Abstract
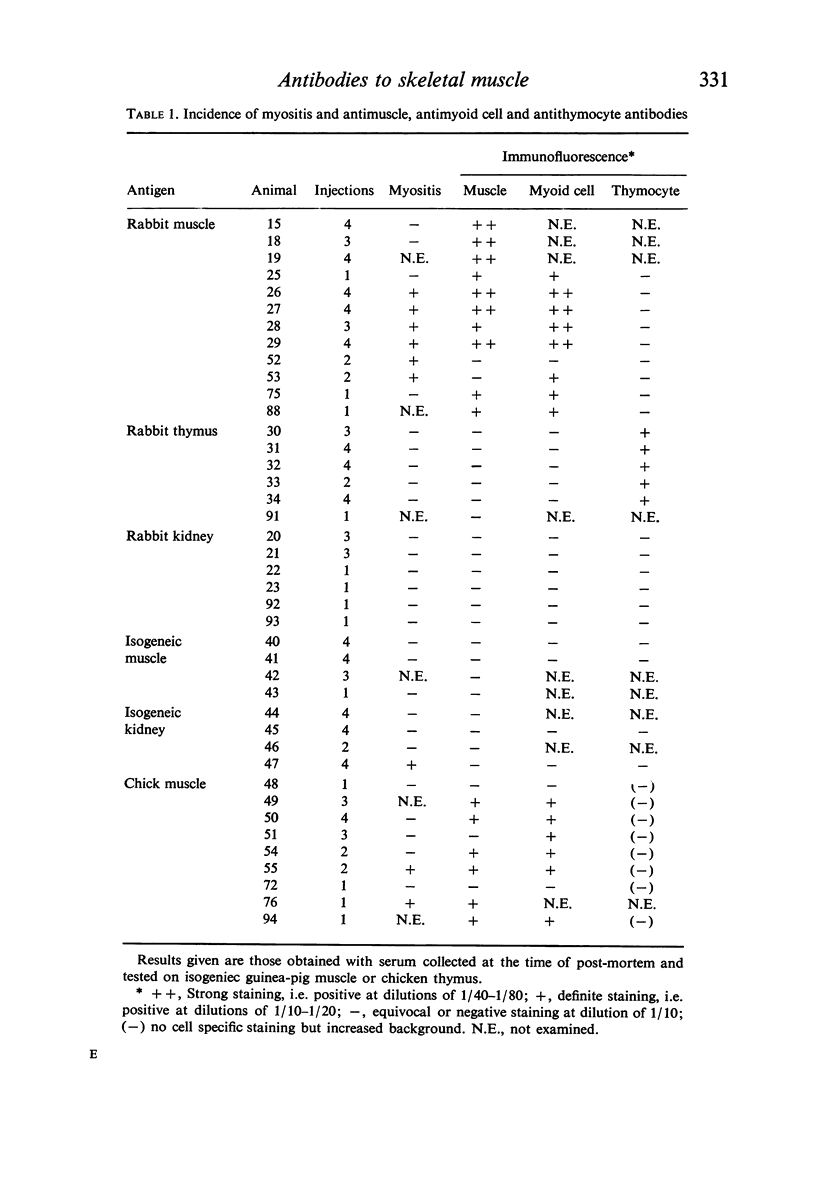
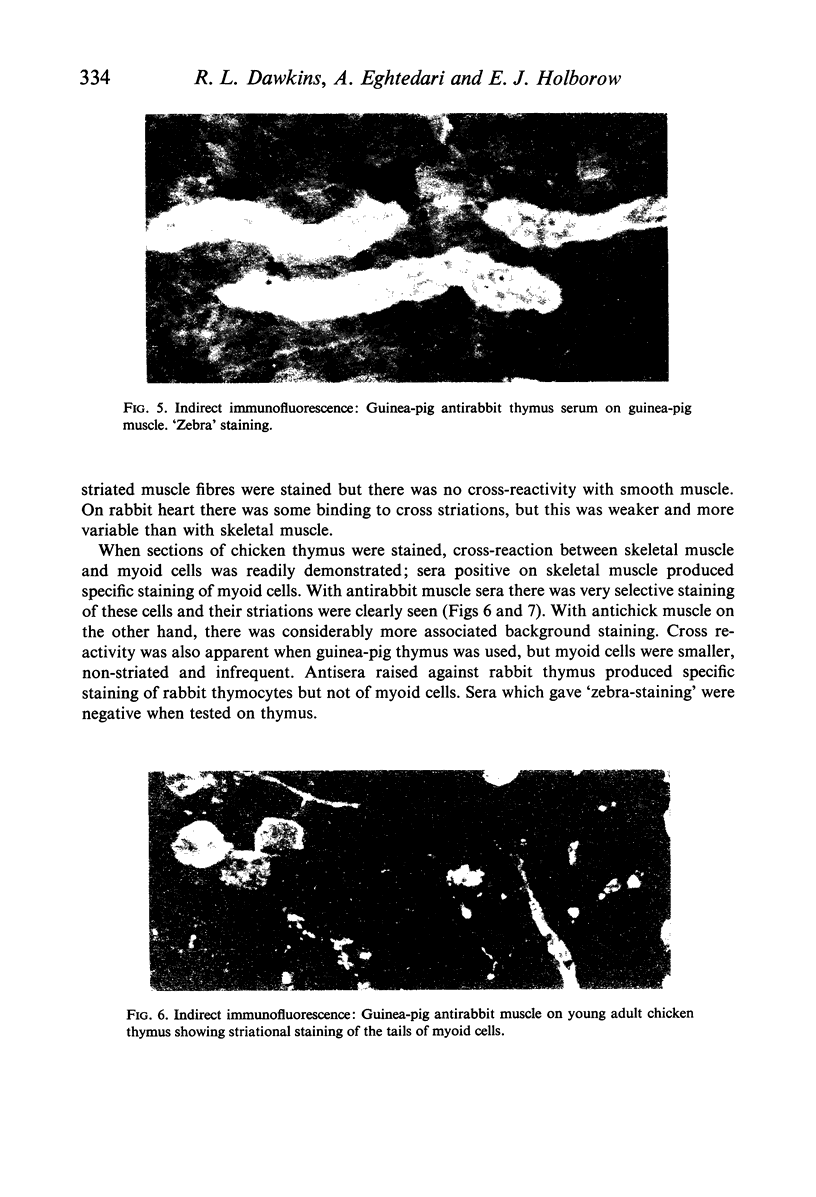
Immunization of inbred guinea-pigs with xenogeneic skeletal muscle can induce the development of a focal segmental myositis associated with the presence of a serum antibody which binds to the cross-striations of isogeneic or xenogeneic skeletal muscle as shown by indirect immunofluorescence. This antibody also produces striational staining of chick myoid cells. The association of striation-binding antibody with some forms of human and animal myositis is emphasized. It is concluded, however, that myositis is unlikely to be directly due to humoral antibody.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feltkamp T. E., Feltkamp-Vroom T. M. Antibodies against the various types of skeletal muscle fibres. Immunology. 1965 Sep;9(3):275–279. [PMC free article] [PubMed] [Google Scholar]
- Fessel W. J., Raas M. C. Autoimmunity in the pathogenesis of muscle disease. Neurology. 1968 Nov;18(11):1137–1139. doi: 10.1212/wnl.18.11.1137. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Whittingham S. Histological and serological features of experimental autoimmune thymitis in guinea-pigs. Clin Exp Immunol. 1967 May;2(3):257–268. [PMC free article] [PubMed] [Google Scholar]
- Kakulas B. A. Destruction of differentiated muscle cultures by sensitised lymphoid cells. J Pathol Bacteriol. 1966 Apr;91(2):495–503. doi: 10.1002/path.1700910225. [DOI] [PubMed] [Google Scholar]
- Kalden J. R., Williamson W. G., Johnston R. J., Irvine W. J. Studies on experimental autoimmune thymitis in guinea-pigs. Clin Exp Immunol. 1969 Oct;5(4):319–340. [PMC free article] [PubMed] [Google Scholar]
- Oosterhuis H. J., Bethlem J., Feltkamp T. E. Muscle pathology, thymoma, and immunological abnormalities in patients with myasthenia gravis. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):460–463. doi: 10.1136/jnnp.31.5.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe F. A. Analysis of antibody staining patterns obtained with striated myofibrils in fluorescence microscopy and electron microscopy. Int Rev Cytol. 1968;24:193–231. doi: 10.1016/s0074-7696(08)61400-x. [DOI] [PubMed] [Google Scholar]
- RUSSELL D. S. Histological changes in the striped muscles in myasthenia gravis. J Pathol Bacteriol. 1953 Apr;65(2):279–289. doi: 10.1002/path.1700650202. [DOI] [PubMed] [Google Scholar]
- Stern G. M., Rose A. L., Jacobs K. Circulating antibodies in polymyositis. J Neurol Sci. 1967 Jul-Aug;5(1):181–183. doi: 10.1016/0022-510x(67)90015-9. [DOI] [PubMed] [Google Scholar]
- Stewart H. L., Snell K. C. Thymomas and thymic hyperplasia in Praomys (Mastomys) natalensis. Concomitant myositis, myocarditis, and sialodacryoadenitis. J Natl Cancer Inst. 1968 Jun;40(6):1135–1159. [PubMed] [Google Scholar]
- Strauss A. J., Kemp P. G., Jr Serum autoantibodies in myasthenia gravis and thymoma: selective affinity for I-bands of striated muscle as a guide to identification of antigen(s). J Immunol. 1967 Nov;99(5):945–953. [PubMed] [Google Scholar]
- Strauss A. J., Snell K. C., Duntley B. J., Soban E. J., Stewart H. L. Spontaneous thymoma, polymyositis, and serum-autoantibodies to striated muscle in the rodent, subgenus Praomys (Mastomys) natalensis. Similarities to thymoma with myasthenia gravis in man. Lancet. 1968 May 25;1(7552):1126–1129. doi: 10.1016/s0140-6736(68)90189-x. [DOI] [PubMed] [Google Scholar]
- Vetters J. M., Simpson J. A., Folkarde A. Experimental myasthenia gravis. Lancet. 1969 Jul 5;2(7610):28–31. doi: 10.1016/s0140-6736(69)92601-4. [DOI] [PubMed] [Google Scholar]