Abstract
Objective: To investigate the expression of maspin in RA synovial tissue and compare it with the expression in osteoarthritis (OA) and normal synovial tissue (NS).
Methods: Using specific primers for maspin, a 237 bp fragment was amplified from cDNA obtained from cultured RA, OA, and normal synovial fibroblasts (SF) by RT-PCR. Additionally, mRNA expression levels were determined quantitatively by real time PCR. mRNA expression of maspin was investigated on snap frozen and paraffin embedded synovial tissue sections by in situ hybridisation. Immunohistochemistry was used to identify the cell type expressing maspin. SDS-PAGE and western blotting were performed to evaluate the protein expression in cultured SF. To confirm protein synthesis in situ, immunohistochemistry with specific anti-maspin antibodies was performed in synovial tissue sections of patients with RA.
Results: RT-PCR showed expression of maspin in all cDNA samples from cultured SF. Maspin mRNA was found to be decreased in RA SF twofold and 70-fold compared with OA SF and NS SF, respectively. Maspin mRNA was expressed in RA, OA, and normal synovial tissue. Importantly, maspin transcripts were also found at sites of invasion into cartilage and bone. At the protein level, maspin could be detected in RA and, less prominently, OA SF. In RA synovial tissue, maspin protein was detected in only a few synovial lining cells.
Conclusion: Maspin is expressed intensively in RA SF at the mRNA level, but only slightly at the protein level, possibly owing to down regulation of maspin; this may contribute to the hyperplasia of synovial tissue in RA.
Full Text
The Full Text of this article is available as a PDF (383.3 KB).
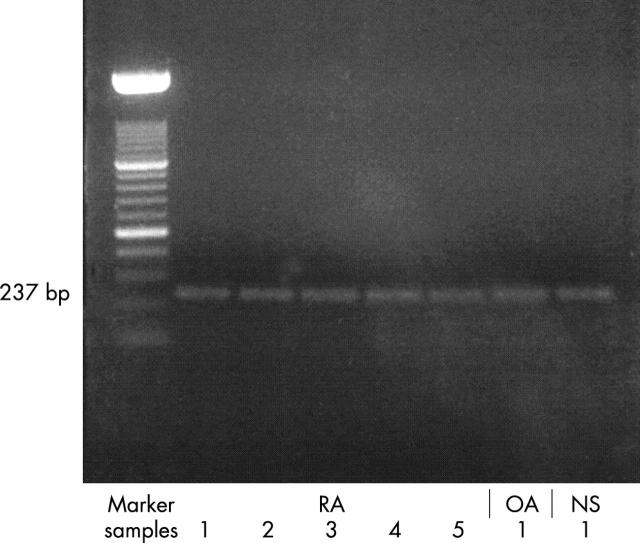
Figure 1.
RT-PCR using maspin-specific primers spanning a 237 bp fragment showed expression of maspin in all synovial fibroblast RNA samples examined (representative RA, OA, normal synovial tissue samples).
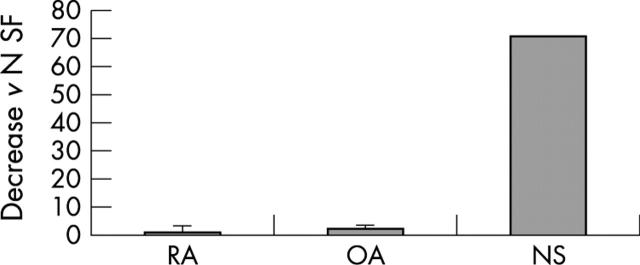
Figure 2.
SF from seven patients with RA, four with OA, and one trauma patient were examined by real time PCR with maspin-specific primers and probe (TaqMan system, PE Biosystems). Less maspin was expressed in RA samples than in OA and normal synovial fibroblast samples (twofold and 70-fold lower in comparison with OA and NS fibroblast samples, respectively).
Figure 3.
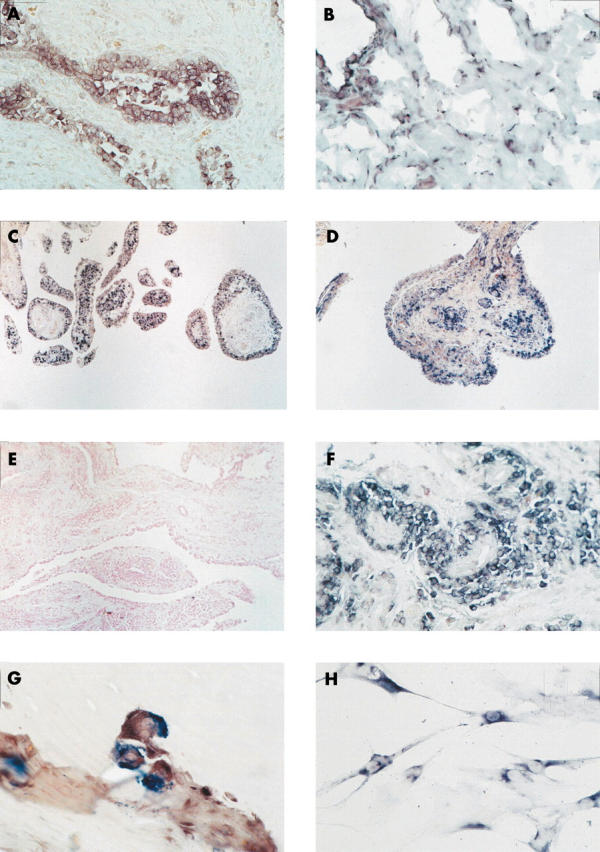
In situ hybridisation on paraffin embedded synovial tissue sections showed maspin mRNA expression in RA, OA, and normal synovial tissue. Whereas the expression of maspin was restricted to SF in lining and sublining in normal and OA synovial tissue (B and C), in RA, maspin mRNA was additionally seen within perivascular infiltrates (D). Prostate tissue was used as positive control showing staining in epithelial cells (A). In situ hybridisation using the sense probe remained negative (negative control; E). Higher magnification disclosed maspin expression in mononuclear cells around vessels (F) as well as in multinucleated cells resembling osteoclasts at sites of invasion of the synovial tissue into cartilage and bone (G). Cultured RA SF on chamber slides also showed a positive signal for maspin (H). Original magnification x100 (E), x200 (B, C, D), x400 (A, F), x630 (G, H).
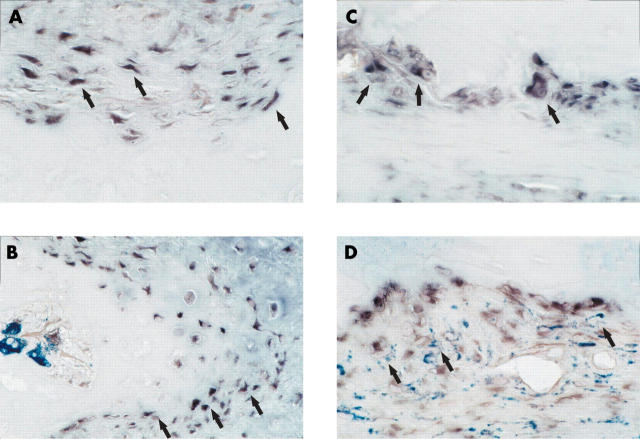
Figure 4.
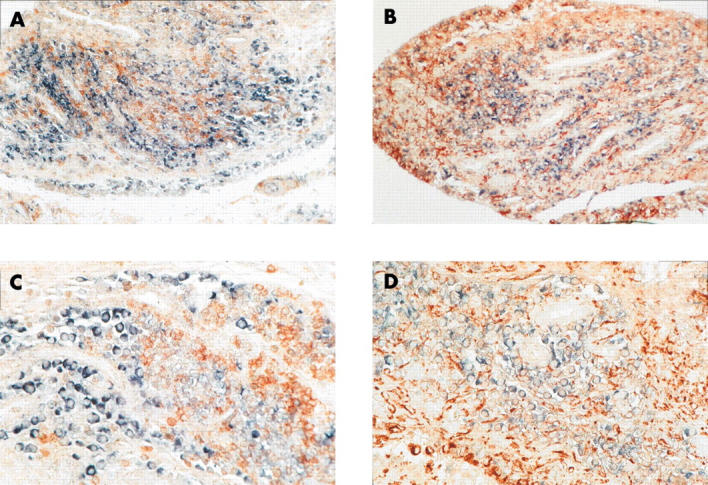
To further characterise the cell type expressing maspin, double labelling with anti-CD68 (A, C) and anti-CD3 antibodies (B, D) was performed after in situ hybridisation with maspin probes on paraffin embedded RA synovial tissue samples. Predominantly, maspin was detected in CD68 and CD3 negative SF. Blue-black colour: maspin transcripts, red-brown colour: anti-CD68 and anti-CD3 positive cells, respectively. Original magnificationx200 (A, C), x400 (B, D).
Figure 5.
Maspin mRNA expression was additionally investigated in RA synovial tissue at sites of cartilage and bone destruction. Maspin positive cells with both fibroblast-like (A) and macrophage-like as well as osteoclast-like morphology (C) were found. Double labelling with anti-CD68 antibodies showed both maspin positive, CD68 negative (figs 5B and 3G) as well as maspin positive, CD68 positive cells (D). Original magnification of all figures x400.
Figure 6.
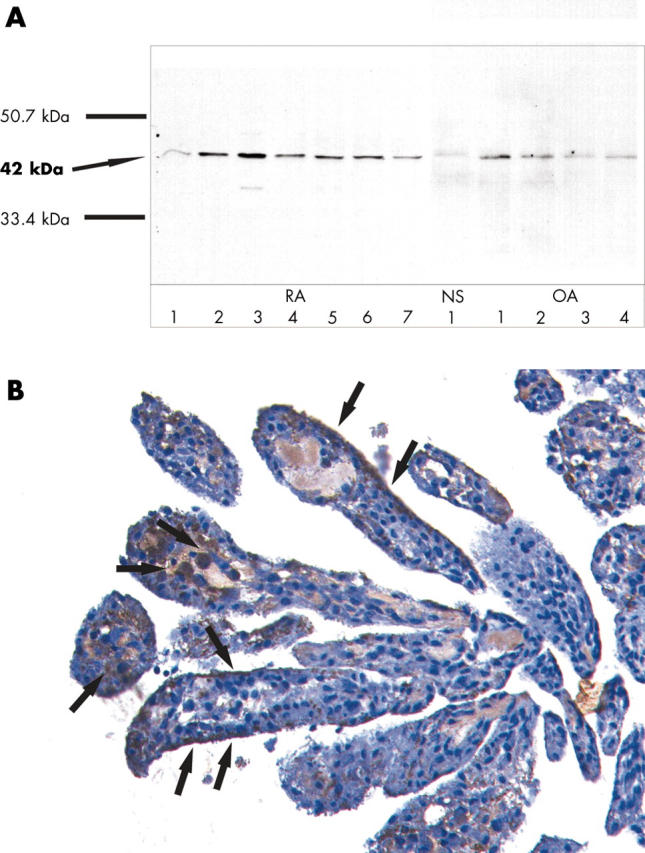
To examine the protein expression of maspin in RA SF, SDS-PAGE and western blot with anti-maspin antibodies was performed in SF of seven patients with RA and four with OA. A positive band of the correct size (42 kDa) was detected in all RA SF and OA SF examined. In normal SF, a discrete band could also be detected (A). To confirm these results in situ, immunohistochemistry using anti-maspin antibodies was performed in synovial tissue samples of four patients with RA. Prostate tissue was used as positive control (result not shown). Maspin could be detected only in a few single cells in the synovial lining as well as single cells in the sublining (B, arrows). Original magnification x200.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Asahara H., Hasumuna T., Kobata T., Yagita H., Okumura K., Inoue H., Gay S., Sumida T., Nishioka K. Expression of Fas antigen and Fas ligand in the rheumatoid synovial tissue. Clin Immunol Immunopathol. 1996 Oct;81(1):27–34. doi: 10.1006/clin.1996.0153. [DOI] [PubMed] [Google Scholar]
- Biliran H., Jr, Sheng S. Pleiotrophic inhibition of pericellular urokinase-type plasminogen activator system by endogenous tumor suppressive maspin. Cancer Res. 2001 Dec 15;61(24):8676–8682. [PubMed] [Google Scholar]
- Busso N., Péclat V., So A., Sappino A. P. Plasminogen activation in synovial tissues: differences between normal, osteoarthritis, and rheumatoid arthritis joints. Ann Rheum Dis. 1997 Sep;56(9):550–557. doi: 10.1136/ard.56.9.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosso M., Fibbi G., Matucci Cerinic M. The urokinase-type plasminogen activator system and inflammatory joint diseases. Clin Exp Rheumatol. 1999 Jul-Aug;17(4):485–498. [PubMed] [Google Scholar]
- Firestein G. S., Yeo M., Zvaifler N. J. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995 Sep;96(3):1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese E. M., Pettit A. R., Lee R., Madore R., Manning C., Tsay A., Gaspar J., Goldring M. B., Goldring S. R., Oettgen P. Angiopoietin-1 is expressed in the synovium of patients with rheumatoid arthritis and is induced by tumour necrosis factor alpha. Ann Rheum Dis. 2003 Feb;62(2):100–107. doi: 10.1136/ard.62.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Boyle D. L., Shi Y., Green D. R., Firestein G. S. Dominant-negative p53 mutations in rheumatoid arthritis. Arthritis Rheum. 1999 Jun;42(6):1088–1092. doi: 10.1002/1529-0131(199906)42:6<1088::AID-ANR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J. De-mystifying the mechanism(s) of maspin. Nat Med. 2000 Apr;6(4):374–376. doi: 10.1038/74624. [DOI] [PubMed] [Google Scholar]
- Kinne R. W., Palombo-Kinne E., Emmrich F. Activation of synovial fibroblasts in rheumatoid arthritis. Ann Rheum Dis. 1995 Jun;54(6):501–504. doi: 10.1136/ard.54.6.501-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R., Remmers E. F., Roberts A. B., Yocum D. E., Sporn M. B., Wilder R. L. Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-beta and retinoids. J Clin Invest. 1989 Apr;83(4):1267–1276. doi: 10.1172/JCI114011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Portek I., Edmonds J., Kirkham B. Synovial membrane p53 protein immunoreactivity in rheumatoid arthritis patients. Ann Rheum Dis. 2000 Feb;59(2):143–145. doi: 10.1136/ard.59.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass N., Hojo T., Zhang M., Sager R., Jonat W., Nagasaki K. Maspin--a novel protease inhibitor with tumor-suppressing activity in breast cancer. Acta Oncol. 2000;39(8):931–934. doi: 10.1080/02841860050215909. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Müller-Ladner U., Gay R. E., Nishioka K., Gay S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J Rheumatol. 1996 Aug;23(8):1345–1352. [PubMed] [Google Scholar]
- Murphy G., Stanton H., Cowell S., Butler G., Knäuper V., Atkinson S., Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999 Jan;107(1):38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Müller-Ladner U., Gay R. E., Gay S. Molecular biology of cartilage and bone destruction. Curr Opin Rheumatol. 1998 May;10(3):212–219. doi: 10.1097/00002281-199805000-00010. [DOI] [PubMed] [Google Scholar]
- Müller-Ladner U., Kriegsmann J., Franklin B. N., Matsumoto S., Geiler T., Gay R. E., Gay S. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996 Nov;149(5):1607–1615. [PMC free article] [PubMed] [Google Scholar]
- Müller-Ladner U., Kriegsmann J., Gay R. E., Gay S. Oncogenes in rheumatoid arthritis. Rheum Dis Clin North Am. 1995 Aug;21(3):675–690. [PubMed] [Google Scholar]
- Müller-Ladner U., Nishioka K. p53 in rheumatoid arthritis: friend or foe? Arthritis Res. 2000 Mar 31;2(3):175–178. doi: 10.1186/ar84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Ladner Ulf, Gay Renate E., Gay Steffen. Role of nuclear factor kappaB in synovial inflammation. Curr Rheumatol Rep. 2002 Jun;4(3):201–207. doi: 10.1007/s11926-002-0066-1. [DOI] [PubMed] [Google Scholar]
- Nickels A., Selter H., Pfreundschuh M., Montenarh M., Koch B. Detection of p53 in inflammatory tissue and lymphocytes using immunohistology and flow cytometry: a critical comment. J Clin Pathol. 1997 Aug;50(8):654–660. doi: 10.1136/jcp.50.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap T., Franz J. K., Hummel K. M., Jeisy E., Gay R., Gay S. Activation of synovial fibroblasts in rheumatoid arthritis: lack of Expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2000;2(1):59–64. doi: 10.1186/ar69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap T., Müller-Ladner U., Gay R. E., Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000 Jun 8;2(5):361–367. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrow P. K., Hummel K. M., Schedel J., Franz J. K., Klein C. L., Müller-Ladner U., Kriegsmann J., Chang P. L., Prince C. W., Gay R. E. Expression of osteopontin messenger RNA and protein in rheumatoid arthritis: effects of osteopontin on the release of collagenase 1 from articular chondrocytes and synovial fibroblasts. Arthritis Rheum. 2000 Jul;43(7):1597–1605. doi: 10.1002/1529-0131(200007)43:7<1597::AID-ANR25>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rinaldi N., Schwarz-Eywill M., Weis D., Leppelmann-Jansen P., Lukoschek M., Keilholz U., Barth T. F. Increased expression of integrins on fibroblast-like synoviocytes from rheumatoid arthritis in vitro correlates with enhanced binding to extracellular matrix proteins. Ann Rheum Dis. 1997 Jan;56(1):45–51. doi: 10.1136/ard.56.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxne T., Lecander I., Geborek P. Plasminogen activators and plasminogen activator inhibitors in synovial fluid. Difference between inflammatory joint disorders and osteoarthritis. J Rheumatol. 1993 Jan;20(1):91–96. [PubMed] [Google Scholar]
- Schedel Jörg, Gay Renate E., Kuenzler Peter, Seemayer Christian, Simmen Beat, Michel Beat A., Gay Steffen. FLICE-inhibitory protein expression in synovial fibroblasts and at sites of cartilage and bone erosion in rheumatoid arthritis. Arthritis Rheum. 2002 Jun;46(6):1512–1518. doi: 10.1002/art.10309. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997 Nov 3;139(3):575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Sheng S., Pemberton P. A., Sager R., Hendrix M. J. maspin suppresses the invasive phenotype of human breast carcinoma. Cancer Res. 1998 Dec 15;58(24):5681–5685. [PubMed] [Google Scholar]
- Sheng S., Truong B., Fredrickson D., Wu R., Pardee A. B., Sager R. Tissue-type plasminogen activator is a target of the tumor suppressor gene maspin. Proc Natl Acad Sci U S A. 1998 Jan 20;95(2):499–504. doi: 10.1073/pnas.95.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z., Koch A. E. Chemokines and angiogenesis. Curr Opin Rheumatol. 2001 May;13(3):202–208. doi: 10.1097/00002281-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Van Ness K., Chobaz-Péclat V., Castellucci M., So A., Busso N. Plasminogen activator inhibitor type-1 deficiency attenuates murine antigen-induced arthritis. Rheumatology (Oxford) 2002 Feb;41(2):136–141. doi: 10.1093/rheumatology/41.2.136. [DOI] [PubMed] [Google Scholar]
- Zhang M., Volpert O., Shi Y. H., Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000 Feb;6(2):196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- Zou Z., Anisowicz A., Hendrix M. J., Thor A., Neveu M., Sheng S., Rafidi K., Seftor E., Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994 Jan 28;263(5146):526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- Zou Z., Gao C., Nagaich A. K., Connell T., Saito S., Moul J. W., Seth P., Appella E., Srivastava S. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem. 2000 Mar 3;275(9):6051–6054. doi: 10.1074/jbc.275.9.6051. [DOI] [PubMed] [Google Scholar]
- van der Laan W. H., Pap T., Ronday H. K., Grimbergen J. M., Huisman L. G., TeKoppele J. M., Breedveld F. C., Gay R. E., Gay S., Huizinga T. W. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of a cell surface-targeted plasmin inhibitor. Arthritis Rheum. 2000 Aug;43(8):1710–1718. doi: 10.1002/1529-0131(200008)43:8<1710::AID-ANR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]