Abstract
Effective delivery of secreted proteins by gene therapy will require a vector that directs stable delivery of a transgene and a regulatory system that permits pharmacologic control over the level and kinetics of therapeutic protein expression. We previously described a regulatory system that enables transcription of a target gene to be controlled by rapamycin, an orally bioavailable drug. Here we demonstrate in vivo regulation of gene expression after intramuscular injection of two separate adenovirus or adeno-associated virus (AAV) vectors, one encoding an inducible human growth hormone (hGH) target gene, and the other a bipartite rapamycin-regulated transcription factor. Upon delivery of either vector system into immunodeficient mice, basal plasma hGH expression was undetectable and was induced to high levels after administration of rapamycin. The precise level and duration of hGH expression could be controlled by the rapamycin dosing regimen. Equivalent profiles of induction were observed after repeated administration of single doses of rapamycin over many months. AAV conferred stable expression of regulated hGH in both immunocompetent and immunodeficient mice, whereas adenovirus-directed hGH expression quickly extinguished in immunocompetent animals. These studies demonstrate that the rapamycin-based regulatory system, delivered intramuscularly by AAV, fulfills many of the conditions necessary for the safe and effective delivery of therapeutic proteins by gene therapy.
Systemic delivery of therapeutic proteins by gene therapy has the potential to improve the efficacy, convenience, and cost-effectiveness of treatment of a variety of diseases by allowing frequent injections of expensive recombinant proteins to be replaced by the infrequent, or one-time, delivery of therapeutic genes. The spectrum of diseases that can be treated may also be expanded by allowing delivery of proteins that cannot be administered safely or effectively by injection because of poor pharmacokinetics or narrow therapeutic windows.
Significant advances have recently been made in the generation of vectors that direct efficient long-term expression of transgenes in vivo. In particular, both replication-deficient adenoviral vectors and recombinant adeno-associated virus (AAV) vectors have been used to deliver therapeutic levels of a number of secreted proteins upon intramuscular (i.m.) injection into mice and nonhuman primates (1–9). Adenoviral vectors are attractive because they can be produced at high titer and transduce postmitotic cells very efficiently in vivo (10). Unique advantages of AAV vectors include their ability to direct stable expression of transgenes without inducing destructive cellular immune responses to the transgene product (4, 11–13). Muscle is a preferred target for gene delivery because it is accessible, well vascularized, and able to express and process secreted proteins (14).
Broad application of gene therapy, however, will require the ability to regulate precisely both the timing and dosage of protein delivery to produce levels of protein that fall within a therapeutic window and to ensure that therapy can be terminated if necessary. A number of regulatory systems have been developed to address this issue. These systems generally rely on the use of a small-molecule drug, such as tetracycline, RU486, or ecdysone, to control the activity of an appropriately engineered transcription factor that in turn regulates expression of the therapeutic gene (15). We have employed a system in which regulation is achieved by expressing the two critical domains of a transcription factor as separate polypeptides that interact only in the presence of rapamycin (16, 17). Rapamycin is an orally bioavailable drug that interacts simultaneously with two cellular polypeptides, FKBP12 and FRAP (18, 19). By fusing FKBP domains to a DNA-binding domain, called ZFHD1, and a portion of FRAP, called FRB, to a transcriptional activation domain from the p65 subunit of transcription factor NF-κB, reconstitution of a functional transcription factor and expression of a target gene can be made dependent on the presence of rapamycin (16). To reduce the potential for immunogenicity in humans, all functional components of the transcription factors, including the DNA-binding domain and activation domain, are derived from human proteins. In cell culture, and in vivo upon transplantation of engineered cells into mice, basal expression of the target gene has been shown to be extremely low and highly inducible in a rapamycin dose-dependent manner (16, 20). Regulated expression of erythropoietin from AAV has been achieved with this system in skeletal muscle of mice and a nonhuman primate (21). In this paper, we explore the potential of this system to enable the precise, long-term regulation of expression of human growth hormone when introduced into mice by i.m. injection of adenoviral and AAV vectors.
MATERIALS AND METHODS
Construction of Vectors and Production of Recombinant Viruses.
TF1 vectors contain a cytomegalovirus (CMV) enhancer/promoter (16, 22) driving expression of a bicistronic transcript that contains an activation domain fusion, an internal ribosome entry sequence (IRES), and a DNA-binding domain fusion followed by an 830-bp portion of the rabbit β-globin (RBG) gene containing the final intron and 3′ untranslated region (UTR; ref. 16). The activation domain fusion (FRB-p65) consists of the FRB fragment of human FRAP (in which the threonine at amino acid 2098 was mutated to leucine), fused to an activation domain derived from the p65 subunit of human NF-κB (16). The IRES is derived from the encephalomyocarditis virus (23). The DNA-binding domain fusion (ZFHD1–3xFKBP) consists of the ZFHD1 DNA-binding domain and three tandemly repeated copies of human FKBP12 (16). The FRB-p65 and ZFHD1–3xFKBP fusion proteins both contain an amino-terminal epitope tag and nuclear localization signal. The reporter construct ZG1 contains 12 ZFHD1 binding sites upstream of a minimal interleukin-2 (IL-2) promoter driving expression of a genomic human growth hormone (hGH) gene (24), followed by a 3′ UTR from the simian virus 40 late gene. This vector was generated by replacing the SEAP gene of Z12-IL2-SEAP (16) with that of hGH. In ZG2, the hGH genomic clone was replaced by an hGH cDNA (obtained by reverse transcription–PCR from cells expressing the hGH genomic clone), and the target gene cassette was followed by a 2844-bp fragment that contains a CMV enhancer driving expression of the activation domain fusion, FRB-p65, with a portion of the RBG gene containing the final intron and 3′ UTR. CMVhGH was constructed by inserting the hGH cDNA downstream of the CMV enhancer/promoter (22).
Recombinant adenoviruses Ad.TF1, Ad.ZG1, and Ad.CMVhGH were generated by cotransfection of ClaI-digested sub360 genome DNA and adenoviral construction vectors carrying TF1, ZG1, or CMVhGH expression cassettes into 293 cells (22). The resulting viral plaques were further purified through three rounds of plaque isolation. Recombinant AAV was generated by cloning TF1, ZG1, or ZG2 expression cassettes into an XbaI-digested pSub 201 backbone (25). The resulting plasmids, pAAV.TF1, pAAV.ZG1, and pAAV.ZG2, were used to produce recombinant AAV as described previously (12).
In Vivo Experiments.
Male BALB/c nude or BALB/c immunocompetent mice (4–5 weeks old) were injected with either recombinant adenovirus or AAV into four i.m. sites (quadriceps and tibialis anterior, bilaterally) at various doses (25 μl per site). Rapamycin was initially dissolved in N,N-dimethylacetamide as a stock solution, then diluted to each specific concentration in a mixture, with the final concentration (vol/vol) of 4% N,N-dimethylacetamide, 10% polyethylene glycol (average molecular weight 400), and 17% polyoxyethylene sorbitan monooleate. The injection was given intraperitoneally (i.p.) in a total volume of 100 μl. Blood samples were taken retroorbitally at various times, and circulating plasma hGH concentrations were measured by ELISA (Boehringer Mannheim).
RESULTS
Regulated Gene Expression in Skeletal Muscle of Immunodeficient Mice with Adenoviral Vectors.
Our strategy was to incorporate the three components of the rapamycin regulatory system into two vectors. One vector expresses the activation domain and DNA-binding domain fusions from a single transcriptional unit with an internal ribosome entry site. The second vector contains the therapeutic gene, in this study hGH, driven by a promoter recognized by the ZFHD1 DNA-binding domain (Fig. 1; TF1 and ZG1). Initial experiments were performed with recombinant adenoviruses. A549 cells were exposed to equal quantities of the two adenoviral vectors at a total multiplicity of infection of 4 (Table 1). Expression in the absence of rapamycin, which was only 5-fold above background, increased more than 100-fold in the presence of rapamycin.
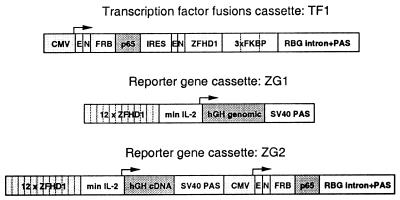
Figure 1.
Schematic diagram of transcription factor fusions and the reporter gene constructs. TF1 vector. The enhancer/promoter from CMV drives expression of a bicistronic transcript in which the first cistron encodes a fusion of the rapamycin binding domain of FRAP (FRB) with the activation domain of the p65 subunit of NF-κB. The second cistron, translated from an internal ribosome entry site (IRES), encodes 3 copies of FKBP fused to the chimeric DNA-binding domain ZFHD1. The 3′ untranslated region consists of an intron and polyadenylation sequence (PAS) from the rabbit β-globin gene (RBG). Both fusion proteins contain an amino-terminal epitope tag (E) and nuclear localization signal (N). ZG1 vector. This target gene vector contains 12 repeats of the recognition site for ZFHD1 upstream of a minimal IL-2 promoter driving expression of hGH, followed by the polyadenylation signal of the simian virus 40 late gene. ZG2 vector. This vector contains two transcription units in series. The ZG1 cassette is followed by all the elements of the TF1 cassette except the IRES and DNA-binding domain fusion.
Table 1.
Rapamycin-induced hGH production by adenoviral and AAV vectors in vitro
| Vectors | moi | hGH production, pg/ml
|
Fold increase | |
|---|---|---|---|---|
| Without rapamycin | With rapamycin | |||
| Adenovirus* | ||||
| Ad.TF1 | 2 | <10 | <10 | — |
| Ad.ZG1 | 2 | 85 ± 7 | 52 ± 16 | 0.6 |
| Ad.TF1 + ZG1 | 2 + 2 | 51 ± 3 | 5,543 ± 2,897 | 107 |
| AAV† | ||||
| AAV.TF1 | 2 | <10 | <10 | — |
| AAV.ZG1 | 2 | <10 | <10 | — |
| AAV.ZG2 | 2 | 576 ± 113 | 1,051 ± 312 | 1.8 |
| AAV.TF1 + ZG1 | 2 + 2 | 42 ± 24 | 7,800 ± 3,527 | 186 |
| AAV.TF1 + ZG2 | 2 + 2 | 3,369 ± 183 | 41,785 ± 2,692 | 12.4 |
A549 cells were infected with adenoviral vectors at the indicated multiplicity of infection (moi; ratio of particles to plaque-forming units = 100).
84-31 cells were infected with the AAV vectors at the indicated moi (ratio of genome copies to infectious particles = 1,000). Rapamycin (50 nM) was added 24 h after infection. Culture medium was harvested 24 h later and hGH concentration was determined by ELISA. The limit of detection is 10 pg/ml. Values are mean ± SEM.
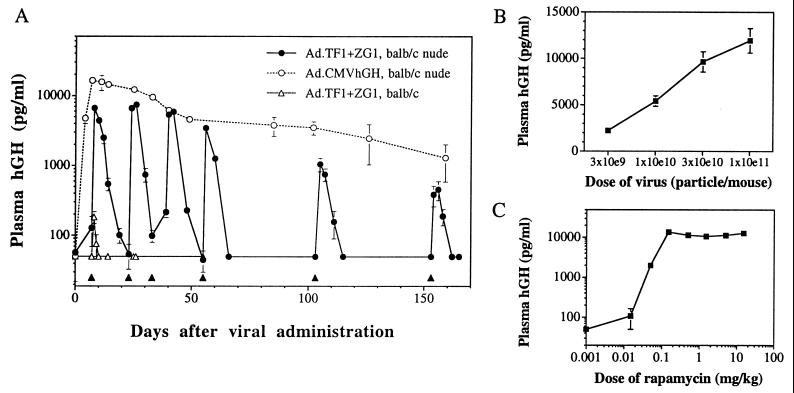
To test the ability of the system to function in vivo, adenoviral vectors were injected i.m. Skeletal muscle is an ideal target tissue because individual muscle fibers are large and multinucleated. These properties would be expected to favor the cotransduction of individual cells with the transcription factor and target gene vectors. In initial experiments, equal quantities of the two adenoviral vectors were injected into the tibialis anterior and quadriceps of immunodeficient mice (Fig. 2A). In the absence of rapamycin, basal plasma levels of hGH were below the detection limit of the assay (50 pg/ml). A single i.p. injection of rapamycin (5 mg/kg) resulted in at least a 100-fold increase in plasma hGH. hGH levels then diminished to baseline over the next 14 days. Similar induction profiles were noted after five subsequent injections of rapamycin administered periodically over 6 months. The peak level of induced hGH was similar to that seen in animals injected with an equal quantity of an adenovirus expressing hGH from the strong constitutive enhancer/promoter of the immediate early gene of CMV; in each case the peak level diminished approximately 10-fold over the 6-month period. The mechanism of this non-immune-mediated extinction of transgene expression is unclear, although instability of the nonintegrated viral chromosome and shut-off of transcription are both possible.
Figure 2.
Regulated hGH secretion in mice receiving i.m. injection of adenovirus. (A) Male BALB/c nude (open and filled circles) or BALB/c immunocompetent mice (open triangles) were injected i.m. with 0.5 × 1011 particles each of Ad.TF1 and Ad.ZG1 or 1 × 1011 particles of Ad.CMVhGH. The filled triangles indicate administration of rapamycin (5 mg/kg, i.p.). Values of plasma hGH are mean ± SEM (n = 4). The lower limit of detection of the assay is 50 pg/ml. (B) Vector dose–response. Male BALB/c nude mice were injected i.m. with a 1:1 mixture of Ad.TF1 and Ad.ZG1 at the indicated total dose (10e9 = 109, etc.). Rapamycin (5 mg/kg, i.p.) was administered 10 days after vector infusion, and plasma samples were collected 24 h later. Values of plasma hGH are mean ± SEM. (C) Rapamycin dose–response. Male BALB/c nude mice were injected i.m. with a 1:1 mixture of Ad.TF1 and Ad.ZG1 at a total dose of 1 × 1011 particles per mouse. The mice were challenged with rapamycin at the indicated doses 10 days after vector infusion, and plasma samples were collected 24 h later. Values of plasma hGH are mean ± SEM (n = 5).
Additional experiments were performed to evaluate the impact of vector dose on peak hGH expression after a single dose of rapamycin. Equal quantities of adenoviral vectors expressing the transcription factors and hGH were injected at decreasing doses into immunodeficient animals. The results presented in Fig. 2B demonstrate a relationship between dose of vector and rapamycin-induced hGH expression that is equivalent to that achieved with the CMV-driven vector (data not shown); peak expression does not decline out of proportion to vector dose over a 30-fold range of vector. These studies suggest the syncytial structure of a muscle fiber enables efficient reconstitution of the regulated system even at lower doses of vector.
Rapamycin Regulation of the Level and Kinetics of hGH Expression in Vivo.
The next set of experiments was designed to test whether rapamycin dosing could be modified to regulate the precise level and duration of hGH delivery in immunodeficient mice transduced with adenoviral vectors. As shown in Fig. 2C, there is a direct relationship between peak hGH levels and the amount of rapamycin administered after a single dose of between 0.01 and 0.25 mg/kg. A similar relationship was observed when rapamycin was administered orally (data not shown), although equivalent levels of hGH release required a 4-fold higher concentration of rapamycin, consistent with its previously reported oral bioavailability of 15–20% (20, 26).
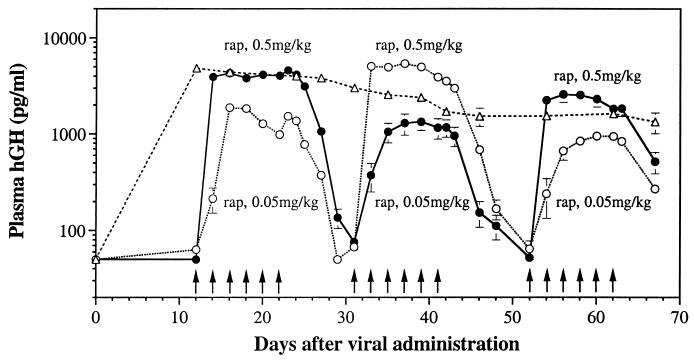
Many applications of this strategy for treatment of diseases will require maintenance of stable protein concentrations within a therapeutic window. hGH is an excellent gene to use in this evaluation because the half-life of the protein is so short (i.e., 4 min; ref. 27). To test the ability of the rapamycin dosing regimen to control the duration as well as the level of protein production, two groups of mice were administered 0.5 or 0.05 mg/kg rapamycin every 2 days over an 11-day period (Fig. 3). In both groups, stable levels of hGH were maintained throughout the dosing period at a concentration that was determined by the dose of rapamycin, with the group that received a 10-fold lower dose of rapamycin showing a 5-fold decrease in steady-state hGH levels. Upon termination of rapamycin administration, hGH levels returned to baseline within 2 weeks. When this dosing regimen was repeated two additional times, each time alternating the dose each group received, identical profiles of hGH induction were observed. Thus, the level and duration of hGH production can be precisely regulated through dosing of rapamycin.
Figure 3.
Control over the level and duration of hGH expression with rapamycin. Male BALB/c nude mice were injected i.m. with 1 × 1011 total particles of Ad.TF1 and Ad.ZG1 in a 1:1 ratio (filled and open circles) or with 5 × 1010 particles of Ad.CMVhGH (open triangles). Mice receiving Ad.TF1 and Ad.ZG1 were divided into two groups. Group 1 (open circles) received six consecutive doses of rapamycin (0.05 mg/kg, i.p.) every 2 days. Plasma samples were taken before each rapamycin dose and every other day after the last dose of rapamycin. The second and third sets of rapamycin challenges (0.5 and 0.05 mg/kg, respectively) were initiated when plasma hGH returned to basal levels from the previous challenges. Group 2 (filled circles) received similar injection and bleeding schedules but a 10-fold higher dose of rapamycin initially (0.5 mg/kg), with subsequent dosing schedules of 0.05 and 0.5 mg/kg. Values of plasma hGH are mean ± SEM of 5 mice for the group receiving Ad-CMVhGH and 10 mice for groups receiving Ad.TF1 and Ad.ZG1. Arrows indicate times of rapamycin dosing. The lower limit of detection of the hGH assay is 50 pg/ml.
Experiments were repeated with the adenoviral vectors in immunocompetent animals to more closely simulate models in larger animals and clinical applications in humans. As shown in Fig. 2A (open triangles), peak levels of hGH after a single administration of rapamycin were 50-fold lower than those observed in immunodeficient animals, and no induction was observed after a second administration of rapamycin. This result is likely caused by destructive cellular and humoral immune responses to the products of each vector (i.e., chimeric human transcription factors, hGH, and/or viral proteins).
Stable Reconstitution of the Regulated System in Immunocompetent Mice by Using AAV.
The rapamycin-inducible system was next evaluated in AAV vectors with the hope of conferring more stable expression and avoiding host immune responses (4, 11–13). Infection of the AAV-permissive cell line 84–31 (28) with equal quantities of the two AAV vectors (TF1 and ZG1; Fig. 1) resulted in full reconstitution of the regulated system with a 186-fold induction after rapamycin treatment (Table 1). Equal quantities of the two AAV vectors were then injected into the tibialis anterior and quadriceps of immunodeficient mice, which were subsequently administered rapamycin at 5 mg/kg. Little induction of hGH was observed (data not shown), possibly because of insufficient expression of the activation domain fusion, which appears to be the limiting factor in cell culture. Enhanced expression of the activation domain fusion was achieved by incorporating a second CMV-FRB-p65 transcriptional unit into the target gene cassette to generate the vector ZG2 (Fig. 1). In vitro studies demonstrated substantial induction of hGH (Table 1), although basal expression in the absence of rapamycin was high, possibly caused by activation of the target gene promoter by binding of cellular transcription factors to the CMV enhancer.
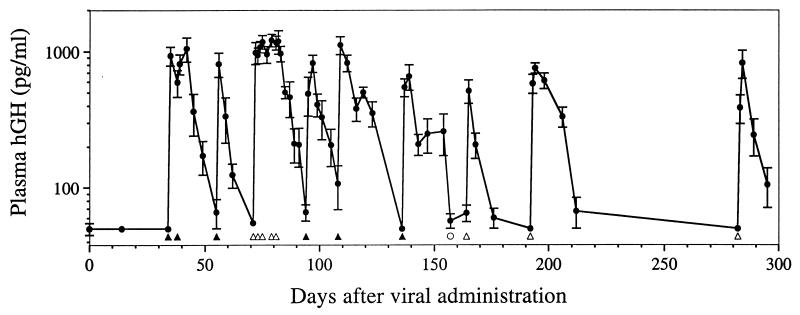
Upon in vivo administration of TF1 and ZG2, however, there was no detectable basal production of hGH (Fig. 4). After administration of rapamycin, plasma hGH levels rose at least 20-fold before returning to baseline levels. Since basal levels of hGH were below the limits of detection of the assay (50 pg/ml), the actual fold induction may be much greater. Moreover, peak levels of induced hGH were high, equivalent to those seen in animals injected with an equal quantity of AAV vector expressing hGH from the strong CMV enhancer/promoter (data not shown). The duration of peak production was once again shown to be determined by the duration of rapamycin administration. In total, mice received nine separate administrations of rapamycin. Peak hGH levels after each administration were remarkably consistent, with no detectable reduction between the first dose and the last dose 10 months after viral injection.
Figure 4.
Long-term rapamycin-induced hGH secretion in mice receiving i.m. injection of AAV. Immunodeficient BALB/c mice were injected i.m. with a total of 2 × 1011 genome copies of AAV.TF1 and AAV.ZG2 in a 1:1 ratio. Rapamycin (5 mg/kg filled triangles, 1 mg/kg open triangles) or a rapamycin analog with 10-fold decreased activity (5 mg/kg open circle) was administered i.p. in single or multiple doses as indicated. Values of plasma hGH are mean ± SEM (n = 5). The lower limit of detection of the hGH assay is 50 pg/ml.
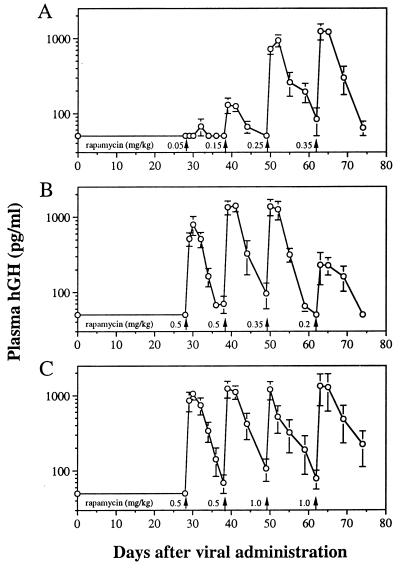
Our previous studies indicate that AAV is capable of evading destructive immune responses to immunogenic transgene products. This appears to be due to the fact that AAV, as opposed to adenovirus or naked DNA, does not efficiently transduce antigen-presenting cells (13). The rapamycin-inducible system in AAV was therefore tested in immunocompetent animals. In this experiment, three groups of mice received various doses of rapamycin. As shown in Fig. 5, the system performed essentially identically in immunocompetent mice as in immunodeficient animals. Once again, basal expression of hGH was below the limits of detection, and rapamycin induced at least a 20-fold increase that was reversible. Induced hGH levels were proportional to the dose of rapamycin in a range from 0.05 to 0.35 mg/kg (Fig. 5 A and B). Peak hGH levels did not decline during four sequential rapamycin injections (Fig. 5C).
Figure 5.
Dose-responsive hGH secretion in immunocompetent mice receiving i.m. injection of AAV. Immunocompetent BALB/c mice were injected i.m. with 2 × 1011 total genome copies of AAV.TF1 and AAV.ZG2 at a 1:1 ratio. The mice were then divided into three groups and challenged with different single doses of rapamycin as indicated. Values of plasma hGH are mean ± SEM (n = 5).
DISCUSSION
Recent progress in the development of vectors that direct efficient, long-term gene delivery in vivo and regulatory systems that allow small-molecule control of gene expression has enabled direct evaluation of the potential for regulated delivery of secreted proteins after in vivo gene transfer (21, 29–31). In several different systems, regulated production of erythropoietin (Epo) has recently been demonstrated after i.m. injection of AAV vectors into mice. For example, it has been shown that Epo expression, and as a result hematocrit, can be induced twice over a 4–7 month period by using tetracycline-based systems (30, 31). However, basal expression of Epo was sufficient to cause a significant elevation of hematocrit. Using the rapamycin-based system, we have also demonstrated repetitive Epo induction, but without detectable basal expression, over 6- and 3-month periods in mice and a rhesus monkey, respectively (21). Here we extend these results by demonstrating precise control over the level and kinetics of hGH production in mice when the rapamycin regulatory system is delivered i.m. by either adenovirus or AAV vectors. Use of an hGH target gene provides a more stringent test of the system because the short half-life of hGH in mice (4 min; ref. 27) relative to that of Epo (8 h; ref. 32) requires that transcription be induced to high levels to produce therapeutic levels of protein. The short half-life of hGH in circulation also allowed us to examine more closely the relationship between the rapamycin dosing regimen and the kinetics of protein production.
In mice transduced with AAV or adenovirus vectors, basal hGH expression is undetectable and is induced to high levels after a single administration of rapamycin. This study and others (20) have demonstrated that peak levels of hGH are observed approximately 16–24 h after administration of rapamycin. Most of this delay presumably reflects the time it takes for newly transcribed transgene mRNA to accumulate to maximal levels. Once fully activated, peak circulating levels are comparable to those seen when hGH expression is driven constitutively by the CMV enhancer/promoter. After withdrawal of rapamycin, hGH levels return to baseline with a half-time of about 1.5 days, a rate that matches the half-life of the hGH mRNA (33). By introducing elements that shorten the mRNA half-life (34), the half-time of plasma hGH decay can be reduced to approximately 4 h, which closely matches the 4.5-h half-life of rapamycin in mice (data not shown). However, peak levels of hGH are also decreased because the short-lived mRNA accumulates to lower levels.
We further demonstrate that circulating levels of hGH can be controlled precisely by the dose of rapamycin and that the duration of hGH expression is determined by the duration of rapamycin administration. Stable circulating plasma hGH levels can be maintained by administration of rapamycin every 2 days. In addition, repeated administration of rapamycin after decay of plasma hGH induces equivalent profiles of hGH induction with both vector systems. However, in adenovirus-infected cells peak hGH levels begin to decrease after 50 days, whereas AAV-infected cells show no reduction in peak levels over at least a 10-month period. This difference is likely due to the fact that the adenovirus genome remains episomal, whereas the AAV genome appears to become integrated (11, 12). A second difference is reflected in the persistence of inducible expression in immunocompetent mice when delivered by AAV but not adenovirus vectors. This is probably explained by the fact that AAV does not transduce antigen-presenting cells and therefore does not induce destructive immune responses to the transcription factors or the transgene product (13). For these reasons, AAV is the preferred delivery vector for long-term systemic protein delivery.
Taken together with the results of Ye et al. (21), these studies demonstrate that the rapamycin-based regulatory system, delivered i.m. by AAV, fulfills many crucial requirements necessary for the safe and efficacious systemic delivery of therapeutic proteins by gene therapy. In this system, expression of a therapeutic protein is induced with an orally bioavailable drug, and the protein can be delivered at the desired circulating concentration by controlling the rapamycin dose. The peak level of protein produced is high, equivalent to that deliverable by using a strong viral enhancer. Depending on the needs of the patient, a pulse of therapeutic protein expression can be induced by administering a single dose of rapamycin, or protein levels can be maintained at a steady level by administering rapamycin chronically. Importantly, in the absence of rapamycin, protein expression is undetectable. Furthermore, the regulated expression persists, without any detectable decrease in inducibility, for at least 10 months in mice. Finally, as the system is made up of human components, the potential for immunogenic response against the transgenes, when administered in humans, is reduced. The system is currently limited by the immunosuppressive activity of rapamycin which results from the inhibition of endogenous FRAP activity (19). To overcome this limitation, we (data not shown) and others (35) have developed nonimmunosuppressive analogs of rapamycin by adding substituents to rapamycin that block its ability to bind endogenous FRAP. By making appropriate modifications to the transcription factor fusions, the ability of these analogs to regulate transcription of a target gene has been maintained. The regulatory and pharmacologic properties of such analogs may be evaluated by using the AAV regulatory system described here.
Acknowledgments
We thank Tim Clackson for helpful comments on the manuscript. Funding was provided by grants from the National Institutes of Health (P30 DK47757-05 and P01 AR-S43648-03 to J.M.W.), ARIAD Pharmaceuticals, a company in which G. R. Crabtree has equity, and Genovo, Inc., a company J.M.W. founded and in which he has equity.
ABBREVIATIONS
- hGH
human growth hormone
- AAV
adeno-associated virus
- CMV
cytomegalovirus
References
- 1.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 2.Svensson E C, Black H B, Dugger D L, Tripathy S K, Goldwasser E, Hao Z, Chu L, Leiden J M. Hum Gene Ther. 1997;8:1797–1806. doi: 10.1089/hum.1997.8.15-1797. [DOI] [PubMed] [Google Scholar]
- 3.Herzog R W, Hagstrom J N, Kung S H, Tai S J, Wilson J M, Fisher K J, High K A. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monahan P E, Samulski R J, Tazelaar J, Xiao X, Nichols T C, Bellinger D A, Read M S, Walsh C E. Gene Ther. 1998;5:40–49. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- 6.Murphy J E, Zhou S, Giese K, Williams L T, Escobedo J A, Dwarki V J. Proc Natl Acad Sci USA. 1997;94:13921–13926. doi: 10.1073/pnas.94.25.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder R O, Spratt S K, Lagarde C, Bohl D, Kaspar B, Sloan B, Cohen L K, Danos O. Hum Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]
- 8.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne B J, Atkinson M, Flotte T R. Proc Natl Acad Sci USA. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou S, Murphy J E, Escobedo J A, Dwarki V J. Gene Ther. 1998;5:665–670. doi: 10.1038/sj.gt.3300648. [DOI] [PubMed] [Google Scholar]
- 10.Kozarsky K F, Wilson J M. Curr Opin Genet Dev. 1993;3:499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- 11.Xiao X, Li J, Samulski R J. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 13.Jooss K, Yang Y, Fisher K J, Wilson J M. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall D J, Leiden J M. Curr Opin Genet Dev. 1998;8:360–365. doi: 10.1016/s0959-437x(98)80094-4. [DOI] [PubMed] [Google Scholar]
- 15.Clackson T. Curr Opin Chem Biol. 1997;1:210–218. doi: 10.1016/s1367-5931(97)80012-9. [DOI] [PubMed] [Google Scholar]
- 16.Rivera V M, Clackson T, Natesan S, Pollock R, Amara J F, Keenan T, Magari S R, Phillips T, Courage N L, Cerasoli F, Jr, et al. Nat Med. 1996;2:1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- 17.Ho S N, Biggar S R, Spencer D M, Schreiber S L, Crabtree G R. Nature (London) 1996;382:822–826. doi: 10.1038/382822a0. [DOI] [PubMed] [Google Scholar]
- 18.Standaert R F, Galat A, Verdine G L, Schreiber S L. Nature (London) 1990;346:671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- 19.Brown E J, Albers M W, Shin T B, Ichikawa K, Keith C T, Lane W S, Schreiber S L. Nature (London) 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 20.Magari S R, Rivera V M, Iuliucci J D, Gilman M, Cerasoli F., Jr J Clin Invest. 1997;100:2865–2872. doi: 10.1172/JCI119835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye X, Rivera V M, Zoltick P, Cerasoli F, Jr, Schnell M A, Gao G, Hughes J V, Gilman M, Wilson J M. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- 22.Ye X, Robinson M B, Batshaw M L, Furth E E, Smith I, Wilson J M. J Biol Chem. 1996;271:3639–3646. doi: 10.1074/jbc.271.7.3639. [DOI] [PubMed] [Google Scholar]
- 23.Rivera V M. Methods. 1998;14:421–429. doi: 10.1006/meth.1998.0596. [DOI] [PubMed] [Google Scholar]
- 24.Selden R F, Howie K B, Rowe M E, Goodman H M, Moore D D. Mol Cell Biol. 1986;6:3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samulski R J, Chang L S, Shenk T. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yatscoff R W. Transplant Proc. 1996;28:970–973. [PubMed] [Google Scholar]
- 27.Peeters S, Friesen H G. Endocrinology. 1977;101:1164–1183. doi: 10.1210/endo-101-4-1164. [DOI] [PubMed] [Google Scholar]
- 28.Fisher K J, Gao G P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burcin M M, Schiedner G, Kochanek S, Tsai S Y, O’Malley B W. Proc Natl Acad Sci USA. 1999;96:355–360. doi: 10.1073/pnas.96.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohl D, Salvetti A, Moullier P, Heard J M. Blood. 1998;92:1512–1517. [PubMed] [Google Scholar]
- 31.Rendahl K G, Leff S E, Otten G R, Spratt S K, Bohl D, Van Roey M, Donahue B A, Cohen L K, Mandel R J, Danos O, Snyder R O. Nat Biotechnol. 1998;16:757–761. doi: 10.1038/nbt0898-757. [DOI] [PubMed] [Google Scholar]
- 32.Erslev A J. N Engl J Med. 1991;324:1339–1344. doi: 10.1056/NEJM199105093241907. [DOI] [PubMed] [Google Scholar]
- 33.Helms S R, Rottman F M. Nucleic Acids Res. 1990;18:255–259. doi: 10.1093/nar/18.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shyu A B, Belasco J G, Greenberg M E. Genes Dev. 1991;5:221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- 35.Liberles S D, Diver S T, Austin D J, Schreiber S L. Proc Natl Acad Sci USA. 1997;94:7825–7830. doi: 10.1073/pnas.94.15.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]