. 5.
PKR is modulated by a number of cellular proteins. The PKR activator PACT, the melanoma differentiation factor MDA7, and the transcription factor E2F-1 are the only known proteins to induce PKR activation, although PACT is the best-characterized PKR activator. However, numerous cellular inhibitors of PKR have been described: p58IPK (the first cellular inhibitor reported) and the RNA-binding protein TRBP are both associated with influenza virus and HIV-1 infection. Other inhibitors of PKR action are the heat shock Hsp90/Hsp70 proteins, NPM, and the glycoprotein p67.
TABLE 1.
Viral products that inhibit PKR activation and/or eIF2α phosphorylation
| Viral product(s) (virus) | Mechanism of action |
|---|---|
| γ134.5 (HSV) | eIF2α dephosphorylation |
| Us11 (HSV) | dsRNA sequestration, direct interaction with PKR and PACT |
| vIRF-2 (KSHV) | Direct interaction with PKR |
| LANA2 (KSHV) | Inhibition of eIF2α phosphorylation |
| EBER RNA (EBV) | PKR pseudoactivator |
| SM (EBV) | dsRNA sequestration, direct interaction with PKR |
| E3L (VV) | dsRNA sequestration, direct interaction with PKR |
| K3L (VV) | PKR pseudosubstrate, direct interaction with PKR |
| NS1 (influenza virus) | dsRNA sequestration, direct interaction with PKR |
| Tat (HIV-1) | PKR pseudosubstrate, direct interaction with PKR |
| NS5A (HCV) | Direct interaction with PKR |
| E2 (HCV) | PKR pseudosubstrate, direct interaction with PKR, |
| σ3, σ4 (reovirus) | dsRNA sequestration |
| NSP3 (rotavirus) | dsRNA sequestration |
| VAI RNAs (adenovirus) | PKR pseudoactivator |
| TRS1, IRS1 (human | |
| cytomegalovirus) | dsRNA sequestration |
Modulation by Cell Proteins
We next describe the characteristics of the cellular inhibitors of PKR that have been identified thus far, with the view that new molecules will emerge in the coming years.
p58IPK.
p58IPK is a member of the tetratricopeptide repeat family and was the first reported cellular inhibitor of PKR (218-220). p58IPK interacts directly with PKR and inhibits its kinase activity by preventing dimerization. Influenza virus partially evades the host antiviral response by recruiting p58IPK to repress PKR-mediated eIF-2α phosphorylation (219, 220). In the absence of viral infection, p58IPK overexpression results in malignant transformation (14). Although the exact mechanism has not been defined, it has been suggested that p58IPK transforms cells by interfering with PKR-regulated pathways. PKR inhibition by p58IPK can stimulate cell growth by disrupting PKR-dependent control of mRNA translation and by blocking PKR-dependent apoptosis (102).
TRBP.
The trans-activation response (TAR) RNA-binding protein (TRBP) is a cellular RNA-binding protein isolated by its ability to bind human immunodeficiency virus type 1 (HIV-1) TAR RNA (110, 111). Proposed TRBP functions include inhibition of PKR activation, regulation of cell proliferation, PKR-independent translational activation, modulation of HIV-1 gene expression through its association with TAR, and the control of mRNA translation. (9, 19, 86, 274, 327). TRBP facilitates VV protein synthesis in cells infected with a virus mutant lacking the E3L gene (274). Like TRBP, PKR also binds TAR RNA. This RNA activates and inhibits PKR at low and high concentrations, respectively. However, why TAR RNA activates PKR in some studies (35, 88, 193, 318) but not in others (135, 136) remains unexplained. These discrepancies are probably related to the purity of the TAR RNA preparations. TRBP is thought to inhibit PKR function by competing for common RNA substrates. In addition, TRBP and PKR form a complex through direct protein-protein interaction through their dsRBDs (60), preventing PKR activation. Recent data indicate that low TRBP levels support an innate HIV-1 resistance in astrocytes by enhancing the PKR antiviral response, which suggests a major role for TRBP in viral expression (268). Like that of the p58 PKR inhibitor, TRBP overexpression results in malignant transformation (19). Interestingly, TRBP is described as a dsRBD protein partner of human Dicer, which is required for optimal RNA silencing mediated by siRNA and endogenous micro RNA (137).
Glycoprotein p67.
The glycoprotein p67 was identified first as a component that copurified with eIF-2 fractions (63) and later as a methionine aminopeptidase 2 (358); recent studies with mammalian cells demonstrate a link between these two properties (65, 68). p67 inhibits eIF-2α phosphorylation from its kinases HRI, PERK, GCN2, and PKR, although the molecular mechanisms involved are still unclear (66). p67 has several O-linked N-acetyl-β-d-glucosamine (GlcNAc) residues that are critical for its inhibition of eIF-2α phosphorylation (67). There is correlative evidence that p67 has a role in control of protein synthesis (52). p67 expression rescues BSC-40 cells from the antiviral effects of PKR induction following VV infection, and the PKR-mediated translational block is specifically abrogated by p67. These effects correlate with p67 protection of eIF-2α phosphorylation and, in part, with inhibition of PKR-mediated activation of NF-κB, supporting the concept that p67 is an in vivo modifier of PKR activity (120).
NPM.
Nucleophosmin (NPM) (also known as B23) is an abundant and ubiquitously expressed nucleolar phosphoprotein implicated in ribosome biogenesis (163, 267, 382). It binds nucleic acids (87), has intrinsic RNase activity (310), and also acts as a molecular chaperone (339) shuttling between the nucleus and cytoplasm (24). NPM has also been implicated in the acute response to environmental stress and controls cell proliferation (44, 163, 382). NPM is frequently overexpressed in tumors of diverse origin (50, 262), and it is translocated in lymphomas and leukemias (96, 250). NPM interacts with PKR, inhibiting eIF-2α phosphorylation and PKR-mediated apoptosis (273). It was suggested that the capacity of NPM to inhibit PKR activation could explain how NPM promotes cell proliferation and suppresses the apoptosis pathway (273). The Arf/mdm2/p53 pathway was recently linked to PKR, since NPM was suggested to mediate the antiviral activity of the tumor suppressor ARF via PKR (108).
MDA7.
Melanoma differentiation-associated gene-7 (Mda7) is a tumor suppressor gene with limited homology to the pleiotropic homodimeric cytokine IL-10 (40, 97). Pataer et al. reported that MDA7 protein interacts physically with PKR, leading to the rapid induction of PKR and activation of its downstream targets, resulting in apoptosis induction in human lung cancer cells (276, 277). Overexpression of Mda7 with an adenoviral vector induces apoptosis in cancer cells, but not in normal cells, through activation of multiple signal transduction pathways (247, 275, 276, 306). Direct interaction between PKR and MDA7 may be important for PKR activation and apoptosis induction, probably through MDA7 phosphorylation or activation of other downstream targets.
Heat shock proteins Hsp90 and Hsp70.
Correct PKR folding depends on the chaperone 90 and its cochaperone p23, but subsequent binding of PKR to Hsp90 has an inhibitory effect on its activation (83). The anticancer drug geldanamycin, an inhibitor of Hsp90, disrupts the interaction of the PKR-Hsp90-p23 complex, allowing PKR activation. Similar to Hsp90, Hsp70 binds to PKR, inhibits PKR phosphorylation, and prevents apoptosis. In stressed cells, Hsp70 binds to the Fanconi's anemia complementation group C (FANCC) protein and forms a ternary complex with PKR. FANCC detaches from the PKR-Hsp70 complex and activates PKR (272). Hematopoietic cells with FANCC mutations or downregulated Hsp70 show constitutive PKR activation and sensitivity to various cell stress signals as well as to IFN therapy.
PACT/RAX.
As we have seen, the list of PKR inhibitors is long. The mouse protein RAX and its human orthologue PACT are nonetheless the only known cellular activators of PKR. PACT is a ubiquitously expressed protein (165, 279) that belongs to the family of dsRNA-binding proteins and has three dsRNA-binding domains. Domains 1 and 2, located at the N-terminal side of the protein, not only mediate dsRNA binding but also are involved in the direct interaction of PKR with the N-terminal domain (282). In contrast, PACT domain 3, located at its C terminus, does not bind dsRNA but binds weakly to the PKR kinase domain and activates it (229). Although in vitro analysis shows that domain 3 of PACT is sufficient to activate PKR, all three domains are needed for efficient PKR activation in vivo (282). As mentioned above, different stresses trigger PACT phosphorylation (20), and only then does PACT bind and activate PKR (165, 278, 282). PACT might thus be involved in PKR activation in uninfected cells. PACT also has a role in viral infection. Newcastle disease virus utilizes PACT as a host factor (166), and some viral proteins inhibit PACT, such as HSV type 1 (HSV-1) Us11 protein (283) and influenza virus protein NS1 (227). Recently, PACT was shown to form a complex with Dicer, among other proteins, raising the possibility that PACT has a role in RNA silencing (312).
Modulation of PKR Activation by Mammalian Viruses
Since the IFN-induced cellular antiviral response is the primary defense mechanism against virus infections, many viruses have developed a means to counteract the induction or effects of IFN (131). Viruses use a number of strategies to counteract dsRNA-dependent pathways and specifically to avoid the deleterious effects of the PKR and 2′-5′ oligoadenylate synthetase/RNase L system. There are viral proteins that interfere with these pathways at different levels, by inhibiting PKR activation, sequestering dsRNA, inhibiting PKR dimerization, synthesizing PKR pseudosubstrates, activating antagonist phosphatases, or degrading PKR. Viral PKR inhibitors of different virus families are described below and listed in Table 1.
These viral inhibitors are normally expressed from the onset of infection to maintain PKR inactive until the virus cycle is completed. Elimination of PKR inhibitors from these viruses generally has a severe impact on virus replication and pathogenesis. Viruses disarmed of PKR inhibitors usually replicate at lower levels than wild-type virus in normal cultured cells and show an attenuated phenotype in animals. These viruses can replicate more efficiently in several tumor cell lines. With defects in the IFN system, however, there is an added advantage for the use of viruses to destroy tumor cells. In some cases, such as in VV infection, elimination of the E3L gene alters virus tissue tropism in mice, indicating that the PKR-based antiviral defense can operate preferentially in specific cell types or tissues (210).
Herpesviruses. (i) HSV-1.
The HSV-1 γ34.5 protein is a critical determinant of in vivo virulence (56, 235, 360). In a strict sense, the γ34.5 protein is not a PKR inhibitor but inhibits PKR downstream effects. γ34.5 has homology to the cellular GADD34 protein (248) and blocks the PKR-mediated translational shutoff. γ34.5 recruits the cellular protein phosphatase 1 and forms a high-molecular-weight complex that dephosphorylates eIF-2α (150-152). Consistent with these findings, an HSV-1 mutant lacking γ34.5 is virulent in PKR knockout mice but not in wild-type mice (222). The premature cessation of protein synthesis observed in cells infected with the γ34.5 null mutant can be prevented by another HSV-1-encoded PKR inhibitor, the Us11 protein. Us11 is a dsRNA-binding (176, 191, 252) ribosome-associated protein of HSV-1 (299) that can interact directly with PKR (38, 287), preventing its activation in response to dsRNA and PACT (283).
(ii) Human herpesvirus 8 (KSHV).
The human herpesvirus 8 (Kaposi's sarcoma herpesvirus [KSHV]) genome encodes two proteins that interfere with PKR action, vIRF-2 and LANA2. The IRF-like protein vIRF-2 inhibits the antiviral effect of IFN and rescues translation of VSV mRNA. vIRF-2 interacts physically with PKR and inhibits its autophosphorylation, thus avoiding the phosphorylation of eIF-2α (33). LANA2 (previously known as vIRF-3) shows homology to cellular IRF-4 and KSHV vIRF-2 (295). LANA2 is a nuclear-cytoplasmic shuttling protein with a CRM1-dependent export signal (255). This protein functions as a dominant negative mutant of both IRF-3 and IRF-7 and inhibits virus-mediated transcriptional activity of the IFNA promoter (234). Moreover, LANA2 expression inhibits apoptosis and the PKR-mediated translational block. No interaction has been observed between LANA2 and PKR. LANA2 inhibits PKR-induced activation of caspase 3 but not of caspase 9, suggesting that the FADD/caspase 8 pathway is affected by LANA2 inhibition of PKR-induced apoptosis (93).
(iii) EBV.
Epstein-Barr virus (EBV) establishes a persistent infection and constitutively expresses several gene products, including EBER-1 and EBER-2 RNAs (321). Several recent studies have addressed the possible mechanisms of action of these nontranslated RNA species, and there is increasing evidence for the importance of EBER-1 as a player in the strategy by which EBV transforms cells (41). EBER-1 inhibits PKR in vitro protein kinase activity because it competes with dsRNA activators for binding to the enzyme (321). Consistent with this mode of action, EBER-1 expression protects against inhibition of protein synthesis, NF-κB activation, and IFN-induced apoptosis (208, 257). Comparison of EBER-positive and -negative viruses demonstrated that the small RNA contributes significantly to the efficiency of EBV growth transformation of B cells (385). Ribosomal protein L22 competes with PKR for binding to RNA, thereby halting the PKR inhibition by EBER-1 (89). As a result of this competition, L22 interferes with the ability of the small RNA to inhibit PKR activation by dsRNA, suggesting that L22 may exert a protective effect in vivo against the transforming potential of EBER-1 and EBV. Moreover, EBV codes for the SM protein, which is expressed early in the EBV lytic cycle and has homology to the dsRNA-binding domain of the HSV-1 Us11 protein. SM protein binds dsRNA and associates physically with PKR, preventing its activation (287).
Poxviruses. (i) VV.
In most cell lines tested, VV is relatively resistant to the antiviral effects of IFN (271). In addition, VV is able to rescue the replication of IFN-sensitive viruses such as VSV and encephalomyocarditis virus (EMCV) following coinfection (270). Earlier findings suggested that VV resistance to IFN was related to an interference between the virus and the IFN system (270, 271). Two virus-induced activities, an ATPase and a phosphatase, were proposed to act as inhibitors of IFN-induced enzymes, PKR and the 2-5A system; the role of ATPase I in VV resistance to interferon was documented with these mutants (81). Later studies showed a more complex situation, with the VV genome encoding secreted proteins that bind to receptors and ligands of cytokines and chemokines (2, 341), and at least two proteins, K3L and E3L, with the ability to inhibit intracellular IFN-induced pathways. The K3L protein is expressed early in VV infection. K3L protein binds directly to PKR in vitro (36, 46), and yeast two-hybrid interaction assays have localized the K3L protein-binding site to the C-terminal half of the PKR kinase domain (45, 105). Competition binding experiments and sequence homology between K3L and the N-terminal one-third of eIF-2α (72% similarity and 28% identity) suggest that PKR recognizes K3L and eIF-2α by a common mechanism (322). In this way, K3L protein inhibits autophosphorylation of PKR (36), blocking the subsequent inhibition of protein synthesis (36, 70, 187).
The role of the E3L gene as an inhibitor of apoptosis was first detected after infection of HeLa cells with an E3L deletion mutant of VV (215). The VV E3L gene encodes two proteins, p25 and p20 (373), expressed early in infection (395). E3L is a host range gene, necessary for efficient VV replication in several cell lines (15), and is required for VV pathogenesis (30). The E3L protein is a dsRNA-binding protein (69). The carboxy-terminal domain of E3L encodes the conserved motif that binds dsRNA. The N-terminal domain, required for neurovirulence (29), is suggested to be involved in the direct inhibition of PKR activation, nuclear localization, and Z-DNA binding (30, 196, 209, 300, 395). E3L inhibits activation of PKR, 2′,5′-oligoadenylate synthetase, and adenosine deaminase A-to-I among other dsRNA-dependent proteins, by binding to and sequestering dsRNA molecules (51, 230, 294, 326). E3L also inhibits PKR by direct interaction with PKR, leading to heterodimer formation (300, 320). Results obtained in our laboratory showed that E3L expression in NIH 3T3 cells conferred antiapoptotic and oncogenic properties (109). The role of E3L in inhibition of the IRF-3 and IRF-7 IFN transcription factors (330), the importance of the Z-DNA-binding domain in E3L activity as a viral transactivator (206), and E3L inhibition of PKR are all important determinants in the pleiotropic effects exerted by E3L.
(ii) MCV.
Unlike most poxviruses, molluscum contagiosum virus (MCV) lacks homologues of the E3L and K3L proteins. MCV nonetheless encodes another set of specific molecules, such as MC159L, that control host defenses. This protein is not a direct inhibitor of PKR, since it does not associate with PKR and cannot block PKR-induced phosphorylation of eIF-2α. MC159L expression nevertheless inhibits apoptosis triggered by PKR through death receptor-mediated pathways. In addition, MC159L expression inhibits NF-κB activation induced in response to PKR (123).
Orthomyxovirus: influenza virus.
A key role has been attributed to the viral NS1 protein in modulating expression of cell and viral proteins during influenza virus infection. The NS1 protein anti-IFN properties map to its dsRNA-binding domain, which is able to inhibit diverse dsRNA-activated antiviral pathways (345, 371). By this mechanism, the NS1 protein prevents IFN-α/β synthesis, as well as activation of the antiviral enzyme PKR (23, 148, 233). It was also suggested that NS1 can inhibit PKR in a dsRNA-independent manner, by forming complexes between the NS1 protein and PKR (348), although direct interaction of NS1 and PKR remains controversial (95). It was recently shown that, through its cellular activator PACT, NS1 can prevent PKR activation (227). NS1 and the cellular p58IPK protein (see above) are the proteins used by influenza virus to inhibit IFN and PKR action. It worthy noting, however, that the protein synthesis shutoff induced by influenza virus infection is independent of PKR activity (402).
Retrovirus: HIV-1.
HIV-1 encodes Tat, a highly conserved transcriptional transactivator, that is expressed early in the viral life cycle and is essential for viral replication and progression to disease (49, 177). Tat activates transcription from the HIV-1 long terminal repeat by binding to the TAR RNA. Productive HIV-1 infection results in a significant decrease in PKR levels (301). HIV-1 Tat protein acts as a substrate homologue of eIF-2α, preventing the phosphorylation of this factor and allowing protein synthesis and viral replication to proceed (28, 34, 243). In addition Tat interacts with and is phosphorylated by PKR, resulting in a tighter binding of Tat protein to TAR RNA, which regulates Tat-mediated transcriptional activity (90). It has also been suggested that Tat-mediated induction of IL-10 is associated with PKR activation (225). These findings indicate the intricate relationship between HIV-1 and PKR.
Flavivirus: HCV.
Previous studies identified the hepatitis C virus (HCV) core protein, E2, and NS5A as IFN antagonists and PKR inhibitors. The nonstructural HCV protein NS5A inhibits IFN antiviral activity by binding to PKR through the IFN sensitivity-determining region (ISDR) and its adjacent region, the PKR-binding region (106, 107, 349). Sensitivity to IFN therapy in HCV patients correlates with the ISDR sequence (91, 92, 311). The selective pressure evoked on HCV quasispecies during IFN therapy appears to vary among patients, however, and the ISDR locus per se does not function in a manner consistent with it having a major role in mediating IFN resistance (103). Moreover, recent studies suggest that initial IFN production may be active when HCV proteins, including NS5A, are expressed (39, 129, 315). The oncogenic potential of HCV has also been attributed to the ability of NS5A to bind PKR and inhibit its PKR (104, 113, 126, 261). An in vitro study showed that a 12-amino-acid sequence in the HCV-1b E2 gene is also able to interact with PKR and inhibit its activity through the sequence homology with the PKR autophosphorylation site and with the eIF-2α phosphorylation site (354). It is postulated that this motif, termed PePHD, is involved in the mechanisms of in vivo resistance to therapy, and its variability may account for cases of positive response. Clinical studies have nonetheless generated controversial data (112, 307, 308). The reasons for these discrepancies are difficult to explain, although differences among studies in patient selection and in viral analyses are considered possible explanations (349).
Reovirus.
Mammalian reoviruses express a dsRNA-binding protein, the S4-encoded major outer capsid σ3 protein, which competes with PKR for the dsRNA activator (22, 168, 394). The resistance conferred by the capsid σ3 protein is certainly incomplete, however, since mammalian reoviruses are not protected against high levels of PKR activity, as found in most untransformed cells and in IFN-treated cells. Avian reoviruses express the σA protein, which has much stronger dsRNA-binding affinity than σ3 and interferes efficiently with PKR function (130, 242), corresponding with lower IFN sensitivity of avian in comparison with mammalian reoviruses (242, 334, 373).
ROLE OF PKR IN APOPTOSIS INDUCTION
As we initially described for the VV system, activation of PKR triggers apoptosis (118, 215). Apoptosis is an intrinsic genetic program in multicellular organisms, which implements the ordered removal of damaged or unwanted cells during development and in adult life. Deregulation of the apoptotic process can lead to pathological conditions such as cancer, autoimmunity, and neurodegeneration (132, 347). Several proteins are implicated in triggering cell death in response to dsRNA and viral infections, among them the 2-5A system and PKR (38a, 399a).
PKR Mediates Apoptosis Induced by Different Stimuli
Induction of apoptosis is a common response to viral infection. Although it may represent an antiviral mechanism that acts by rapidly eliminating infected cells and preventing viral spreading, virus-induced apoptosis can also have important pathological implications. The first evidence that PKR was involved in apoptosis was obtained with HeLa cells by using a VV recombinant vector that expressed the enzyme under inducible conditions (215). The role of PKR in apoptosis was reinforced by studies carried out with 3T3 cells expressing a noncatalytic mutant PKR or using MEF derived from PKR−/− mice (77, 333).
Since then, it has been clearly demonstrated that PKR mediates the apoptosis induced by several viruses. In the case of poxviruses, dsRNA produced as a result of symmetrical transcription from late genes during VV infection is the most probable inducer of apoptosis (192). As mentioned above, however, several dsRNA-dependent pathways (the 2-5A system among them) can induce apoptosis in response to dsRNA or viral infection. PKR and the 2-5A system can induce apoptosis independently, as RNase L is able to induce apoptosis in PKR+/+ and PKR−/− cells (82). Although PKR expression induces RNase L activation through an unknown mechanism (294), it can also induces apoptosis in RNase L−/− cells. A role has also been suggested for PKR in influenza-triggered apoptosis. HeLa cells expressing dominant negative PKR mutants are more resistant to cell death during influenza virus infection (344). Influenza virus infection of HeLa cells causes upregulation of Fas transcription, resulting in apoptosis induction (370). NF-IL-6 appears to be involved in the upregulation of Fas mRNA. NF-IL-6 activity increases after influenza virus infection, and the Fas promoter has several NF-IL-6 sites (344). PKR also mediates the cell death provoked by infection with the picornavirus EMCV. U937 cells stably expressing an antisense mRNA targeting PKR are less susceptible to cell death caused by EMCV infection (391). PKR downregulation in cells transformed from highly cytolytic to persistent EMCV infection further indicates the importance of PKR in defining pathogenic outcome after viral infection (185).
PKR also regulates apoptosis induced in the absence of viral infection. PKR was shown to mediate the apoptosis observed during Alzheimer's disease (269), induced by oncogene products such as IRF-1 or E2F-1 (21, 269) or triggered in response to dsRNA, TNF-α, LPS, tunicamycin, serum starvation, or IL-3 withdrawal in hematopoietic cells. The role of PKR in apoptosis in the absence of viral infection was first noted using cells derived from PKR−/− mice. PKR-defective MEF are more resistant that wild-type MEF to LPS-, TNF-α-, or dsRNA-induced apoptosis (390). A role in TNF-α-induced apoptosis was first hypothesized as a result of experiments with U937 cells (392) and 3T3 cells expressing a dominant negative version of PKR (K296R) (333). IL-3 withdrawal from the IL-3-dependent NFS/N1.H7 cell line induces PKR autophosphorylation and eIF-2α phosphorylation and correlates with increased cell death by apoptosis (164), suggesting PKR involvement in apoptosis induced by growth factor withdrawal. Genetic screening for genes involved in endoplasmic reticulum stress-mediated apoptosis, using a randomized ribozyme library, also uncovered a role for PKR in tunicamycin-induced apoptosis and Alzheimer's disease (269). Analysis of autopsied brain tissues from Alzheimer's disease patients showed accumulation of activated PKR in nuclei. Many of the stimuli that trigger PKR-dependent apoptosis in the absence of viral infection rely on PACT/RAX activation; PACT/RAX mediates PKR activation and subsequent apoptosis in response not only to cytokines and serum withdrawal but also to chemotherapy, ethanol, and viral infection (20). Alterations in levels of any of the array of PKR viral (and cellular) inhibitors (see the next section) also have a profound impact on apoptosis induction (64, 351).
Role of PKR Effectors in Apoptosis Induction
Analysis of the role of PKR effectors in mediating cell death suggests an intricate pathway. To distinct degrees, at least eIF-2α, NF-κB, ATF-3, and p53 have been implicated in mediating PKR-induced apoptosis.
The alpha subunit of eIF-2, eIF-2α, is the best characterized PKR substrate. Initial evidence that PKR phosphorylation of eIF-2α is involved in apoptosis induction came from studies showing that PKR-mediated apoptosis can be inhibited by expressing an eIF-2α dominant negative mutant (eIF-2α 51A) (117, 333). In this context, expression of the initiation factor mutant partially blocked apoptosis induced by TNF-α and serum deprivation in NIH 3T3 cells (333). It was also shown that expression of an eIF-2α 51D mutant, in which the aspartic acid residue mimics a phosphorylated serine, causes apoptosis in COS cells. Using the VV expression system, we also showed that apoptosis induced by PKR expression is prevented by coexpression of an eIF-2α 51A mutant (117). Another study that took advantage of cell lines expressing PKR combined with microarray analysis reported that the eIF-2α phosphorylation by PKR is involved in cell death induction (84). These observations stress the role of eIF-2α phosphorylation in mediating PKR-induced apoptosis. Interestingly, cells lacking another eIF-2α kinase, PERK, show decreased eIF-2α phosphorylation but increased apoptosis in response to diverse stimuli (8a). The integration among transcriptional and translational regulatory pathways, and not only the eIF-2α phosphorylation state, thus ultimately dictates cell fate.
NF-κB activation by PKR is also involved in apoptosis induction. Using proteasome inhibitors that block IκBα degradation, or by coexpressing dominant negative forms of IκBα, both of which prevent PKR-induced NF-κB activation, we demonstrated that NF-κB activity is involved in PKR-triggered apoptosis (117) These observations can appear paradoxical, as NF-κB is often classified as a prosurvival factor that prevents apoptosis. There is considerable evidence for the context dependence of NF-κB as a pro- or antiapoptotic factor, related to the stimulus that triggers apoptosis. For example, NF-κB is a proapoptotic factor in response to viral infection; a prototypic case is the apoptosis triggered by HSV-1 replication. It was demonstrated that HSV-1-induced apoptosis depends on PKR-mediated activation of NF-κB (342); moreover, it has been shown unequivocally that cells lacking NF-κB are resistant to HSV-1-induced apoptosis (342). It is tempting to speculate that in circumstances in which viruses are not present, the role of NF-κB switches to that of a prosurvival factor, as has been described previously (84). It would be of interest to understand which NF-κB-regulated genes are involved in mediating PKR-induced apoptosis. Candidates include the FasL (182), Fas (17), IRF-1, caspase 1 (37), and p53 (384) genes. ATF-3 involvement in PKR-induced apoptosis was recently shown by gene expression profiling (134). PKR activation induces and activates the ATF-3 gene, a stress-inducible gene that encodes a member of the ATF/CREB transcription factor family (139, 140). Other potential mediators of PKR-induced apoptosis are the components of the Arf/p53 pathway. The p53 pathway is a critical regulator of apoptosis (328), and the relationship between PKR and this pathway has been established at several levels, as outlined above. IRF-1 is another PKR target involved in apoptosis induction. The mechanism of IRF-1 activation by PKR is proposed to involve modulation of IRF-1 DNA-binding activity through IRF-1 phosphorylation (202). Indeed, this regulation of IRF-1 by PKR is suggested to be responsible for reduced apoptosis in PKR−/− MEF in response to LPS, TNF-α, and dsRNA (202). IRF-1 expression induces apoptosis and exerts oncogenic effects (379) in a PKR-dependent manner (197).
Connections between PKR and the Apoptotic Machinery
To understand how the activation of PKR effectors regulates apoptosis induction, it is necessary to understand how the apoptotic machinery integrates these signals. PKR-induced apoptosis involves mainly the FADD/caspase 8 pathway (6, 119), although APAF/caspase 9 activation also is observed during PKR-induced apoptosis (121). Interestingly, the FADD/caspase 8 pathway is also activated by type I IFN during virus infection and contributes to the potentiation of cell death in that context. Since caspase 8 is the initiator caspase involved in death receptor-induced apoptosis, this suggests that PKR might engage a death receptor. This hypothesis is reinforced by the observation that pIC-induced apoptosis is deficient in FADD knockout cells (6). Expression of a FADD dominant negative mutant or MC159L (from MCV) by using VV recombinants blocks PKR-induced apoptosis and decreases caspase 8 activity, showing that PKR triggers apoptosis through FADD-mediated activation of caspase 8 (119). By using murine cell lines expressing wild-type PKR or a catalytically inactive PKR variant (PKRΔ6) in a tetracycline-inducible manner, PKR was found to induce expression of the proapoptotic proteins Fas and Bax. In contrast, cells expressing PKRΔ6 had low levels of Fas, TNFR1, FADD, FLICE, Bad, and Bax transcripts and high levels of some antiapoptotic proteins such as Bcl2 (6).
The nature of the connection between PKR induction and FADD activation of caspase 8 remains to be clarified. As noted above, several reports have shown that the Fas death receptor is upregulated during PKR-induced apoptosis (6). In several studies, however, no connection was found between different death receptors and PKR-dependent apoptosis (6, 119). Untested death receptors might still be involved, or, alternatively, an atypical pathway could engage FADD during PKR-induced apoptosis. Novel roles for FADD have been recently reported (5), although they do not appear to involve PKR. PKR activation might promote the formation of a death-inducing signaling complex, enhancing Fas, FADD, and caspase 8 levels; as a result, PKR cannot activate apoptosis if one of them is absent (as in FADD−/− cells) (6).
Initial studies showed that apoptosis induced by PKR overexpression is prevented by overexpression of Bcl-2 or Bcl-2 homologues such as African swine fever virus A179L (31, 217). Bcl-2 proteins act at the mitochondrial level; consequently, those results suggest that the mitochondrial APAF/caspase 9 pathway also has a role in PKR-induced apoptosis. PKR expression by VV recombinants induces caspase 9 activation, which correlates with Bax protein translocation to the mitochondria and cytochrome c release to the cytoplasm, resulting in mitochondrion depolarization (121). The PKR-induced caspase 9 biochemical process occurs downstream of caspase 8 activation, as treating cells with an inhibitor of caspase 9 results in partial prevention of PKR-induced apoptosis (121, 161).
PKR REGULATION OF CELL GROWTH AND DIFFERENTIATION
The multiple effects of PKR in translation, transcription, and apoptosis, described in the preceding section, have an impact on cell growth. The first evidence that PKR controls cell growth, and consequently may function as an inhibitor of cell proliferation, was obtained after expression of PKR, which suppresses growth in mammalian, insect, and yeast cells (55, 246). Conversely, expression of several PKR mutants leads to malignant transformation of NIH 3T3 cells and causes tumorigenesis in nude mice (13, 85, 198). PKR also controls cell differentiation, and its own levels oscillate during the cell cycle, as we discuss below.
Despite the many implications of PKR in cell growth and differentiation, PKR knockout mice do not develop spontaneous tumors or display aberrant cell growth, although they show defects in IFN- and dsRNA-mediated signaling (202). However, MEF derived from two PKR knockout mouse strains were shown to express truncated PKR proteins that retain the active catalytic or regulatory domain (8).
Regulation of PKR Activity during the Cell Cycle
PKR activity varies during the cell cycle, with highest levels in early G1 and a second peak at the G1/S boundary. These changes in PKR activity do not correlate with changes in protein levels, suggesting that posttranslational regulation is operating (284, 396). Cells expressing the catalytic inactive form of PKR or lacking PKR have a prolonged G1 phase, with fewer cells engaged in S phase. These results suggest that PKR may have a role in cell cycle progression (284, 396).
PKR Control of Cell Differentiation
PKR regulates cell differentiation to different lineages. Inhibition of endogenous PKR activity by a dominant negative mutant of PKR interferes with myogenesis of murine C2C12 cells (303). In contrast, PKR overexpression during myogenesis causes induction of the cyclin-dependent kinase inhibitor p21WAF1 and an increase in the level of the underphosphorylated active form of the tumor suppressor protein pRb, together with cyclin D1 and c-myc downregulation (199). Osteoblast differentiation and calcification through modulation of STAT1α and/or Runx2 expression by PKR has been also described (249).
In the skin, PKR is constitutively expressed in epidermal and epithelial keratinocytes. PKR is necessary for keratinocyte differentiation, since loss of PKR in these cells induces increased cell proliferation and altered keratinocyte differentiation (204). Transient PKR expression in mouse embryonic 3T3-F442A fibroblasts induces adipocyte differentiation, concomitant with G0/G1 growth arrest at confluence prior to differentiation (380).
PKR also controls the growth of mature T lymphocytes (178), indicating an influence of PKR on the immune system. Since dsRNA is produced during virus infection and in normal cells, PKR activation is predicted to have a role in the expression of immunomodulatory molecules. In fact, noncoding regulatory small RNAs have been observed in stimulated lymphocytes, which activate PKR and the NF-κB pathway, inducing cytotoxic T-lymphocyte and IFN-γ responses (372).
Effects of PKR-Inhibitory Proteins on Cell Growth
The role of PKR in controlling cell proliferation is further indicated by the dramatic effects caused by overexpression of different PKR inhibitors. This was first shown in p58IPK-overexpressing cell lines that are transformed (14); almost all PKR inhibitors studied have similarly been shown to cause oncogenic transformation. For example, TRBP overexpression also results in malignant transformation (19). The viral protein NS5A, encoded by HCV, has oncogenic potential, and this property has been attributed to its ability to bind to PKR and inhibit PKR function (104, 113, 126, 261). The VV-encoded E3L protein also inhibits PKR and transforms NIH 3T3-E3L cells. Cells expressing E3L grow more rapidly than control cells and cooperate with H-Ras in a focus formation assay. Transformed NIH 3T3-E3L cells form solid tumors when injected into nude mice (109). The EBER RNA encoded by Epstein-Barr virus also inhibits PKR and contributes significantly to the oncogenic properties of Epstein-Barr virus (385).
Tumor Suppressors and Oncogenes That Regulate PKR Action
PKR is regulated by several tumor suppressors and is consequently involved in mediating their action. For example, MDA7 induces apoptosis in a wide range of cancer cells through the activation of various signal transduction pathways (247, 275, 276, 306), one of which is involved in PKR activation. The 3′ untranslated regions of tropomyosin, troponin, and cardiac actin function as tumor suppressors. It was recently proposed that these mRNAs activate PKR and that this is the mechanism behind their tumor suppressor activity (71, 263). IRF-1 is deleted in leukemias and acts as a tumor suppressor in mouse models (21). PKR is suggested to mediate the growth-inhibitory activities of IRF-1 (77, 197, 202). Finally, PKR could also regulate some p53 functions. PKR regulates TNF-α-induced p53-dependent apoptosis in U937 cells; in addition, PKR can interact directly with p53 and phosphorylate it. Although the precise mechanism involved in PKR-p53 regulation is still unknown, the ability of p53 to impose cell cycle arrest and regulate transcription of its target genes is impaired in PKR−/− MEF. p53 activation in response to adriamycin or gamma irradiation is also inhibited in PKR knockout cells (47).
Several oncogenes inhibit PKR action. NPM is an oncogene product that inhibits PKR through direct interaction (273); a clear example is seen in Karpas 299 cells isolated from a non-Hodgkin's T-cell lymphoma harboring the NPM/ALK translocation, in which PKR is kept repressed (99a). As discussed previously, NPM also links PKR with the tumor suppressor Arf (108). Hsp90 functions as a repressor of PKR and is overexpressed in different cancer cells (32). Ras and v-mos can inhibit PKR apoptotic activity. Ras inhibits PKR autophosphorylation, by a mechanism still under study (336). A Ras-inducible PKR-inhibitory activity termed RIKI, which prevents PKR activation through dephosphorylation of the enzyme, has been identified (253). Raveh et al. demonstrated the existence of a link between PKR and c-Myc suppression (292). Further experiments indicated that PKR is directly involved in c-Myc downregulation, which is responsible for the IFN-induced inhibition of cell growth (319).
PKR Regulation of Transcription Factors
The transcription factor E2F-1 induces cell cycle progression but if deregulated can cause cell death. PKR was recently identified as an E2F-1-induced gene product with a role in regulating E2F-1-mediated apoptosis (369). Concurring with these results, E2F-1 overexpression induces PKR expression and autophosphorylation (Fig. 5), leading to phosphorylation of its downstream target eIF-2α and to apoptotic cell death in a variety of carcinoma cells (367, 369). PKR can interact with STAT1 to regulate its DNA-binding activity (381); PKR also can interact with and activate STAT3 in response to the cell growth regulator factor PDGF (73). PKR activates NF-κB, which regulates diverse genes encoding cytokines, growth factors, cell adhesion molecules, and pro- and antiapoptotic proteins (115). Finally, PKR induces and activates the transcription factor ATF-3, a stress-inducible factor that promotes cell death and cell arrest, and is able to suppress Ras-mediated tumorigenesis (231).
Role of PKR in Human Tumorigenesis
The PKR gene is located in human chromosome 2p21-22, which is frequently rearranged in myeloproliferative diseases such as leukemia, myelodysplastic syndromes, and malignant lymphomas (12, 25, 149). For example, Jurkat cells express high levels of a truncated version of PKR (PKRΔE7) resulting from an alternative spliced form of PKR (228). Several leukemia-derived cell lines do not have active PKR (1, 226, 256). Furthermore, a number of human leukemias and myelodysplasias have reduced PKR expression levels (21). A recent study with patients with chronic lymphocyte leukemia (B-CLL) showed that of 28 patient samples examined, 21 had no PKR activity despite the presence of a full-length protein; PKR from these patients could not be activated or phosphorylate substrates (154). It should be determined whether these cells have increased levels of some PKR inhibitor. Increased PKR levels have been observed in a broad range of human tumors (142-145), although it is not known whether loss of PKR activity by inactivating mutations or overexpression of PKR inhibitors in these tumors resulted in higher kinase levels. Other studies have reported increased PKR expression and activity levels in colon and breast cancer, as well as in melanoma and hepatocellular carcinoma (75, 194, 195, 264). In peripheral adenocarcinoma of the lung, patients with high-grade PKR expression had significantly shorter survival periods than those with low-grade PKR expression (298). In lymph node-negative rectal cancer, PKR expression levels were associated with disease recurrence (205). Further studies showed that increased expression of PKR correlates with better patient prognosis for certain tumors, and normal tissues tend to have lower PKR levels than their neoplastic counterparts (323, 356, 357, 400).
PKR as a Target in Cancer Therapy
Several cancer drugs act by modulating PKR action. Geldamycin and radicicol, anticancer drugs that have been studied in phase I clinical trials, are able to inhibit the chaperone Hsp90, allowing PKR activation (83). This is proposed to be one mechanism by which these drugs exert their antitumor effects. Clotrimazole is an anticancer drug that functions by depletion of intracellular Ca2+ stores and has antitumorigenic action, caused in part by activation of PKR, phosphorylation of eIF-2α, and inhibition of translational initiation (288, 332). Gene therapy with an adenoviral vector allows high local doses of TNF-α that function as antitumoral in esophageal cancer cell lines by inducing apoptosis. It is suggested that adenovirus-TNF-α-mediated apoptosis is dependent, in part, on PKR upregulation (366). The regulatory B16 RNA, obtained from lymphocytes of animals immunized with the metastatic melanoma B16 cell line, exert antitumor effects by activating NF-κB through PKR activation (325).
Several strategies have been designed to take advantage of the tumor suppressor properties of PKR. Recent work showed that dsRNA induction using replicase-based plasmids during vaccination disrupts immune tolerance. PKR and RNase L could be involved in stimulating the immune system. PKR activation could thus serve as a valuable adjuvant for strategies involving vaccination against tumors (223). Several studies have exploited antisense RNA complementary to fragments flanking genes deleted in cancer. This method allows the production of dsRNA that activates PKR only in tumor cells. PKR activation by this mechanism causes apoptosis in glioma cells (324, 325). A lentiviral vector expressing the 39-nucleotide antisense sequence strongly inhibited glioblastoma growth in mouse brain when injected after tumor cell implantation. This PKR-mediated killing strategy may be useful in treating many cancers that express a unique RNA species. Several phase I clinical studies used defective viruses able to grow only in PKR inactive cells, such as herpes simplex virus defective in ICP 34.5 protein, for specific regression of malignant glioma (241, 291). This is an open field that deserves future exploration.
CONCLUDING REMARKS
Whereas IFN exert many effects on cells, from antiviral to antitumor, since their discovery it was anticipated that these effects were due to the action of products encoded by various IFN-induced genes. PKR is among those identified thus far that fulfill the broad antiviral and anticellular actions of IFN. Extensive biochemical and genetic work has been carried out with this serine/threonine kinase enzyme in the last 20 years. The recent observations of tyrosine phosphorylation on PKR open new avenues for exploration of biochemical functions. With the recent elucidation of the three-dimensional structure of PKR in association with its main substrate, eIF-2α, it has become clear that this enzyme is a critical player in translational control. Because eIF-2 levels are limited in cells, PKR phosphorylation of eIF-2α is a rate-limiting step that tells the cell to stop protein synthesis. PKR is thus a “vigilante” for manufacturing the desirable proteins in the cell. When eIF-2α is phosphorylated, the cell discontinues translation of most mRNAs, and synthesis of undesirable proteins, as during virus infection, will stop. The cell either survives if the initial infection is controlled or commits suicide (apoptosis) if there is continuous activation of PKR, as during a productive virus infection.
The dsRNA molecule acts as a trigger for PKR autophosphorylation. While it has been established that dsRNAs are by-products during virus infection, that TLR3 is the receptor for dsRNA, and that PKR is also a sensor of dsRNA, it remains to be defined how dsRNA molecules are produced in normal cells, what is the inducer, and whether there is a common pathway for dsRNA transport. The use of specific monoclonal dsRNA antibodies will help unravel the localization of dsRNA molecules in normal cells and during virus infections. Similarly, antibodies to phosphorylated PKR can be used to colocalize both dsRNA and activated PKR by confocal microscopy. dsRNA size is critical for PKR activation; this is particularly important in experiments with siRNA, in which PKR can be activated nonspecifically by small RNAs. While eIF-2α is undoubtedly the main PKR substrate, other substrates have been identified, such as phosphatase 2A. Future studies should characterize all PKR substrates, since PKR interacts physically with a variety of molecules involved in cell signaling and apoptosis, as described in this review.
The fact that most animal viruses have developed strategies to counteract PKR action highlights the relevance of this unique enzyme in host defense. We have reviewed the RNA and DNA virus families that use viral genes to subvert PKR effects. While some viruses use similar anti-PKR mechanisms, i.e., sequestering PKR or the activator dsRNA, others use different strategies, such as encoding eIF-2α decoys, enhancing PKR degradation, or overexpressing small RNA inhibitors. It is notable that the large DNA-containing viruses, such as poxviruses and herpesviruses, use an array of genes to counteract PKR and IFN. New findings will most likely emerge in the near future, broadening the spectrum of viral gene effects that antagonize PKR and IFN effects, as we obtain further insights into the PKR signaling mechanisms. This is an active area of research.
While many genes are implicated in the signaling of PKR action, it is predicted that microarray studies, genetic approaches, and biochemical assignment of functions will define common pathways. The genome-wide profiling analysis of host genes that are induced or repressed under different PKR activation conditions will provide the means to identify new genes in PKR signaling pathways. The recent identification by microarray analysis of ATF-3 as being involved in PKR-induced apoptosis coincides with this prediction. How many pathways are involved in PKR action? From the findings described in this review, it is clear that PKR intervenes in various pathways involving TLR1, -3, and -4 (Fig. 3 and 4). The differential interaction of PKR with adapter molecules such as TRAF2, -3, and -6 might influence gene transactivation through AP1, NF-κB, or IRF-3/7. Why PKR interacts with so many molecules is unclear, but might be due to a selective requirement marked by the host response to a given stimulus. It is recognized that PKR can be activated by host factors such as PACT, and maybe others, but PACT is the only well-characterized molecule that induces PKR activation (Fig. 5). Obviously, the cell reacts to PKR activation through recruitment of host proteins that antagonize PKR effects (Fig. 5). Further studies are needed to define how these antagonists act on PKR and whether new host molecules can be identified in PKR-activated cells of various origins.
While PKR is a potent inducer of apoptosis, it has been established that apoptosis induction requires FADD/caspase 8 and mitochondrial APAF/caspase 9 pathways. The nature of the connection between PKR induction and FADD activation of caspase 8 needs further clarification. It has been proposed that caspase 8 and 9 pathways are triggered as a result of a translational block by eIF-2 phosphorylation and through NF-κB activation. Since NF-κB is a transcriptional factor that acts as an antiapoptotic trigger, it cannot be excluded that under stress conditions, such as a virus infection, NF-κB is activated by PKR. This in turn triggers transcription of apoptotic genes, as proposed for cells expressing PKR and infected with VV. When PKR is activated in normal cells, NF-κB is induced and antiapoptotic genes that counteract PKR induction of apoptosis are expressed. Both mechanisms might operate in cells, depending on the stimuli. This issue should be resolved using MEF derived from mice deficient in various genes of the signaling/apoptotic cascade. There is also the issue of whether PKR catalytic activity is needed for NF-κB activation, as experiments giving results supporting and opposing this possibility have been reported. The apparent differences might be due to the cell systems used, i.e., uninfected versus virus infected. Another issue is to what extent PKR, TLR3, and RIGI contribute to IFN type I induction in different cells and tissues. Further work is needed in these areas.
A role has been recognized for PKR in the immune system, since it controls the growth of mature T lymphocytes. These effects could be mediated by dsRNA produced during virus infection and by noncoding regulatory small RNA as described for stimulated lymphocytes from uninfected cells. Active PKR and the NF-κB pathway were shown to trigger cytotoxic T-lymphocyte and IFN-γ responses after treatment with regulatory small RNA. PKR activation of immune cells is predicted to lead to expression of immunomodulatory molecules. This important area of research should be exploited in the vaccine and gene therapy fields.
A question that has not yet been answered is why PKR knockout mice do not develop tumors, while cells expressing a catalytically inactive PKR are transformed and can induce tumors in nude mice. It was proposed that PKR acts as a tumor suppressor, and experiments with results supporting and opposing this possibility have been discussed in this review. It is significant that almost all PKR inhibitors studied were shown to cause oncogenic transformation and that several tumor suppressors regulate PKR. Reduced PKR activity has been associated with human tumorigenesis, although other studies showed increased PKR expression in different tumors. Undoubtedly, more studies with human tissue are needed to clarify the role of PKR in tumor progression. Indeed, PKR could serve as a surrogate marker in cancer. This will require examination of the levels of PKR and its activity throughout tumor development to ascertain whether there is a relationship between tumor growth and PKR activity. PKR is considered a target for cancer therapy. The concept is to selectively activate PKR to induce apoptosis and destroy tumor cells.
While from in vitro and in vivo systems PKR has been shown to have a clear role in the control of virus infections, it remains to be defined whether resistance and sensitivity of humans to viral infections (whether lytic or chronic such as in HIV, hepatitis, and other diseases) are linked to the levels and/or presence of an active or inactive PKR in target tissues. Alternatively, PKR action could be suppressed with inhibitors to allow cell growth, as might be needed, for example, to maintain the viability of cells in the central nervous system. The finding of activators and inhibitors of PKR action is an area of interest to the pharmaceutical industry and should be developed using libraries of chemical compounds. The use of cytolytic viruses that grow preferentially in tumor cells with low PKR activity levels is also a promising approach for selective destruction of tumor cells without hampering growth of normal cells, as these cells have active PKR and virus replication will be halted. This area of research is being pursued actively, and we will learn the results in the near future.
Overall, PKR is a crucial determinant of the host defense mechanism, acting at various levels and sensing whether the cell lives or dies. Emerging studies will provide further insights into the mode of PKR action, as well as its potential utility as an antiviral and antitumor effector molecule.
Acknowledgments
We are thankful to Catherine Mark for excellent editorial assistance.
M. Esteban is funded by grant BIO2005-06264, J. Gil by the Medical Research Council, and C. Rivas by the Spanish Ministry of Education and Science. We are indebted to Fundación Botín for financial support.
REFERENCES
- 1.Abraham, N., M. L. Jaramillo, P. I. Duncan, N. Methot, P. L. Icely, D. F. Stojdl, G. N. Barber, and J. C. Bell. 1998. The murine PKR tumor suppressor gene is rearranged in a lymphocytic leukemia. Exp. Cell Res. 244:394-404. [DOI] [PubMed] [Google Scholar]
- 2.Alcami, A., and G. L. Smith. 1995. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J. Virol. 69:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 4.Arch, R. H., R. W. Gedrich, and C. B. Thompson. 1998. Tumor necrosis factor receptor-associated factors (TRAFs)-a family of adapter proteins that regulates life and death. Genes Dev. 12:2821-2830. [DOI] [PubMed] [Google Scholar]
- 5.Balachandran, S., and G. N. Barber. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5:51-65. [DOI] [PubMed] [Google Scholar]
- 6.Balachandran, S., C. N. Kim, W. C. Yeh, T. W. Mak, K. Bhalla, and G. N. Barber. 1998. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 17:6888-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 8.Baltzis, D., S. Li, and A. E. Koromilas. 2002. Functional characterization of pkr gene products expressed in cells from mice with a targeted deletion of the N terminus or C terminus domain of PKR. J. Biol. Chem. 277:38364-38372. [DOI] [PubMed] [Google Scholar]
- 8a.Baltzis, D., L.-K. Qu, S. Papadopaulou, J. D. Blais, J. C. Bell, N. Sonenberg, and A. E. Koromilas. 2004. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2α kinases PERK and PKR. J. Virol. 78:12747-12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bannwarth, S., and A. Gatignol. 2005. HIV-1 TAR RNA: the target of molecular interactions between the virus and its host. Curr. HIV Res. 3:61-71. [DOI] [PubMed] [Google Scholar]
- 10.Barber, G. N. 2005. The dsRNA-dependent protein kinase, PKR and cell death. Cell Death Differ. 12:563-570. [DOI] [PubMed] [Google Scholar]
- 11.Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. [DOI] [PubMed] [Google Scholar]
- 12.Barber, G. N., S. Edelhoff, M. G. Katze, and C. M. Disteche. 1993. Chromosomal assignment of the interferon-inducible double-stranded RNA-dependent protein kinase (PRKR) to human chromosome 2p21-p22 and mouse chromosome 17 E2. Genomics 16:765-767. [DOI] [PubMed] [Google Scholar]
- 13.Barber, G. N., R. Jagus, E. F. Meurs, A. G. Hovanessian, and M. G. Katze. 1995. Molecular mechanisms responsible for malignant transformation by regulatory and catalytic domain variants of the interferon-induced enzyme RNA-dependent protein kinase. J. Biol. Chem. 270:17423-17428. [DOI] [PubMed] [Google Scholar]
- 14.Barber, G. N., S. Thompson, T. G. Lee, T. Strom, R. Jagus, A. Darveau, and M. G. Katze. 1994. The 58-kilodalton inhibitor of the interferon-induced double-stranded RNA-activated protein kinase is a tetratricopeptide repeat protein with oncogenic properties. Proc. Natl. Acad. Sci. USA 91:4278-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beattie, E., E. Paoletti, and J. Tartaglia. 1995. Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L− and E3L− mutant viruses. Virology 210:254-263. [DOI] [PubMed] [Google Scholar]
- 16.Beere, H. M., B. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. [DOI] [PubMed] [Google Scholar]
- 17.Behrmann, I., H. Walczak, and P. H. Krammer. 1994. Structure of the human APO-1 gene. Eur. J. Immunol. 24:3057-3062. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Asouli, Y., Y. Banai, Y. Pel-Or, A. Shir, and R. Kaempfer. 2002. Human interferon-gamma mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR. Cell 108:221-232. [DOI] [PubMed] [Google Scholar]
- 19.Benkirane, M., C. Neuveut, R. F. Chun, S. M. Smith, C. E. Samuel, A. Gatignol, and K. T. Jeang. 1997. Oncogenic potential of TAR RNA binding protein TRBP and its regulatory interaction with RNA-dependent protein kinase PKR. EMBO J. 16:611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett, R. L., W. L. Blalock, and W. S. May. 2004. Serine 18 phosphorylation of RAX, the PKR activator, is required for PKR activation and consequent translation inhibition. J. Biol. Chem. 279:42687-42693. [DOI] [PubMed] [Google Scholar]
- 21.Beretta, L., M. Gabbay, R. Berger, S. M. Hanash, and N. Sonenberg. 1996. Expression of the protein kinase PKR in modulated by IRF-1 and is reduced in 5q-associated leukemias. Oncogene 12:1593-1596. [PubMed] [Google Scholar]
- 22.Bergeron, J., T. Mabrouk, S. Garzon, and G. Lemay. 1998. Characterization of the thermosensitive ts453 reovirus mutant: increased dsRNA binding of sigma 3 protein correlates with interferon resistance. Virology 246:199-210. [DOI] [PubMed] [Google Scholar]
- 23.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74:6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertwistle, D., M. Sugimoto, and C. J. Sherr. 2004. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol. Cell. Biol. 24:985-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Beutler, B. 2004. Inferences, questions, and possibilities in Toll-like receptor signalling. Nature 430:257-263. [DOI] [PubMed] [Google Scholar]
- 25.Billstrom, R., S. Heim, U. Kristoffersson, N. Mandahl, and F. Mitelman. 1990. Structural chromosomal abnormalities of 3q in myelodysplastic syndrome/acute myeloid leukaemia with Sweet's syndrome. Eur. J. Haematol. 45:150-152. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet, M. C., R. Weil, E. Dam, A. G. Hovanessian, and E. F. Meurs. 2000. PKR stimulates NF-κB irrespective of its kinase function by interacting with the IκB kinase complex. Mol. Cell. Biol. 20:4532-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bottone, F. G., Jr., Y. Moon, J. S. Kim, B. Alston-Mills, M. Ishibashi, and T. E. Eling. 2005. The anti-invasive activity of cyclooxygenase inhibitors is regulated by the transcription factor ATF3 (activating transcription factor 3). Mol. Cancer Ther. 4:693-703. [DOI] [PubMed] [Google Scholar]
- 28.Brand, S. R., R. Kobayashi, and M. B. Mathews. 1997. The Tat protein of human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced, virally activated protein kinase, PKR. J. Biol. Chem. 272:8388-8395. [DOI] [PubMed] [Google Scholar]
- 29.Brandt, T., M. C. Heck, S. Vijaysri, G. M. Jentarra, J. M. Cameron, and B. L. Jacobs. 2005. The N-terminal domain of the vaccinia virus E3L-protein is required for neurovirulence, but not induction of a protective immune response. Virology 333:263-270. [DOI] [PubMed] [Google Scholar]
- 30.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brun, A., C. Rivas, M. Esteban, J. M. Escribano, and C. Alonso. 1996. African swine fever virus gene A179L, a viral homologue of bcl-2, protects cells from programmed cell death. Virology 225:227-230. [DOI] [PubMed] [Google Scholar]
- 32.Buchner, J. 1999. Hsp90 & Co. —a holding for folding. Trends Biochem. Sci. 24:136-141. [DOI] [PubMed] [Google Scholar]
- 33.Burysek, L., and P. M. Pitha. 2001. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase. J. Virol. 75:2345-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai, R., B. Carpick, R. F. Chun, K. T. Jeang, and B. R. Williams. 2000. HIV-I TAT inhibits PKR activity by both RNA-dependent and RNA-independent mechanisms. Arch. Biochem. Biophys. 373:361-367. [DOI] [PubMed] [Google Scholar]
- 35.Carpick, B. W., V. Graziano, D. Schneider, R. K. Maitra, X. Lee, and B. R. Williams. 1997. Characterization of the solution complex between the interferon-induced, double-stranded RNA-activated protein kinase and HIV-I trans-activating region RNA. J. Biol. Chem. 272:9510-9516. [DOI] [PubMed] [Google Scholar]
- 36.Carroll, K., O. Elroy-Stein, B. Moss, and R. Jagus. 1993. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2 alpha-specific protein kinase. J. Biol. Chem. 268:12837-12842. [PubMed] [Google Scholar]
- 37.Casano, F. J., A. M. Rolando, J. S. Mudgett, and S. M. Molineaux. 1994. The structure and complete nucleotide sequence of the murine gene encoding interleukin-1 beta converting enzyme (ICE). Genomics 20:474-481. [DOI] [PubMed] [Google Scholar]
- 38.Cassady, K. A., and M. Gross. 2002. The herpes simplex virus type 1 US11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J. Virol. 76:2029-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Castelli, J. C., B. A. Hassel, A. Maran, J. Paranjape, J. A. Hewitt, X. L. Li, Y. T. Hsu, R. H. Silverman, and R. J. Youle. 1998. The role of 2′-5′ oligoadenylate-activated ribonuclease L in apoptosis. Cell Death Differ. 5:313-320. [DOI] [PubMed] [Google Scholar]
- 39.Castet, V., C. Fournier, A. Soulier, R. Brillet, J. Coste, D. Larrey, D. Dhumeaux, P. Maurel, and J. M. Pawlotsky. 2002. Alpha interferon inhibits hepatitis C virus replication in primary human hepatocytes infected in vitro. J. Virol. 76:8189-8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caudell, E. G., J. B. Mumm, N. Poindexter, S. Ekmekcioglu, A. M. Mhashilkar, X. H. Yang, M. W. Retter, P. Hill, S. Chada, and E. A. Grimm. 2002. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J. Immunol. 168:6041-6046. [DOI] [PubMed] [Google Scholar]
- 41.Clemens, M. J. 2006. Epstein-Barr virus: inhibition of apoptosis as a mechanism of cell transformation. Int. J. Biochem. Cell Biol. 38:164-169. [DOI] [PubMed] [Google Scholar]
- 42.Clemens, M. J. 1997. PKR—a protein kinase regulated by double-stranded RNA. Int. J. Biochem. Cell Biol. 29:945-949. [DOI] [PubMed] [Google Scholar]
- 43.Clemens, M. J., J. W. Hershey, A. C. Hovanessian, B. C. Jacobs, M. G. Katze, R. J. Kaufman, P. Lengyel, C. E. Samuel, G. C. Sen, and B. R. Williams. 1993. PKR: proposed nomenclature for the RNA-dependent protein kinase induced by interferon. J. Interferon Res. 13:241. [DOI] [PubMed] [Google Scholar]
- 44.Colombo, E., J. C. Marine, D. Danovi, B. Falini, and P. G. Pelicci. 2002. Nucleophosmin regulates the stability and transcriptional activity of p53. Nat. Cell Biol. 4:529-533. [DOI] [PubMed] [Google Scholar]
- 45.Cosentino, G. P., S. Venkatesan, F. C. Serluca, S. R. Green, M. B. Mathews, and N. Sonenberg. 1995. Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo. Proc. Natl. Acad. Sci. USA 92:9445-9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craig, A. W., G. P. Cosentino, O. Donze, and N. Sonenberg. 1996. The kinase insert domain of interferon-induced protein kinase PKR is required for activity but not for interaction with the pseudosubstrate K3L. J. Biol. Chem. 271:24526-24533. [DOI] [PubMed] [Google Scholar]
- 47.Cuddihy, A. R., S. Li, N. W. Tam, A. H. Wong, Y. Taya, N. Abraham, J. C. Bell, and A. E. Koromilas. 1999. Double-stranded-RNA-activated protein kinase PKR enhances transcriptional activation by tumor suppressor p53. Mol. Cell. Biol. 19:2475-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuddihy, A. R., A. H. Wong, N. W. Tam, S. Li, and A. E. Koromilas. 1999. The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene 18:2690-2702. [DOI] [PubMed] [Google Scholar]
- 49.Cullen, B. R. 1993. Does HIV-1 Tat induce a change in viral initiation rights? Cell 73:417-420. [DOI] [PubMed] [Google Scholar]
- 50.Chan, W. Y., Q. R. Liu, J. Borjigin, H. Busch, O. M. Rennert, L. A. Tease, and P. K. Chan. 1989. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry 28:1033-1039. [DOI] [PubMed] [Google Scholar]
- 51.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatterjee, M., N. Chatterjee, R. Datta, B. Datta, and N. K. Gupta. 1998. Expression and activity of p67 are induced during heat shock. Biochem. Biophys. Res. Commun. 249:113-117. [DOI] [PubMed] [Google Scholar]
- 53.Reference deleted.
- 54.Cheshire, J. L., B. R. Williams, and A. S. Baldwin, Jr. 1999. Involvement of double-stranded RNA-activated protein kinase in the synergistic activation of nuclear factor-kappaB by tumor necrosis factor-alpha and gamma-interferon in preneuronal cells. J. Biol. Chem. 274:4801-4806. [DOI] [PubMed] [Google Scholar]
- 55.Chong, K. L., L. Feng, K. Schappert, E. Meurs, T. F. Donahue, J. D. Friesen, A. G. Hovanessian, and B. R. Williams. 1992. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 11:1553-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 57.Chu, W. M., R. Ballard, B. W. Carpick, B. R. Williams, and C. W. Schmid. 1998. Potential Alu function: regulation of the activity of double-stranded RNA-activated kinase PKR. Mol. Cell. Biol. 18:58-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu, W. M., D. Ostertag, Z. W. Li, L. Chang, Y. Chen, Y. Hu, B. Williams, J. Perrault, and M. Karin. 1999. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity 11:721-731. [DOI] [PubMed] [Google Scholar]
- 59.Chung, J. Y., Y. C. Park, H. Ye, and H. Wu. 2002. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115:679-688. [DOI] [PubMed] [Google Scholar]
- 60.Daher, A., M. Longuet, D. Dorin, F. Bois, E. Segeral, S. Bannwarth, P. L. Battisti, D. F. Purcell, R. Benarous, C. Vaquero, E. F. Meurs, and A. Gatignol. 2001. Two dimerization domains in the trans-activation response RNA-binding protein (TRBP) individually reverse the protein kinase R inhibition of HIV-1 long terminal repeat expression. J. Biol. Chem. 276:33899-33905. [DOI] [PubMed] [Google Scholar]
- 61.Dar, A. C., T. E. Dever, and F. Sicheri. 2005. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell 122:887-900. [DOI] [PubMed] [Google Scholar]
- 62.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 63.Datta, B., D. Chakrabarti, A. L. Roy, and N. K. Gupta. 1988. Roles of a 67-kDa polypeptide in reversal of protein synthesis inhibition in heme-deficient reticulocyte lysate. Proc. Natl. Acad. Sci. USA 85:3324-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Datta, B., and R. Datta. 1999. Induction of apoptosis due to lowering the level of eukaryotic initiation factor 2-associated protein, p67, from mammalian cells by antisense approach. Exp. Cell Res. 246:376-383. [DOI] [PubMed] [Google Scholar]
- 65.Datta, B., and R. Datta. 2003. Mutation at the acidic residue-rich domain of eukaryotic initiation factor 2 (eIF2alpha)-associated glycoprotein p67 increases the protection of eIF2alpha phosphorylation during heat shock. Arch. Biochem. Biophys. 413:116-122. [DOI] [PubMed] [Google Scholar]
- 66.Datta, B., R. Datta, A. Ghosh, and A. Majumdar. 2004. Eukaryotic initiation factor 2-associated glycoprotein, p67, shows differential effects on the activity of certain kinases during serum-starved conditions. Arch. Biochem. Biophys. 427:68-78. [DOI] [PubMed] [Google Scholar]
- 67.Datta, B., M. K. Ray, D. Chakrabarti, D. E. Wylie, and N. K. Gupta. 1989. Glycosylation of eukaryotic peptide chain initiation factor 2 (eIF-2)-associated 67-kDa polypeptide (p67) and its possible role in the inhibition of eIF-2 kinase-catalyzed phosphorylation of the eIF-2 alpha-subunit. J. Biol. Chem. 264:20620-20624. [PubMed] [Google Scholar]
- 68.Datta, R., R. Tammali, and B. Datta. 2003. Negative regulation of the protection of eIF2alpha phosphorylation activity by a unique acidic domain present at the N-terminus of p67. Exp. Cell Res. 283:237-246. [DOI] [PubMed] [Google Scholar]
- 69.Davies, M. V., H. W. Chang, B. L. Jacobs, and R. J. Kaufman. 1993. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J. Virol. 67:1688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies, M. V., O. Elroy-Stein, R. Jagus, B. Moss, and R. J. Kaufman. 1992. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J. Virol. 66:1943-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis, S., and J. C. Watson. 1996. In vitro activation of the interferon-induced, double-stranded RNA-dependent protein kinase PKR by RNA from the 3′ untranslated regions of human alpha-tropomyosin. Proc. Natl. Acad. Sci. USA 93:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deb, A., S. J. Haque, T. Mogensen, R. H. Silverman, and B. R. Williams. 2001. RNA-dependent protein kinase PKR is required for activation of NF-kappa B by IFN-gamma in a STAT1-independent pathway. J. Immunol. 166:6170-6180. [DOI] [PubMed] [Google Scholar]
- 73.Deb, A., M. Zamanian-Daryoush, Z. Xu, S. Kadereit, and B. R. Williams. 2001. Protein kinase PKR is required for platelet-derived growth factor signaling of c-fos gene expression via Erks and Stat3. EMBO J. 20:2487-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Haro, C., R. Mendez, and J. Santoyo. 1996. The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 10:1378-1387. [DOI] [PubMed] [Google Scholar]
- 75.Delhem, N., A. Sabile, R. Gajardo, P. Podevin, A. Abadie, M. A. Blaton, D. Kremsdorf, L. Beretta, and C. Brechot. 2001. Activation of the interferon-inducible protein kinase PKR by hepatocellular carcinoma derived-hepatitis C virus core protein. Oncogene 20:5836-5845. [DOI] [PubMed] [Google Scholar]
- 76.Der, S. D., and A. S. Lau. 1995. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc. Natl. Acad. Sci. USA 92:8841-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Der, S. D., Y. L. Yang, C. Weissmann, and B. R. Williams. 1997. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:3279-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dever, T. E. 2002. Gene-specific regulation by general translation factors. Cell 108:545-556. [DOI] [PubMed] [Google Scholar]
- 79.Dever, T. E., L. Feng, R. C. Wek, A. M. Cigan, T. F. Donahue, and A. G. Hinnebusch. 1992. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68:585-596. [DOI] [PubMed] [Google Scholar]
- 80.Dey, M., C. Cao, A. C. Dar, T. Tamura, K. Ozato, F. Sicheri, and T. E. Dever. 2005. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell 122:901-913. [DOI] [PubMed] [Google Scholar]
- 81.Diaz-Guerra, M., J. S. Kahn, and M. Esteban. 1993. A mutation of the nucleoside triphosphate phosphohydrolase I (NPH-I) gene confers sensitivity of vaccinia virus to interferon. Virology 197:485-491. [DOI] [PubMed] [Google Scholar]
- 82.Diaz-Guerra, M., C. Rivas, and M. Esteban. 1997. Activation of the IFN-inducible enzyme RNase L causes apoptosis of animal cells. Virology 236:354-363. [DOI] [PubMed] [Google Scholar]
- 83.Donze, O., T. Abbas-Terki, and D. Picard. 2001. The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR. EMBO J. 20:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Donze, O., J. Deng, J. Curran, R. Sladek, D. Picard, and N. Sonenberg. 2004. The protein kinase PKR: a molecular clock that sequentially activates survival and death programs. EMBO J. 23:564-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Donze, O., R. Jagus, A. E. Koromilas, J. W. Hershey, and N. Sonenberg. 1995. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 14:3828-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dorin, D., M. C. Bonnet, S. Bannwarth, A. Gatignol, E. F. Meurs, and C. Vaquero. 2003. The TAR RNA-binding protein, TRBP, stimulates the expression of TAR-containing RNAs in vitro and in vivo independently of its ability to inhibit the dsRNA-dependent kinase PKR. J. Biol. Chem. 278:4440-4448. [DOI] [PubMed] [Google Scholar]
- 87.Dumbar, T. S., G. A. Gentry, and M. O. Olson. 1989. Interaction of nucleolar phosphoprotein B23 with nucleic acids. Biochemistry 28:9495-9501. [DOI] [PubMed] [Google Scholar]
- 88.Edery, I., R. Petryshyn, and N. Sonenberg. 1989. Activation of double-stranded RNA-dependent kinase (dsl) by the TAR region of HIV-1 mRNA: a novel translational control mechanism. Cell 56:303-312. [DOI] [PubMed] [Google Scholar]
- 89.Elia, A., J. Vyas, K. G. Laing, and M. J. Clemens. 2004. Ribosomal protein L22 inhibits regulation of cellular activities by the Epstein-Barr virus small RNA EBER-1. Eur. J. Biochem. 271:1895-1905. [DOI] [PubMed] [Google Scholar]
- 90.Endo-Munoz, L., T. Warby, D. Harrich, and N. A. McMillan. 2005. Phosphorylation of HIV Tat by PKR increases interaction with TAR RNA and enhances transcription. Virol. J. 2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Enomoto, N., I. Sakuma, Y. Asahina, M. Kurosaki, T. Murakami, C. Yamamoto, N. Izumi, F. Marumo, and C. Sato. 1995. Comparison of full-length sequences of interferon-sensitive and resistant hepatitis C virus 1b. Sensitivity to interferon is conferred by amino acid substitutions in the NS5A region. J. Clin. Investig. 96:224-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Enomoto, N., I. Sakuma, Y. Asahina, M. Kurosaki, T. Murakami, C. Yamamoto, Y. Ogura, N. Izumi, F. Marumo, and C. Sato. 1996. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N. Engl. J. Med. 334:77-81. [DOI] [PubMed] [Google Scholar]
- 93.Esteban, M., M. A. Garcia, E. Domingo-Gil, J. Arroyo, C. Nombela, and C. Rivas. 2003. The latency protein LANA2 from Kaposi's sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J. Gen. Virol. 84:1463-1470. [DOI] [PubMed] [Google Scholar]
- 94.Reference deleted.
- 95.Falcon, A. M., P. Fortes, R. M. Marion, A. Beloso, and J. Ortin. 1999. Interaction of influenza virus NS1 protein and the human homologue of Staufen in vivo and in vitro. Nucleic Acids Res. 27:2241-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Falini, B., C. Mecucci, E. Tiacci, M. Alcalay, R. Rosati, L. Pasqualucci, R. La Starza, D. Diverio, E. Colombo, A. Santucci, B. Bigerna, R. Pacini, A. Pucciarini, A. Liso, M. Vignetti, P. Fazi, N. Meani, V. Pettirossi, G. Saglio, F. Mandelli, F. Lo-Coco, P. G. Pelicci, and M. F. Martelli. 2005. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 352:254-266. [DOI] [PubMed] [Google Scholar]
- 97.Fickenscher, H., S. Hor, H. Kupers, A. Knappe, S. Wittmann, and H. Sticht. 2002. The interleukin-10 family of cytokines. Trends Immunol. 23:89-96. [DOI] [PubMed] [Google Scholar]
- 98.Flati, V., S. J. Haque, and B. R. Williams. 1996. Interferon-alpha-induced phosphorylation and activation of cytosolic phospholipase A2 is required for the formation of interferon-stimulated gene factor three. EMBO J. 15:1566-1571. [PMC free article] [PubMed] [Google Scholar]
- 99.Friedman, R. M., D. H. Metz, R. M. Esteban, D. R. Tovell, L. A. Ball, and I. M. Kerr. 1972. Mechanism of interferon action: inhibition of viral messenger ribonucleic acid translation in L-cell extracts. J. Virol. 10:1184-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99a.Freidrich, I., H. Ben-Bassat, and A. Levitzki. 2005. Activation of dsRNA dependent protein kinase PKR in Karpas299 does not lead to cell death. Cancer Biol. Ther. 4:734-739. [DOI] [PubMed] [Google Scholar]
- 100.Gaestel, M. 2006. MAPKAP kinases—MKs—two's company, three's a crowd. Nat. Rev. Mol. Cell Biol. 7:120-130. [DOI] [PubMed] [Google Scholar]
- 101.Galabru, J., and A. Hovanessian. 1987. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J. Biol. Chem. 262:15538-15544. [PubMed] [Google Scholar]
- 102.Gale, M., Jr., C. M. Blakely, A. Darveau, P. R. Romano, M. J. Korth, and M. G. Katze. 2002. P52rIPK regulates the molecular cochaperone P58IPK to mediate control of the RNA-dependent protein kinase in response to cytoplasmic stress. Biochemistry 41:11878-11887. [DOI] [PubMed] [Google Scholar]
- 103.Gale, M., Jr., C. M. Blakely, B. Kwieciszewski, S. L. Tan, M. Dossett, N. M. Tang, M. J. Korth, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 18:5208-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gale, M., Jr., B. Kwieciszewski, M. Dossett, H. Nakao, and M. G. Katze. 1999. Antiapoptotic and oncogenic potentials of hepatitis C virus are linked to interferon resistance by viral repression of the PKR protein kinase. J. Virol. 73:6506-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gale, M., Jr., S. L. Tan, M. Wambach, and M. G. Katze. 1996. Interaction of the interferon-induced PKR protein kinase with inhibitory proteins P58IPK and vaccinia virus K3L is mediated by unique domains: implications for kinase regulation. Mol. Cell. Biol. 16:4172-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gale, M. J., Jr., M. J. Korth, and M. G. Katze. 1998. Repression of the PKR protein kinase by the hepatitis C virus NS5A protein: a potential mechanism of interferon resistance. Clin. Diagn. Virol. 10:157-162. [DOI] [PubMed] [Google Scholar]
- 107.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 108.Garcia, M. A., M. Collado, C. Munoz-Fontela, A. Matheu, L. Marcos-Villar, J. Arroyo, M. Esteban, M. Serrano, and C. Rivas. 2006. Antiviral action of the tumor suppressor ARF. EMBO J. 25:4284-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia, M. A., S. Guerra, J. Gil, V. Jimenez, and M. Esteban. 2002. Anti-apoptotic and oncogenic properties of the dsRNA-binding protein of vaccinia virus, E3L. Oncogene 21:8379-8387. [DOI] [PubMed] [Google Scholar]
- 110.Gatignol, A., A. Buckler-White, B. Berkhout, and K. T. Jeang. 1991. Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR. Science 251:1597-1600. [DOI] [PubMed] [Google Scholar]
- 111.Gatignol, A., A. Kumar, A. Rabson, and K. T. Jeang. 1989. Identification of cellular proteins that bind to the human immunodeficiency virus type 1 trans-activation-responsive TAR element RNA. Proc. Natl. Acad. Sci. USA 86:7828-7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gerotto, M., F. Dal Pero, P. Pontisso, F. Noventa, A. Gatta, and A. Alberti. 2000. Two PKR inhibitor HCV proteins correlate with early but not sustained response to interferon. Gastroenterology 119:1649-1655. [DOI] [PubMed] [Google Scholar]
- 113.Ghosh, A. K., R. Steele, K. Meyer, R. Ray, and R. B. Ray. 1999. Hepatitis C virus NS5A protein modulates cell cycle regulatory genes and promotes cell growth. J. Gen. Virol. 80:1179-1183. [DOI] [PubMed] [Google Scholar]
- 114.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-kappaB puzzle. Cell 1 09(Suppl):S81-S96. [DOI] [PubMed] [Google Scholar]
- 115.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 116.Gil, J., J. Alcami, and M. Esteban. 2000. Activation of NF-kappa B by the dsRNA-dependent protein kinase, PKR, involves the I kappa B kinase complex. Oncogene 19:1369-1378. [DOI] [PubMed] [Google Scholar]
- 117.Gil, J., J. Alcami, and M. Esteban. 1999. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-κB. Mol. Cell. Biol. 19:4653-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gil, J., and M. Esteban. 2000. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis 5:107-114. [DOI] [PubMed] [Google Scholar]
- 119.Gil, J., and M. Esteban. 2000. The interferon-induced protein kinase (PKR) triggers apoptosis through FADD-mediated activation of caspase 8 in a manner independent of Fas and TNF-alpha receptors. Oncogene 19:3665-3674. [DOI] [PubMed] [Google Scholar]
- 120.Gil, J., M. Esteban, and D. Roth. 2000. In vivo regulation of the dsRNA-dependent protein kinase PKR by the cellular glycoprotein p67. Biochemistry 39:16016-16025. [DOI] [PubMed] [Google Scholar]
- 121.Gil, J., M. A. Garcia, and M. Esteban. 2002. Caspase 9 activation by the dsRNA-dependent protein kinase, PKR: molecular mechanism and relevance. FEBS Lett. 529:249-255. [DOI] [PubMed] [Google Scholar]
- 122.Gil, J., M. A. Garcia, P. Gomez-Puertas, S. Guerra, J. Rullas, H. Nakano, J. Alcami, and M. Esteban. 2004. TRAF family proteins link PKR with NF-κB activation. Mol. Cell. Biol. 24:4502-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gil, J., J. Rullas, J. Alcami, and M. Esteban. 2001. MC159L protein from the poxvirus molluscum contagiosum virus inhibits NF-kappaB activation and apoptosis induced by PKR. J. Gen. Virol. 82:3027-3034. [DOI] [PubMed] [Google Scholar]
- 124.Gil, J., J. Rullas, M. A. Garcia, J. Alcami, and M. Esteban. 2001. The catalytic activity of dsRNA-dependent protein kinase, PKR, is required for NF-kappaB activation. Oncogene 20:385-394. [DOI] [PubMed] [Google Scholar]
- 125.Gil, J., and M. Esteban. 2004. Vaccinia virus recombinants as a model system to analyze IFN-induced pathways. J. Interferon Cytokine Res. 24:637-646. [DOI] [PubMed] [Google Scholar]
- 126.Gimenez-Barcons, M., S. Franco, Y. Suarez, X. Forns, S. Ampurdanes, F. Puig-Basagoiti, A. Sanchez-Fueyo, J. M. Barrera, J. M. Llovet, J. Bruix, J. M. Sanchez-Tapias, J. Rodes, and J. C. Saiz. 2001. High amino acid variability within the NS5A of hepatitis C virus (HCV) is associated with hepatocellular carcinoma in patients with HCV-1b-related cirrhosis. Hepatology 34:158-167. [DOI] [PubMed] [Google Scholar]
- 127.Goh, K. C., M. J. deVeer, and B. R. Williams. 2000. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 19:4292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gohda, J., T. Matsumura, and J. Inoue. 2004. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J. Immunol. 173:2913-2917. [DOI] [PubMed] [Google Scholar]
- 129.Gomez, C. E., A. M. Vandermeeren, M. A. Garcia, E. Domingo-Gil, and M. Esteban. 2005. Involvement of PKR and RNase L in translational control and induction of apoptosis after hepatitis C polyprotein expression from a vaccinia virus recombinant. Virol. J. 2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gonzalez-Lopez, C., J. Martinez-Costas, M. Esteban, and J. Benavente. 2003. Evidence that avian reovirus sigmaA protein is an inhibitor of the double-stranded RNA-dependent protein kinase. J Gen. Virol. 84:1629-1639. [DOI] [PubMed] [Google Scholar]
- 131.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 132.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 133.Guerra, S., L. A. Lopez-Fernandez, R. Conde, A. Pascual-Montano, K. Harshman, and M. Esteban. 2004. Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells. J. Virol. 78:5820-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guerra, S., L. A. Lopez-Fernandez, M. A. Garcia, A. Zaballos, and M. Esteban. 2006. Human gene profiling in response to the active protein kinase, interferon-induced serine/threonine protein kinase (PKR), in infected cells. Involvement of the transcription factor ATF-3 IN PKR-induced apoptosis. J. Biol. Chem. 281:18734-18745. [DOI] [PubMed] [Google Scholar]
- 135.Gunnery, S., S. R. Green, and M. B. Mathews. 1992. Tat-responsive region RNA of human immunodeficiency virus type 1 stimulates protein synthesis in vivo and in vitro: relationship between structure and function. Proc. Natl. Acad. Sci. USA 89:11557-11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gunnery, S., A. P. Rice, H. D. Robertson, and M. B. Mathews. 1990. Tat-responsive region RNA of human immunodeficiency virus 1 can prevent activation of the double-stranded-RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 87:8687-8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haase, A. D., L. Jaskiewicz, H. Zhang, S. Laine, R. Sack, A. Gatignol, and W. Filipowicz. 2005. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 6:961-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hacker, H., V. Redecke, B. Blagoev, I. Kratchmarova, L. C. Hsu, G. G. Wang, M. P. Kamps, E. Raz, H. Wagner, G. Hacker, M. Mann, and M. Karin. 2006. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439:204-207. [DOI] [PubMed] [Google Scholar]
- 139.Hai, T., and M. G. Hartman. 2001. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 273:1-11. [DOI] [PubMed] [Google Scholar]
- 140.Hai, T., C. D. Wolfgang, D. K. Marsee, A. E. Allen, and U. Sivaprasad. 1999. ATF3 and stress responses. Gene Expr. 7:321-335. [PMC free article] [PubMed] [Google Scholar]
- 141.Haines, G. K., III, S. Becker, G. Ghadge, M. Kies, H. Pelzer, and J. A. Radosevich. 1993. Expression of the double-stranded RNA-dependent protein kinase (p68) in squamous cell carcinoma of the head and neck region. Arch. Otolaryngol. Head Neck Surg. 119:1142-1147. [DOI] [PubMed] [Google Scholar]
- 142.Haines, G. K., III, R. J. Panos, P. M. Bak, T. Brown, M. Zielinski, J. Leyland, and J. A. Radosevich. 1998. Interferon-responsive protein kinase (p68) and proliferating cell nuclear antigen are inversely distributed in head and neck squamous cell carcinoma. Tumour Biol. 19:52-59. [DOI] [PubMed] [Google Scholar]
- 143.Haines, G. K., R. Cajulis, R. Hayden, R. Duda, M. Talamonti, and J. A. Radosevich. 1996. Expression of the double-stranded RNA-dependent protein kinase (p68) in human breast tissues. Tumour Biol. 17:5-12. [DOI] [PubMed] [Google Scholar]
- 144.Haines, G. K., G. Ghadge, B. Thimmappaya, and J. A. Radosevich. 1992. Expression of the protein kinase p-68 recognized by the monoclonal antibody TJ4C4 in human lung neoplasms. Virchows Arch. B 62:151-158. [DOI] [PubMed] [Google Scholar]
- 145.Haines, G. K., G. D. Ghadge, S. G. Combs, W. Leslie, B. Thimmappaya, and J. A. Radosevich. 1992. Immunohistochemical detection of double-stranded-RNA-dependent protein kinase (p68) with a novel monoclonal antibody TJ4C4. A case report of an AIDS-associated Kaposi's sarcoma treated with alpha-interferon. Tumour Biol. 13:324-329. [DOI] [PubMed] [Google Scholar]
- 146.Harding, H. P., I. Novoa, Y. Zhang, H. Zeng, R. Wek, M. Schapira, and D. Ron. 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6:1099-1108. [DOI] [PubMed] [Google Scholar]
- 147.Hartman, M. G., D. Lu, M. L. Kim, G. J. Kociba, T. Shukri, J. Buteau, X. Wang, W. L. Frankel, D. Guttridge, M. Prentki, S. T. Grey, D. Ron, and T. Hai. 2004. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol. Cell. Biol. 24:5721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hatada, E., S. Saito, and R. Fukuda. 1999. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J. Virol. 73:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Haus, O. 2000. The genes of interferons and interferon-related factors: localization and relationships with chromosome aberrations in cancer. Arch. Immunol. Ther. Exp. (Warsz) 48:95-100. [PubMed] [Google Scholar]
- 150.He, B., J. Chou, R. Brandimarti, I. Mohr, Y. Gluzman, and B. Roizman. 1997. Suppression of the phenotype of γ134.5− herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the α47 gene. J. Virol. 71:6049-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He, B., M. Gross, and B. Roizman. 1998. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737-20743. [DOI] [PubMed] [Google Scholar]
- 152.He, B., M. Gross, and B. Roizman. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hershey, J. W. 1991. Translational control in mammalian cells. Annu. Rev. Biochem. 60:717-755. [DOI] [PubMed] [Google Scholar]
- 154.Hii, S. I., L. Hardy, T. Crough, E. J. Payne, K. Grimmett, D. Gill, and N. A. McMillan. 2004. Loss of PKR activity in chronic lymphocytic leukemia. Int. J. Cancer 109:329-335. [DOI] [PubMed] [Google Scholar]
- 155.Horng, T., G. M. Barton, and R. Medzhitov. 2001. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2:835-841. [DOI] [PubMed] [Google Scholar]
- 156.Hovanessian, A. G. 1989. The double stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J. Interferon Res. 9:641-647. [DOI] [PubMed] [Google Scholar]
- 157.Hovanessian, A. G., and J. Galabru. 1987. The double-stranded RNA-dependent protein kinase is also activated by heparin. Eur. J. Biochem. 167:467-473. [DOI] [PubMed] [Google Scholar]
- 158.Hovanessian, A. G., and I. M. Kerr. 1979. The (2′-5′) oligoadenylate (pppA2′-5′A2′-5′A) synthetase and protein kinase(s) from interferon-treated cells. Eur. J. Biochem. 93:515-526. [DOI] [PubMed] [Google Scholar]
- 159.Hsu, L. C., J. M. Park, K. Zhang, J. L. Luo, S. Maeda, R. J. Kaufman, L. Eckmann, D. G. Guiney, and M. Karin. 2004. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature 428:341-345. [DOI] [PubMed] [Google Scholar]
- 160.Icely, P. L., P. Gros, J. J. Bergeron, A. Devault, D. E. Afar, and J. C. Bell. 1991. TIK, a novel serine/threonine kinase, is recognized by antibodies directed against phosphotyrosine. J. Biol. Chem. 266:16073-16077. [PubMed] [Google Scholar]
- 161.Iordanov, M. S., O. P. Ryabinina, P. Schneider, and B. E. Magun. 2005. Two mechanisms of caspase 9 processing in double-stranded RNA- and virus-triggered apoptosis. Apoptosis 10:153-166. [DOI] [PubMed] [Google Scholar]
- 162.Isaacs, A., and J. Lindenmann. 1957. Virus interference. I. The interferon. Proc. R Soc. London B 147:258-267. [DOI] [PubMed] [Google Scholar]
- 163.Itahana, K., K. P. Bhat, A. Jin, Y. Itahana, D. Hawke, R. Kobayashi, and Y. Zhang. 2003. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol. Cell 12:1151-1164. [DOI] [PubMed] [Google Scholar]
- 164.Ito, T., R. Jagus, and W. S. May. 1994. Interleukin 3 stimulates protein synthesis by regulating double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 91:7455-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ito, T., M. Yang, and W. S. May. 1999. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J. Biol. Chem. 274:15427-15432. [DOI] [PubMed] [Google Scholar]
- 166.Iwamura, T., M. Yoneyama, N. Koizumi, Y. Okabe, H. Namiki, C. E. Samuel, and T. Fujita. 2001. PACT, a double-stranded RNA binding protein acts as a positive regulator for type I interferon gene induced by Newcastle disease virus. Biochem. Biophys. Res. Commun. 282:515-523. [DOI] [PubMed] [Google Scholar]
- 167.Iwamura, T., M. Yoneyama, K. Yamaguchi, W. Suhara, W. Mori, K. Shiota, Y. Okabe, H. Namiki, and T. Fujita. 2001. Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells 6:375-388. [DOI] [PubMed] [Google Scholar]
- 168.Jacobs, B. L., and J. O. Langland. 1998. Reovirus sigma 3 protein: dsRNA binding and inhibition of RNA-activated protein kinase. Curr. Top. Microbiol. Immunol. 233:185-196. [DOI] [PubMed] [Google Scholar]
- 169.Jacquemont, B., and B. Roizman. 1975. RNA synthesis in cells infected with herpes simplex virus. X. Properties of viral symmetric transcripts and of double-stranded RNA prepared from them. J. Virol. 15:707-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jagus, R., and M. M. Gray. 1994. Proteins that interact with PKR. Biochimie 76:779-791. [DOI] [PubMed] [Google Scholar]
- 171.Jagus, R., B. Joshi, and G. N. Barber. 1999. PKR, apoptosis and cancer. Int. J. Biochem. Cell Biol. 31:123-138. [DOI] [PubMed] [Google Scholar]
- 172.Jeffrey, I. W., S. Kadereit, E. F. Meurs, T. Metzger, M. Bachmann, M. Schwemmle, A. G. Hovanessian, and M. J. Clemens. 1995. Nuclear localization of the interferon-inducible protein kinase PKR in human cells and transfected mouse cells. Exp. Cell Res. 218:17-27. [DOI] [PubMed] [Google Scholar]
- 173.Jiang, H. Y., S. A. Wek, B. C. McGrath, D. Lu, T. Hai, H. P. Harding, X. Wang, D. Ron, D. R. Cavener, and R. C. Wek. 2004. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol. Cell. Biol. 24:1365-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jiang, Z., M. Zamanian-Daryoush, H. Nie, A. M. Silva, B. R. Williams, and X. Li. 2003. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J. Biol. Chem. 278:16713-16719. [DOI] [PubMed] [Google Scholar]
- 175.Jindal, S., and R. A. Young. 1992. Vaccinia virus infection induces a stress response that leads to association of Hsp70 with viral proteins. J. Virol. 66:5357-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jing, X., M. Cerveny, K. Yang, and B. He. 2004. Replication of herpes simplex virus 1 depends on the gamma 134.5 functions that facilitate virus response to interferon and egress in the different stages of productive infection. J. Virol. 78:7653-7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jones, K. A., and B. M. Peterlin. 1994. Control of RNA initiation and elongation at the HIV-1 promoter. Annu. Rev. Biochem. 63:717-743. [DOI] [PubMed] [Google Scholar]
- 178.Kadereit, S., H. Xu, T. M. Engeman, Y. L. Yang, R. L. Fairchild, and B. R. Williams. 2000. Negative regulation of CD8+ T cell function by the IFN-induced and double-stranded RNA-activated kinase PKR. J. Immunol. 165:6896-6901. [DOI] [PubMed] [Google Scholar]
- 179.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kaempfer, R. 2003. RNA sensors: novel regulators of gene expression. EMBO Rep. 4:1043-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kang, D. C., R. V. Gopalkrishnan, Q. Wu, E. Jankowsky, A. M. Pyle, and P. B. Fisher. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 99:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kasibhatla, S., T. Brunner, L. Genestier, F. Echeverri, A. Mahboubi, and D. R. Green. 1998. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol. Cell 1:543-551. [DOI] [PubMed] [Google Scholar]
- 183.Katze, M. G. 2002. Interferon, PKR, virology, and genomics: what is past and what is next in the new millennium? J. Interferon Cytokine Res. 22:283-286. [DOI] [PubMed] [Google Scholar]
- 184.Katze, M. G. 1995. Regulation of the interferon-induced PKR: can viruses cope? Trends Microbiol. 3:75-78. [DOI] [PubMed] [Google Scholar]
- 185.Kaufman, R. J. 1999. Double-stranded RNA-activated protein kinase mediates virus-induced apoptosis: a new role for an old actor. Proc. Natl. Acad. Sci. USA 96:11693-11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaufman, R. J., M. V. Davies, V. K. Pathak, and J. W. Hershey. 1989. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol. Cell. Biol. 9:946-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kawagishi-Kobayashi, M., J. B. Silverman, T. L. Ung, and T. E. Dever. 1997. Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIF2α. Mol. Cell. Biol. 17:4146-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kawakubo, K., K. L. Kuhen, J. W. Vessey, C. X. George, and C. E. Samuel. 1999. Alternative splice variants of the human PKR protein kinase possessing different 5′-untranslated regions: expression in untreated and interferon-treated cells and translational activity. Virology 264:106-114. [DOI] [PubMed] [Google Scholar]
- 189.Kawauchi, J., C. Zhang, K. Nobori, Y. Hashimoto, M. T. Adachi, A. Noda, M. Sunamori, and S. Kitajima. 2002. Transcriptional repressor activating transcription factor 3 protects human umbilical vein endothelial cells from tumor necrosis factor-alpha-induced apoptosis through down-regulation of p53 transcription. J. Biol. Chem. 277:39025-39034. [DOI] [PubMed] [Google Scholar]
- 190.Kazemi, S., S. Papadopoulou, S. Li, Q. Su, S. Wang, A. Yoshimura, G. Matlashewski, T. E. Dever, and A. E. Koromilas. 2004. Control of α subunit of eukaryotic translation initiation factor 2 (eIF2α) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2α-dependent gene expression and cell death. Mol. Cell. Biol. 24:3415-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190a.Kerr, I. M., R. E. Brown, and L. A. Ball. 1974. Increased sensitivity of cell-free protein synthesis to double-stranded RNA after interferon treatment. Nature 250:57-59. [DOI] [PubMed] [Google Scholar]
- 191.Khoo, D., C. Perez, and I. Mohr. 2002. Characterization of RNA determinants recognized by the arginine- and proline-rich region of Us11, a herpes simplex virus type 1-encoded double-stranded RNA binding protein that prevents PKR activation. J. Virol. 76:11971-11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kibler, K. V., T. Shors, K. B. Perkins, C. C. Zeman, M. P. Banaszak, J. Biesterfeldt, J. O. Langland, and B. L. Jacobs. 1997. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J. Virol. 71:1992-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim, I., C. W. Liu, and J. D. Puglisi. 2006. Specific recognition of HIV TAR RNA by the dsRNA binding domains (dsRBD1-dsRBD2) of PKR. J. Mol. Biol. 358:430-442. [DOI] [PubMed] [Google Scholar]
- 194.Kim, S. H., A. P. Forman, M. B. Mathews, and S. Gunnery. 2000. Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene 19:3086-3094. [DOI] [PubMed] [Google Scholar]
- 195.Kim, S. H., S. Gunnery, J. K. Choe, and M. B. Mathews. 2002. Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon-inducible protein kinase, PKR. Oncogene 21:8741-8748. [DOI] [PubMed] [Google Scholar]
- 196.Kim, Y. G., M. Muralinath, T. Brandt, M. Pearcy, K. Hauns, K. Lowenhaupt, B. L. Jacobs, and A. Rich. 2003. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc. Natl. Acad. Sci. USA 100:6974-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kirchhoff, S., A. E. Koromilas, F. Schaper, M. Grashoff, N. Sonenberg, and H. Hauser. 1995. IRF-1 induced cell growth inhibition and interferon induction requires the activity of the protein kinase PKR. Oncogene 11:439-445. [PubMed] [Google Scholar]
- 198.Koromilas, A. E., S. Roy, G. N. Barber, M. G. Katze, and N. Sonenberg. 1992. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science 257:1685-1689. [DOI] [PubMed] [Google Scholar]
- 199.Kronfeld-Kinar, Y., S. Vilchik, T. Hyman, F. Leibkowicz, and S. Salzberg. 1999. Involvement of PKR in the regulation of myogenesis. Cell Growth Differ. 10:201-212. [PubMed] [Google Scholar]
- 200.Kuhen, K. L., X. Shen, E. R. Carlisle, A. L. Richardson, H. U. Weier, H. Tanaka, and C. E. Samuel. 1996. Structural organization of the human gene (PKR) encoding an interferon-inducible RNA-dependent protein kinase (PKR) and differences from its mouse homolog. Genomics 36:197-201. [DOI] [PubMed] [Google Scholar]
- 201.Kumar, A., J. Haque, J. Lacoste, J. Hiscott, and B. R. Williams. 1994. Double-stranded RNA-dependent protein kinase activates transcription factor NF-kappa B by phosphorylating I kappa B. Proc. Natl. Acad. Sci. USA 91:6288-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kumar, A., Y. L. Yang, V. Flati, S. Der, S. Kadereit, A. Deb, J. Haque, L. Reis, C. Weissmann, and B. R. Williams. 1997. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-kappaB. EMBO J. 16:406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kumar, K. U., S. P. Srivastava, and R. J. Kaufman. 1999. Double-stranded RNA-activated protein kinase (PKR) is negatively regulated by 60S ribosomal subunit protein L18. Mol. Cell. Biol. 19:1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kuyama, M., G. Nakanishi, J. Arata, K. Iwatsuki, and W. Fujimoto. 2003. Expression of double-stranded RNA-activated protein kinase in keratinocytes and keratinocytic neoplasia. J. Dermatol. 30:579-589. [DOI] [PubMed] [Google Scholar]
- 205.Kwon, H. C., C. H. Moon, S. H. Kim, H. J. Choi, H. S. Lee, M. S. Roh, T. H. Hwang, J. S. Kim, and H. J. Kim. 2005. Expression of double-stranded RNA-activated protein kinase (PKR) and its prognostic significance in lymph node negative rectal cancer. Jpn. J. Clin. Oncol. 35:545-550. [DOI] [PubMed] [Google Scholar]
- 206.Kwon, J. A., and A. Rich. 2005. Biological function of the vaccinia virus Z-DNA-binding protein E3L: gene transactivation and antiapoptotic activity in HeLa cells. Proc. Natl. Acad. Sci. USA 102:12759-12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kyriakis, J. M., and J. Avruch. 1996. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays 18:567-577. [DOI] [PubMed] [Google Scholar]
- 208.Laing, K. G., A. Elia, I. Jeffrey, V. Matys, V. J. Tilleray, B. Souberbielle, and M. J. Clemens. 2002. In vivo effects of the Epstein-Barr virus small RNA EBER-1 on protein synthesis and cell growth regulation. Virology 297:253-269. [DOI] [PubMed] [Google Scholar]
- 209.Langland, J. O., and B. L. Jacobs. 2004. Inhibition of PKR by vaccinia virus: role of the N- and C-terminal domains of E3L. Virology 324:419-429. [DOI] [PubMed] [Google Scholar]
- 210.Langland, J. O., and B. L. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299:133-141. [DOI] [PubMed] [Google Scholar]
- 211.Laurent, A. G., B. Krust, J. Galabru, J. Svab, and A. G. Hovanessian. 1985. Monoclonal antibodies to an interferon-induced Mr 68,000 protein and their use for the detection of double-stranded RNA-dependent protein kinase in human cells. Proc. Natl. Acad. Sci. USA 82:4341-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Reference deleted.
- 213.Lee, J. H., E. J. Park, O. S. Kim, H. Y. Kim, E. H. Joe, and I. Jou. 2005. Double-stranded RNA-activated protein kinase is required for the LPS-induced activation of STAT1 inflammatory signaling in rat brain glial cells. Glia 50:66-79. [DOI] [PubMed] [Google Scholar]
- 214.Lee, S. B., R. Bablanian, and M. Esteban. 1996. Regulated expression of the interferon-induced protein kinase p68 (PKR) by vaccinia virus recombinants inhibits the replication of vesicular stomatitis virus but not that of poliovirus. J. Interferon Cytokine Res. 16:1073-1078. [DOI] [PubMed] [Google Scholar]
- 215.Lee, S. B., and M. Esteban. 1994. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology 199:491-496. [DOI] [PubMed] [Google Scholar]
- 216.Lee, S. B., Z. Melkova, W. Yan, B. R. Williams, A. G. Hovanessian, and M. Esteban. 1993. The interferon-induced double-stranded RNA-activated human p68 protein kinase potently inhibits protein synthesis in cultured cells. Virology 192:380-385. [DOI] [PubMed] [Google Scholar]
- 217.Lee, S. B., D. Rodriguez, J. R. Rodriguez, and M. Esteban. 1997. The apoptosis pathway triggered by the interferon-induced protein kinase PKR requires the third basic domain, initiates upstream of Bcl-2, and involves ICE-like proteases. Virology 231:81-88. [DOI] [PubMed] [Google Scholar]
- 218.Lee, T. G., N. Tang, S. Thompson, J. Miller, and M. G. Katze. 1994. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol. Cell. Biol. 14:2331-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lee, T. G., J. Tomita, A. G. Hovanessian, and M. G. Katze. 1992. Characterization and regulation of the 58,000-dalton cellular inhibitor of the interferon-induced, dsRNA-activated protein kinase. J. Biol. Chem. 267:14238-14243. [PubMed] [Google Scholar]
- 220.Lee, T. G., J. Tomita, A. G. Hovanessian, and M. G. Katze. 1990. Purification and partial characterization of a cellular inhibitor of the interferon-induced protein kinase of Mr 68,000 from influenza virus-infected cells. Proc. Natl. Acad. Sci. USA 87:6208-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lehner, P. J., and P. Cresswell. 1996. Processing and delivery of peptides presented by MHC class I molecules. Curr. Opin. Immunol. 8:59-67. [DOI] [PubMed] [Google Scholar]
- 222.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Leitner, W. W., L. N. Hwang, M. J. deVeer, A. Zhou, R. H. Silverman, B. R. Williams, T. W. Dubensky, H. Ying, and N. P. Restifo. 2003. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 9:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lengyel, P. 1993. Tumor-suppressor genes: news about the interferon connection. Proc. Natl. Acad. Sci. USA 90:5893-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li, J. C., D. C. Lee, B. K. Cheung, and A. S. Lau. 2005. Mechanisms for HIV Tat upregulation of IL-10 and other cytokine expression: kinase signaling and PKR-mediated immune response. FEBS Lett. 579:3055-3062. [DOI] [PubMed] [Google Scholar]
- 226.Li, S., and A. E. Koromilas. 2001. Dominant negative function by an alternatively spliced form of the interferon-inducible protein kinase PKR. J. Biol. Chem. 276:13881-13890. [DOI] [PubMed] [Google Scholar]
- 227.Li, S., J. Y. Min, R. M. Krug, and G. C. Sen. 2006. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 349:13-21. [DOI] [PubMed] [Google Scholar]
- 228.Li, S., K. Nagai, and A. E. Koromilas. 2000. A diminished activation capacity of the interferon-inducible protein kinase PKR in human T lymphocytes. Eur. J. Biochem. 267:1598-1606. [DOI] [PubMed] [Google Scholar]
- 229.Li, S., G. A. Peters, K. Ding, X. Zhang, J. Qin, and G. C. Sen. 2006. Molecular basis for PKR activation by PACT or dsRNA. Proc. Natl. Acad. Sci. USA 103:10005-10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu, Y., K. C. Wolff, B. L. Jacobs, and C. E. Samuel. 2001. Vaccinia virus E3L interferon resistance protein inhibits the interferon-induced adenosine deaminase A-to-I editing activity. Virology 289:378-387. [DOI] [PubMed] [Google Scholar]
- 231.Lu, D., C. D. Wolfgang, and T. Hai. 2006. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J. Biol. Chem. 281:10473-10481. [DOI] [PubMed] [Google Scholar]
- 232.Lu, P. D., H. P. Harding, and D. Ron. 2004. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 234.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.MacLean, A. R., M. ul-Fareed, L. Robertson, J. Harland, and S. M. Brown. 1991. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J Gen. Virol. 72:631-639. [DOI] [PubMed] [Google Scholar]
- 236.Majumdar, R., and U. Maitra. 2005. Regulation of GTP hydrolysis prior to ribosomal AUG selection during eukaryotic translation initiation. EMBO J. 24:3737-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Malathi, K., J. M. Paranjape, E. Bulanova, M. Shim, J. M. Guenther-Johnson, P. W. Faber, T. E. Eling, B. R. Williams, and R. H. Silverman. 2005. A transcriptional signaling pathway in the IFN system mediated by 2′-5′-oligoadenylate activation of RNase L. Proc. Natl. Acad. Sci. USA 102:14533-14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Manche, L., S. R. Green, C. Schmedt, and M. B. Mathews. 1992. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol. Cell. Biol. 12:5238-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Maran, A., R. K. Maitra, A. Kumar, B. Dong, W. Xiao, G. Li, B. R. Williams, P. F. Torrence, and R. H. Silverman. 1994. Blockage of NF-kappa B signaling by selective ablation of an mRNA target by 2-5A antisense chimeras. Science 265:789-792. [DOI] [PubMed] [Google Scholar]
- 240.Maran, A., and M. B. Mathews. 1988. Characterization of the double-stranded RNA implicated in the inhibition of protein synthesis in cells infected with a mutant adenovirus defective for VA RNA. Virology 164:106-113. [DOI] [PubMed] [Google Scholar]
- 241.Markert, J. M., M. D. Medlock, S. D. Rabkin, G. Y. Gillespie, T. Todo, W. D. Hunter, C. A. Palmer, F. Feigenbaum, C. Tornatore, F. Tufaro, and R. L. Martuza. 2000. Conditionally replicating herpes simplex virus mutant, G207, for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 7:867-874. [DOI] [PubMed] [Google Scholar]
- 242.Martinez-Costas, J., C. Gonzalez-Lopez, V. N. Vakharia, and J. Benavente. 2000. Possible involvement of the double-stranded RNA-binding core protein σA in the resistance of avian reovirus to interferon. J. Virol. 74:1124-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.McMillan, N. A., R. F. Chun, D. P. Siderovski, J. Galabru, W. M. Toone, C. E. Samuel, T. W. Mak, A. G. Hovanessian, K. T. Jeang, and B. R. Williams. 1995. HIV-1 Tat directly interacts with the interferon-induced, double-stranded RNA-dependent kinase, PKR. Virology 213:413-424. [DOI] [PubMed] [Google Scholar]
- 244.Metz, D. H., and M. Esteban. 1972. Interferon inhibits viral protein synthesis in L cells infected with vaccinia virus. Nature 238:385-388. [DOI] [PubMed] [Google Scholar]
- 245.Meurs, E., K. Chong, J. Galabru, N. S. Thomas, I. M. Kerr, B. R. Williams, and A. G. Hovanessian. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62:379-390. [DOI] [PubMed] [Google Scholar]
- 246.Meurs, E. F., J. Galabru, G. N. Barber, M. G. Katze, and A. G. Hovanessian. 1993. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 90:232-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mhashilkar, A. M., A. L. Stewart, K. Sieger, H. Y. Yang, A. H. Khimani, I. Ito, Y. Saito, K. K. Hunt, E. A. Grimm, J. A. Roth, R. E. Meyn, R. Ramesh, and S. Chada. 2003. MDA-7 negatively regulates the beta-catenin and PI3K signaling pathways in breast and lung tumor cells. Mol. Ther. 8:207-219. [DOI] [PubMed] [Google Scholar]
- 248.Mohr, I., and Y. Gluzman. 1996. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 15:4759-4766. [PMC free article] [PubMed] [Google Scholar]
- 249.Morimoto, H., A. Ozaki, H. Okamura, K. Yoshida, S. Kitamura, and T. Haneji. 2005. Okadaic acid induces tyrosine phosphorylation of IkappaBalpha that mediated by PKR pathway in human osteoblastic MG63 cells. Mol. Cell. Biochem. 276:211-217. [DOI] [PubMed] [Google Scholar]
- 250.Morris, S. W., M. N. Kirstein, M. B. Valentine, K. G. Dittmer, D. N. Shapiro, D. L. Saltman, and A. T. Look. 1994. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 263:1281-1284. [DOI] [PubMed] [Google Scholar]
- 251.Mosser, D. D., A. W. Caron, L. Bourget, A. B. Meriin, M. Y. Sherman, R. I. Morimoto, and B. Massie. 2000. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 20:7146-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mulvey, M., J. Poppers, D. Sternberg, and I. Mohr. 2003. Regulation of eIF2α phosphorylation by different functions that act during discrete phases in the herpes simplex virus type 1 life cycle. J. Virol. 77:10917-10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mundschau, L. J., and D. V. Faller. 1994. Endogenous inhibitors of the dsRNA-dependent eIF-2 alpha protein kinase PKR in normal and ras-transformed cells. Biochimie 76:792-800. [DOI] [PubMed] [Google Scholar]
- 254.Mundschau, L. J., and D. V. Faller. 1995. Platelet-derived growth factor signal transduction through the interferon-inducible kinase PKR. Immediate early gene induction. J. Biol. Chem. 270:3100-3106. [DOI] [PubMed] [Google Scholar]
- 255.Munoz-Fontela, C., M. Collado, E. Rodriguez, M. A. Garcia, A. Alvarez-Barrientos, J. Arroyo, C. Nombela, and C. Rivas. 2005. Identification of a nuclear export signal in the KSHV latent protein LANA2 mediating its export from the nucleus. Exp. Cell Res. 311:96-105. [DOI] [PubMed] [Google Scholar]
- 256.Murad, J. M., L. G. Tone, L. R. de Souza, and F. L. De Lucca. 2005. A point mutation in the RNA-binding domain I results in decrease of PKR activation in acute lymphoblastic leukemia. Blood Cells Mol. Dis. 34:1-5. [DOI] [PubMed] [Google Scholar]
- 257.Nanbo, A., K. Inoue, K. Adachi-Takasawa, and K. Takada. 2002. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt's lymphoma. EMBO J. 21:954-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nanduri, S., B. Carpick, Y. Yang, B. R. Williams, and J. Qin. 1998. 1H, 13C, 15N resonance assignment of the 20 kDa double stranded RNA binding domain of PKR. J. Biomol. NMR 12:349-351. [DOI] [PubMed] [Google Scholar]
- 259.Nanduri, S., B. W. Carpick, Y. Yang, B. R. Williams, and J. Qin. 1998. Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J. 17:5458-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nawa, T., M. T. Nawa, M. T. Adachi, I. Uchimura, R. Shimokawa, K. Fujisawa, A. Tanaka, F. Numano, and S. Kitajima. 2002. Expression of transcriptional repressor ATF3/LRF1 in human atherosclerosis: colocalization and possible involvement in cell death of vascular endothelial cells. Atherosclerosis 161:281-291. [DOI] [PubMed] [Google Scholar]
- 261.Noguchi, T., S. Satoh, T. Noshi, E. Hatada, R. Fukuda, A. Kawai, S. Ikeda, M. Hijikata, and K. Shimotohno. 2001. Effects of mutation in hepatitis C virus nonstructural protein 5A on interferon resistance mediated by inhibition of PKR kinase activity in mammalian cells. Microbiol. Immunol. 45:829-840. [DOI] [PubMed] [Google Scholar]
- 262.Nozawa, Y., N. Van Belzen, A. C. Van der Made, W. N. Dinjens, and F. T. Bosman. 1996. Expression of nucleophosmin/B23 in normal and neoplastic colorectal mucosa. J. Pathol. 178:48-52. [DOI] [PubMed] [Google Scholar]
- 263.Nussbaum, J. M., S. Gunnery, and M. B. Mathews. 2002. The 3′-untranslated regions of cytoskeletal muscle mRNAs inhibit translation by activating the double-stranded RNA-dependent protein kinase PKR. Nucleic Acids Res. 30:1205-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nussbaum, J. M., M. Major, and S. Gunnery. 2003. Transcriptional upregulation of interferon-induced protein kinase, PKR, in breast cancer. Cancer Lett. 196:207-216. [DOI] [PubMed] [Google Scholar]
- 265.Offermann, M. K., J. Zimring, K. H. Mellits, M. K. Hagan, R. Shaw, R. M. Medford, M. B. Mathews, S. Goodbourn, and R. Jagus. 1995. Activation of the double-stranded-RNA-activated protein kinase and induction of vascular cell adhesion molecule-1 by poly (I). poly (C) in endothelial cells. Eur. J. Biochem. 232:28-36. [DOI] [PubMed] [Google Scholar]
- 266.Oganesyan, G., S. K. Saha, B. Guo, J. Q. He, A. Shahangian, B. Zarnegar, A. Perry, and G. Cheng. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439:208-211. [DOI] [PubMed] [Google Scholar]
- 267.Olson, M. O., M. O. Wallace, A. H. Herrera, L. Marshall-Carlson, and R. C. Hunt. 1986. Preribosomal ribonucleoprotein particles are a major component of a nucleolar matrix fraction. Biochemistry 25:484-491. [DOI] [PubMed] [Google Scholar]
- 268.Ong, C. L., J. C. Thorpe, P. R. Gorry, S. Bannwarth, A. Jaworowski, J. L. Howard, S. Chung, S. Campbell, H. S. Christensen, G. Clerzius, A. J. Mouland, A. Gatignol, and D. F. Purcell. 2005. Low TRBP levels support an innate human immunodeficiency virus type 1 resistance in astrocytes by enhancing the PKR antiviral response. J. Virol. 79:12763-12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Onuki, R., Y. Bando, E. Suyama, T. Katayama, H. Kawasaki, T. Baba, M. Tohyama, and K. Taira. 2004. An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer's disease. EMBO J. 23:959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Paez, E., and M. Esteban. 1984. Nature and mode of action of vaccinia virus products that block activation of the interferon-mediated ppp(A2′p)nA-synthetase. Virology 134:29-39. [DOI] [PubMed] [Google Scholar]
- 271.Paez, E., and M. Esteban. 1984. Resistance of vaccinia virus to interferon is related to an interference phenomenon between the virus and the interferon system. Virology 134:12-28. [DOI] [PubMed] [Google Scholar]
- 272.Pang, Q., T. A. Christianson, W. Keeble, T. Koretsky, and G. C. Bagby. 2002. The anti-apoptotic function of Hsp70 in the interferon-inducible double-stranded RNA-dependent protein kinase-mediated death signaling pathway requires the Fanconi anemia protein, FANCC. J. Biol. Chem. 277:49638-49643. [DOI] [PubMed] [Google Scholar]
- 273.Pang, Q., T. A. Christianson, T. Koretsky, H. Carlson, L. David, W. Keeble, G. R. Faulkner, A. Speckhart, and G. C. Bagby. 2003. Nucleophosmin interacts with and inhibits the catalytic function of eukaryotic initiation factor 2 kinase PKR. J. Biol. Chem. 278:41709-41717. [DOI] [PubMed] [Google Scholar]
- 274.Park, H., M. V. Davies, J. O. Langland, H. W. Chang, Y. S. Nam, J. Tartaglia, E. Paoletti, B. L. Jacobs, R. J. Kaufman, and S. Venkatesan. 1994. TAR RNA-binding protein is an inhibitor of the interferon-induced protein kinase PKR. Proc. Natl. Acad. Sci. USA 91:4713-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pataer, A., S. Chada, K. K. Hunt, J. A. Roth, and S. G. Swisher. 2003. Adenoviral melanoma differentiation-associated gene 7 induces apoptosis in lung cancer cells through mitochondrial permeability transition-independent cytochrome c release. J. Thorac. Cardiovasc. Surg. 125:1328-1335. [DOI] [PubMed] [Google Scholar]
- 276.Pataer, A., S. A. Vorburger, G. N. Barber, S. Chada, A. M. Mhashilkar, H. Zou-Yang, A. L. Stewart, S. Balachandran, J. A. Roth, K. K. Hunt, and S. G. Swisher. 2002. Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via up-regulation of the double-stranded RNA-dependent protein kinase (PKR). Cancer Res. 62:2239-2243. [PubMed] [Google Scholar]
- 277.Pataer, A., S. A. Vorburger, S. Chada, S. Balachandran, G. N. Barber, J. A. Roth, K. K. Hunt, and S. G. Swisher. 2005. Melanoma differentiation-associated gene-7 protein physically associates with the double-stranded RNA-activated protein kinase PKR. Mol. Ther. 11:717-723. [DOI] [PubMed] [Google Scholar]
- 278.Patel, C. V., I. Handy, T. Goldsmith, and R. C. Patel. 2000. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J. Biol. Chem. 275:37993-37998. [DOI] [PubMed] [Google Scholar]
- 279.Patel, R. C., and G. C. Sen. 1998. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 17:4379-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Peel, A. L. 2004. PKR activation in neurodegenerative disease. J. Neuropathol. Exp. Neurol. 63:97-105. [DOI] [PubMed] [Google Scholar]
- 281.Pestka, S., C. D. Krause, and M. R. Walter. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202:8-32. [DOI] [PubMed] [Google Scholar]
- 282.Peters, G. A., R. Hartmann, J. Qin, and G. C. Sen. 2001. Modular structure of PACT: distinct domains for binding and activating PKR. Mol. Cell. Biol. 21:1908-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Peters, G. A., D. Khoo, I. Mohr, and G. C. Sen. 2002. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J. Virol. 76:11054-11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Petryshyn, R. A., J. Li, and R. Judware. 1994. Activation of the dsRNA-dependent kinase. Prog. Mol. Subcell. Biol. 14:1-14. [DOI] [PubMed] [Google Scholar]
- 285.Reference deleted.
- 286.Pomerantz, J. L., and D. Baltimore. 2002. Two pathways to NF-κB. Mol. Cell 10:693-695. [DOI] [PubMed] [Google Scholar]
- 287.Poppers, J., M. Mulvey, C. Perez, D. Khoo, and I. Mohr. 2003. Identification of a lytic-cycle Epstein-Barr virus gene product that can regulate PKR activation. J. Virol. 77:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Prostko, C. R., J. N. Dholakia, M. A. Brostrom, and C. O. Brostrom. 1995. Activation of the double-stranded RNA-regulated protein kinase by depletion of endoplasmic reticular calcium stores. J. Biol. Chem. 270:6211-6215. [DOI] [PubMed] [Google Scholar]
- 289.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Derijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ramana, C. V., N. Grammatikakis, M. Chernov, H. Nguyen, K. C. Goh, B. R. Williams, and G. R. Stark. 2000. Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. EMBO J. 19:263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rampling, R., G. Cruickshank, V. Papanastassiou, J. Nicoll, D. Hadley, D. Brennan, R. Petty, A. MacLean, J. Harland, E. McKie, R. Mabbs, and M. Brown. 2000. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 7:859-866. [DOI] [PubMed] [Google Scholar]
- 292.Raveh, T., A. G. Hovanessian, E. F. Meurs, N. Sonenberg, and A. Kimchi. 1996. Double-stranded RNA-dependent protein kinase mediates c-Myc suppression induced by type I interferons. J. Biol. Chem. 271:25479-25484. [DOI] [PubMed] [Google Scholar]
- 293.Rhoads, R. E. 1993. Regulation of eukaryotic protein synthesis by initiation factors. J. Biol. Chem. 268:3017-3020. [PubMed] [Google Scholar]
- 294.Rivas, C., J. Gil, Z. Melkova, M. Esteban, and M. Diaz-Guerra. 1998. Vaccinia virus E3L protein is an inhibitor of the interferon (i.f.n.)-induced 2-5A synthetase enzyme. Virology 243:406-414. [DOI] [PubMed] [Google Scholar]
- 295.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Roberts, W. K., A. Hovanessian, R. E. Brown, M. J. Clemens, and I. M. Kerr. 1976. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature 264:477-480. [DOI] [PubMed] [Google Scholar]
- 297.Roff, M., J. Thompson, M. S. Rodriguez, J. M. Jacque, F. Baleux, F. Arenzana-Seisdedos, and R. T. Hay. 1996. Role of IkappaBalpha ubiquitination in signal-induced activation of NFkappaB in vivo. J. Biol. Chem. 271:7844-7850. [DOI] [PubMed] [Google Scholar]
- 298.Roh, M. S., J. Y. Kwak, S. J. Kim, H. W. Lee, H. C. Kwon, T. H. Hwang, P. J. Choi, and Y. S. Hong. 2005. Expression of double-stranded RNA-activated protein kinase in small-size peripheral adenocarcinoma of the lung. Pathol. Int. 55:688-693. [DOI] [PubMed] [Google Scholar]
- 299.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Romano, P. R., F. Zhang, S. L. Tan, M. T. Garcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Roy, S., M. G. Katze, N. T. Parkin, I. Edery, A. G. Hovanessian, and N. Sonenberg. 1990. Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product. Science 247:1216-1219. [DOI] [PubMed] [Google Scholar]
- 302.Ruvolo, P. P., F. Gao, W. L. Blalock, X. Deng, and W. S. May. 2001. Ceramide regulates protein synthesis by a novel mechanism involving the cellular PKR activator RAX. J. Biol. Chem. 276:11754-11758. [DOI] [PubMed] [Google Scholar]
- 303.Salzberg, S., S. Vilchik, S. Cohen, A. Heller, and Y. Kronfeld-Kinar. 2000. Expression of a PKR dominant-negative mutant in myogenic cells interferes with the myogenic process. Exp. Cell Res. 254:45-54. [DOI] [PubMed] [Google Scholar]
- 304.Samuel, C. E. 1991. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology 183:1-11. [DOI] [PubMed] [Google Scholar]
- 305.Samuel, C. E., K. L. Kuhen, C. X. George, L. G. Ortega, R. Rende-Fournier, and H. Tanaka. 1997. The PKR protein kinase—an interferon-inducible regulator of cell growth and differentiation. Int. J. Hematol. 65:227-237. [DOI] [PubMed] [Google Scholar]
- 306.Sarkar, D., Z. Z. Su, I. V. Lebedeva, M. Sauane, R. V. Gopalkrishnan, K. Valerie, P. Dent, and P. B. Fisher. 2002. mda-7 (IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc. Natl. Acad. Sci. USA 99:10054-10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sarrazin, C., M. Bruckner, E. Herrmann, B. Ruster, K. Bruch, W. K. Roth, and S. Zeuzem. 2001. Quasispecies heterogeneity of the carboxy-terminal part of the E2 gene including the PePHD and sensitivity of hepatitis C virus 1b isolates to antiviral therapy. Virology 289:150-163. [DOI] [PubMed] [Google Scholar]
- 308.Sarrazin, C., I. Kornetzky, B. Ruster, J. H. Lee, B. Kronenberger, K. Bruch, W. K. Roth, and S. Zeuzem. 2000. Mutations within the E2 and NS5A protein in patients infected with hepatitis C virus type 3a and correlation with treatment response. Hepatology 31:1360-1370. [DOI] [PubMed] [Google Scholar]
- 309.Saunders, L. R., and G. N. Barber. 2003. The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J. 17:961-983. [DOI] [PubMed] [Google Scholar]
- 310.Savkur, R. S., and M. O. Olson. 1998. Preferential cleavage in pre-ribosomal RNA byprotein B23 endoribonuclease. Nucleic Acids Res. 26:4508-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Schiappa, D. A., C. Mittal, J. A. Brown, and B. P. Mika. 2002. Relationship of hepatitis C genotype 1 NS5A sequence mutations to early phase viral kinetics and interferon effectiveness. J. Infect. Dis. 185:868-877. [DOI] [PubMed] [Google Scholar]
- 312.Schlee, M., V. Hornung, and G. Hartmann. 2006. siRNA and isRNA: two edges of one sword. Mol. Ther. 14:463-470. [DOI] [PubMed] [Google Scholar]
- 313.Sedger, L., and J. Ruby. 1994. Heat shock response to vaccinia virus infection. J. Virol. 68:4685-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Sekellick, M. J., S. A. Carra, A. Bowman, D. A. Hopkins, and P. I. Marcus. 2000. Transient resistance of influenza virus to interferon action attributed to random multiple packaging and activity of NS genes. J. Interferon Cytokine Res. 20:963-970. [DOI] [PubMed] [Google Scholar]
- 315.Sen, A., R. Steele, A. K. Ghosh, A. Basu, R. Ray, and R. B. Ray. 2003. Inhibition of hepatitis C virus protein expression by RNA interference. Virus Res. 96:27-35. [DOI] [PubMed] [Google Scholar]
- 316.Sen, G. C., and P. Lengyel. 1992. The interferon system. A bird's eye view of its biochemistry. J. Biol. Chem. 267:5017-5020. [PubMed] [Google Scholar]
- 317.Sen, G. C., H. Taira, and P. Lengyel. 1978. Interferon, double-stranded RNA, and protein phosphorylation. Characteristics of a double-stranded RNA-activated protein kinase system partially purified from interferon treated Ehrlich ascites tumor cells. J. Biol. Chem. 253:5915-5921. [PubMed] [Google Scholar]
- 318.SenGupta, D. N., B. Berkhout, A. Gatignol, A. M. Zhou, and R. H. Silverman. 1990. Direct evidence for translational regulation by leader RNA and Tat protein of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 87:7492-7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Shang, Y., C. R. Baumrucker, and M. H. Green. 1998. c-Myc is a major mediator of the synergistic growth inhibitory effects of retinoic acid and interferon in breast cancer cells. J. Biol. Chem. 273:30608-30613. [DOI] [PubMed] [Google Scholar]
- 320.Sharp, T. V., F. Moonan, A. Romashko, B. Joshi, G. N. Barber, and R. Jagus. 1998. The vaccinia virus E3L gene product interacts with both the regulatory and the substrate binding regions of PKR: implications for PKR autoregulation. Virology 250:302-315. [DOI] [PubMed] [Google Scholar]
- 321.Sharp, T. V., M. Schwemmle, I. Jeffrey, K. Laing, H. Mellor, C. G. Proud, K. Hilse, and M. J. Clemens. 1993. Comparative analysis of the regulation of the interferon-inducible protein kinase PKR by Epstein-Barr virus RNAs EBER-1 and EBER-2 and adenovirus VAI RNA. Nucleic Acids Res. 21:4483-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Sharp, T. V., J. E. Witzel, and R. Jagus. 1997. Homologous regions of the alpha subunit of eukaryotic translational initiation factor 2 (eIF2alpha) and the vaccinia virus K3L gene product interact with the same domain within the dsRNA-activated protein kinase (PKR). Eur. J. Biochem. 250:85-91. [DOI] [PubMed] [Google Scholar]
- 323.Shimada, A., G. Shiota, H. Miyata, T. Kamahora, H. Kawasaki, K. Shiraki, S. Hino, and T. Terada. 1998. Aberrant expression of double-stranded RNA-dependent protein kinase in hepatocytes of chronic hepatitis and differentiated hepatocellular carcinoma. Cancer Res. 58:4434-4438. [PubMed] [Google Scholar]
- 324.Shir, A., I. Friedrich, and A. Levitzki. 2003. Tumor specific activation of PKR as a non-toxic modality of cancer treatment. Semin. Cancer Biol. 13:309-314. [DOI] [PubMed] [Google Scholar]
- 325.Shir, A., and A. Levitzki. 2002. Inhibition of glioma growth by tumor-specific activation of double-stranded RNA-dependent protein kinase PKR. Nat. Biotechnol. 20:895-900. [DOI] [PubMed] [Google Scholar]
- 326.Shors, T., K. V. Kibler, K. B. Perkins, R. Seidler-Wulff, M. P. Banaszak, and B. L. Jacobs. 1997. Complementation of vaccinia virus deleted of the E3L gene by mutants of E3L. Virology 239:269-276. [DOI] [PubMed] [Google Scholar]
- 327.Siffroi, J. P., A. Pawlak, M. F. Alfonsi, F. Troalen, G. Guellaen, and J. P. Dadoune. 2001. Expression of the TAR RNA binding protein in human testis. Mol. Hum. Reprod. 7:219-225. [DOI] [PubMed] [Google Scholar]
- 328.Sionov, R. V., and Y. Haupt. 1999. The cellular response to p53: the decision between life and death. Oncogene 18:6145-6157. [DOI] [PubMed] [Google Scholar]
- 329.Sledz, C. A., M. Holko, M. J. de Veer, R. H. Silverman, and B. R. Williams. 2003. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5:834-839. [DOI] [PubMed] [Google Scholar]
- 330.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or Ikappa B kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 331.Squire, J., E. F. Meurs, K. L. Chong, N. A. McMillan, A. G. Hovanessian, and B. R. Williams. 1993. Localization of the human interferon-induced, ds-RNA activated p68 kinase gene (PRKR) to chromosome 2p21-p22. Genomics 16:768-770. [DOI] [PubMed] [Google Scholar]
- 332.Srivastava, S. P., M. V. Davies, and R. J. Kaufman. 1995. Calcium depletion from the endoplasmic reticulum activates the double-stranded RNA-dependent protein kinase (PKR) to inhibit protein synthesis. J. Biol. Chem. 270:16619-16624. [DOI] [PubMed] [Google Scholar]
- 333.Srivastava, S. P., K. U. Kumar, and R. J. Kaufman. 1998. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 273:2416-2423. [DOI] [PubMed] [Google Scholar]
- 333a.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 334.Stewart, M. J., M. A. Blum, and B. Sherry. 2003. PKR's protective role in viral myocarditis. Virology 314:92-100. [DOI] [PubMed] [Google Scholar]
- 335.Stojdl, D. F., N. Abraham, S. Knowles, R. Marius, A. Brasey, B. D. Lichty, E. G. Brown, N. Sonenberg, and J. C. Bell. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 74:9580-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Strong, J. E., M. C. Coffey, D. Tang, P. Sabinin, and P. W. Lee. 1998. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17:3351-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Su, Q., S. Wang, D. Baltzis, L. K. Qu, A. H. Wong, and A. E. Koromilas. 2006. Tyrosine phosphorylation acts as a molecular switch to full-scale activation of the eIF2alpha RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 103:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sudhakar, A., A. Ramachandran, S. Ghosh, S. E. Hasnain, R. J. Kaufman, and K. V. Ramaiah. 2000. Phosphorylation of serine 51 in initiation factor 2 alpha (eIF2 alpha) promotes complex formation between eIF2 alpha(P) and eIF2B and causes inhibition in the guanine nucleotide exchange activity of eIF2B. Biochemistry 39:12929-12938. [DOI] [PubMed] [Google Scholar]
- 339.Sugimoto, M., M. L. Kuo, M. F. Roussel, and C. J. Sherr. 2003. Nucleolar Arf tumor suppressor inhibits ribosomal RNA processing. Mol. Cell 11:415-424. [DOI] [PubMed] [Google Scholar]
- 340.Syed, V., K. Mukherjee, J. Lyons-Weiler, K. M. Lau, T. Mashima, T. Tsuruo, and S. M. Ho. 2005. Identification of ATF-3, caveolin-1, DLC-1, and NM23-H2 as putative antitumorigenic, progesterone-regulated genes for ovarian cancer cells by gene profiling. Oncogene 24:1774-1787. [DOI] [PubMed] [Google Scholar]
- 341.Symons, J. A., A. Alcami, and G. L. Smith. 1995. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 81:551-560. [DOI] [PubMed] [Google Scholar]
- 342.Taddeo, B., T. R. Luo, W. Zhang, and B. Roizman. 2003. Activation of NF-kappaB in cells productively infected with HSV-1 depends on activated protein kinase R and plays no apparent role in blocking apoptosis. Proc. Natl. Acad. Sci. USA 100:12408-12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 344.Takizawa, T., K. Ohashi, and Y. Nakanishi. 1996. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J. Virol. 70:8128-8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Tamura, K., B. Hua, S. Adachi, I. Guney, J. Kawauchi, M. Morioka, M. Tamamori-Adachi, Y. Tanaka, Y. Nakabeppu, M. Sunamori, J. M. Sedivy, and S. Kitajima. 2005. Stress response gene ATF3 is a target of c-myc in serum-induced cell proliferation. EMBO J. 24:2590-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Tan, S., Y. Sagara, Y. Liu, P. Maher, and D. Schubert. 1998. The regulation of reactive oxygen species production during programmed cell death. J. Cell Biol. 141:1423-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Tan, S. L., and M. G. Katze. 1998. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J. Interferon Cytokine Res. 18:757-766. [DOI] [PubMed] [Google Scholar]
- 349.Tan, S. L., and M. G. Katze. 2001. How hepatitis C virus counteracts the interferon response: the jury is still out on NS5A. Virology 284:1-12. [DOI] [PubMed] [Google Scholar]
- 350.Tanaka, H., and C. E. Samuel. 1994. Mechanism of interferon action: structure of the mouse PKR gene encoding the interferon-inducible RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 91:7995-7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tang, N. M., M. J. Korth, M. Gale, Jr., M. Wambach, S. D. Der, S. K. Bandyopadhyay, B. R. Williams, and M. G. Katze. 1999. Inhibition of double-stranded RNA- and tumor necrosis factor alpha-mediated apoptosis by tetratricopeptide repeat protein and cochaperone P58(IPK). Mol. Cell. Biol. 19:4757-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Taniguchi, T., and A. Takaoka. 2002. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14:111-116. [DOI] [PubMed] [Google Scholar]
- 353.Taylor, D. R., S. B. Lee, P. R. Romano, D. R. Marshak, A. G. Hinnebusch, M. Esteban, and M. B. Mathews. 1996. Autophosphorylation sites participate in the activation of the double-stranded-RNA-activated protein kinase PKR. Mol. Cell. Biol. 16:6295-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Taylor, D. R., S. T. Shi, P. R. Romano, G. N. Barber, and M. M. Lai. 1999. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 285:107-110. [DOI] [PubMed] [Google Scholar]
- 355.Taylor, S. S., N. M. Haste, and G. Ghosh. 2005. PKR and eIF2alpha: integration of kinase dimerization, activation, and substrate docking. Cell 122:823-825. [DOI] [PubMed] [Google Scholar]
- 356.Terada, T., H. Maeta, K. Endo, and T. Ohta. 2000. Protein expression of double-stranded RNA-activated protein kinase in thyroid carcinomas: correlations with histologic types, pathologic parameters, and Ki-67 labeling. Hum. Pathol. 31:817-821. [DOI] [PubMed] [Google Scholar]
- 357.Terada, T., J. Ueyama, Y. Ukita, and T. Ohta. 2000. Protein expression of double-stranded RNA-activated protein kinase (PKR) in intrahepatic bile ducts in normal adult livers, fetal livers, primary biliary cirrhosis, hepatolithiasis and intrahepatic cholangiocarcinoma. Liver 20:450-457. [DOI] [PubMed] [Google Scholar]
- 358.Turk, B. E., E. C. Griffith, S. Wolf, K. Biemann, Y. H. Chang, and J. O. Liu. 1999. Selective inhibition of amino-terminal methionine processing by TNP-470 and ovalicin in endothelial cells. Chem. Biol. 6:823-833. [DOI] [PubMed] [Google Scholar]
- 359.Ung, T. L., C. Cao, J. Lu, K. Ozato, and T. E. Dever. 2001. Heterologous dimerization domains functionally substitute for the double-stranded RNA binding domains of the kinase PKR. EMBO J. 20:3728-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Valyi-Nagy, T., M. U. Fareed, J. S. O'Keefe, R. M. Gesser, A. R. MacLean, S. M. Brown, J. G. Spivack, and N. W. Fraser. 1994. The herpes simplex virus type 1 strain 17+ gamma 34.5 deletion mutant 1716 is avirulent in SCID mice. J Gen. Virol. 75:2059-2063. [DOI] [PubMed] [Google Scholar]
- 361.Reference deleted.
- 362.Reference deleted.
- 363.Ventoso, I., M. A. Sanz, S. Molina, J. J. Berlanga, L. Carrasco, and M. Esteban. 2006. Translational resistance of late alphavirus mRNA to eIF2alpha phosphorylation: a strategy to overcome the antiviral effect of protein kinase PKR. Genes Dev. 20:87-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Verma, I. M., J. K. Stevenson, E. M. Schwarz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 9:2723-2735. [DOI] [PubMed] [Google Scholar]
- 365.Visvanathan, K. V., and S. Goodbourn. 1989. Double-stranded RNA activates binding of NF-kappa B to an inducible element in the human beta-interferon promoter. EMBO J. 8:1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.von Holzen, U., D. Bocangel, A. Pataer, S. A. Vorburger, Y. Liu, X. Lu, K. K. Hunt, and S. G. Swisher. 2005. Role for the double-stranded RNA-activated protein kinase PKR in Ad-TNF-alpha gene therapy in esophageal cancer. Surgery 138:261-268. [DOI] [PubMed] [Google Scholar]
- 367.Vorburger, S. A., N. Hetrakul, W. Xia, M. Wilson-Heiner, N. Mirza, R. E. Pollock, B. Feig, S. G. Swisher, and K. K. Hunt. 2005. Gene therapy with E2F-1 up-regulates the protein kinase PKR and inhibits growth of leiomyosarcoma in vivo. Mol. Cancer Ther. 4:1710-1716. [DOI] [PubMed] [Google Scholar]
- 368.Vorburger, S. A., A. Pataer, S. G. Swisher, and K. K. Hunt. 2004. Genetically targeted cancer therapy: tumor destruction by PKR activation. Am. J. Pharmacogenomics 4:189-198. [DOI] [PubMed] [Google Scholar]
- 369.Vorburger, S. A., A. Pataer, K. Yoshida, G. N. Barber, W. Xia, P. Chiao, L. M. Ellis, M. C. Hung, S. G. Swisher, and K. K. Hunt. 2002. Role for the double-stranded RNA activated protein kinase PKR in E2F-1-induced apoptosis. Oncogene 21:6278-6288. [DOI] [PubMed] [Google Scholar]
- 370.Wada, N., M. Matsumura, Y. Ohba, N. Kobayashi, T. Takizawa, and Y. Nakanishi. 1995. Transcription stimulation of the Fas-encoding gene by nuclear factor for interleukin-6 expression upon influenza virus infection. J. Biol. Chem. 270:18007-18012. [DOI] [PubMed] [Google Scholar]
- 371.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Watanabe, M. A., L. R. de Souza, J. M. Murad, and F. L. De Lucca. 2005. Activation of the RNA-dependent protein kinase (PKR) of lymphocytes by regulatory RNAs: implications for immunomodulation in HIV infection. Curr. HIV Res. 3:329-337. [DOI] [PubMed] [Google Scholar]
- 373.Watson, J. C., H. W. Chang, and B. L. Jacobs. 1991. Characterization of a vaccinia virus-encoded double-stranded RNA-binding protein that may be involved in inhibition of the double-stranded RNA-dependent protein kinase. Virology 185:206-216. [DOI] [PubMed] [Google Scholar]
- 374.Wesche, H., W. J. Henzel, W. Shillinglaw, S. Li, and Z. Cao. 1997. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7:837-847. [DOI] [PubMed] [Google Scholar]
- 375.Wilson, J. E., T. V. Pestova, C. U. Hellen, and P. Sarnow. 2000. Initiation of protein synthesis from the A site of the ribosome. Cell 102:511-520. [DOI] [PubMed] [Google Scholar]
- 376.Williams, B. R. 1999. PKR; a sentinel kinase for cellular stress. Oncogene 18:6112-6120. [DOI] [PubMed] [Google Scholar]
- 377.Williams, B. R. 1997. Role of the double-stranded RNA-activated protein kinase (PKR) in cell regulation. Biochem. Soc. Trans. 25:509-513. [DOI] [PubMed] [Google Scholar]
- 378.Williams, B. R. 2001. Signal integration via PKR. Sci. STKE 2001:RE2. [DOI] [PubMed] [Google Scholar]
- 379.Willman, C. L., C. E. Sever, M. G. Pallavicini, H. Harada, N. Tanaka, M. L. Slovak, H. Yamamoto, K. Harada, T. C. Meeker, A. F. List, et al. 1993. Deletion of IRF-1, mapping to chromosome 5q31.1, in human leukemia and preleukemic myelodysplasia. Science 259:968-971. [DOI] [PubMed] [Google Scholar]
- 380.Woldehawariat, G., S. Nekhai, and R. Petryshyn. 1999. Differential phosphorylation of PKR associates with deregulation of eIF-2alpha phosphorylation and altered growth characteristics in 3T3-F442A fibroblasts. Mol. Cell. Biochem. 198:7-17. [DOI] [PubMed] [Google Scholar]
- 381.Wong, A. H., N. W. Tam, Y. L. Yang, A. R. Cuddihy, S. Li, S. Kirchhoff, H. Hauser, T. Decker, and A. E. Koromilas. 1997. Physical association between STAT1 and the interferon-inducible protein kinase PKR and implications for interferon and double-stranded RNA signaling pathways. EMBO J. 16:1291-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wu, M. H., and B. Y. Yung. 2002. UV stimulation of nucleophosmin/B23 expression is an immediate-early gene response induced by damaged DNA. J. Biol. Chem. 277:48234-48240. [DOI] [PubMed] [Google Scholar]
- 383.Wu, S., K. U. Kumar, and R. J. Kaufman. 1998. Identification and requirement of three ribosome binding domains in dsRNA-dependent protein kinase (PKR). Biochemistry 37:13816-13826. [DOI] [PubMed] [Google Scholar]
- 384.Wu, X., and A. J. Levine. 1994. p53 and E2F-1 cooperate to mediate apoptosis. Proc. Natl. Acad. Sci. USA 91:3602-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Yajima, M., T. Kanda, and K. Takada. 2005. Critical role of Epstein-Barr virus (EBV)-encoded RNA in efficient EBV-induced B-lymphocyte growth transformation. J. Virol. 79:4298-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Yaman, I., J. Fernandez, H. Liu, M. Caprara, A. A. Komar, A. E. Koromilas, L. Zhou, M. D. Snider, D. Scheuner, R. J. Kaufman, and M. Hatzoglou. 2003. The zipper model of translational control: a small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell 113:519-531. [DOI] [PubMed] [Google Scholar]
- 387.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 388.Yan, C., M. S. Jamaluddin, B. Aggarwal, J. Myers, and D. D. Boyd. 2005. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol. Cancer Ther. 4:233-241. [PubMed] [Google Scholar]
- 389.Yan, C., D. Lu, T. Hai, and D. D. Boyd. 2005. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. EMBO J. 24:2425-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Yeung, M. C., D. L. Chang, R. E. Camantigue, and A. S. Lau. 1999. Inhibitory role of the host apoptogenic gene PKR in the establishment of persistent infection by encephalomyocarditis virus in U937 cells. Proc. Natl. Acad. Sci. USA 96:11860-11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Yeung, M. C., J. Liu, and A. S. Lau. 1996. An essential role for the interferon-inducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc. Natl. Acad. Sci. USA 93:12451-12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 394.Yue, Z., and A. J. Shatkin. 1997. Double-stranded RNA-dependent protein kinase (PKR) is regulated by reovirus structural proteins. Virology 234:364-371. [DOI] [PubMed] [Google Scholar]
- 395.Yuwen, H., J. H. Cox, J. W. Yewdell, J. R. Bennink, and B. Moss. 1993. Nuclear localization of a double-stranded RNA-binding protein encoded by the vaccinia virus E3L gene. Virology 195:732-744. [DOI] [PubMed] [Google Scholar]
- 396.Zamanian-Daryoush, M., S. D. Der, and B. R. Williams. 1999. Cell cycle regulation of the double stranded RNA activated protein kinase, PKR. Oncogene 18:315-326. [DOI] [PubMed] [Google Scholar]
- 397.Zamanian-Daryoush, M., T. H. Mogensen, J. A. DiDonato, and B. R. Williams. 2000. NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol. Cell. Biol. 20:1278-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Zhang, C., J. Kawauchi, M. T. Adachi, Y. Hashimoto, S. Oshiro, T. Aso, and S. Kitajima. 2001. Activation of JNK and transcriptional repressor ATF3/LRF1 through the IRE1/TRAF2 pathway is implicated in human vascular endothelial cell death by homocysteine. Biochem. Biophys. Res. Commun. 289:718-724. [DOI] [PubMed] [Google Scholar]
- 399.Zhang, F., P. R. Romano, T. Nagamura-Inoue, B. Tian, T. E. Dever, M. B. Mathews, K. Ozato, and A. G. Hinnebusch. 2001. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J. Biol. Chem. 276:24946-24958. [DOI] [PubMed] [Google Scholar]
- 399a.Zhou, A., J. M. Paranjape, B. A. Hassel, H. Nie, S. Shah, B. Galinski, and R. H. Silverman. 1998. Impact of RNase L overexpression on viral and cellular growth and death. J. Interferon Cytokine Res. 18:953-961. [DOI] [PubMed] [Google Scholar]
- 400.Zhou, Y., A. Gobl, S. Wang, M. B. Jacobsen, E. T. Janson, G. K. Haines III, J. A. Radosevich, and K. Oberg. 1998. Expression of p68 protein kinase and its prognostic significance during IFN-alpha therapy in patients with carcinoid tumours. Eur. J. Cancer 34:2046-2052. [DOI] [PubMed] [Google Scholar]
- 401.Zhu, S., P. R. Romano, and R. C. Wek. 1997. Ribosome targeting of PKR is mediated by two double-stranded RNA-binding domains and facilitates in vivo phosphorylation of eukaryotic initiation factor-2. J. Biol. Chem. 272:14434-14441. [DOI] [PubMed] [Google Scholar]
- 402.Zurcher, T., R. M. Marion, and J. Ortin. 2000. Protein synthesis shut-off induced by influenza virus infection is independent of PKR activity. J. Virol. 74:8781-8784. [DOI] [PMC free article] [PubMed] [Google Scholar]