Abstract
Arabidopsis halleri and lyrata have three different major centromeric satellite sequences, a unique finding for a diploid Arabidopsis species. Since centromeric histones coevolve with centromeric satellites, these proteins would be predicted to show signs of selection when new centromere satellites have recently arisen. We isolated centromeric protein genes from A. halleri and lyrata and found that one of them, HTR12 (CENP-A), is duplicated, while CENP-C is not. Phylogenetic analysis indicates that the HTR12 duplication occurred after these species diverged from A. thaliana. Genetic mapping shows that HTR12 copy B has the same genomic location as the A. thaliana gene; the other copy (A, at the other end of the same chromosome) is probably the new copy. To test for selection since the duplication, we surveyed diversity at both HTR12 loci within A. lyrata. Overall, there is no strong evidence for an “evolutionary arms race” causing multiple replacement substitutions. The A. lyrata HTR12B sequences fall into three classes of haplotypes, apparently maintained for a long time, but they all encode the same amino acid sequence. In contrast, HTR12A has low diversity, but many variants are amino acid replacements, possibly due to independent selective sweeps within populations of the species.
IT is thought that variation in the sequence-binding specificity of centromere-specific histone H3-like proteins and evolutionary changes in centromere sequences are causally related. When a centromere sequence mutates to a sequence with a competitive advantage over the existing one, conferring preferential segregation (Pardo-Manuel de Villena and Sapienza 2001), this could induce a selection pressure on the genes encoding binding proteins to resist preferential segregation (Henikoff et al. 2000, 2001; Malik and Henikoff 2002). Centromere evolution in Drosophila seems to involve adaptive evolution of one of the centromere-specific histone H3-like proteins, Cid, the Drosophila CENP-A (Henikoff et al. 2001; Malik and Henikoff 2001; Malik et al. 2002). An excess of amino-acid-changing substitutions is observed in the Cid sequence between different Drosophila species, suggesting adaptive evolution, consistent with these changes being driven by differences in centromeric satellite sequences between species (Malik and Henikoff 2001; Malik et al. 2002). The “arms race” theory of centromere evolution is, however, still controversial (e.g., Sullivan 2002). First, it is not yet clear whether specific DNA sequences are essential for centromere functions. Second, CENP-A proteins of different species can sometimes replace one another (in transgenic experiments), proving that they are not specialized to function only with their own satellite sequences.
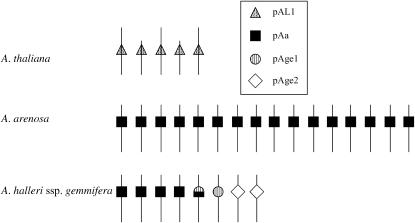
Recent work in the genus Arabidopsis has found evidence of adaptive evolution in the centromere-specific histone genes of these species also (Talbert et al. 2002). In Arabidopsis thaliana, the single-copy HTR12 gene (At1g01370) is thought to encode the protein with the equivalent centromere-binding function and to represent the CENP-A gene of this species. The A. thaliana HTR12 gene has nine exons and encodes a protein of 178 amino acids, which localizes to centromere regions (Talbert et al. 2002) and binds the A. thaliana centromeric satellite sequence (Nagaki et al. 2003). Like the centromeric histone H3 proteins of other organisms, the HTR12 protein has two domains, an N-terminal tail and a histone core domain. The histone core domain, which forms histone octamer nucleosomes, has ∼60% amino acid identity to A. thaliana histone H3 proteins. A. thaliana and A. arenosa each have a single major satellite sequence (with some minor variants), which is highly repeated at the centromeres of all the chromosomes (Kamm et al. 1995); in A. thaliana, this is called pAL1, and in A. arenosa (an autotetraploid species), the centromere sequences differ and are called pAa (see Figure 1). Studies of HTR12 suggest adaptive evolution between A. thaliana and A. arenosa (Talbert et al. 2002), and a recent study of seven more Arabidopsis species (including three tetraploids) inferred that two regions, the N-terminal tail and loop I of the histone core domain, show evidence of adaptive evolution (Cooper and Henikoff 2004).
Figure 1.—
Schematic of the centromeric satellite sequence families of A. thaliana, A. arenosa, and A. halleri ssp. gemmifera (from Kamm et al. 1995 and Kawabe and Nasuda 2005). The centromere satellite families present on each individual chromosome have not yet been identified in A. halleri.
In addition to CENP-A genes, CENP-C, another non-sequence-specific DNA-binding protein, has been reported to evolve adaptively. This has been inferred especially in mammals and grasses, whose CENP-A genes show no signatures of adaptive evolution (Talbert et al. 2004). In contrast, in Drosophila species, there is evidence of positive selection on CENP-A (Cid; Henikoff et al. 2001; Malik and Henikoff 2001; Malik et al. 2002), but not on CENP-C (Heeger et al. 2005). Comparisons between distant Drosophila species indicated adaptive evolution in CENP-C (Heeger et al. 2005; Richards et al. 2005), suggesting that different species may have experienced adaptive evolution in different centromere-binding protein genes. Comparisons of sequences of regions of the CENP-C from Arabidopsis species identified regions that appear to have undergone adaptive evolution on the basis of high Ka/Ks ratios between A. thaliana and A. arenosa (Talbert et al. 2004). These, however, are mainly caused by low synonymous divergence values (the same is true for the subregion studied here, see below). Furthermore, since it is based on comparison of sequences from only two species, it was not determined in which lineages positive selection occurred, since comparisons including additional species are needed to allow determination of the lineages in which substitutions occurred.
In A. halleri ssp. gemmifera, one of the closest relatives of A. thaliana (Miyashita et al. 1998; Kawabe and Miyashita 2002a), there are three major centromeric satellite families with different sequences in different chromosomes: the pAa satellite found in A. arenosa, plus two different sequences, pAge1 and -2 (Kawabe and Nasuda 2005; see also Figure 1). The other A. halleri subspecies (ssp. halleri) and the closely related A. lyrata (both subspecies lyrata and petraea) also have the same three major centromeric satellite families (A. Kawabe, unpublished observations).
If competition for binding to centromeric proteins with different DNA-binding specificity (or competition in relation to some other centromere function) caused the rapid change in centromere satellite sequences across all the chromosomes since the divergence from A. thaliana, we might expect these species to respond to the selection pressure outlined above, which will exist as long as multiple satellite DNA sequences are present at centromeres. The existence of multiple major centromere satellite sequences in A. halleri and A. lyrata suggests that these species may be in a transient stage, undergoing the proposed competitive centromere protein evolution, and it is thus interesting to study centromere-specific proteins (CENP-A and CENP-C) in these species to test whether they also vary, i.e., whether there are different alleles at these loci that might interact differently with the different satellite DNA families, and whether there is evidence of recently arisen new alleles, suggesting recent selective sweeps caused by the spread of advantageous alleles. Here, we describe results from the CENP-A (HTR12) and CENP-C genes of species of plants related to A. thaliana.
The CENP-C gene is single copy, as in the related species, but the CENP-A gene is not just polymorphic with different HTR12 alleles, but the A. halleri and A. lyrata genomes have more than one gene copy encoding CENP-A protein, i.e., paralogous genes, and one of them is highly polymorphic. Among species of plants related to A. thaliana, duplication of centromeric histone H3 genes has previously been reported only in polyploids (Cooper and Henikoff 2004). It therefore seems significant that these species with clear signs of centromere satellite sequence evolution also have multiple centromeric histone H3 genes. We describe the evidence that the new copies arose in the lineage leading to A. halleri and A. lyrata and that both are functional genes, findings that are consistent with the new CENP-A sequence having evolved because it was able to associate with a new centromeric satellite sequence in these species. The coexistence of both multiple centromere satellite families and centromeric histone H3 genes (HTR12 in Arabidopsis) in A. lyrata and A. halleri suggests possible multiple satellite–protein interactions in the “arms race” of centromere evolution. A major purpose of this study therefore was to further test this, by testing for signatures of selection in the derived HTR12 gene copy of A. halleri and A. lyrata, using both divergence between the species and diversity within them. Surprisingly, we failed to detect any evidence for fixation of advantage mutations in either locus in these species, or in their CENP-C.
MATERIALS AND METHODS
Plant materials and DNA isolation:
To investigate the HTR12 gene sequences of the study species and to estimate divergence between them, we used the following individual plants from the following species: an A. halleri ssp. gemmifera plant from Ohtani (Kasumi, Hyogo, Japan), an A. halleri ssp. halleri individual (AH-P1-13, from Pontresina, Switzerland), an A. lyrata ssp. lyrata individual (Ontario4, from Ontario, Canada, given to us by. B. K. Mable, University of Glasgow), an A. lyrata ssp. petraea plant (99R11-2, from Mount Esja, Iceland), and an A. glabra plant from Ohmi-Shirahama (Takashima, Shiga, Japan). The sample used for diversity estimates is described below. Total DNA was isolated from dried leaves by a modified CTAB method or using a FastDNA kit (Q-BIOgene) according to the manufacturer's instructions.
Isolation of centromeric histone H3 (HTR12) and CENP-C genes:
Primers were designed on the basis of the sequence of the HTR12 (At1g01370) gene of the A. thaliana strain Col-0. The primers ATC1672+ (5′-TAA AAA TCA ATG GCG AGA AC-3′) and ATC3536− (5′-CGA AAA GCA GAT AGA AAC AC-3′) were designed for the 5′ region, including the initiation codon, and the 3′ flanking region of the At1g01370 sequence, respectively. PCR reactions yielded two different sequences from genomic DNAs of A. halleri ssp. gemmifera. As described later, these two sequences appear to represent two loci, denoted in what follows by HTR12A and HTR12B. Primers specific for each putative locus were then designed (a 5′ primer ATC1672+ for both loci, and 3′ primers for loci A and B: HTR12a−: 5′-ATT CCG CTT TCC AGT TAT GTT T-3′ and HTR12b−: 5′-GGA TCC TAG ATA TTG TTA ACT ATT C-3′). These primers yielded two sequences from A. halleri ssp. halleri, A. lyrata ssp. lyrata, and A. lyrata ssp. petraea and from whole young shoot cDNA of A. halleri ssp. gemmifera. The exon–intron junctions were determined by comparing the HTR12 sequences from cDNA and genomic DNA. For CENP-C amplification, two primers designed in exon 6 (5′-AAA AGG AAA AGA GGT AGA TGT GC-3′) and exon 11 (5′-ATG CCG ATA ACA GTA GTC AAA C-3′) were used. The sequences were deposited in the DDBJ and GenBank databases under accession nos. AB081500–AB081505, DQ450543–DQ450605, and DQ987606–DQ987610.
Genotyping of the mapping family:
A set of 99 F2 mapping family plants (Kuittinen et al. 2004; Hansson et al. 2006) was used to determine the chromosomal locations of the duplicated loci in A. lyrata ssp. petraea. Four F2 plants were chosen to determine partial sequences of the two HTR12 genes, using primers HTR1229+ (5′-GCC CCT CCC CAA ATC AAT C-3′) and either HTR12a− or HTR12b− (see above). Polymorphic restriction enzyme recognition sites found in the sequenced regions among the F2 plants were then analyzed by PCR–RFLP (HinfI was used for scoring the HTR12A genotypes, and HaeIII for HTR12B).
Sequence diversity analysis:
A total of 21 plants from five populations of A. lyrata ssp. petraea and 4 plants from a single population of A. lyrata ssp. lyrata were sampled and used to survey DNA polymorphism in the HTR12 loci (Table 1). For these plants, sequences of at least three clones were determined for each sample and consensus sequences were used in the analyses below. If one clone differed from the others, more clones were sequenced to obtain both alleles of each plant. In total, 30 HTR12A and 30 HTR12B sequences were obtained from A. lyrata. Coding regions were assigned using cDNA information from A. halleri ssp. gemmifera obtained in this study and A. thaliana (Talbert et al. 2002).
TABLE 1.
Sources and numbers of A. lyrata plants used in the diversity study
| No. of plants
|
|||
|---|---|---|---|
| Population | Country | Sequence and PCR–RFLP | PCR–RFLP only |
| Subspecies petraea | |||
| Mount Esja | Iceland | 7 | 18 |
| Reykjanes | Iceland | 0 | 2 |
| Stubbsund | Sweden | 4 | 3 |
| Plech | Germany | 4 | 4 |
| Karhumaki | Russia | 4 | 5 |
| Spiterstulen | Norway | 0 | 8 |
| Lom | Norway | 0 | 8 |
| Clogwyn D'ur Arddu | Wales | 2 | 2 |
| Glaslyn | Wales | 0 | 8 |
| Subspecies lyrata | |||
| Ontario | Canada | 4 | 0 |
| Indiana | United States | 0 | 4 |
Sequence analyses:
The sequences were aligned manually. The DnaSP version 3.5 (Rozas and Rozas 1999) and MEGA2 (Kumar et al. 2000) programs were used to analyze divergence and diversity (using nucleotide diversity, π, as the estimate of intraspecific polymorphism) and population differentiation, to test linkage disequilibrium (LD), and to apply several tests for neutrality (see below), including McDonald–Kreitman tests (McDonald and Kreitman 1991). The other tests for neutrality were those of HKA (Hudson et al. 1987), Tajima's D (Tajima 1989), Fu and Li's D (Fu and Li 1993), Fu's Fs (Fu 1997), and Fay and Wu's H (Fay and Wu 2000). Wall's B and Q (Wall 1999), and Kelly's ZnS (Kelly 1997) were used to test for LD, using only parsimony informative sites. Recombination rates were estimated as R per site (R/site) and as Rm, the minimum number of recombination events, using DNAsp.
Trees were constructed from the sequences by the neighbor-joining method with Jukes–Cantor distances, using the MEGA2 program (Kumar et al. 2000). Synonymous and replacement divergence values were estimated by Nei and Gojobori's method with Jukes–Cantor correction, using MEGA2. The directions of mutations in A. halleri and lyrata were determined by parsimony with the A. thaliana sequence as the outgroup. When the nucleotides in both species differed from that in A. thaliana, or when the site was in a sequence gap in the A. thaliana sequence, the site was excluded from this analysis. For the CENP-C gene, mutations were assigned to individual branches by parsimony and then DN, DS, and Dintron values were calculated for each branch by dividing the number of mutations by the numbers of sites using Jukes–Cantor correction.
PCR–RFLP:
Three sequence types were found at the HTR12B locus (see below). To estimate the haplotype frequencies in samples from natural populations, we used PCR–RFLP. Two HTR12B primers (HTR1229+: 5′-GCC CCT CCC CAA ATC AAT C-3′ and HTR12b−) were used to obtain a PCR product of ∼550 bp, which was digested by either EcoRV or PstI and separated on 1.5% agarose gels. The PstI recognition site is specific to sequence type 1 and the EcoRV recognition site is present in both types 1 and 2. Thus, the three sequence types can be distinguished.
RESULTS
Duplicated HTR12 genes:
Unlike other diploid animals and plants, two apparently distinct HTR12 loci, HTR12A and HTR12B, were found in the genomes of all four A. halleri and lyrata subspecies, and in cDNA. The CENP-C gene is single copy, as in other related species, and will be discussed below. The exon–intron structures of the HTR12A and HTR12B genes of A. lyrata are the same as for the previously reported Arabidopsis HTR12 genes (Talbert et al. 2002). In both A. halleri subspecies, however, the HTR12A gene has a 16-bp deletion relative to either the A. halleri ssp. gemmifera HTR12B or the A. thaliana HTR12 sequence. This deletion is located near the 3′-end of intron 1 and includes the putative 3′ AG splice site. There is a new functional splice site 22 bp upstream of the usual splice site, so that the A. halleri HTR12A exon 2 is two amino acids longer than the other HTR12 loci. Both duplicates are probably functional, since this product was obtained from cDNA from A. halleri ssp. gemmifera whole young shoots. Furthermore, mean site divergence values per replacement site (Ka) are low between the A and B copies, compared with silent-site divergence values (Ka = 0.020, Ks = 0.078, Ksilent = 0.069), and between each of them and the A. thaliana HTR12 sequence (Ka = 0.064, Ks = 0.132, Ksilent = 0.102).
Chromosomal locations of the duplicated HTR12 genes:
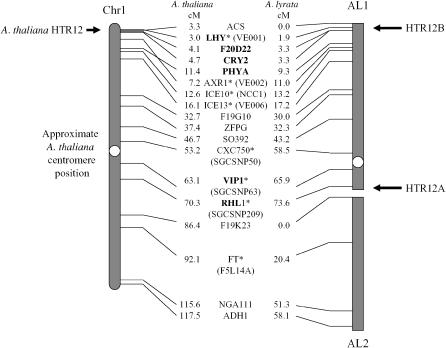
To test the interpretation that the two types of HTR12 sequences represent a duplication, we used genetic mapping using polymorphisms in a mapping family of A. lyrata ssp. petraea (Kuittinen et al. 2004). Because one parent was homozygous for HTR12B, and the other for HTR12A, linkage of the duplicated loci could not be tested directly, but we infer that they are unlinked from their positions in the genetic map (Figure 2). In A. thaliana, the marker closest to the single HTR12 locus (At1g01370) is PVV4 (At1g01480), at 3.2 cM, near the tip of the short arm of chromosome 1. HTR12B is probably located at the same position as the A. thaliana HTR12, as it is closely linked (in the A. lyrata linkage group 1) to several genes that, in A. thaliana, are near HTR12: LHY (MIPS code At1g01060, one recombinant/82 meioses scored), F20D22 (At1g04120, one recombinant/83 meioses), CRY2 (At1g04400, two recombinants/80 meioses), and PHYA (At1g09570, four recombinants/87 meioses). In contrast, HTR12A is closely linked to the following genes in the long arm of A. thaliana chromosome 1, also in the A. lyrata linkage group 1: VIP1 (At1g43700, eight recombinants/81 meioses) and RHL1 (At1g48380, one recombinant/75 meioses). The locations of closely linked genes in A. thaliana, together with the general conservation of gene order between the A. thaliana and A. lyrata maps of this chromosome (Kuittinen et al. 2004; Koch and Kiefer 2005; Yogeeswaran et al. 2005; Hansson et al. 2006), indicate that the duplicate HTR12 genes are probably at opposite ends of the chromosome corresponding to the A. lyrata linkage group 1.
Figure 2.—
Genetic maps of A. thaliana chromosome 1 and the homologous A. lyrata linkage groups 1 and 2 (AL1 and -2), showing the map positions of the A. thaliana HTR12 gene and the duplicated HTR12 loci in A. lyrata ssp. petraea. The names of markers showing close linkage to the duplicated HTR12 loci in A. lyrata are in boldface type. Map positions of markers in the two species are centimorgan values in the A. thaliana recombinant inbred (RI) genetic map in the The Arabidopsis Information Resource database (http://www.arabidopsis.org/) and the A. lyrata map of Kuittinen et al. (2004). Seven markers in the A. lyrata map (indicated by asterisks after the marker names) correspond to markers that were not mapped in the A. thaliana RI genetic map. The homologous A. thaliana markers were assumed to be located close to the positions of mapped markers that are <100 kb away in the complete genome sequence. These marker names are shown in parentheses. More map details are described in Hansson et al. (2006).
The origin of the duplication:
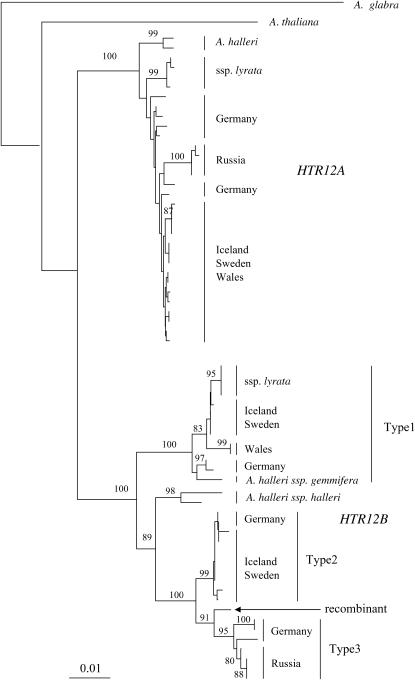
The divergence between the A. thaliana sequence and the duplicated HTR12 loci is much larger than between the duplicated loci in A. halleri and A. lyrata, suggesting that the duplication occurred after the ancestor of A. halleri and A. lyrata split from that of A. thaliana, assuming that mutations accumulated in the two lineages at similar rates. The respective mean divergence values for all sites are 0.0916 vs. 0.0557; the silent-site divergence from the A. thaliana sequence is also about twice that between the duplicated loci (see above). In A. glabra, moreover, we found only a single HTR12 gene, supporting the conclusion that the duplication occurred after the split of A. thaliana from the lineage leading to A. halleri and A. lyrata. The phylogeny, with A. glabra as an outgroup species, is shown in Figure 3 (the diversity results within A. lyrata are described below).
Figure 3.—
Neighbor-joining tree based on the nucleotide differences between the HTR12 sequences, based on the entire region. Bootstrap values >80% are shown by the relevant nodes. A distance bar is shown below the trees.
These findings seem to rule out the alternative possibility, suggested by the location of the HTR12A locus, that the duplication is an ancient one that was lost from A. thaliana as part of the fusion event that created its chromosome 1, which is the derived state (Koch and Kiefer 2005). HTR12B is probably the ancestral copy, because of its identical location to A. thaliana HTR12 and HTR12A, a recently duplicated copy in the lineage leading to A. halleri and A. lyrata. Since HTR12A has an exon–intron structure in A. lyrata identical to that of other HTR12 genes, the duplication event was not via a messenger RNA intermediate, but probably involved a transposition of genomic sequence.
Diversity in the duplicated HTR12 loci:
To test for the action of selection, we also obtained data on diversity within species for the duplicate loci.
Within-population diversity:
A. lyrata HTR12A is considerably less polymorphic than HTR12B. For the entire region sequenced, the nucleotide diversity estimates, π, are 0.8 and 2.6%, respectively (Table 2; similar values of 0.7 and 2.5%, respectively, are found in A. lyrata ssp. petraea), but the difference is nonsignificant by a HKA test (P = 0.187). In addition to 57 single nucleotide polymorphisms, there are 41 length variants in the HTR12A locus, 4 of these indels being in the coding region. In the HTR12B locus, there are 114 polymorphic sites and 29 length variants (only 1 in the coding region). Two plants from the Plech population have an identical large deletion in HTR12B (101 bp), starting in intron 2; this causes loss of the intron–exon junction and part of exon 3 and shifts the reading frame, so this sequence is probably an inactive allele. Alternatively, since this deletion causes loss of the splicesome recognition site in intron 2, part of the intron 2 sequence may be included in the mRNA. The transcript of this allele would then include 10 bp of intron 2 sequence, but would have lost 28 bp of exon 3, forming a protein 6 amino acids shorter than that encoded by the nondeletion alleles, and with no homology between the 7 amino acids changed in the deletion alleles and the presumably wild-type sequence (13 amino acids), although we could not test this hypothesis by analyzing cDNAs because only dried leaves were available, from which mRNA could not be extracted.
TABLE 2.
Nucleotide variants and diversity estimates (π) in the HTR12 loci
| Coding region
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entire region
|
Synonymous
|
Nonsynonymous
|
Noncoding
|
||||||||||
| Taxon | N | No. of sites | Sn | π | No. of sites | Sn | π | No. of sites | Sn | π | No. of sites | Sn | π |
| HTR12A | |||||||||||||
| All | 30 | 1600 | 57 (60) | 0.0085 | 129.32 | 3 | 0.0072 | 377.68 | 8 | 0.0062 | 1087 | 46 (49) | 0.0095 |
| ssp. petraea | 26 | 1626 | 50 (53) | 0.0076 | 129.27 | 3 | 0.0056 | 377.73 | 6 | 0.0048 | 1113 | 41 (44) | 0.0088 |
| ssp. lyrata | 4 | 1767 | 3 | 0.0009 | 139.67 | 0 | 0 | 406.33 | 1 | 0.0012 | 1218 | 2 | 0.0009 |
| HTR12B | |||||||||||||
| All | 30 | 1546 | 114 (115) | 0.0262 | 124.33 | 12 | 0.0374 | 361.67 | 3 | 0.0033 | 1055 | 98 (99) | 0.0328 |
| ssp. petraea | 26 | 1546 | 111 (112) | 0.0247 | 124.33 | 11 | 0.0340 | 361.67 | 2 | 0.0024 | 1055 | 97 (98) | 0.0312 |
| ssp. lyrata | 4 | 1776 | 0 | 0 | 136.67 | 0 | 0 | 391.33 | 0 | 0 | 1245 | 0 | 0 |
Numbers of mutations are shown in parentheses. Sn, number of polymorphic sites.
The diversity estimates (Table 2) are similar to those for other nuclear genes in A. lyrata ssp. petraea (Wright et al. 2003; Wright 2003; Ramos-Onsins et al. 2004). The mean silent site π-value based on 30 non-HTR12 loci is 1.4%, excluding PgiC, whose diversity is very high, but may be duplicated (see Kawabe and Miyashita 2002b).
The higher polymorphism in HTR12B is due mainly to the presence of three divergent types of sequences with high synonymous and noncoding site divergence (6/12 synonymous and 47/99 noncoding variations were fixed between sequence types, although, with our small sample size, the numbers of fixed sites will be overestimated), but there are only three nonsynonymous variants, and these are not fixed between the sequence types, so they appear not to represent functionally different alleles. Two of the three types have diversity similar to that of the entire set of HTR12A sequences. The three types are not simply local variants, since all are widely distributed geographically, and some populations include several (the Plech population from Germany has all three). Type 1 is found in the North American subspecies, A. lyrata ssp. lyrata, and in four European A. lyrata ssp. petraea populations, as well as in A. halleri ssp. gemmifera (Figure 3). Sequence type 2 is much less polymorphic than the other types (or than HTR12A) and was found in three geographically distant European ssp. petraea populations: Mount Esja (Iceland), Stubbsund (Sweden), and Plech (Figure 3). All the sequences from the Karhumaki population (Russia) are of type 3, together with some from Plech. Further evidence for long-term persistence of the three different HTR12B sequence types is that there are many shared polymorphisms between A. lyrata and A. halleri. The sequences of an A. halleri ssp. halleri plant from Switzerland cluster separately from the other sequences, but are most similar to sequence types 2 and 3, whereas the A. halleri ssp. gemmifera sequence is similar to sequence type 1. These results suggest that the different HTR12B sequence types originated before A. halleri and A. lyrata split from one another.
In contrast, the HTR12A sequences of A. lyrata and A. halleri cluster separately from one another, and even sequences from the two A. lyrata subspecies, lyrata and petraea, can be distinguished in the phylogenetic tree (with 99% bootstrap support; Figure 3). In both HTR12A and HTR12B trees, sequences from the populations from Wales, Karhumaki, and Ontario form clusters, supporting previous suggestions of some isolation between these geographically distant A. lyrata populations (Wright et al. 2003; Ramos-Onsins et al. 2004). However, all these populations have very low within-population polymorphism that could cause separate clustering.
FST for HTR12A exceeds 70% for 10 of 15 between-population comparisons, and the mean for all populations is 0.71 (average FST in subspecies petraea is 0.62, similar to the value based on 18 reference loci studied in the same populations, which yielded a mean of 0.58, including ssp. lyrata and 0.54 for the European ssp. petraea populations; A. Kawabe, S. I. Wright, A. Forrest and D. Charlesworth, unpublished data).
Frequencies of the different HTR12B sequence types:
Because our samples are small, they only poorly estimate the frequency distribution of the three HTR12B sequence types. To estimate frequencies, we therefore also did PCR–RFLP analyses for 99 plants from 11 populations from seven countries (Table 3). Eight of the populations were found to be monomorphic for one sequence type, mostly type 1 (the only exception is the Karhumaki population, fixed for sequence type 3). However, three populations showed polymorphism by PCR–RFLP, where two or three sequence types were found by sequence analyses. All three polymorphic populations have intermediate frequencies of each sequence type. In the Mount Esja population, the genotype frequencies do not deviate from Hardy–Weinberg equilibrium frequencies (χ2 = 0.877). However, Plech has an excess of heterozygotes and deviates significantly (11 heterozygotes of 14 plants, χ2 = 7.942, P < 0.01), while none of the 13 plants in the Stubbsund sample was heterozygous (χ2 = 10.692, P < 0.01).
TABLE 3.
Frequencies of the three major HTR12B sequence types determined by PCR–RFLP analyses
| Genotypes
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Homozygotes
|
Heterozygotes
|
Sequence types
|
Frequencies of sequence types
|
|||||||||||
| Population | Country | No. of plants | 1 | 2 | 3 | 1/2 | 1/3 | 2/3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Mount Esja | Iceland | 25 | 8 | 12 | 5 | 21 | 29 | 0 | 0.420 | 0.580 | 0 | |||
| Reykjanes | Iceland | 2 | 2 | 4 | 0 | 0 | 1 | 0 | 0 | |||||
| Clogwyn D'ur Arddu | Wales | 4 | 4 | 8 | 0 | 0 | 1 | 0 | 0 | |||||
| Glaslyn | Wales | 8 | 8 | 16 | 0 | 0 | 1 | 0 | 0 | |||||
| Plech | Germany | 14 | 1 | 2 | 3 | 3 | 5 | 8 | 10 | 8 | 0.308 | 0.385 | 0.308 | |
| Lom | Norway | 8 | 8 | 16 | 0 | 0 | 1 | 0 | 0 | |||||
| Spiterstulen | Norway | 8 | 8 | 16 | 0 | 0 | 1 | 0 | 0 | |||||
| Stubbsund | Sweden | 13 | 10 | 3 | 20 | 6 | 0 | 0.769 | 0.231 | 0 | ||||
| Karhumaki | Russia | 9 | 9 | 0 | 0 | 18 | 0 | 0 | 1 | |||||
| Ontario | Canada | 4 | 4 | 8 | 0 | 0 | 1 | 0 | 0 | |||||
| Indiana | United States | 4 | 4 | 8 | 0 | 0 | 1 | 0 | 0 | |||||
| Total | 99 | 57 | 17 | 9 | 8 | 3 | 5 | 125 | 45 | 26 | 0.638 | 0.230 | 0.133 | |
See materials and methods.
Patterns of polymorphism at the HTR12 loci:
Despite the highly divergent HTR12B sequences, only Fu's Fs statistic (Fu 1997) for the HTR12A locus suggests a significant deviation from neutrality (Table 4). This statistic is highly sensitive to hitchhiking events and population growth. Thus this locus may have experienced a recent bottleneck event. This result remains significant for just the A. lyrata ssp. petraea sequences.
TABLE 4.
Tests of neutrality and results of estimates of linkage disequilibrium and recombination
|
HTR12A
|
HTR12B
|
||||
|---|---|---|---|---|---|
| Locus and taxa | All | ssp. petraea | ssp. lyrata | All | ssp. petraea |
| Sample size (alleles) | 30 | 26 | 4 | 30 | 26 |
| Tajima's D | −0.389 | −0.444 | 0.168 | 1.518 | 1.172 |
| Fu and Li's Da | 0.063 | 0.009 | −0.368 | 0.997 | 0.986 |
| Fu's Fs | −7.962** | −5.664* | −2.181 | 4.271 | 2.987 |
| Fay's Ha | −0.055 | −2.055 | 0.667 | −1.113 | −5.772 |
| LD | |||||
| No. of parsimony informative sites | 38 | 31 | 1 | 98 | 95 |
| Significant pairs (P < 0.05 by Fisher's exact test) | 215/703 | 169/465 | NA | 2831/4753 | 2326/4465 |
| Significant pairs after Bonferroni correction | 44 (6.26%) | 32 (6.88%) | NA | 1296 (27.27%) | 707 (15.83%) |
| Kelly's ZnS | 0.173 | 0.229 | NA | 0.374 | 0.376 |
| Wall's B | 0.162 | 0.167 | NA | 0.433* | 0.457* |
| Wall's Q | 0.263 | 0.29 | NA | 0.602* | 0.632* |
| R/site | 0.0090 | 0.0045 | NA | 0.0025 | 0.0021 |
| Rm | 11 | 10 | NA | 7 | 7 |
HTR12B was not analyzed separately for A. lyrata ssp. lyrata because there were no polymorphic sites. *P < 5%; **P < 0.01.
Estimated using A. thaliana sequence as the outgroup.
We found a high proportion of significant linkage disequilibria between pairs of sites in HTR12B data sets (27% of site pairs are significant after Bonferroni correction). Wall's B and Q statistics are also both significant for the HTR12B sequences (Table 4). Nevertheless, many recombination events are detectable in both HTR12A and HTR12B (Table 4).
In the A. lyrata sequences, HTR12A has eight replacement polymorphisms and three synonymous ones, while for HTR12B the ratio of replacement-to-synonymous polymorphisms is 3:12, a significant difference. A McDonald–Kreitman test using divergence between the duplicated loci in A. lyrata gave a significant excess of replacement polymorphisms in HTR12A, whereas HTR12B showed no significant difference from neutrality. The only form of selection suggested by these results is purifying selection in HTR12B since the gene duplication. Using divergence from the A. thaliana sequence, a McDonald–Kreitman test was not significant for HTR12A, but was significant (P < 0.05) for the HTR12B locus, which shows an excess of fixed replacement variants, but few replacement polymorphisms (Table 5), suggesting the action of directional selection. Previous studies of HTR12 also found unusually high replacement divergence between several Arabidopsis species (Talbert et al. 2002; Cooper and Henikoff 2004). To infer when these substitutions (and thus the selection) occurred, we determined the direction of each mutation by parsimony using the sequence of the single copy in A. glabra. This analysis placed seven synonymous and 13 replacement substitutions in the A. thaliana lineage, while four synonymous and 5 replacement substitutions are assigned to the A. lyrata lineage before the duplication event (for two of the replacement sites that differ between the Arabidopsis species, we cannot determine the lineage, because there is a sequence gap in A. glabra). These differences between substitutions before and after the duplication are not significant by a G-test. The replacement fixations are therefore probably not specifically related to the recent duplication. Overall, there is little evidence for selectively driven substitutions in either A. lyrata gene.
TABLE 5.
McDonald–Kreitman tests
| Polymorphisms within A. lyrata
|
Fixed differences from A. thaliana
|
Fixed differences from other locus
|
||||||
|---|---|---|---|---|---|---|---|---|
| Locus | Synonymous | Replacement | Synonymous | Replacement | Significance | Synonymous | Replacement | Significance |
| HTR12A | 3 | 8 | 15 | 21 | NS | 6 | 2 | |
| HTR12B | 12 | 3 | 12 | 19 | * | 6 | 2 | NS |
| Polymorphisms within A. lyrata
|
||||||||
| Lineageb | Synonymous | Replacement | ||||||
| A. thaliana | 7 | 13 | ||||||
| A. lyrata | ||||||||
| Before duplication | 4 | 5 | ||||||
| HTR12A | 4 | 1 | ||||||
| HTR12B | 2 | 1 | ||||||
Note that the number of polymorphic sites differs between the comparisons because of alignment gaps. *P < 0.05.
One replacement polymorphic site in HTR12A is found in polymorphic indel variation in HTR12B. If this mutation is excluded, the comparison between 3:7 vs. 6:2 is not significant (P = 0.063).
Directions of mutations are determined by parsimony with A. glabra sequence. Two replacements found only in A. thaliana sequence locate in a sequence gap in A. glabra. One synonymous and two replacement changes in the A. thaliana lineage are in the polymorphic indel variation in HTR12B.
CENP-C gene divergence:
To test for selection acting on the other centromere protein gene, CENP-C, we sequenced the gene in A. lyrata and A. halleri and analyzed divergence from A. thaliana. We determined sequences for one plant from each A. halleri and A. lyrata subspecies for this genomic region for which adaptive evolution was suggested between A. thaliana and A. arenosa (Talbert et al. 2004); the region extends from exon 6 to intron 10 and its length is ∼1.4 kb. Using the A. glabra sequence as an outgroup, most mutations could be assigned to individual branches by parsimony. In the coding region analyzed (∼600 bp), the high DN/DS values tend to be found for branches that have high DS. The highest DN/DS ratio is inferred in the A. glabra lineage (1.423), and the lowest in the A. halleri lineage (0.184). Except in the A. glabra and A. thaliana lineages, DN values are lower than DS or Dintron values.
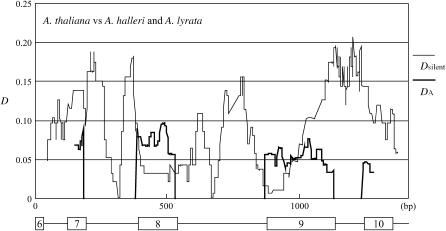
Although relatively high DN/DS ratios are seen in CENP-C, in almost all species comparisons this is mainly due to low synonymous divergence. Exons 8 and 9, which are a putative region undergoing adaptive evolution (Talbert et al. 2004), have no synonymous substitutions, even in comparison with the A. glabra sequence. On the other hand, there are many substitutions at replacement and intron sites (Figure 4).
Figure 4.—
Sliding-window analyses for the CENP-C gene. Sliding-window plot for divergence between A. thaliana and A. halleri–A. lyrata is shown. The exon–intron structure is indicated below the plot. Divergence values are plotted in 1-bp steps in 50- and 100-bp windows for silent (thin line) and replacement (thick line) sites, respectively.
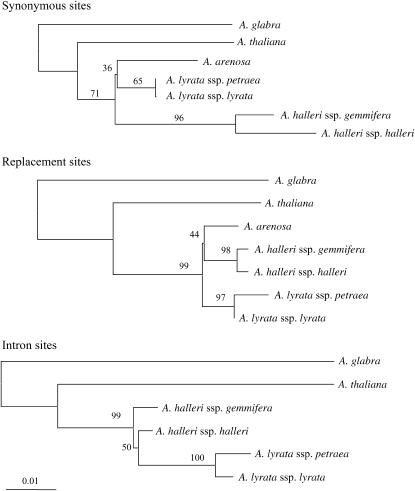
Among the taxa that have multiple centromere satellite families (A. halleri and A. lyrata), divergence estimates for this gene are not unusually low (DS = 0.042, DN = 0.018, and Dintron = 0.032 between the species), ruling out a recent selective sweep before the split of the two species, such as could be due to selection associated with the fixation of centromere satellite sequences shared between these two species. The sequences from the two subspecies within both A. lyrata and A. halleri have very few differences (Figure 5; note that no intron sequence is available for A. arenosa). Thus an interpretation involving independent selective sweeps in each species after their split is possible, but it is evident that neither species has maintained different CENP-C alleles that could have different DNA–protein interactions. The gene trees for synonymous and replacement sites also suggest a divergence time for CENP-C sequences of A. lyrata and A. halleri very close to these species' split from the A. arenosa sequence. Two major centromere satellite families, pAge1 and pAge2, are shared by A. lyrata and A. halleri but not found in A. arenosa. If their appearance has led to replacement of CENP-C by new, functionally different alleles since these species split, one would expect the A. lyrata and A. halleri sequences to be much more similar to each other than to A. arenosa. Our results do not, therefore, support a conclusion of adaptive changes in CENP-C.
Figure 5.—
Neighbor-joining tree based on the nucleotide differences between the CENP-C sequences. Trees based on the synonymous sites, replacement sites, and intronic region are shown. Bootstrap values are shown by the relevant nodes. All trees have the same scale (see the distance bar below the trees).
DISCUSSION
Duplication of the HTR12 gene in A. halleri and A. lyrata:
This is the first known case of duplication of the centromeric histone H3 (CenpA) gene in a diploid Arabidopsis species. Talbert et al. (2002) suggested that, in allopolyploid species, the two CENP-A proteins from the different parent species might form heterodimers, sometimes allowing coexistence of both species' centromeric satellite sequences. However, this state probably does not persist in the long term. In Zea mays, which is a fairly recent allotetraploid (due to an event estimated to be ∼11 MYA; Gaut and Doebley 1997), there is now only one centromeric histone H3 gene copy (Zhong et al. 2002). The only other known case of duplication is in the nematode Caenorhabditis elegans, which has two CENP-A loci, while the related species C. briggsae has just one copy, but one copy is not expressed, or is expressed very weakly (Monen et al. 2005). Duplication and maintenance of two or more copies of CENP-A thus appears to be rare in both plant and animal species. A. halleri and A. lyrata may be in a situation similar to that in the early stage of allopolyploid formation; the HTR12 proteins encoded by the duplicate genes have diverged in sequence, and it remains to be tested in the future whether they now colocalize at different centromere regions and bind different centromere repeats. Since there are three major satellite families, but only two HTR12 genes, there is not a simple one-to-one relationship between HTR12 and centromeric satellite families. A. halleri and A. lyrata may thus represent a transient stage with protein–DNA interactions whose specificity is not stringent.
Evidence for selection:
If centromere evolution drives the evolution of differences in centromeric histone H3 genes (HTR12 in Arabidopsis) to acquire altered binding specificities to prevent distorted segregation of different centromere satellite variants (Henikoff et al. 2001; Malik and Henikoff 2001), the two HTR12 loci may be expected to have experienced different selective pressures. For example, one of the duplicates might experience an evolutionary “arms race” with a centromere and should then have many fixed replacement substitutions (and low within-locus diversity may be found, if a selective sweep has occurred recently). Neither of the two duplicated HTR12 loci in A. lyrata shows clear evidence of the expected multiple replacement substitutions fixed in their lineages even though relaxed selective constraint might be expected for duplicated genes.
The HTR12A locus:
Polymorphism in A. lyrata is low for the duplicated HTR12A locus, which might suggest a recent selective sweep, and we find a significant result from one test that can detect such events (Fu's Fs test, which detects an unusually low frequency of old alleles, i.e., an excess of variants at frequencies lower than predicted by a constant population size and neutral model, indicating recent mutations on the external branches). The low HTR12A locus polymorphism, and also the high relative number of replacement polymorphisms, could be due to a recent bottleneck affecting either this species or just this gene, since a prolonged bottleneck of low population effective size can hinder the elimination of slightly deleterious replacement mutations. The significant Fs value is seen only for HTR12A, whereas a recent strong bottleneck should affect all loci (e.g., the one inferred in A. halleri ssp. gemmifera, which was detected at four loci; see Kawabe and Miyashita 2003). However, data from other A. lyrata loci do not suggest a bottleneck in population size (only 2 of 18 reference loci showed significantly negative Fs values, A. Kawabe, S. I. Wright, A. Forrest and D. Charlesworth, unpublished results). In the future, it will be interesting to analyze more loci, including genes near the HTR12A locus.
If bottlenecks are ruled out, a selective sweep in the HTR12A locus in A. lyrata may be a possible explanation for our results for this locus, although there is certainly no evidence for an “arms race”-driven substitution process and no significant excess of replacement substitutions.
The HTR12B locus:
An excess of replacement substitutions was found for HTR12B, which is probably the ancestral copy, but this was due to substitutions in the A. thaliana lineage, not in HTR12B. Moreover, this gene has high diversity in A. lyrata and shows evidence of long-term maintenance of several different haplotypes, clearly dating to before the split between A. lyrata and A. halleri. Our segregation analyses establish that the different sequence types are allelic. HTR12B has therefore not undergone the repeated selective sweeps expected under an “arms race” scenario.
It is possible that persistence of these sequence types is related to the presence of the different major centromere sequences in our study species. The linkage disequilibrium that we observe is consistent with long-term balancing selection, but none of the tests that we applied detects significant deviations from neutrality. A low recombination frequency (or a high degree of selfing, implying a low effective recombination rate) can also cause linkage disequilibria (Nordborg et al. 2000). However, HTR12B is not in a genomic region of low recombination rate (Hansson et al. 2006), and A. lyrata is an outcrossing plant in most populations; of the populations studies here, only the Ontario population (population TC in Mable et al. 2005) may be partially inbreeding. Larger samples from individual populations may be needed to detect a signature of balancing selection by Tajima's test, since pooled data from different populations can be misleading (see Schierup et al. 2000). However, the fact that no replacement sites are fixed between the three HTR12B sequence types suggests that they may not represent functionally different types. We also cannot exclude the possibility that a gene very closely linked to HTR12B experiences balancing selection and influences the HTR12B locus polymorphism. The surrounding genome region should then show linkage disequilibrium with HTR12B and should also have high diversity (Charlesworth et al. 1997; Takahata and Satta 1998; Schierup et al. 2000; Navarro and Barton 2002); these are testable predictions, but have not yet been tested.
Although the reasons for the maintenance of the divergent HTR12B alleles are thus unclear, it is clear that this locus has not been the target of a centromere satellite–protein coevolutionary arms race. The only clear evidence that we have found for selection suggests that the HTR12B locus is under purifying selection. The HTR12B locus results suggest purifying selection, while HTR12A has low polymorphism and might possibly have undergone a selective sweep. HTR12B might have maintained interactions with the ancestral satellite sequence families, while HTR12A evolved to interact with new satellite families. However, the evidence is weak that HTR12A has been repeatedly selected for functional changes involving interaction with the new centromere sequences, because there is no evidence of an “arms race” since we do not observe multiple amino acid substitutions at this locus.
If these species have undergone a centromere “evolutionary arms race,” it might perhaps have involved the other centromere protein, CENP-C, which, as mentioned above, has been reported as acting as a player of the centromere “arms race” evolution in both plants and animals (Talbert et al. 2004), including Arabidopsis species. Our finding of high divergence between A. halleri and A. lyrata, and low divergence within these species, does not, however, support CENP-C evolution associated with the multiple centromere satellite families shared between A. halleri and A. lyrata. The high DN/DS ratios between many species appear to be due to low synonymous changes, whose cause is not understood, and not to adaptive evolution or relaxed selective constraints. The cause of the reduced rate of synonymous change in the CENP-C gene of Arabidopsis is not understood, but constraints affecting synonymous sites are reported in other organisms and can cause local heterogeneity of synonymous divergence, and consequently high DN/DS ratios in certain regions of some genes (e.g., Pond and Muse 2005; Charmary et al. 2006).
Acknowledgments
We thank H. Kuittinen and O. Savolainen for DNA samples from the mapping family, B. K. Mable for plant material, S. Preuss for isolation of genomic DNAs, and the Institute of Evolutionary Biology, Edinburgh University Sequencing service for sequencing.
References
- Chamary, J. V., J. L. Parmley and L. D. Hurst, 2006. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat. Rev. Genet. 7: 98–108. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., M. Nordborg and D. Charlesworth, 1997. The effects of local selection, balanced polymorphism and background selection on equilibrium patterns of genetic diversity in subdivided inbreeding and outcrossing populations. Genet. Res. 70: 155–174. [DOI] [PubMed] [Google Scholar]
- Cooper, J. L., and S. Henikoff, 2004. Adaptive evolution of the histone fold domain in centromeric histones. Mol. Biol. Evol. 21: 1712–1718. [DOI] [PubMed] [Google Scholar]
- Fay, J. C., and C.-I Wu, 2000. Hitchhiking under positive Darwinian selection. Genetics 155: 1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y.-X., 1997. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y.-X., and W.-H. Li, 1993. Statistical tests of neutrality of mutations. Genetics 133: 693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut, B. S., and J. F. Doebley, 1997. DNA sequence evidence for the segmental allotetraploid origin of maize. Proc. Natl. Acad. Sci. USA 94: 6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, B., A. Kawabe, S. Preuss, H. Kuittinen and D. Charlesworth, 2006. Comparative gene mapping in Arabidopsis lyrata chromosomes 1 and 2 and the corresponding A. thaliana chromosome 1: recombination rates, rearrangements and centromere location. Genet. Res. 87: 75–85. [DOI] [PubMed] [Google Scholar]
- Heeger, S., O. Leismann, R. Schittenhelm, O. Schraidt, S. Heidmann et al., 2005. Genetic interactions of separase regulatory subunits reveal the diverged Drosophila Cenp-C homolog. Genes Dev. 19: 2041–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff, S., K. Ahmad, J. S. Platero and B. Vansteensel, 2000. Heterochromatic deposition of centromeric histone H3-like proteins. Proc. Natl. Acad. Sci. USA 97: 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff, S., K. Ahmad and H. S. Malik, 2001. The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293: 1098–1102. [DOI] [PubMed] [Google Scholar]
- Hudson, R. R., M. Kreitman and M. Aguade, 1987. A test of neutral molecular evolution based on nucleotide data. Genetics 116: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm, A., I. Glasso, T. Schmidt and J. S. Heslop-Harrison, 1995. Analysis of a repetitive DNA family from Arabidopsis arenosa and relationship between Arabidopsis species. Plant Mol. Biol. 27: 853–862. [DOI] [PubMed] [Google Scholar]
- Kawabe, A., and N. T. Miyashita, 2002. a DNA variation in the acidic chitinase locus (ChiA) region in Arabis gemmifera and among its related species. Genes Genet. Syst. 77: 167–175. [DOI] [PubMed] [Google Scholar]
- Kawabe, A., and N. T. Miyashita, 2002. b Characterization of duplicated two cytosolic phosphoglucose isomerase (PgiC) loci in Arabidopsis halleri ssp. gemmifera. Genes Genet. Syst. 77: 159–165. [DOI] [PubMed] [Google Scholar]
- Kawabe, A., and N. T. Miyashita, 2003. DNA polymorphism in active gene and pseudogene of the cytosolic phosphoglucose isomerase (PgiC) loci in Arabidopsis halleri ssp. gemmifera. Mol. Biol. Evol. 20: 1043–1050. [DOI] [PubMed] [Google Scholar]
- Kawabe, A., and S. Nasuda, 2005. Structure and genomic organization of centromeric repeats in Arabidopsis species. Mol. Genet. Genomics 272: 593–602. [DOI] [PubMed] [Google Scholar]
- Kelly, J. K., 1997. A test of neutrality based on interlocus associations. Genetics 146: 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, M., and M. Kiefer, 2005. Genome evolution among Cruciferous plants: a lecture from the comparison of the genetic map of three diploid species: Capsella rubella, Arabidopsis lyrata subsp. petraea, and A. thaliana. Am. J. Bot. 92: 761–767. [DOI] [PubMed] [Google Scholar]
- Kuittinen, H., A. A. De Haan, C. Vogl, S. Oikarinen, J. Leppälä et al., 2004. Comparing the linkage map of the close relatives Arabidopsis lyrata and A. thaliana. Genetics 168: 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., K. Tamura, I. Jacobsen and M. Nei, 2000. MEGA2: Molecular Evolutionary Genetics Analysis, Version 2.0. Pennsylvania State University/Arizona State University, University Park, PA/Tempe, AZ.
- Mable, B. K., A. V. Robertson, S. Dart, C. D. Berardo and L. Witham, 2005. Breakdown of self-incompatibility in the perennial Arabidopsis lyrata (Brassicaceae) and its genetic consequences. Evolution 59: 1437–1448. [PubMed] [Google Scholar]
- Malik, H. S., and S. Henikoff, 2001. Adaptive evolution of Cid, a centromere-specific histone in Drosophila. Genetics 157: 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, H. S., and S. Henikoff, 2002. Conflict begets complexity: the evolution of centromeres. Curr. Opin. Genet. Dev. 12: 711–718. [DOI] [PubMed] [Google Scholar]
- Malik, H. S., D. Vermaak and S. Henikoff, 2002. Recurrent evolution of DNA-binding motif in the Drosophila centromeric histone. Proc. Natl. Acad. Sci. USA 99: 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. H., and M. Kreitman, 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351: 652–654. [DOI] [PubMed] [Google Scholar]
- Miyashita, N. T., A. Kawabe, H. Innan and R. Terauchi, 1998. Intra- and interspecific DNA variation and codon bias of alcohol dehydrogenase (Adh) locus in Arabis and Arabidopsis species. Mol. Biol. Evol. 15: 1420–1429. [DOI] [PubMed] [Google Scholar]
- Monen, J., P. S. Maddox, F. Hyndman, K. Oegema and A. Desai, 2005. Differential role of CENP-A in the segregation of holocentric C. elegans chromosomes during meiosis and mitosis. Nat. Cell Biol. 7: 1248–1255. [DOI] [PubMed] [Google Scholar]
- Nagaki, K., P. B. Talbert, C. X. Zhong, R. K. Dawe, S. Henikoff et al., 2003. Chromatin immunoprecipitation reveals that the 180-bp satellite repeat is the key functional DNA element of Arabidopsis thaliana centromeres. Genetics 163: 1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, A., and N. H. Barton, 2002. The effects of multilocus balancing selection on neutral variability. Genetics 161: 849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg, M., 2000. Linkage disequilibrium, gene trees, and selfing: an ancestral recombination graph with partial self-fertilization. Genetics 154: 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Manuel de Villena, F., and C. Sapienza, 2001. Female meiosis drives karyotypic evolution in mammals. Genetics 159: 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond, S. K., and S. V. Muse, 2005. Site-to-site variation of synonymous substitution rates. Mol. Biol. Evol. 22: 2375–2385. [DOI] [PubMed] [Google Scholar]
- Ramos-Onsins, S. E., B. E. Stranger, T. Mitchell-Olds and M. Aguade, 2004. Multilocus analysis of variation and speciation in the closely related species Arabidopsis halleri and A. lyrata. Genetics 166: 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, S., Y. Liu, B. R. Betterncourt, P. Hradecky, S. Letovsky et al., 2005. Comparative genome sequencing of Drosophila pseudoobscura chromosomal, gene and cis-element evolution. Genome Res. 15: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas, J., and R. Rozas, 1999. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15: 174–175. [DOI] [PubMed] [Google Scholar]
- Schierup, M. H., X. Vekemans and D. Charlesworth, 2000. The effect of hitch-hiking on genes linked to a balanced polymorphism in a subdivided population. Genet. Res. 76: 63–73. [DOI] [PubMed] [Google Scholar]
- Sullivan, B. A., 2002. Centromere round-up at the heterochromatin corral. Trends Biotechnol. 3: 89–92. [DOI] [PubMed] [Google Scholar]
- Tajima, F., 1989. Statistical test for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata, N., and Y. Satta, 1998. Footprints of intragenic recombination at HLA loci. Immunogenetics 47: 430–441. [DOI] [PubMed] [Google Scholar]
- Talbert, P. B., R. Masuelli, A. P. Tyagi, L. Comai and S. Henikoff, 2002. Centromeric localization and adaptive evolution of an Arabidopsis histone H3 variant. Plant Cell 14: 1053–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert, P. B., T. D. Bryson and S. Henikoff, 2004. Adaptive evolution of centromere protein in plants and animals. J. Biol. 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall, J. D., 1999. Recombination and the power of statistical tests of neutrality. Genet. Res. 74: 65–79. [Google Scholar]
- Wright, S. I., 2003. Effects of recombination rate and mating system on genome evolution and diversity in Arabidopsis lyrata. Ph. D. Thesis, University of Edinburgh, Edinburgh, United Kingdom.
- Wright, S. I., B. Lauga and D. Charlesworth, 2003. Subdivision and haplotype structure in natural populations of Arabidopsis lyrata. Mol. Ecol. 12: 1247–1263. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran, K., A. Frary, T. L. York, A. Amenta, A. H. Lesser et al., 2005. Comparative genome analyses of Arabidopsis spp.: inferring chromosomal rearrangement events in the evolutionary history of A. thaliana. Genome Res. 15: 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, C. X., J. B. Marshall, C. Topp, R. Mroczek, A. Kato et al., 2002. Centromeric retroelement and satellites interact with maize kinetochore protein CENH3. Plant Cell 14: 2825–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]