Abstract
The [PSI+] determinant of Saccharomyces cerevisiae, consisting of the cytosolic translation termination factor Sup35, is a prion-type genetic element that induces an inheritable conformational change and converts the Sup35 protein into amyloid fibers. The molecular chaperone Hsp104 is required to maintain self-replication of [PSI+]. We observe in vitro that addition of catalytic amounts of Hsp104 to the prion-determining region of the NM domain of Sup35, Sup355–26, results in the dissociation of oligomeric Sup35 into monomeric species. Several intermediates of Sup355–26 could be detected during this process. Strong interactions are found between Hsp104 and hexameric/tetrameric Sup355–26, whereas the intermediate and monomeric “release” forms show a decreased affinity with respect to Hsp104, as monitored by saturation transfer difference and diffusion-ordered NMR spectroscopic experiments. Interactions are mediated mostly by the side chains of Gln, Asn, and Tyr residues in Sup355–26. No interaction can be detected between Hsp104 and higher oligomeric states (≥8) of Sup355–26. Taking into account the fact that Hsp104 is required for maintenance of [PSI+], we suggest that low-oligomeric-weight species of Sup35 are important for prion propagation in yeast.
The [PSI+] factor of Saccharomyces cerevisiae is a protein-based genetic element that consists of the cytosolic translation termination factor Sup35. In [psi–] cells, Sup35 is a soluble protein, but in [PSI+] cells, most Sup35 protein is found in a prion-like aggregated conformation that can be propagated onto the daughter cells. The occurrence of this phenotype is modulated by the chaperone Hsp104, first identified as a protein important for the thermotolerance of yeast (1, 2). Like its homologue, ClpB from Escherichia coli, Hsp104 forms ring-shaped oligomers and is thought to be involved in the dissociation of protein complexes and aggregates (3, 4). Both deletion of the gene encoding Hsp104 and overexpression of Hsp104 can cure yeast cells from [PSI+] and return Sup35 to its soluble state (5, 6). The N-terminal domain of Sup35, which is rich in glutamine and asparagine, was attributed a critical role in the process of prion formation and propagation (7), hence the name prion domain (PrD). Especially mutations in the N-terminal part of PrD not only alter the fibril formation properties but can also cause curing of wild-type aggregates in vivo. In nonprion neurodegenerative disorders like Huntington's disease, similar expansions of polyglutamine repeats appear to be responsible for amyloid formation and neurotoxicity (8, 9). Fusion proteins consisting of huntingtin-type polyglutamine repeats and green fluorescent protein were found to form aggregates when expressed in yeast cells. Deletion of the Hsp104 gene, however, resulted in elimination of aggregation (10).
Two models have been proposed for [PSI+] inheritance. Lindquist and coworkers (5, 6) suggest a model in which Hsp104 is required to put the polyglutamine repeats into an “aggregation competent” state, thereby inducing prion formation. Glutamine residues are a determining factor in interactions with Hsp104 (5, 6), which is thought to be necessary for the formation or maintenance (or both) of a partially unfolded form of Sup35. Therefore, an insufficient amount of the chaperone would prevent prion formation. If the amount of chaperone were too high, however, the prion “template” would dissociate from the unfolded intermediate, leading to the loss of the protein-modifying activity. Similarly, Cohen and Prusiner (11) have suggested that the conformational conversion from the wild-type to the prion form may require a partially unfolded intermediate. A prion “template” is supposed to bind to this intermediate, converting it into the prion conformation. Alternatively, Ter-Avanesyan and coworkers (12, 13) propose a model in which Hsp104 cleaves Sup35 [PSI+] aggregates into smaller pieces, which is necessary for their stable segregation during cell division.
So far, the only biochemical evidence that soluble Sup35 directly interacts with Hsp104 comes from CD experiments (14). In this manuscript, we present NMR data that give a more detailed picture of how the interaction between Sup35 and Hsp104 occurs. In our experiments, we used the peptide Sup355–26, which corresponds to residues 5–26 of Sup35, instead of the full-length protein. Our results show that in solution, Sup355–26 forms various oligomers of different sizes, which could be characterized in terms of both their structure and binding properties.
Methods
Peptide Synthesis and Protein Expression. Sup355–26 was obtained as a custom synthesis from BioSource International (Camarillo, CA). Hsp104 was overproduced in S. cerevisiae W303 carrying a plasmid encoding a constitutively expressed Hsp104 gene. The protein was purified in three steps, as described previously (15).
Diffusion-Ordered Spectroscopy (DOSY) NMR Experiments. DOSY NMR (16) were carried out to determine the molecular weight of Sup355–26. In these experiments, the size of a molecule can be estimated by encoding the diffusion of a molecule in a gradient echo. The measured diffusion constant D = (kBT)/(6πhFrS) is related via the Stokes–Einstein relation to the hydrodynamic radius rS of the molecule and thus to the molecular weight at a given viscosity η of the solution. kB denotes Boltzmann's constant, T the absolute temperature, and F the dimensionless Perrin factor. Only molecules that are not diffusing along a given axis are detected. The decay of magnetization can be analyzed in an analytical way, using I/I0 = exp{–D(Δ-δ/3)q2}, where q = γδg. Δ refers to the separation of the gradient echo, δ to the duration of the gradient, γ to the gyromagnetic ratio of the nucleus, and g to the strength of the gradient. Further information is published as supporting information on the PNAS web site, www.pnas.org.
Saturation Transfer Difference (STD) NMR Spectroscopy. STD was successfully used in the past to screen compound mixtures for binding to a receptor protein (17). Furthermore, it was used to characterize interactions between membrane channel proteins and neurotoxic peptides (18). In this way, it is possible to identify the chemical groups involved in ligand binding. The experiment relies on the selective saturation of the 1H resonances of the target protein. If a ligand binds weakly, saturation is transferred via cross relaxation to the bound ligand. An attenuated signal is then observed for the free ligand after dissociation. The method works best for small ligands (with fast correlation times) and large complexes, where the reorientation process is slow enough to give rise to large cross-relaxation rates. The STD amplification factor α is defined as α = (I0 – Isat/I0)*c, where I0 and Isat correspond to the intensity of the signal during off- and on-resonance irradiation, respectively. c refers to the relative concentration of the ligand with respect to the enzyme. Further information is published as supporting information.
Results
No structural information on the interaction between the yeast prion protein Sup35 and the molecular chaperone Hsp104 is available so far. In this work, we present solution-state NMR experiments that show that the peptide Sup355–26,
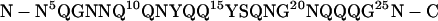 |
corresponding to residues 5–26 of Sup35, interacts with Hsp104. This peptide was chosen because mutation studies reveal differences of prion formation propensity on mutations in the respective section of the prion domain (7). More importantly, deletion of this fragment cures yeast cells from [PSI+]. It was therefore hypothesized that binding of Sup35 to Hsp104 involves glutamine and asparagine side-chains of Sup355–26. Although it was shown that other parts of Sup35 have an influence on prion formation as well (19, 20), this peptide constitutes a good model system to investigate the interaction of Sup35 with Hsp104 by NMR. The aim of our studies is to identify the chemical groups involved in interactions with the chaperone. We find that the peptide exists as a complex mixture of different oligomeric states ranging from monomers to octamers. On addition of Hsp104, the formation of a monomeric species can be observed that shows only weak interactions with Hsp104.
Aggregation of Sup355–26 Is Suppressed by Hsp104. On dissolution of freeze-dried Sup355–26 in buffer, the peptide aggregates quickly, as shown by the rapid decay of observable 1H NMR resonances (Fig. 1, triangle up). Only signals originating from soluble peptide molecules are visible in the spectrum. At a peptide concentration of 1.5 mM and T = 27°C, the characteristic time τagg, after which 50% of the peptide became insoluble, was found to be ≈80 min. At 12°C, aggregation was too fast to be monitored with this experimental setup. A possible explanation for this counterintuitive temperature-dependent behavior is that the peptide consists of an ensemble of species with differing aggregation probabilities. At higher temperature, this distribution is shifted toward forms that have a smaller tendency to aggregate. Also, the aggregates may be less stable at elevated temperatures. This idea is supported by the observation that aggregated peptide could be resolubilized by heating the sample to 60°C (data not shown). Importantly, the aggregated peptide strongly increased the fluorescence of Thioflavin T, a dye commonly used to detect amyloids (data not shown).
Fig. 1.
Time dependence of the intensity of the 1H NMR resonance line of tyrosine Hδ in Sup355–26 for different molar ratios [Hsp104]:[Sup355–26] [triangles: without Hsp104 (T = 27°C); squares: 1:100 (T = 12°C); circles: 1:50 (T = 12°C), inverted triangles: 1:100 (T = 27°C)]. The initial concentration of Sup355–26 was 1.5 mM in all experiments. The peptide was dissolved in 50 mM Na-phosphate buffer, pH 7.7. Sup355–26 and Hsp104 were mixed 3 min before recording the first data point.
Addition of Hsp104 substantially reduces the rate of aggregation (Fig. 1). Furthermore, aggregation is no longer quantitative in the presence of Hsp104. At a molar ratio of Hsp104 to Sup355–26 of 1:50 (monomer:monomer, T = 12°C), ≈35% of the peptide remains soluble after incubating the sample for 800 min (Fig. 1, filled circles). τagg was determined to be in the range of 400 min. NMR resonances of protons stemming from Hsp104 are not observable in these experiments due to the low concentration and the large molecular weight of the hexameric Hsp104.
Hsp104 Induces Conformational Changes in Sup355–26. To obtain a more detailed view of the interaction between Hsp104 and Sup355–26, we recorded 1D 1H spectra at 27°C and a molar ratio of Hsp104 to Sup355–26 of 1:25. Under these conditions, the peptide apparently can no longer aggregate. The intensities of nonexchangeable protons are constant throughout the experiment. After 300 min, however, new signals appear in the spectrum that cannot be found in the reference spectrum recorded in the absence of Hsp104 or in the experiments recorded at lower temperature (12°C) in the presence of Hsp104. Matrix-assisted laser desorption ionization–MS was carried out to verify that the peptide had not become degraded or chemically modified during the experiment (data not shown). The new signals must therefore reflect a change in the conformation of Sup355–26. A detailed view of the Tyr aromatic region is displayed in Fig. 2. The decay of the initial resonance line (black) is accompanied by the rise of two intermediate species (red). At later times, a fourth conformer (blue) accumulates. The full spectrum is published as supporting information. The absolute intensities of the four different species are shown as a function of time in Fig. 3. Sup355–26 contains two tyrosines. The scalar coupling between Hδ and Hε causes a splitting of the signal into a doublet. For clarity, the intensities of the doublets for intermediate 2 and the final conformer are divided by two, to take into account the overlap of the two resonance lines. The decay of the initial species occurs with a time constant of 1,600 min. Concomitantly, intermediate 1 becomes populated and reaches its maximum concentration after ≈2,000 min. After 5,000 min, the prevailing species of Sup355–26 is intermediate 2. At this point, the final conformer is populated only with≈25%. One hundred percent population of this final Sup355–26 conformer was found after only 7 days.
Fig. 2.
1D 1H NMR spectra displaying only the tyrosine Hδ spectral region. Spectra recorded between 0 and 5,500 min are represented in equidistant steps. Transiently populated species are indicated by bold lines.
Fig. 3.
Time dependence of the 1H signal of tyrosine Hδ in Sup355–26 for the molar ratios [Hsp104]:[Sup355–26] = 1:25 at 27°C for the initial (circles), intermediate (triangles), and final (squares) set of tyrosine resonances.
Sup355–26 Is a Mixture of Various Oligomeric Species. The differences in the aggregation behavior at 12 and 27°C (Fig. 1) and the conformational changes observed in the presence of Hsp104 (Fig. 2) indicate that Sup355–26 consists of a variety of species with differing aggregation properties and affinities for Hsp104. To further characterize this ensemble, we determined the molecular mass of Sup355–26 with DOSY NMR experiments (16). Fig. 4 shows the time dependence of the calculated molecular weight of Sup355–26 at 12°C (Fig. 4a) and at 27°C (Fig. 4b), both in the presence of Hsp104. The experimental relative signal intensities as a function of gradient strength are published as supporting information. It is apparent that the average molecular weight of Sup355–26 changes due to a shift in equilibrium between different oligomeric states. At 12°C, the molecular mass decreases from ≈22 kDa (t = 0 min; I = I0) to 10 kDa (t = 280 min; I = 0.8–0.4 I0). Under these conditions, ≈65% of the peptide molecules precipitate (see Fig. 1). Apparently, the initial distribution is centered around an octameric species. On longer incubation, the majority of the molecules form insoluble aggregates, whereas a fraction dissociates into smaller species that remain soluble. At a molar ratio of 1:25 for [Hsp104]:[Sup355–26]at T = 27°C, we observe a different behavior: Initially, the average molecular weight of Sup355–26 is ≈14 kDa, corresponding to a population of a predominantly hexameric species. With longer incubation, this species dissociates into smaller oligomeric states, and the apparent molecular mass decreases (≈8 kDa). Importantly, the chemical shift of the Tyr Hδ proton remains the same, indicating that the environment of this proton does not change during this process. From the spectra displayed in Fig. 2, we deduce that Sup355–26 is slowly converting into new species, indicated by changes in the chemical shifts. Using signals specific for the new species, their molecular weight could be determined in the same DOSY experiment. Intermediate 1 is mainly tetrameric at the beginning of the experiment. On incubation with Hsp104, the equilibrium is shifted to smaller oligomeric states, and Sup355–26 is slowly converted into a dimeric species. Intermediate 2 as well as the final product are monomeric throughout the experiment.
Fig. 4.
NMR diffusion data (DOSY) for Sup355–26 in the presence of Hsp104 at a molar ratio of [Hsp104]:[Sup355–26] = 1:50, T = 12°C (a) and 1:25 and T = 27°C (b); calculated molecular weight of Sup355–26 as a function of time (according to Eq. 1). Diffusion data were always recorded in parallel with the STD experiments to monitor the oligomeric state of the Sup355–26 complex. Representative of the other resonances, only DOSY data for the tyrosine resonance are shown. (a) The molecular mass is shifted from ≈23 kDa (t = 0 min; I = I0)to10kDa(t = 280 min; I = 0.8–0.4 I0) in the course of the experiment. (b) The calculated molecular mass shifts from ≈15 kDa (t = 0 min; I = I0) to 5.0 kDa (t = 4,000 min; I = 0.2 I0).
These results suggest that at 27°C, the Sup355–26 peptide adopts a range of oligomeric states centered around hexameric species. On interaction with Hsp104, these hexamers are slowly converted to structurally closely related tetramers. Subsequently, these tetramers dissociate into monomers in a two-step process, which is accompanied by characteristic changes in the 1D 1H NMR spectrum.
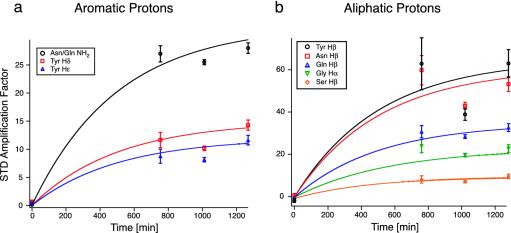
Binding of Sup355–26 to Hsp104. To identify the chemical groups of Sup35 that interact with Hsp104, we carried out STD experiments. The time dependence of the STD amplification factor for Sup355–26 in the presence of Hsp104 at a molar ratio of 50:1 at 12°C is shown in Fig. 5. At 12°C, no STD signal is observed in the beginning, indicating that the initial ensemble of Sup355–26 species shows no interaction with Hsp104. Taking into account the results of the DOSY experiment, this observation suggests that octameric Sup355–26 does not bind to Hsp104. With further incubation, however, STD signals are increasing monotonously, suggesting that species are formed that bind to Hsp104. Side-chain resonances of Asn and Gln as well as Tyr show the fastest STD buildup. This may be expected, because mutation studies show that these residues are essential for prion propagation and interaction with Hsp104 (7). On average, the time constant of the process monitored in the STD experiment is similar to the time constant observed for the decay of magnetization shown in Fig. 1 (≈400 min). Because no new 1H resonances can be observed under these conditions, the decrease in signal intensity is therefore purely due to aggregation. Apparently, the molecular chaperone Hsp104 modulates an equilibrium between different conformers of Sup355–26. Initially, the Sup35 species that interact with Hsp104 are not populated, and therefore no STD signal can be observed. Binding of Hsp104 to these molecules, however, could shift the distribution toward these conformers by the law of mass action. Their concentrations increase, and hence we observe an STD signal.
Fig. 5.
Time dependence of the STD signal for Sup355–26 in the presence of Hsp104 for a molar ratio of [Hsp104]:[Sup355–26] = 1:50 at T = 12°C. The assignment for the STD amplification factor for the various protons is given. Side chain protons of Asn, Gln, and Tyr show the fastest buildup, corresponding to the strongest interaction with Hsp104. At this temperature, no interaction between Hsp104 and Sup355–26 is observed at t = 0. As a consequence, the affinity between high oligomeric states of Sup355–26 and Hsp104 must be small.
At 27°C, Sup355–26 behaves strikingly different. Fig. 6 displays the STD amplification factors of the tyrosine Hδ resonance lines for the initial, intermediate, and final species of Sup355–26 (as indicated in Fig. 2). Other resonance lines show qualitatively the same behavior. The tyrosine signal has been selected in the analysis, because it is not overlapping with other 1H resonances. The initial species (circles) shows the strongest interaction with Hsp104, whereas the interaction of the intermediates 1 and 2 (triangles) is reduced. The final species (squares) shows only very weak binding to the chaperone. Error bars are based on the signal-to-noise ratio of the respective data sets. Generally, the error bars for the initial species are increasing in the course of the experiment, whereas the error bars for the final species are decreasing, due to the changing populations of the two states.
Fig. 6.
Time dependence of the STD signal for Sup355–26 in the presence of Hsp104 for a molar ratio of [Hsp104]:[Sup355–26] = 1:25 and T = 27°C. Open circles, squares, and triangles indicate the STD amplification factor of the Hδ tyrosine signal of the initial, intermediate, and final conformer. In contrast to Fig. 5, first a decay and, after t = 2,000 min, an increase of the STD amplification factor is observed. During the experiment, the equilibrium between high and low oligomeric states of Sup355–26 is shifted toward the low oligomeric state of Sup355–26 (circles), both of which can interact with hexameric Hsp104. The initial species shows strong interactions with Hsp104, whereas the “release” form shows a decreased interaction with Hsp104. The time constant of the rate of change is in agreement with the decay of magnetization due to the formation of a new species. Error bars indicate the uncertainty in the determination of the STD amplification factor.
The STD amplification factor for the initial conformer shows a characteristic minimum after ≈1,500 min. Thereafter, the signal is slowly increasing and finally dropping again at the end of the experiment. Combining the intensity data, to which all conformational states at a specific chemical shift contribute (Fig. 3), and the DOSY results (Fig. 4) allows us to estimate the population of the individual Sup355–26 oligomer at a given time (assuming there is conformational averaging between two predominantly populated species): The population of the initial hexameric conformer is decaying with a time constant of ≈1,500 min, whereas the population for intermediate 1 is first increasing and finally decaying at the end of the experiment. Because the relative STD amplification factor is related to the population of an individual species in a nonlinear manner, superposition of one exponentially decaying and one increasing STD curve leads to the observed amplification factor with an absolute minimum after ≈1,500 min. We conclude, therefore, that a hexamer as well as a tetramer of Sup355–26 can interact with Hsp104 at this temperature (circles).
A similar dip-type curve would also be expected for the STD amplification factor of intermediate 1 (triangles up), which consists of a tetrameric and a dimeric species (see above). The total population of this intermediate increases in the beginning and then decreases after 2,000 min (Fig. 3). At the same time, the distribution is shifted from the tetrameric to the dimeric species, as monitored by DOSY experiments. Qualitatively, the STD signal of intermediate 1 (Fig. 6) is comparable to the curve shape of the initial species. The absolute intensity, however, is much less pronounced, indicating weaker binding affinity of intermediate 1 compared with the initial conformer of Sup355–26 with respect to Hsp104. In principle, an increase of the STD amplification factor for t < 800 min would be expected initially. However, these time points are not accessible, because this species is not populated at the beginning of the experiment. For the second intermediate of Sup355–26, only a decrease in the STD amplification factor can be detected. Simultaneously, small changes in the apparent molecular weight for this conformer are observed, which correspond to a shift of equilibrium from a dimeric to monomeric species of Sup355–26. Again, the initial points in the DOSY experiments for intermediate 2 are difficult to access for a reliable interpretation due to the low signal-to-noise ratio for intermediate 2 for t < 1,500 min. No STD signal is observed for the resonance lines of the final state of Sup355–26 (squares), indicating that there is negligible interaction between the release form of Sup355–26 and Hsp104.
The focus of the current study was to characterize the oligomeric states of Sup355–26 that can interact with Hsp104. However, care was taken to study these interactions under defined oligomeric conditions for Hsp104. Biochemical experiments show that Hsp104 is hexameric at the conditions used for the NMR studies (data not shown). Gradient SDS/PAGE was performed at different concentrations of Hsp104, as well as with and without addition of peptide. In all cases, Hsp104 could be quantitatively cross-linked to a complex with a molecular mass of ≈600 kDa, corresponding to a hexamer, in agreement with other studies (21). Additionally, dynamic light-scattering experiments were carried out to obtain information about the degree of oligomerization of Hsp104. We found that Hsp104 is hexameric under the conditions used in the NMR experiments (data not shown). Assuming a globular structure for Hsp104, a hydrodynamic radius of ≈3.42 nm for the monomer is expected¶ (22). The observed hydrodynamic radius of ≈7.0 nm corresponds relatively well to a hexameric complex of Hsp104 at the two temperatures investigated (12 and 27°C).
Discussion
We could demonstrate that the presence of catalytic amounts of the chaperone Hsp104 significantly reduces the aggregation of Sup355–26. At lower temperatures (T = 12°C), only the rate and extent of conversion from the soluble into the aggregated form are reduced. At higher temperatures (27°C), we find that Hsp104 also induces structural changes in Sup355–26. It has been shown that temperature can affect the equilibrium between different oligomeric states of Sup35 (23). Our observations suggest that Hsp104 may facilitate this process by either lowering the activation barrier or energetically favoring an alternatively folded state of Sup355–26.
A model of the different equilibria between monomeric and oligomeric species of Sup355–26 and their interaction with Hsp104 is depicted in Fig. 7. After dissolution, Sup355–26 quickly forms oligomers. These oligomers may be related to nuclei that precede fibril formation or aggregation (24). Ultimately, aggregation of Sup355–26 leads to the formation of high oligomeric structures that bind the amyloid-specific dye Thioflavin T. Their molecular weight is too large to be detected by solution-state NMR spectroscopy; this process is responsible for the loss of signal shown in Fig. 1.
Fig. 7.
Interaction model for Hsp104 and Sup35. Sup355–26 can exist in various oligomeric states. At 12°C, Hsp104 induces an increase of population of smaller oligomeric states of Sup355–26. Higher oligomeric states (n > 6) of Sup355–26 cannot interact with Hsp104. At 27°C, refolding of an intially hexameric form of Sup355–26 to a monomeric form via two intermediate species is observed. This is reflected by a characteristic chemical shift change of the resonances of Sup355–26. The initial hexameric species of Sup355–26 display the largest interaction with Hsp104, whereas the lower oligomeric states show a reduced interaction with the chaperone. The implicated oligomeric states of Sup355–26 and Hsp104 are verified experimentally by DOSY NMR and DLS experiments.
Substoichiometric Amounts of Hsp104 Suppress the Aggregation of Sup355–26. How can we explain the observation that the addition of small amounts of Hsp104 to a solution of Sup355–26 strongly reduces the aggregation of the peptide? Our data suggest that Hsp104 may simply bind to the nuclei and thus block further polymerization. Alternatively, the chaperone may be able to dissolve nuclei and/or aggregated peptide, even in the absence of ATP. A third model, in which Hsp104 binds to monomeric peptide and prevents its incorporation into aggregates, is less probable because, according to our data, binding of monomeric Sup355–26 to Hsp104 is relatively weak and Hsp104 is active in substoichiometric amounts.
Oligomeric Species of Sup355–26 Dissociate in the Presence of Hsp104. Our results obtained with DOSY-NMR suggest that at 27°C, the freshly dissolved peptide is mainly hexameric (Fig. 7). It is not known whether these molecules or some other species in the initial oligomer distribution represent the nuclei for the subsequent polymerization process. However, the concentration of nuclei must be high to account for the high rate of aggregation.
After addition of Hsp104 to Sup355–26, the hexameric state is slowly converted to smaller species in a multistep process. The different intermediates are characterized by their specific chemical shifts. A characterization of the exact oligomeric state of Sup355–26 is difficult. First, multiple oligomeric states are likely to be in fast exchange. Second, we cannot assume that Sup355–26 is a spherical molecule. Therefore, only weighted averages of diffusion constants and approximated molecular weights can be determined.
Eventually, all soluble peptide becomes monomeric. This observation indicates that, under the conditions of the experiment, the monomeric peptide may be more stable than the oligomeric species. The 1D-NMR spectra thus monitor the establishment of the equilibrium distribution, and Hsp104 may serve as a catalyst in this process. This is difficult to asses because the reaction can be observed only in the presence of the chaperone. According to this notion, the initial distribution of Sup355–26 does not reflect the equilibrium but is highly enriched in oligomeric species that serve as seeds for rapid aggregation. After its synthesis, the peptide was purified by reverse-phase HPLC and subsequently lyophilized. It is possible that organic solvents stabilize the oligomeric states of Sup355–26 and are thus responsible for the overrepresentation of these species in the starting material. Furthermore, the dissolution process will generate high local concentrations of peptide, which may in turn lead to the formation of metastable oligomers. Thus, one would expect that the “equilibrated” peptide solution shows a decreased tendency to aggregate, because it no longer contains nuclei. This is indeed what we observe: after dialysis of the Sup355–26/Hsp104 solution, Sup355–26 no longer aggregates, even in the absence of Hsp104 (data not shown).
Like many other chaperones, Hsp104 requires the hydrolysis of ATP to carry out its biological function (3, 25). It would thus be obvious to investigate whether the presence of ATP changes the way in which Hsp104 interacts with Sup355–26. However, this turned out to be very difficult. The turnover rate of Hsp104 for ATP is in the order of kcat ≈ 70 min–1 (21). Thus, the rate of ATP hydrolysis would be ≈4 mM/min–1 in cases where [Hsp104]:[Sup355–26] = 1:25. The required concentration of ATP is not amenable to an NMR experiment, which takes several minutes to hours. Therefore, we focused on the intrinsic binding properties of Sup35 to Hsp104 in the present study. It has been shown for a number of ATP-dependent molecular chaperones that some features of their mechanism, e.g., substrate specificity, can be studied in the absence of nucleotide (26, 27). Experiments have been carried out by using a nonhydrolyzable ATP analogon (AMP-PNP) to study the influence of ATP on binding of Sup35 to Hsp104 (data not shown). These experiments show quantitatively the same behavior as is the case without AMP-PNP.
Interactions Between Sup355–26 and Hsp104. The chemical groups on the peptide involved in the interaction with Hsp104 could be identified by using the STD NMR technique. STD signals were observed for the side-chains of Asn/Gln and Tyr. Because binding kinetics are presumably fast compared with the time required for recording an STD experiment, a change in the STD signal over time can be caused only by changes in the concentration or in the affinity of the ligand. Our data show that Sup355–26 remains soluble throughout the experiment, and thus its total concentration is constant. We can conclude from DOSY experiments and 1D-NMR spectra that both the degree of oligomerization and the structure of the peptide change over time. Therefore, the time dependence of the STD signal can be attributed to the interconversion of the various Sup355–26 species. Moreover, conclusions can be drawn about the relative affinities of these species toward Hsp104. The data recorded at 27°C suggest that Hsp104 preferentially binds to oligomeric peptide species. Although we have currently no structural information on the peptide-binding site(s) of the Hsp104 hexamer, this is a rather intriguing result. It indicates that peptide binding may be cooperative, i.e., the peptide oligomer behaves like a multivalent ligand that binds to several Hsp104 subunits simultaneously (Fig. 7). This idea is in agreement with recent results of Lindquist and coworkers, who showed that polylysine stimulates ATP-hydrolysis by Hsp104 in a cooperative manner (28). Multivalent peptide binding has also been observed for the molecular chaperone GroEL from E. coli, which consists of two rings of seven subunits each (29). Another interesting observation in this context is that the octameric species, which dominates the initial distribution at 12°C, does not bind to Hsp104. One can speculate whether this molecule is not recognized because of its size or because of its inappropriate symmetry.
Structural Changes in Sup355–26 on Interaction with Hsp104. The monotonously decaying STD intensity of intermediate 2 indicates that the monomeric peptide does not interact with Hsp104. The observation that signals arising from exchangeable protons disappear in the course of the 1H 1D spectra of Sup355–26, whereas the intensity of protons from nonexchangeable sites remain constant, suggests that hydrogen bonds, in which amide protons are protected from exchange are lost. This conclusion is supported by the NMR diffusion data. Moreover, the chemical shift dispersion for the two tyrosine aromatic resonance lines becomes degenerate, indicating a loss of structure in both intermediate 2 and the release state of Sup355–26. A structural characterization of the various intermediates is currently in progress in our laboratory. Preliminary results obtained with CD spectroscopy indicate that the oligomeric species present in freshly prepared peptide solutions are rich in β structure (data not shown).
Implications for Fibril Assembly and Prion Propagation. The proposed model for the interaction between Sup35 and Hsp104 also has possible implications on the assembly process of amyloid fibrils, as indicated by dashed lines in Fig. 7. It is plausible to assume that monomeric Sup35 associates with small oligomers and eventually larger species that may serve as nuclei for amyloid fibril formation or aggregation. In the absence of Hsp104, these intermediates are short-lived and cannot be detected by NMR. However, in the presence of the chaperone, they are stabilized and therefore amenable for a structural investigation (23).
With respect to prion propagation, our observations favor a model in which Hsp104 interacts only with low oligomeric state (4 ≤ n ≤ 6) species of Sup355–26 as has been postulated by Lindquist and coworkers (5, 6). No interaction between Hsp104 and Sup355–26 (n > 6) could be detected. The latter observation comes from experiments that were carried out at 12°C, because species with n > 6 are stable only at lower temperatures. We cannot, however, rule out the possibility that Hsp104 also interacts with fibrillized Sup355–26. At 27°C, fibrils are not formed: the total intensity (Fig. 3) is approximately constant during the experiment. At 12°C, however, a significant amount of Sup355–26 is aggregating and is presumably forming fibrils, which are too large to be detected by solution-state NMR. The state-of-the-art detection limit is in the order of M < 900 kDa (30), which would correspond to an oligomer comprising ≈350 Sup355–26 subunits. Scheibel and Lindquist (23) reported that the nucleus that precedes fibril elongation consists of 10–30 subunits of the N + M domain of Sup35. Therefore, we exclude the possibility that interactions between Hsp104 and a nucleus of this size are not detected. To characterize interactions between fibrillized Sup355–26 and Hsp104, solid-state NMR experiments are envisaged. Such interactions have recently been reported by Yoshida (31). However, their importance with respect to prion propagation is so far not clear.
Supplementary Material
Acknowledgments
This research was supported by the Deutsche Forschungsgemeinschaft (SFB594, Teilprojekt A3). B.R. and S.N. acknowledge support from Deutsche Forschungsgemeinschaft Grant Re 1435/2. We are grateful to Profs. S. Weinkauf and N. Neumeier, Technische Universität München, for performing the dynamic light scattering experiments on Hsp104. We thank H. Krause, B. Cordes (Technische Universität München) and Dr. E. Krause (Forschungsinstitut für Molekulare Pharmakologie Berlin) for MS analysis of Sup355–26. We thank Prof. H. Schwalbe (University of Frankfurt) and Profs. H. Kessler and J. Buchner (Technische Universität München) for helpful comments on the manuscript.
Abbreviations: DOSY, diffusion-ordered spectroscopy; STD, saturation transfer difference.
Footnotes
The empirical relation between the number of residues N and the hydrodynamic radius of a native state protein is given as: rH = 4.75 N0.29 Å.
References
- 1.Sanchez, Y. & Lindquist, S. L. (1996) Science 248, 1112–1114. [DOI] [PubMed] [Google Scholar]
- 2.Lindquist, S. & Kim, G. (1996) Proc. Natl. Acad. Sci. USA 93, 5301–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsell, D. A., Kowal, A. S., Singer, M. A. & Lindquist, S. (1994) Nature 372, 475–478. [DOI] [PubMed] [Google Scholar]
- 4.Kim, K. I., Cheong, G. W., Park, S. C., Ha, J. S., Woo, K. M., Choi, S. J. & Chung, C. H. (2000) J. Mol. Biol. 303, 655–666. [DOI] [PubMed] [Google Scholar]
- 5.Chernoff, Y. O., Lindquist, S. L., Ono, B., Inge-Vechtomov, S. G. & Liebman, S. W. (1995) Science 268, 880–884. [DOI] [PubMed] [Google Scholar]
- 6.Patino, M. M., Liu, J. J., Glover, J. R. & Lindquist, S. (1996) Science 273, 622–626. [DOI] [PubMed] [Google Scholar]
- 7.DePace, A. H., Santoso, A., Hillner, P. & Weissman, J. S. (1998) Cell 93, 1241–1252. [DOI] [PubMed] [Google Scholar]
- 8.Reddy, P. S. & Houseman, D. E. (1997) Curr. Opin. Cell Biol. 9, 364–372. [DOI] [PubMed] [Google Scholar]
- 9.Ross, C. A. (1997) Neuron 19, 1147–1150. [DOI] [PubMed] [Google Scholar]
- 10.Krobitsch, S. & Lindquist, S. (2000) Proc. Natl. Acad. Sci. USA 97, 1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen, F. E. & Prusiner, S. B. (1998) Annu. Rev. Biochem. 67, 793–819. [DOI] [PubMed] [Google Scholar]
- 12.Paushkin, S. V., Kushnirov, V. V., Smirnov, V. N. & Ter-Avanesyan, M. D. (1996) EMBO J. 15, 3127–3134. [PMC free article] [PubMed] [Google Scholar]
- 13.Kushnirov, V. V. & Ter-Avanesyan, M. D. (1998) Cell 94, 13–16. [DOI] [PubMed] [Google Scholar]
- 14.Schirmer, E. C. & Lindquist, S. (1997) Proc. Natl. Acad. Sci. USA 94, 13932–13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schirmer, E. C. & Lindquist, S. (1998) in Methods in Enzymology, eds. Lorimer, G. H. & Baldwin, T. (Academic, San Diego), Vol. 290, pp. 430–444. [DOI] [PubMed] [Google Scholar]
- 16.Stejskal, E. O. & Tanner, J. E. (1965) J. Chem. Phys. 42, 288–292. [Google Scholar]
- 17.Mayer, M. & Meyer, B. (1999) Angew. Chem. Int. Ed. 38, 1784–1788. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi, H., Nakanishi, T., Kami, K., Arata, Y. & Shimada, I. (2000) Nat. Struct. Biol. 7, 220–223. [DOI] [PubMed] [Google Scholar]
- 19.Borchsenius, A. S., Wegrzyn, R. D., Newnam, G. P., Inge-Vechtomov, S. G. & Chernoff, Y. O. (2001) EMBO J. 20, 6683–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J.-J., Sondheimer, N. & Lindquist, S. L. (2002) Proc. Natl. Acad. Sci. USA 99, 16446–16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattendorf, D. A. & Lindquist, S. L. (2002) EMBO J. 21, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkins, D. K., Grimshaw, S. B., Receveur, V., Dobson, C. M., Jones, J. A. & Smith, L. J. (1999) Biochem. J. 38, 16424–16431. [DOI] [PubMed] [Google Scholar]
- 23.Scheibel, T. & Lindquist, S. L. (2002) Nat. Struct. Biol. 8, 958–962. [DOI] [PubMed] [Google Scholar]
- 24.Podlisny, M. B., Walsh, D. M., Amarante, P., Ostaszewski, B. L., Stimson, E. R., Maggio, J. E., Teplow, D. B. & Selkoe, D. J. (1998) Biochem. J. 37, 3602–3611. [DOI] [PubMed] [Google Scholar]
- 25.Glover, J. R. & Lindquist, S. (1998) Cell 94, 73–82. [DOI] [PubMed] [Google Scholar]
- 26.Rudiger, S., Germeroth, L., Schneider-Mergener, J. & Bukau, B. (1997) EMBO J. 16, 1501–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter, S., Lorimer, G. H. & Schmid, F. X. (1996) Proc. Natl. Acad. Sci. USA 93, 9425–9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cashikar, A. G., Schirmer, E. C., Hattendorf, D. A., Glover, R., Ramakrishnan, M. S., Ware, D. M. & Lindquist, S. L. (2002) Mol. Cell 9, 751–760. [DOI] [PubMed] [Google Scholar]
- 29.Chen, L. & Sigler, P. B. (1999) Cell 99, 757–768. [DOI] [PubMed] [Google Scholar]
- 30.Fiaux, J., Bertelsen, E. B., Horwich, A. L. & Wüthrich, K. (2002) Nature 418, 207–210. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida, M. (2002) FASEB Summer Research Conference on Protein Folding in the Cell), (FASEB, Bethesda).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.