Abstract
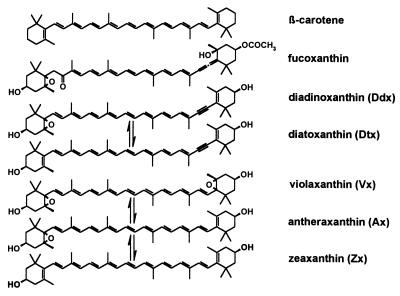
According to general agreement, all photosynthetic organisms using xanthophyll cycling for photoprotection contain either the violaxanthin (Vx) cycle or the diadinoxanthin (Ddx) cycle instead. Here, we report the temporal accumulation of substantial amounts of pigments of the Vx cycle under prolonged high-light stress in several microalgae thought to possess only the Ddx cycle. In the diatom Phaeodactylum tricornutum, used as a model organism, these pigments also participate in xanthophyll cycling, and their accumulation depends on de novo synthesis of carotenoids and on deepoxidase activity. Furthermore, our data strongly suggest a biosynthetic sequence from Vx via Ddx to fucoxanthin in P. tricornutum. This gives experimental support to the long-stated hypothesis that Vx is a common precursor of all carotenoids with an allenic or acetylenic group, including the main light-harvesting carotenoids in most chlorophyll a/c-containing algae. Thus, another important function for xanthophyll cycling may be to optimize the biosynthesis of light-harvesting xanthophylls under fluctuating light conditions.
Fluctuating light intensities pose a serious problem to all photosynthetic organisms: in low light (LL), it is highly desirable to collect photons as efficiently as possible, but when light intensities become supersaturating for photosynthesis, there must be ways to protect the organism from potential damage by excess energy absorption. Carotenoids fulfill important functions in both situations, in light harvesting as well as in photoprotection (for reviews, see refs. 1–3).
Within the last decade (4, 5), it has been recognized that in higher plants, the reversible conversion of the xanthophylls violaxanthin (Vx), antheraxanthin (Ax), and zeaxanthin (Zx; see Fig. 1) in the so-called xanthophyll cycle (6, 7) is intimately related to the ability of the plants to regulate the dissipation of surplus absorbed light energy on a short-term time scale. Zx, which is formed from Vx by enzymatic deepoxidation when the pH in the thylakoids drops below a critical level (8), is thought to enhance thermal dissipation, and two main mechanisms have been proposed (9–12). Recently the isolation of mutants defective in synthesis or cycling of xanthophylls has offered new possibilities for studying the influence of different carotenoids on energy dissipation in vivo (13, 14).
Figure 1.
Molecular structures of the carotenoids mentioned in the text. Arrows between pigments denote enzymatic conversion caused by xanthophyll cycling.
In several algal groups such as the diatoms, the dinophytes, and the haptophytes, which together are largely responsible for oceanic primary production (15), the Vx cycle is replaced by a xanthophyll-cycle alternating diadinoxanthin (Ddx) with diatoxanthin (Dtx) (16). This cycle comprises a single deepoxidation step, because only one of the ionon rings of Ddx carries an epoxide group. Epoxidation of the second ionon ring by the respective xanthophyll-cycle epoxidase does not occur, probably because of steric constraints caused by the acetylenic bond at C-7′ (Fig. 1). As is the case with Zx, the formation of Dtx correlates with a higher ability for nonradiative relaxation of singlet chlorophyll (Chl) (17–20) and is assumed to act by the same mechanisms (21). Other xanthophylls of Chl a/c-containing algae, e.g., fucoxanthin (Fig. 1) in diatoms and brown algae or peridinin in the dinophytes, play a major role in light harvesting, thus replacing Chl b of higher plants and green algae (22, 23). These xanthophylls share an allenic group adjacent to one of the ionon rings as a common structural element. Despite their ecophysiological significance, knowledge about the biosynthesis of xanthophylls with an allenic or acetylenic group is still scarce (24). Considering feasible chemical reactions, it has been postulated that Vx is a common precursor of all these carotenoids (25, 26), but experimental evidence for this is still lacking (26). Although for a dinophyte it has been shown that Zx is a precursor of Ddx and peridinin (27), the involvement of an epoxide-xanthophyll such as Vx remains to be demonstrated (28).
We have been studying how pigment pools and the Ddx cycle in diatoms acclimate under periodically fluctuating light conditions. For this purpose, we used continuous cultures of the diatom Phaeodactylum tricornutum, which were illuminated with 6-h high light (HL) and 18-h LL for several days. We observed a transient accumulation of substantial amounts of new pigments during each HL period and set out to elucidate the nature of this phenomenon.
MATERIALS AND METHODS
Algal Strains and Culture Conditions.
Most experiments were performed with the diatom P. tricornutum, axenic strain 1090–1a of the Sammlung von Algenkulturen (SAG, Göttingen, Germany) (29), grown aseptically in a turbidostat as described previously (30). Continuous illumination with LL had a photon flux density (PFD) of 40 μmol photons m−2⋅s−1 as measured in the empty culture vessels. The vessels (3.5 cm diameter, 365 ml volume) were submerged in a temperature-controlled water bath (20.0 ± 0.5°C) and aerated with a flux of 1 liter per min. Chl a concentrations were kept at 1.8 mg per liter by automatic dilution. Growth rates were calculated by dividing the dilution volume per time of the turbidostat system by the volume of the culture vessels (31).
For screening of xanthophyll-cycle pigments, batch cultures of the following algae were used [strain numbers of SAG (29) in parentheses]: Cyclotella meneghiniana (1020–1a), Prymnesium parvum (127.79), Pavlova lutheri (926.1), Pleurochloris meiringensis (860–3), Mischococcus sphaerocephalus (847–1), and Amphidinium carterae (37.80). All algae were kept at 20°C with a 16-h light (incident PFD of 40 μmol m−2⋅s−1)/8-h dark cycle. The culture media used were: (i) ASP-2 medium (ref. 32, modified) for P. tricornutum, P. meiringensis, and M. sphaerocephalus; (ii) ASP-2 medium plus silica (1 mM) for C. meneghiniana, and (iii) ASWP-Medium plus ASW-vitamin stock (33) for A. carterae, P. parvum, and P. lutheri. All cultures except those of the latter three algae were shaken at 120 rpm. Fresh cultures were inoculated every 2 wk. All cultures were frequently examined by means of a light microscope to identify contaminating algae, which, however, we never observed.
HL Treatment.
The turbidostat cultures of P. tricornutum were operated in LL for at least 1 mo and frequently checked to remain unialgal by means of a microscope and HPLC. They were then exposed to a minimum of six cycles of 6-h HL, either with a PFD of 700 μmol m−2⋅s−1 (HL1) or with 1,000 μmol m−2⋅s−1 (HL2), followed by 18-h LL, to allow acclimation before the experiments started (30). For each experiment, 150 ml was transferred from the turbidostat vessel into a separate vessel and further incubated in the temperature-controlled water bath under aeration. As a consequence, the cell suspensions under examination were no longer diluted. For screening of the Vx cycle in other algal taxa, the corresponding batch cultures during exponential growth were used for a single HL1 treatment of 6 h by using the same incubation conditions.
The light-intensity dependence of the relative accumulation of Zx and Dtx in P. tricornutum was determined on aliquots of 15 ml placed into aerated test tubes at various distances from a slide projector equipped with an infrared filter. Loss of water from the tubes during 6-h aeration was below 1%, as determined by weight of the contents.
Inhibitor Treatment.
DTT was applied from a 500-mM aqueous stock solution. Addition of norflurazon to a final concentration of 5 μM from a 2.5-mM methanolic stock solution resulted in a methanol concentration of only 0.2% (addition of methanol alone had no adverse effect on pigment synthesis). Norflurazon was a gift from Peter Böger (Konstanz, Germany).
Pigment Analysis.
General precautions for work with pigments were taken, and standard methods for purification of pigments were applied (see ref. 34). The algae were collected on glass fiber filters under mild suction, frozen in liquid N2 within 25 s of withdrawal of samples, and then stored at −80°C. After freeze drying, the samples were extracted in methanol/ethyl acetate/water (8:1:1) buffered with 0.2 M ammonium acetate, as described previously (35). Cell debris was removed by centrifugation (14,500 × g, 2 min) before RP-HPLC analysis on a Waters 600-MS chromatography system equipped with a Waters 717 autosampler and a Waters 996 photodiode-array detector (see ref. 35 for further details). Pigments were separated on a Nucleosil wide-pore C18-column (Macherey-Nagel, Düren, Germany) by applying a gradient according to ref. 36 and quantified as described in ref. 35. Each of the algae examined displayed only the set of pigment peaks typical for the respective algal group (with the exception of the previously not-observed pigments of the Vx cycle), thus corroborating that the cultures were unialgal.
Identification of Vx, Ax, and Zx.
Spinach (Spinacia oleracea, var. Matador) grown under a PFD of 150 μmol photons m−2⋅s−1, treated with HL before extraction, was used as a source for the reference pigments Vx, Ax, and Zx. Pigments used for photometry and MS were isolated from saponified extracts of spinach and P. tricornutum by HPLC and further purified on a silica column. Separation of Zx and lutein from spinach was achieved on TLC plates according to ref. 37.
The corresponding pigments from P. tricornutum and spinach showed identical behavior in two chromatographic systems [HPLC and TLC, by using Silica Gel 60 plates (Merck) with 60 ml diethyl ether/0.3 ml H2O as mobile phase] and had the same absorption properties in two different solvents (HPLC gradient and 100% ethanol). On acidification with hydrochloric acid, Vx and Ax showed the typical blue shift in absorption of 40 nm and 20 nm, respectively, because of furanoid rearrangement of their 5,6-epoxide groups (38). Molecular weights, as determined by matrix-assisted laser desorption ionization/time-of-flight-MS (MALDI/TOF-MS) on a Perkin–Elmer Voyager-DE System by using 4-hydroxy-α-cyano-cinnamic acid as matrix, were also in agreement with the proposed identity of the pigments (data are published as a supplemental table on the PNAS web site, www.pnas.org). Thus the minimum criteria for identification of these pigments as proposed in ref. 34 were met. Xanthophyll-cycle pigments in the other algae were identified by HPLC by using retention times and on-line spectra. Additionally, saponified pigment extracts were acidified to induce furanoid rearrangement and then analyzed by HPLC, proving the existence of 5,6-epoxide groups in the pigments designated as Ddx, Ax, and Vx.
RESULTS
The Diatom P. tricornutum Accumulates High Amounts of Pigments of the Vx Cycle During Periodic HL Stress.
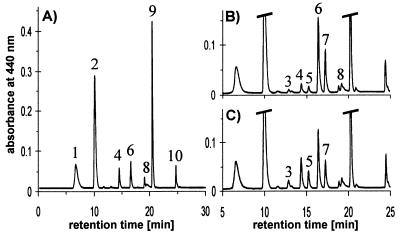
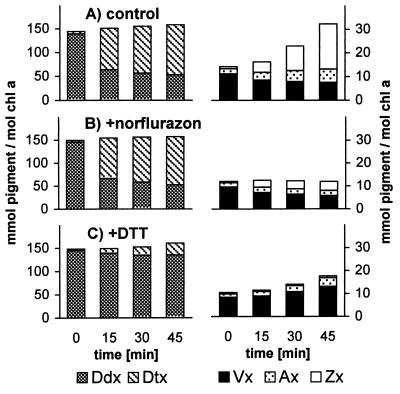
The turbidostat cultures of P. tricornutum exposed to a periodic light cycle consisting of 6 h HL1 (700 μmol m−2⋅s−1) and 18 h LL (40 μmol m−2⋅s−1) showed a daily growth rate μ of 0.67. When the algae were sampled at the beginning of a HL period, HPLC analysis confirmed the presence of pigments typical for diatoms, namely the Chls c1 and c2 (not resolved in our chromatographic system), Chl a, and the carotenoids fucoxanthin, Ddx, and β-carotene (see Fig. 1 for molecular structure of carotenoids). Illumination of the algae with HL1 for 10 min resulted in partial deepoxidation of Ddx to Dtx (Fig. 2A). After 6 h of HL1, more than 90% of Ddx had been converted into Dtx, and additionally the pigments of the Vx cycle became detectable (Fig. 2B) with Zx being the dominant pigment. Subsequent shift from HL1 back to LL lead to an almost immediate onset of reconversion of Dtx to Ddx, and Zx was transformed into Ax and Vx (Fig. 2C), as would be expected if these pigments also participate in xanthophyll cycling.
Figure 2.
HPLC analysis of pigment extracts from the diatom P. tricornutum growing under periodic HL stress. Extract from cells (A) after 10-min illumination with HL1 (700 μmol m−2⋅s−1), (B) after 6-h HL1, and (C) after 6-h HL1 followed by 10-min LL (40 μmol m−2⋅s−1). 1, Chl c1+ c2; 2, fucoxanthin; 3, Vx; 4, Ddx; 5, Ax; 6, Dtx; 7, Zx; 8, derivatives of Chl a (two peaks); 9, Chl a; 10, β-carotene.
Zx Can Also Be Accumulated in Other Chl a/c-Containing Algae Possessing the Ddx Cycle.
To check whether the ability to accumulate Zx is restricted to P. tricornutum or represents a more widespread phenomenon, we exposed several other algae possessing the Ddx cycle to a single treatment with HL1 for 6 h. All algae examined were capable of Zx accumulation (Table 1). Some algae, such as M. sphaerocephalus, P. parvum, and C. meneghiniana, accumulated large amounts of Zx. In particular, M. sphaerocephalus, which contained appreciable amounts of Vx even before applying HL, formed as much Zx as Dtx in 6-h HL. When the algae were transferred back to LL, all species showed an immediate onset of epoxidation of Dtx to Ddx and of Zx to Vx via Ax as well, thus demonstrating the parallel action of both xanthophyll cycles during the LL recovery (data not shown).
Table 1.
Xanthophyll cycle pigments before (LL) and after (HL) a single treatment with HL1 for 6 h in several Chl a/c-containing algae which possess the Ddx/Dtx cycle
| Algal species | Light | Ddx | Dtx | Sum | Vx | Ax | Zx | Sum |
|---|---|---|---|---|---|---|---|---|
| C. meneghiniana (Bacillariophyceae) | LL/HL | 157/53 | 20/211 | 177/264 | 7/7 | ND/12 | ND/93 | 7/112 |
| M. sphaerocephalus (Xanthophyceae) | LL/HL | 185/64 | 4/138 | 189/202 | 44/26 | 2/19 | 4/141 | 50/186 |
| P. meiringensis (Xanthophyceae) | LL/HL | 210/84 | 2/162 | 212/246 | 15/15 | ND/12 | 3/57 | 18/84 |
| P. lutheri (Haptophyta) | LL/HL | 401/40 | 18/460 | 419/500 | 3/8 | ND/1 | ND/19 | 3/28 |
| P. parvum (Haptophyta) | LL/HL | 230/65 | 5/351 | 235/416 | 8/49 | ND/4 | 1/63 | 9/116 |
| A. carterae (Dinophyta) | LL/HL | 309/197 | 2/168 | 311/365 | 4/4 | ND/20 | ND/53 | 4/77 |
All data are given as mmol pigment per mol Chl a, representing averages of two separate samples. Values of a sample pair differed by not more than 2 mmol/mol Chl a (ND, not detectable).
In P. tricornutum, the Deepoxidation Rate of Ddx Is Four Times Higher than That of Vx, Whereas Epoxidation Kinetics of Dtx and Zx Are Equal.
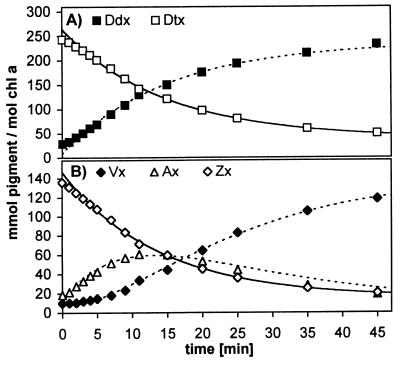
Using P. tricornutum as a model system, we further investigated the factors responsible for the accumulation of Zx. As a first step, the kinetics of epoxidation and deepoxidation after the HL treatment were analyzed for the five xanthophylls mentioned above. Typical epoxidation patterns for Dtx and Zx/Ax are shown in Fig. 3. The rate constants of three independent experiments are summarized in Table 2. Epoxidation rates of pigments increase in the order Dtx, Zx, Ax, but the rates differ by no more than 30%, and the difference between the epoxidation kinetics of Dtx and Zx is only about 10%.
Figure 3.
Kinetics of epoxidation in P. tricornutum in LL (40 μmol m−2⋅s−1) after 6-h illumination with HL1. Pigments are normalized to Chl a, because changes in Chl a concentrations within the 45-min LL recovery were negligible. (A) Epoxidation of Dtx to Ddx; (B) epoxidation of Zx via Ax to Vx. Solid lines represent fit to monoexponential decay (first three data points of each pigment were omitted); dashed lines were calculated assuming stoichiometric conversion between pigments of the corresponding xanthophyll cycle.
Table 2.
Rate constants of epoxidation and deepoxidation in P. tricornutum, determined from independent experiments as described for Figs. 3 and 4
| Exp. | Epoxidation
|
Exp. | Deepoxidation
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Dtx → Ddx, k1 [min−1] | Zx →Ax, k2 [min−1] | Ax → Vx, k3 [min−1] | k1/k2 | Ddx → Dtx, k−1 [min−1] | Vx → Ax, k−3 [min−1] | Ax → Zx, k−2 [min−1] | k−1/k−3 | ||
| 1 | 0.049 | 0.058 | 0.070 | 0.85 | 4 | 0.256 | 0.061 | 0.80 | 4.19 |
| 2 | 0.068 | 0.073 | 0.076 | 0.92 | 5 | 0.249 | 0.066 | 0.70 | 3.77 |
| 3 | 0.067 | 0.073 | 0.076 | 0.92 | 6 | 0.280 | 0.073 | 0.70 | 3.83 |
| Average: | 0.90 | 3.93 | |||||||
The kinetics of deepoxidation were determined in cells incubated for 6 h with HL1 followed by 45-min recovery in LL (Fig. 4). Thus deepoxidation starts at the end of the time scale in Fig. 3. When comparing the kinetics with those of the epoxidation, a different picture emerges: the deepoxidation rate of Ddx is about four times higher than that of Vx (Table 2), and the deepoxidation rate of Ax appears to be even 10 times faster. The difference between the increase in Zx as calculated (Fig. 4B, dashed line) and the experimental values indicates that not all Vx is converted into Zx. Instead the Ddx/Dtx content rises (Fig. 4A), suggesting that part of Vx or Ax is converted into Ddx or Dtx, respectively.
Figure 4.
Kinetics of deepoxidation in P. tricornutum in HL1 (700 μmol m−2⋅s−1) after 6-h HL1 followed by 45-min LL. Pigments are normalized to Chl a because during 30-min HL1, no increase in Chl a occurred. (A) Deepoxidation of Ddx to Dtx; (B) deepoxidation of Vx via Ax to Zx. Solid lines represent fit to monoexponential decay; dashed lines were calculated assuming stoichiometric conversion between pigments of the corresponding xanthophyll cycle.
Accumulation of Zx Can Be Prevented Either by Blocking de Novo Carotenoid Biosynthesis or by the Deepoxidase Inhibitor DTT.
We examined the significance of deepoxidase activity and of de novo synthesis of carotenoids for this accumulation by using norflurazon, an inhibitor of phytoene desaturase (39), to prevent de novo formation of carotenoids and the disulfide-reducing agent DTT to inhibit the deepoxidase activity (40). To exclude long-term secondary effects of the inhibitors, we monitored only pigment changes under HL for the first 45 min after their addition.
In control cells without inhibitors during 45 min of HL2 (1,000 μmol m−2⋅s−1), about two-thirds of the Ddx present was converted into Dtx. This conversion was paralleled by a substantial increase in Zx content (Fig. 5A). Addition of norflurazon did not affect the deepoxidation of Ddx to Dtx, and part of the Vx already present was converted into Zx (Fig. 5B). Importantly, no net increase in (Vx + Ax + Zx) took place, showing that the Zx increase in the control is not originating from the Ddx/Dtx pool but instead depends on de novo synthesis of carotenoids.
Figure 5.
Changes of xanthophyll-cycle pigments in P. tricornutum during the first 45 min of incubation with HL2 (1,000 μmol m−2⋅s−1). (A) Control without additives; (B) addition of norflurazon (5 μM) or (C) addition of DTT (500 μM) before HL2 incubation. The concentration of β-carotene increased slightly during 45-min HL in the control, remained unchanged when DTT was present, and fell slightly with norflurazon present (not shown).
DTT (500 μM), on the other hand, led to an 80% inhibition of deepoxidation of Ddx to Dtx (Fig. 5C). Nevertheless, a total increase in (Vx + Ax + Zx) was observed, now because of an accumulation of Vx, although this increase was notably smaller than that of Zx in the control. Increasing the concentration of DTT to 5 mM completely blocked deepoxidation and also inhibited de novo synthesis of carotenoids (not shown). Thus it seems likely that already at 500 μM DTT a partial inhibition of carotenoid synthesis occurred. In addition, part of the newly synthesized Vx has probably been consumed as a precursor for other xanthophylls.
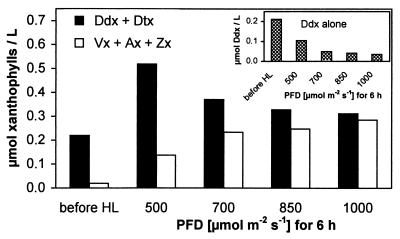
Light Intensity Modulates the Allocation of Newly Synthesized Carotenoids Between Dtx and Zx.
The importance of deepoxidase activity for the accumulation of Zx also can be demonstrated when modulating its activity by applying different HL intensities (Fig. 6). Cell suspensions of P. tricornutum exposed to different PFDs between 500 and 1,000 μmol m−2⋅s−1 for 6 h showed some characteristic responses irrespective of the PFD applied: the accumulation of Chl a and Chls c1/c2 as well as of fucoxanthin almost ceased, the concentration of β-carotene increased slightly by 10%, and total concentration of xanthophyll-cycle pigments (Ddx + Dtx + Vx + Ax + Zx) increased in all cases by a factor of about 2.5. However, the distribution of newly synthesized xanthophylls between the two pools Ddx/Dtx and Vx/Ax/Zx changed in favor of the latter with increasing PFD, resulting in the accumulation of almost equal amounts of both pools at the highest PFD (Fig. 6). As an indication of intensified deepoxidase activity, Ddx showed a higher degree of deepoxidation with increasing PFD (Fig. 6 Inset).
Figure 6.
Allocation of newly synthesized carotenoids between the two xanthophyll-cycle pools in a cell suspension of P. tricornutum after 6-h incubation with HL of different PFDs. (Inset) Degree of deepoxidation of Ddx depending on PFD. For each light intensity, two samples were analyzed; differences between values of a sample pair did not exceed 1%.
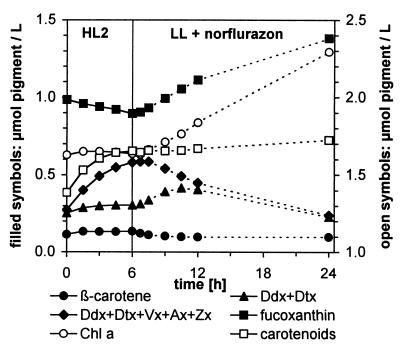
In Prolonged LL, Vx Is Converted into Ddx, Which Subsequently Gives Rise to Fucoxanthin.
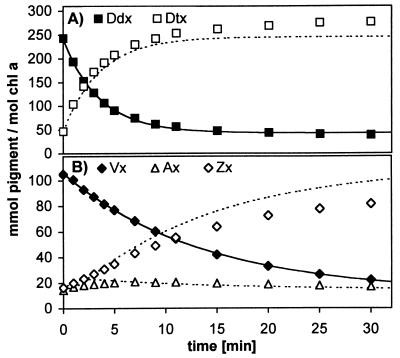
The fate of the Vx/Ax/Zx pool accumulated under HL becomes evident when monitoring the pigment changes in the algal suspension during the subsequent recovery in LL. To this end, we used HL2 (1,000 μmol m−2⋅s−1) leading to the accumulation of almost as much (Vx + Ax + Zx) as (Ddx + Dtx) within 6 h (Figs. 6 and 7). During the subsequent LL period, the de novo synthesis of carotenoids was prevented by using norflurazon. Thus any increase in a carotenoid species must be caused by the transformation of another carotenoid species already present in the algae.
Figure 7.
Time course of pigment changes in a cell suspension of P. tricornutum during 6-h illumination with HL2 (solid lines) and transformations of carotenoids in subsequent LL after addition of norflurazon (5 μM; dashed lines). Note that instead of the Vx cycle pool alone, the sum of both pools is depicted; the quantity of (Vx + Ax + Zx) then is represented by the area between (Ddx + Dtx) and the bulk of both pools.
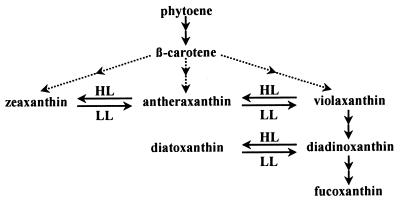
Synthesis of Chls was not affected by norflurazon for the first 6 h of LL when compared with a control without the inhibitor (see below). The fastest conversions between carotenoids occurred within the first hour of LL, namely the epoxidation of Dtx to Ddx and of Zx to Vx (Fig. 3). Within the next 5 h, two major changes in carotenoid concentrations took place. The Vx/Ax/Zx pool—now mainly represented by Vx—almost completely disappeared, and concentrations of the Ddx/Dtx pool (now mainly present as Ddx) and of fucoxanthin increased (Fig. 7), implying that Vx is converted into Ddx and fucoxanthin. During the next 12 h, the absolute amounts of Ddx in the suspension also decreased while only the increase in fucoxanthin continued, indicative of Ddx as a precursor of fucoxanthin. Together, these results strongly suggest a biosynthetic sequence from Vx via Ddx to fucoxanthin in P. tricornutum, as depicted in Fig. 8. The changes in β-carotene are far too small to be of any significance for the observed transformations of the other carotenoids.
Figure 8.
Hypothetical coupling between xanthophyll-cycle pools and the biosynthetic pathway leading to fucoxanthin in diatoms.
It is important to note that essentially the same pattern of pigment changes was observed without addition of norflurazon. There was almost no difference during the first 6 h of LL. However, in the period between 6 h and 18 h of LL, samples lacking the inhibitor showed a much stronger increase in Chl a as well as fucoxanthin, and the concentrations of Ddx and β-carotene also increased (see Fig. 9, which is published as supplemental data on the PNAS web site, www.pnas.org). Nevertheless, the pigments of the Vx cycle were present only in very small amounts after 18 h of LL.
DISCUSSION
This is, to our knowledge, the first description of the transient occurrence of substantial amounts of Vx, Ax, and Zx in algae that have been thought to contain only the Ddx cycle. It seems surprising that an accumulation of pigments of the Vx cycle in these algae has not been reported so far. As will become clear from the discussion below, the accumulation of the pigments of the Vx cycle can be observed best on algae displaying a high rate of de novo carotenoid synthesis in combination with high deepoxidase activity. With respect to P. tricornutum, our culture system and light conditions appeared to be particularly suitable for providing these conditions. In former investigations, the algae were often precultured under relatively high PFDs (18–20), probably resulting in partial HL adaptation, and HL periods lasted 2 h at most (17–20), thus being too short for accumulation of significant amounts of Zx, or there was no shift between LL and HL at all (17, 41), i.e., no stimulation of the deepoxidation.
Which factors are responsible for the accumulation of the Vx-cycle pigments? Our results suggest that Vx is an obligate precursor of Ddx and is permanently present in small amounts. Because Vx is a substrate of the deepoxidase, in HL enzymatic deepoxidation can compete with the otherwise favored conversion of Vx to Ddx (Fig. 8). As a consequence, increasing amounts of newly synthesized Vx are converted into Zx instead of Ddx as is demonstrated by the light-intensity dependence of the distribution of newly synthesized carotenoids between the two xanthophyll-cycle pools (Fig. 6). The precursor status of Vx becomes evident when the de novo synthesis of carotenoids is inhibited: the Vx already present is converted to Ddx (Fig. 7); however, in HL, no Vx or Zx is formed from other xanthophylls (Fig. 5B). Furthermore, the general occurrence of Vx in algae possessing the Ddx cycle (Table 1) strongly corroborates its proposed role as a precursor. Hence there is experimental evidence for the pivotal role of Vx as a key intermediate in the biosynthesis of all carotenoids with allenic or acetylenic conformation at C-7′, as has been proposed by several authors (25, 26).
In P. tricornutum in prolonged LL, the Ddx formed from Vx is used as a precursor of fucoxanthin (Fig. 7). The same observation has been made for C. meneghiniana (42) and is substantiated further by identical conclusions drawn from pigment-labeling studies in another diatom (43). As a consequence, in diatoms the pigments Vx and Ddx represent two facultative branching points in the biosynthetic pathway to fucoxanthin (Fig. 8). This finding has important implications regarding a regulatory role of xanthophyll cycling in the biosynthetic pathway of light-harvesting carotenoids in Chl a/c-containing algae. We propose that the xanthophyll cycles in these algae not only are important for photoprotection but also enable them to further synthesize xanthophylls under conditions of excess light, which can be converted into light-harvesting pigments in subsequent LL with almost no additional metabolic costs. To substantiate this hypothesis, the coupling between the xanthophyll-cycle pigments and the respective light-harvesting carotenoids in the other algae examined by us has to be demonstrated. We will address this question in a forthcoming paper. Furthermore, the conversion from Vx to Ddx probably requires more than one enzymatic step (25), as does the transformation of Ddx into fucoxanthin. It will be of major importance to identify the enzymes that are responsible for these transformations.
Moreover, we intend to have a closer look at how Vx is derived from β-carotene in the algae we examined. In principle, we can suggest two different pathways. Hydroxylation of β-carotene would lead to Zx first, which then is epoxidized to Vx; this is the case in higher plants (44). Alternatively, the ionon rings of β-carotene initially could be epoxidized, hydroxylation being the second step. As will be presented elsewhere, we made some observations on P. tricornutum that appear to be in conflict with the higher plant pathway. Therefore, Fig. 8 depicts several hypothetic pathways from β-carotene to Vx. However, these alternatives leave all the conclusions unchanged that have been reached here.
Is the Vx cycle in algae that possess the Ddx cycle of any significance for photoprotection? We have not studied this question yet, and it also remains to be shown whether the algae accumulate significant amounts of Zx under field conditions. The results gained on the Ddx cycle so far imply that this cycle exerts sufficient influence on thermal energy dissipation to protect the algae from photodamage (18–20). Results on two diatoms (18, 19) and energetic calculations on the isolated pigments (21) indicate that Dtx mediates energy quenching less efficiently than Zx, but the lower efficiency may be compensated by a larger pool size of the Ddx cycle (Table 1) compared with the Vx cycle in higher plants (45). Thus, in algae possessing the Ddx cycle the temporal accumulation of Zx in HL may be just an unavoidable side reaction.
On the other hand it seems justified to assume that Zx in principle could act as a photoprotectant in the algae we examined. The brown algae (Phaeophyceae) and the chrysophytes possess no Ddx cycle but use the Vx cycle for photoprotection (46, 47). Still, they do contain fucoxanthin as light-harvesting pigment, suggesting that Ddx is not an obligate precursor of fucoxanthin. So why has the Ddx cycle then been evolved at all and why is it used by the predominant microalgae in the oceans? Currently we can only speculate on these questions, but in view of the photoprotective role of xanthophyll cycles, an attractive explanation could be the faster kinetics of deepoxidation and epoxidation in the Ddx cycle compared with the Vx cycle (Table 2). All carotenoids that are deepoxidized in P. tricornutum have been shown in vitro to be substrates of the enzyme Vx deepoxidase (VDE) purified from lettuce (48). Notably, Ddx was deepoxidized two times faster by lettuce-VDE than was Vx, despite the fact that the former pigment is not present in lettuce. This finding favors the assumption that only one deepoxidase in P. tricornutum is acting on both substrates. It also suggests that Ddx is in general a better substrate for the deepoxidase than Vx, making it more favorable for short-term regulation. The epoxidation in turn could be achieved faster because the one-step reaction of Dtx into Ddx has an advantage over the two-step sequence from Vx to Zx via Ax on condition of almost equal kinetic rate constants for all substrates (Fig. 3, Table 2). Accelerated xanthophyll cycling may be of benefit in the highly variable underwater light climates, which require high flexibility, in particular for microalgae (49).
Supplementary Material
Acknowledgments
We thank Prof. Dr. H. Paulsen, Dr. V. H. R. Schmid, and Dr. J. R. Harris (all at Universität Mainz) for critically reading the manuscript and two anonymous reviewers for helpful comments. We are grateful to Dr. D. Haferburg (Universität Leipzig) for the matrix-assisted laser desorption ionization/time-of-flight–MS measurements. This work is part of the Ph.D. thesis of M.L. at the Universität Mainz and was supported by a Deutsche Forschungsgemeinschaft graduate scholarship.
ABBREVIATIONS
- Ax
antheraxanthin
- Chl
chlorophyll
- Ddx
diadinoxanthin
- Dtx
diatoxanthin
- HL
high light
- LL
low light
- PFD
photon flux density
- Vx
violaxanthin
- Zx
zeaxanthin
References
- 1.Siefermann-Harms D. Physiol Plant. 1987;69:561–568. [Google Scholar]
- 2.Frank H A, Cogdell R J. Photochem Photobiol. 1996;63:257–264. doi: 10.1111/j.1751-1097.1996.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H Y, Bassi R. In: Oxygenic Photosynthesis: The Light Reactions. Ort D R, Yocum C F, editors. Dordrecht, the Netherlands: Kluwer; 1996. pp. 539–563. [Google Scholar]
- 4.Demmig B, Winter K, Krüger A, Czygan F-C. Plant Physiol. 1987;84:218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demmig-Adams B, Gilmore A M, Adams W W., III FASEB J. 1996;10:403–412. doi: 10.1096/fasebj.10.4.8647339. [DOI] [PubMed] [Google Scholar]
- 6.Sapozhnikov D I, Krasovskaya T A, Mayevskaya A N. Dokl Akad Nauk SSSR. 1957;113:456–467. [Google Scholar]
- 7.Yamamoto H Y, Nakayama T O M, Chichester C O. Arch Biochem Biophys. 1962;97:168–173. doi: 10.1016/0003-9861(62)90060-7. [DOI] [PubMed] [Google Scholar]
- 8.Hager A. Planta. 1969;89:224–243. doi: 10.1007/BF00385028. [DOI] [PubMed] [Google Scholar]
- 9.Horton P, Ruban A V, Rees D, Pascal A A, Noctor G, Young A J. FEBS Lett. 1991;292:1–4. doi: 10.1016/0014-5793(91)80819-o. [DOI] [PubMed] [Google Scholar]
- 10.Owens T G. In: Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field. Baker N R, Bowyer J R, editors. Oxford: BIOS; 1994. pp. 95–109. [Google Scholar]
- 11.Frank H A, Cua A, Chynwat V, Young A, Gosztola D, Wasielewski M R. Photosynth Res. 1994;41:389–395. doi: 10.1007/BF02183041. [DOI] [PubMed] [Google Scholar]
- 12.Ruban A V, Phillip D, Young A J, Horton P. Biochemistry. 1997;36:7855–7859. doi: 10.1021/bi9630725. [DOI] [PubMed] [Google Scholar]
- 13.Niyogi K K, Björkman O, Grossman A R. Proc Natl Acad Sci USA. 1997;94:14162–14167. doi: 10.1073/pnas.94.25.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niyogi K K, Grossman A R, Björkman O. Plant Cell. 1998;10:1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons T R, Takahashi M, Hargrave B. Biological Oceanographic Processes. 3rd Ed. Oxford: Pergamon; 1984. [Google Scholar]
- 16.Stransky H, Hager A. Arch Mikrobiol. 1970;73:315–323. [PubMed] [Google Scholar]
- 17.Demers S, Roy S, Gagnon R, Vignault C. Mar Ecol Prog Ser. 1991;76:185–193. [Google Scholar]
- 18.Olaizola M, Yamamoto H Y. J Phycol. 1994;30:606–612. [Google Scholar]
- 19.Olaizola M, LaRoche J, Kolber Z, Falkowski P G. Photosynth Res. 1994;41:357–370. doi: 10.1007/BF00019413. [DOI] [PubMed] [Google Scholar]
- 20.Arsalane W, Rousseau B, Duval J-C. Photochem Photobiol. 1994;60:237–243. [Google Scholar]
- 21.Frank H A, Cua A, Chynwat V, Young A, Gosztola D, Wasielewski M R. Biochim Biophys Acta. 1996;1277:243–252. doi: 10.1016/s0005-2728(96)00106-5. [DOI] [PubMed] [Google Scholar]
- 22.Anderson J M, Barrett J. Encycl Plant Physiol New Ser. 1986;19:269–285. [Google Scholar]
- 23.Wilhelm C. Plant Physiol Biochem. 1990;28:293–306. [Google Scholar]
- 24.Britton G. In: Carotenoids in Photosynthesis. Young A, Britton G, editors. London: Chapman & Hall; 1993. pp. 96–126. [Google Scholar]
- 25.Swift I E, Milborrow B V. Biochem J. 1981;199:69–74. doi: 10.1042/bj1990069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Bjørnland T, Liaaen-Jensen S. In: The Chromophyte Algae: Problems and Perspectives. Green J C, Leadbeater B S C, Diver W L, editors. Oxford: Clarendon; 1989. pp. 37–61. [Google Scholar]
- 27.Swift I E, Milborrow B V, Jeffrey S W. Phytochemistry. 1982;21:2859–2864. [Google Scholar]
- 28.Milborrow B V. In: Carotenoid Chemistry and Biochemistry. Britton G, Goodwin T W, editors. Oxford: Pergamon; 1982. pp. 279–295. [Google Scholar]
- 29.Schlösser U G. Bot Acta. 1994;107:113–186. [Google Scholar]
- 30.Wilhelm C, Bida J, Domin A, Lohr M. In: Photosynthesis: From Light to Biosphere. Mathis P, editor. Vol. 5. Dordrecht, the Netherlands: Kluwer; 1995. pp. 809–812. [Google Scholar]
- 31.Phillips J N, Jr, Myers J. Plant Physiol. 1953;29:148–152. doi: 10.1104/pp.29.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provasoli L, McLaughlin J J A, Droop M R. Arch Mikrobiol. 1957;25:392–428. doi: 10.1007/BF00446694. [DOI] [PubMed] [Google Scholar]
- 33.The Culture Collection of Algae and Protozoa. CCAP Catalogue of Strains. Ambleside, U.K.: Institute of Freshwater Ecology; 1995. [Google Scholar]
- 34.Schiedt K, Liaaen-Jensen S. In: Carotenoids, Vol. 1A: Isolation and Analysis. Britton G, Liaaen-Jensen S, Pfander H, editors. Basel: Birkhäuser; 1995. pp. 81–108. [Google Scholar]
- 35.Wilhelm C, Volkmar P, Lohmann C, Becker A, Meyer M. Aqua (London) 1995;44:132–141. [Google Scholar]
- 36.Kraay G W, Zapata M, Veldhuis M J W. J Phycol. 1992;28:708–712. [Google Scholar]
- 37.Hager A, Meyer-Bertenrath T. Planta. 1966;69:198–217. doi: 10.1007/BF00384873. [DOI] [PubMed] [Google Scholar]
- 38.Eugster C H. In: Carotenoids, Vol. 1A: Isolation and Analysis. Britton G, Liaaen-Jensen S, Pfander H, editors. Basel: Birkhäuser; 1995. pp. 71–80. [Google Scholar]
- 39.Sandmann G, Linden H, Böger P. Z Naturforsch. 1989;44c:787–790. [Google Scholar]
- 40.Yamamoto H Y, Kamite L. Biochim Biophys Acta. 1972;267:538–543. doi: 10.1016/0005-2728(72)90182-x. [DOI] [PubMed] [Google Scholar]
- 41.Willemoës M, Monas E. Physiol Plant. 1991;83:449–456. [Google Scholar]
- 42.Lohr M, Wilhelm C. In: Photosynthesis: Mechanisms and Effects. Garab G, editor. Vol. 3. Dordrecht, the Netherlands: Kluwer; 1998. pp. 2313–2316. [Google Scholar]
- 43.Goericke R, Welschmeyer N A. J Phycol. 1992;28:507–517. [Google Scholar]
- 44.Cunningham F X, Jr, Gantt E. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:557–583. doi: 10.1146/annurev.arplant.49.1.557. [DOI] [PubMed] [Google Scholar]
- 45.Thayer S S, Björkman O. Photosynth Res. 1990;23:331–343. doi: 10.1007/BF00034864. [DOI] [PubMed] [Google Scholar]
- 46.Uhrmacher S, Hanelt D, Nultsch W. Mar Biol (Berlin) 1995;123:159–165. [Google Scholar]
- 47.Lichtlé C, Arsalane W, Duval J C, Passaquet C. J Phycol. 1995;31:380–387. [Google Scholar]
- 48.Yamamoto H Y, Higashi R M. Arch Biochem Biophys. 1978;190:514–522. doi: 10.1016/0003-9861(78)90305-3. [DOI] [PubMed] [Google Scholar]
- 49.Larkum A W D, Barrett J. Adv Bot Res. 1983;10:1–219. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.