Abstract
Chromosome-specific DNA markers provide a powerful approach for studying complex problems in human genetics and offer an opportunity to begin understanding the human genome at the molecular level. The approach described here for isolating and characterizing DNA markers specific to human chromosome 15 involved construction of a partial chromosome-15 phage library from a human/Chinese hamster cell hybrid with a single human chromosome 15. Restriction fragments that identified unique- and low-copy loci on chromosome 15 were isolated from the phage inserts. These fragments were regionally mapped to the chromosome by three methods, including Southern analysis with a mapping panel of cell hybrids, in situ hybridization to metaphase chromosomes, and quantitative hybridization or dosage analysis. A total of 42 restriction fragments of unique- and low-copy sequences were identified in 14 phage. The majority of the fragments that have been characterized so far exhibited the hybridization pattern of a unique locus on chromosome 15. Regional mapping assigned these markers to specific locations on chromosome 15, including q24-25, q21-23, q13-14, q11-12, and q11. RFLP analysis revealed that several markers displayed polymorphisms at frequencies useful for genetic linkage analysis. The markers mapped to the proximal long arm of chromosome 15 are particularly valuable for the molecular analysis of Prader-Willi syndrome, which maps to this region. Polymorphic markers in this region may also be useful for definitively establishing linkage with one form of dyslexia. DNA probes in this chromosomal region should facilitate molecular structural analysis for elucidation of the nature of instability in this region, which is frequently associated with chromosomal aberrations.
Full text
PDF




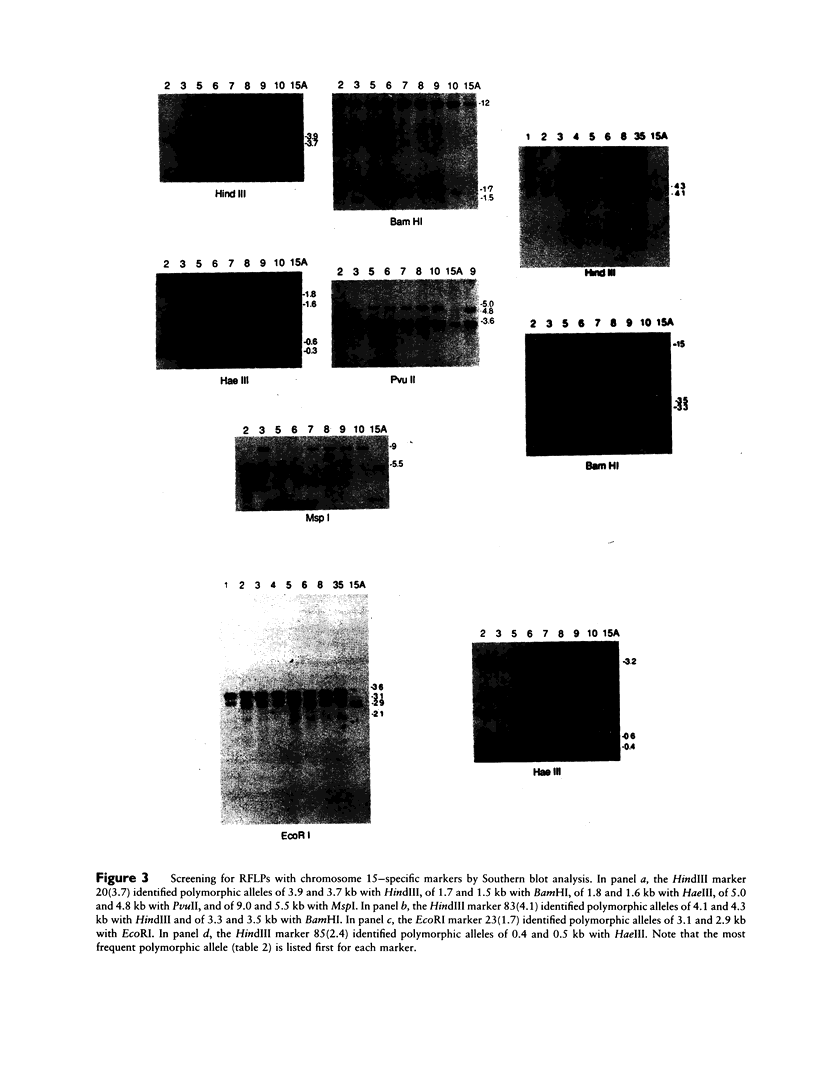




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Butler M. G., Meaney F. J., Palmer C. G. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986 Mar;23(3):793–809. doi: 10.1002/ajmg.1320230307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. G., Palmer C. G. Parental origin of chromosome 15 deletion in Prader-Willi syndrome. Lancet. 1983 Jun 4;1(8336):1285–1286. doi: 10.1016/s0140-6736(83)92745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W., Leach R., Mohandas T., Pearson P., White R. Isolation and regional localization of DNA segments revealing polymorphic loci from human chromosome 13. Am J Hum Genet. 1984 Jan;36(1):10–24. [PMC free article] [PubMed] [Google Scholar]
- Croce C. M. Loss of mouse chromosomes in somatic cell hybrids between HT-1080 human fibrosarcoma cells and mouse peritioneal macrophages. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3248–3252. doi: 10.1073/pnas.73.9.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlon T. A., Lalande M., Wyman A., Bruns G., Latt S. A. Isolation of molecular probes associated with the chromosome 15 instability in the Prader-Willi syndrome. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4408–4412. doi: 10.1073/pnas.83.12.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D., Davies K., Hartley D., Mandel J. L., Camerino G., Williamson R., White R. Genetic mapping of the human X chromosome by using restriction fragment length polymorphisms. Proc Natl Acad Sci U S A. 1984 May;81(9):2836–2839. doi: 10.1073/pnas.81.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. H., Lister C. K., Kellett E., Finnegan D. J. Transposable elements controlling I-R hybrid dysgenesis in D. melanogaster are similar to mammalian LINEs. Cell. 1986 Dec 26;47(6):1007–1015. doi: 10.1016/0092-8674(86)90815-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher J. H., Gusella J. F., Scoggin C. H. Molecular hybridization under conditions of high stringency permits cloned DNA segments containing reiterated DNA sequences to be assigned to specific chromosomal locations. Proc Natl Acad Sci U S A. 1984 Jan;81(2):520–524. doi: 10.1073/pnas.81.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Keys C., VarsanyiBreiner A., Kao F. T., Jones C., Puck T. T., Housman D. Isolation and localization of DNA segments from specific human chromosomes. Proc Natl Acad Sci U S A. 1980 May;77(5):2829–2833. doi: 10.1073/pnas.77.5.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J., Varsanyi-Breiner A., Kao F. T., Jones C., Puck T. T., Keys C., Orkin S., Housman D. Precise localization of human beta-globin gene complex on chromosome 11. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5239–5242. doi: 10.1073/pnas.76.10.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Monaco A. P., Feener C. C., Kunkel L. M. Conservation of the Duchenne muscular dystrophy gene in mice and humans. Science. 1987 Oct 16;238(4825):347–350. doi: 10.1126/science.3659917. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Hartz J. A., Law M. L., Davidson J. N. Isolation and chromosomal localization of unique DNA sequences from a human genomic library. Proc Natl Acad Sci U S A. 1982 Feb;79(3):865–869. doi: 10.1073/pnas.79.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Johnson R. T., Puck T. T. Complementation analysis on virus-fused Chinese hamster cells with nutritional markers. Science. 1969 Apr 18;164(3877):312–314. doi: 10.1126/science.164.3877.312. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Jones C., Puck T. T. Genetics of somatic mammalian cells: genetic, immunologic, and biochemical analysis with Chinese hamster cell hybrids containing selected human chromosomes. Proc Natl Acad Sci U S A. 1976 Jan;73(1):193–197. doi: 10.1073/pnas.73.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Law M. L., Hartz J., Jones C., Zhang X. L., Dewji N., O'Brien J. S., Wenger D. A. Regional localization of the gene coding for sphingolipid activator protein SAP-1 on human chromosome 10. Somat Cell Mol Genet. 1987 Nov;13(6):685–688. doi: 10.1007/BF01534489. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnit D. M. Satellite DNA and heterochromatin variants: the case for unequal mitotic crossing over. Hum Genet. 1979 Mar 12;47(2):169–186. doi: 10.1007/BF00273199. [DOI] [PubMed] [Google Scholar]
- Law M. L., Cai G. Y., Hartz J., Kao F. T., Hogg D., Breitman M. L., Tsui L. C. Localization of a beta-crystallin gene, Hu beta A3/A1 (gene symbol: CRYB1), to the long arm of human chromosome 17. Cytogenet Cell Genet. 1986;42(4):202–207. doi: 10.1159/000132279. [DOI] [PubMed] [Google Scholar]
- Ledbetter D. H., Riccardi V. M., Airhart S. D., Strobel R. J., Keenan B. S., Crawford J. D. Deletions of chromosome 15 as a cause of the Prader-Willi syndrome. N Engl J Med. 1981 Feb 5;304(6):325–329. doi: 10.1056/NEJM198102053040604. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lejeune J., Maunoury C., Prieur M., Van den Akker J. Translocation sauteuse (5p;15q), (8q;15q), (12q;15q). Ann Genet. 1979;22(4):210–213. [PubMed] [Google Scholar]
- Mattei M. G., Souiah N., Mattei J. F. Chromosome 15 anomalies and the Prader-Willi syndrome: cytogenetic analysis. Hum Genet. 1984;66(4):313–334. doi: 10.1007/BF00287636. [DOI] [PubMed] [Google Scholar]
- McKusick V. A. The human gene map 15 April 1986. Clin Genet. 1986 Jun;29(6):545–588. doi: 10.1111/j.1399-0004.1986.tb00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn M. L., Mayall B. H., Bogart E., Moore D. H., 2nd, Perry B. H. DNA content and DNA-based centromeric index of the 24 human chromosomes. Science. 1973 Mar 16;179(4078):1126–1129. doi: 10.1126/science.179.4078.1126. [DOI] [PubMed] [Google Scholar]
- Miklos G. L., John B. Heterochromatin and satellite DNA in man: properties and prospects. Am J Hum Genet. 1979 May;31(3):264–280. [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Middlesworth W., Colletti C. A., Aldridge J., Fischbeck K. H., Bartlett R., Pericak-Vance M. A., Roses A. D., Kunkel L. M. Detection of deletions spanning the Duchenne muscular dystrophy locus using a tightly linked DNA segment. 1985 Aug 29-Sep 4Nature. 316(6031):842–845. doi: 10.1038/316842a0. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Oliver N., Francke U., Pellegrino M. A. Regional assignment of genes for mannose phosphate isomerase, pyruvate kinase-3, and beta 2-microglobulin expression on human chromosome 15 by hybridization of cells from a t(15;22) (q14;q13.3) translocation carrier. Cytogenet Cell Genet. 1978;22(1-6):506–510. doi: 10.1159/000131009. [DOI] [PubMed] [Google Scholar]
- Ray P. N., Belfall B., Duff C., Logan C., Kean V., Thompson M. W., Sylvester J. E., Gorski J. L., Schmickel R. D., Worton R. G. Cloning of the breakpoint of an X;21 translocation associated with Duchenne muscular dystrophy. Nature. 1985 Dec 19;318(6047):672–675. doi: 10.1038/318672a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scalenghe F., Turco E., Edström J. E., Pirrotta V., Melli M. Microdissection and cloning of DNA from a specific region of Drosophila melanogaster polytene chromosomes. Chromosoma. 1981;82(2):205–216. doi: 10.1007/BF00286105. [DOI] [PubMed] [Google Scholar]
- Smith S. D., Kimberling W. J., Pennington B. F., Lubs H. A. Specific reading disability: identification of an inherited form through linkage analysis. Science. 1983 Mar 18;219(4590):1345–1347. doi: 10.1126/science.6828864. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vellutino F. R. Dyslexia. Sci Am. 1987 Mar;256(3):34–41. doi: 10.1038/scientificamerican0387-34. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- White R., Leppert M., Bishop D. T., Barker D., Berkowitz J., Brown C., Callahan P., Holm T., Jerominski L. Construction of linkage maps with DNA markers for human chromosomes. Nature. 1985 Jan 10;313(5998):101–105. doi: 10.1038/313101a0. [DOI] [PubMed] [Google Scholar]
- Wyman A. R., Wolfe L. B., Botstein D. Propagation of some human DNA sequences in bacteriophage lambda vectors requires mutant Escherichia coli hosts. Proc Natl Acad Sci U S A. 1985 May;82(9):2880–2884. doi: 10.1073/pnas.82.9.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]