Abstract
Inheritance of animal mtDNA is almost exclusively maternal, most likely because sperm-derived mitochondria are actively eliminated from the ovum, either at or soon after fertilization. How such elimination occurs is currently unknown. We asked whether similar behavior could be detected in somatic cells, by following the fate of mitochondria and mtDNAs after entry of human sperm into transformed cells containing mitochondria but lacking endogenous mtDNAs (rho0 cells). We found that a high proportion (10%-20%) of cells contained functioning sperm mitochondria soon after sperm entry. However, under selective conditions permitting only the survival of cells harboring functional mtDNAs, only approximately 1/10(5) cells containing sperm mitochondria survived and proliferated. These data imply that mitochondria in sperm can enter somatic cells relatively easily, but they also suggest that mechanisms exist to eliminate sperm-derived mtDNA from somatic cells, mechanisms perhaps similar to those presumed to operate in the fertilized oocyte.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Ankel-Simons F., Cummins J. M. Misconceptions about mitochondria and mammalian fertilization: implications for theories on human evolution. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13859–13863. doi: 10.1073/pnas.93.24.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida E. R., Scofield V. L. Lymphocyte major histocompatibility complex-encoded class II structures may act as sperm receptors. Proc Natl Acad Sci U S A. 1987 May;84(10):3395–3399. doi: 10.1073/pnas.84.10.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr G. F., Engler W. F. Considerations of volume, mass, DNA, and arrangement of mitochondria in the midpiece of bull spermatozoa. Exp Cell Res. 1970 Jun;60(3):338–340. doi: 10.1016/0014-4827(70)90526-4. [DOI] [PubMed] [Google Scholar]
- Bendich A., Borenfreund E., Sternberg S. S. Penetration of somatic mammalian cells by sperm. Science. 1974 Mar 1;183(4127):857–859. doi: 10.1126/science.183.4127.857. [DOI] [PubMed] [Google Scholar]
- Birky C. W., Jr Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11331–11338. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P., de Muys J. M., Morais R. An established avian fibroblast cell line without mitochondrial DNA. Somat Cell Mol Genet. 1986 Mar;12(2):133–139. doi: 10.1007/BF01560660. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Blanc H., Cann H. M., Wallace D. C. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U., Wharton D., Josefsson A., Wilson A. C. Paternal inheritance of mitochondrial DNA in mice. Nature. 1991 Jul 18;352(6332):255–257. doi: 10.1038/352255a0. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Borenfreund E., Bendich A. Appearance of foetal antigens in somatic cells after interaction with heterologous sperm. Nature. 1975 Oct 9;257(5526):488–489. doi: 10.1038/257488a0. [DOI] [PubMed] [Google Scholar]
- Hiraoka J., Hirao Y. Fate of sperm tail components after incorporation into the hamster egg. Gamete Res. 1988 Apr;19(4):369–380. doi: 10.1002/mrd.1120190408. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Newbold J. E., Potter S. S., Edgell M. H. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974 Oct 11;251(5475):536–538. doi: 10.1038/251536a0. [DOI] [PubMed] [Google Scholar]
- Kaneda H., Hayashi J., Takahama S., Taya C., Lindahl K. F., Yonekawa H. Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4542–4546. doi: 10.1073/pnas.92.10.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- King M. P., Attardi G. Injection of mitochondria into human cells leads to a rapid replacement of the endogenous mitochondrial DNA. Cell. 1988 Mar 25;52(6):811–819. doi: 10.1016/0092-8674(88)90423-0. [DOI] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. P. Use of ethidium bromide to manipulate ratio of mutated and wild-type mitochondrial DNA in cultured cells. Methods Enzymol. 1996;264:339–344. doi: 10.1016/s0076-6879(96)64032-4. [DOI] [PubMed] [Google Scholar]
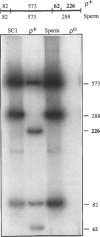
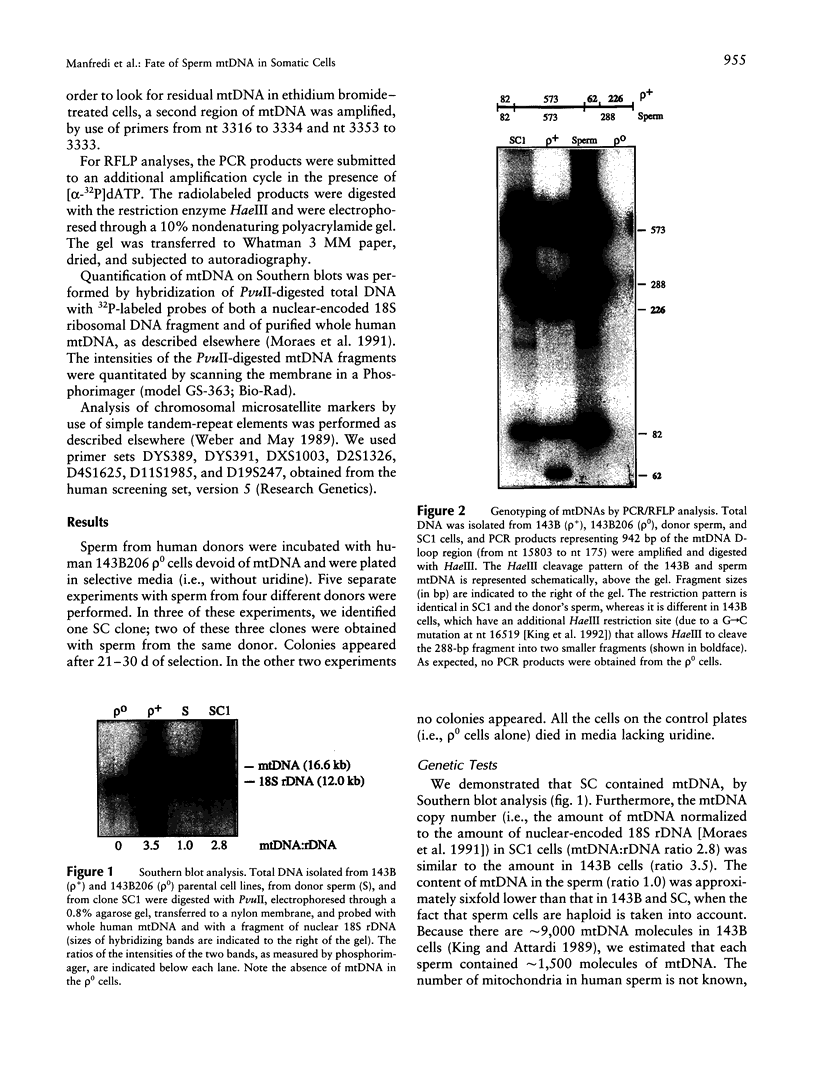
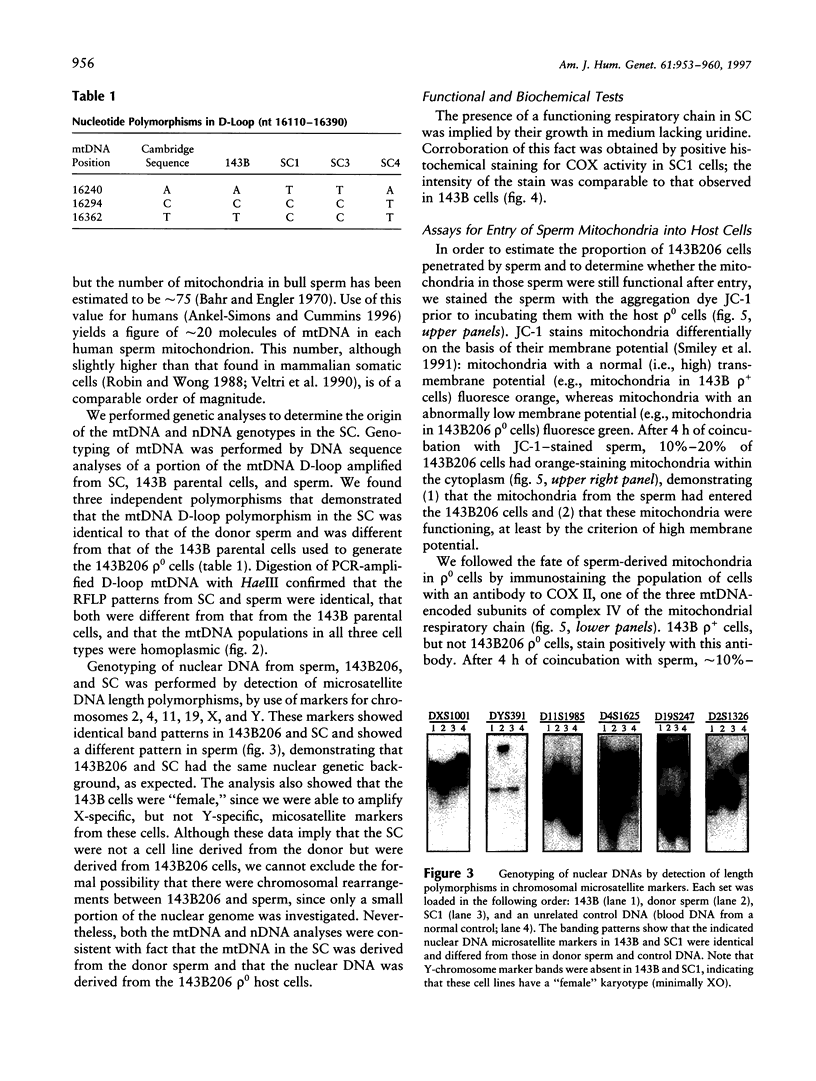
- Moraes C. T., Shanske S., Tritschler H. J., Aprille J. R., Andreetta F., Bonilla E., Schon E. A., DiMauro S. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991 Mar;48(3):492–501. [PMC free article] [PubMed] [Google Scholar]
- Morais R., Desjardins P., Turmel C., Zinkewich-Péotti K. Development and characterization of continuous avian cell lines depleted of mitochondrial DNA. In Vitro Cell Dev Biol. 1988 Jul;24(7):649–658. doi: 10.1007/BF02623602. [DOI] [PubMed] [Google Scholar]
- Robin E. D., Wong R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol. 1988 Sep;136(3):507–513. doi: 10.1002/jcp.1041360316. [DOI] [PubMed] [Google Scholar]
- Seligman A. M., Karnovsky M. J., Wasserkrug H. L., Hanker J. S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol. 1968 Jul;38(1):1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalgi R., Magnus A., Jones R., Phillips D. M. Fate of sperm organelles during early embryogenesis in the rat. Mol Reprod Dev. 1994 Mar;37(3):264–271. doi: 10.1002/mrd.1080370304. [DOI] [PubMed] [Google Scholar]
- Smiley S. T., Reers M., Mottola-Hartshorn C., Lin M., Chen A., Smith T. W., Steele G. D., Jr, Chen L. B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P., Navara C. S., Schatten G. Fate of the sperm mitochondria, and the incorporation, conversion, and disassembly of the sperm tail structures during bovine fertilization. Biol Reprod. 1996 Dec;55(6):1195–1205. doi: 10.1095/biolreprod55.6.1195. [DOI] [PubMed] [Google Scholar]
- Szollosi D. The fate of sperm middle-piece mitochondria in the rat egg. J Exp Zool. 1965 Aug;159(3):367–377. doi: 10.1002/jez.1401590309. [DOI] [PubMed] [Google Scholar]
- Tritschler H. J., Bonilla E., Lombes A., Andreetta F., Servidei S., Schneyder B., Miranda A. F., Schon E. A., Kadenbach B., DiMauro S. Differential diagnosis of fatal and benign cytochrome c oxidase-deficient myopathies of infancy: an immunohistochemical approach. Neurology. 1991 Feb;41(2 ):300–305. doi: 10.1212/wnl.41.2_part_1.300. [DOI] [PubMed] [Google Scholar]
- Veltri K. L., Espiritu M., Singh G. Distinct genomic copy number in mitochondria of different mammalian organs. J Cell Physiol. 1990 Apr;143(1):160–164. doi: 10.1002/jcp.1041430122. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- ZUCKER-FRANKLIN D., HIRSCH J. G. ELECTRON MICROSCOPE STUDIES ON THE DEGRANULATION OF RABBIT PERITONEAL LEUKOCYTES DURING PHAGOCYTOSIS. J Exp Med. 1964 Oct 1;120:569–576. doi: 10.1084/jem.120.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]