Abstract
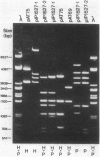
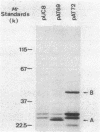
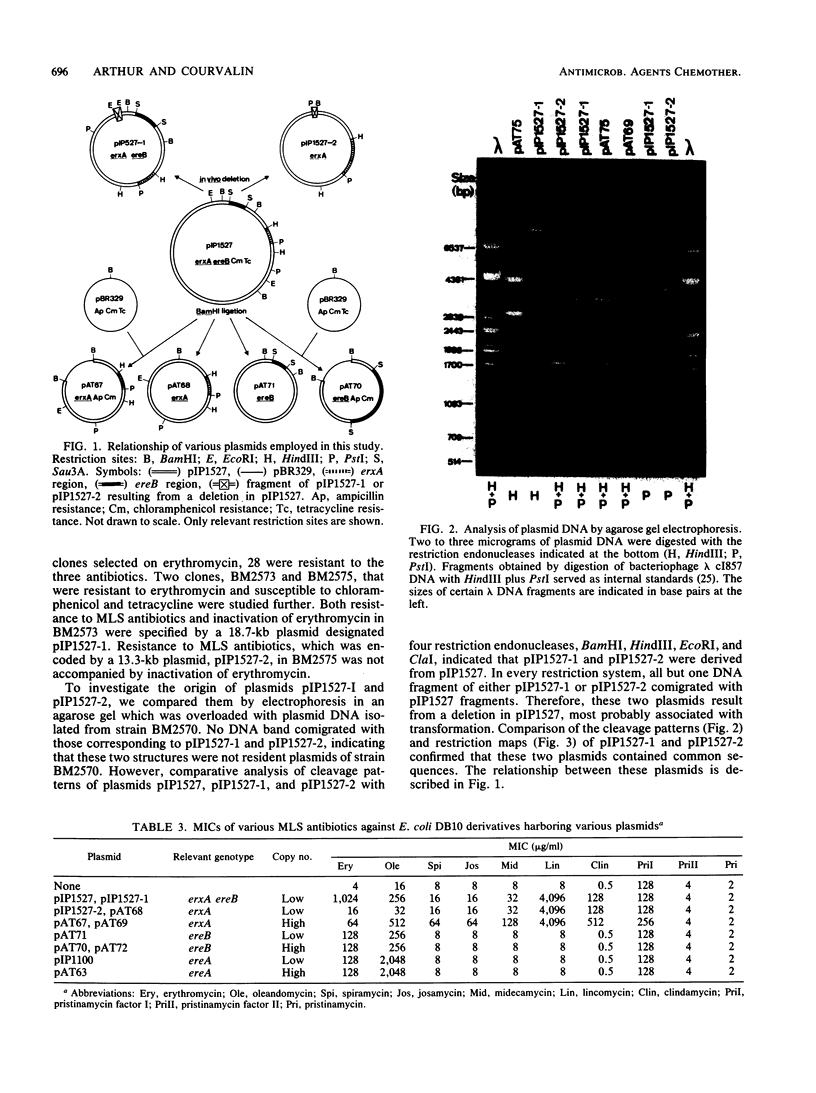
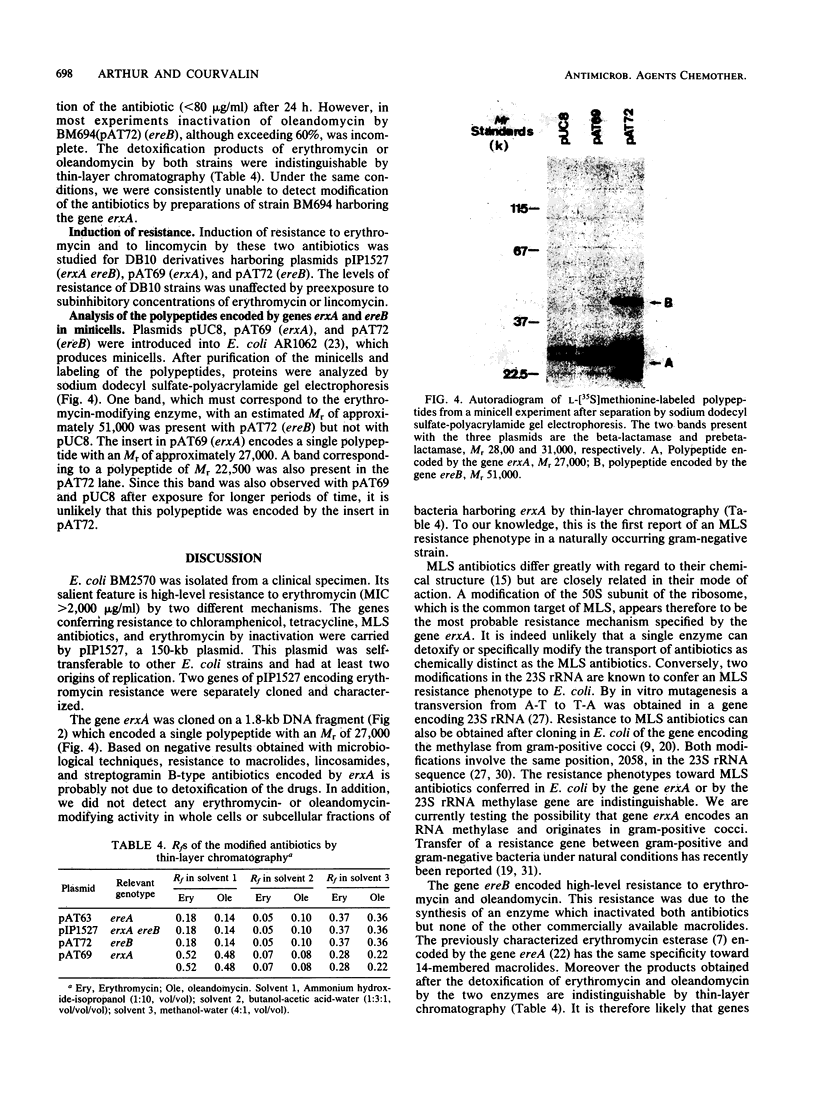
Escherichia coli BM2570 was resistant to high levels of erythromycin by two different mechanisms. The two genes conferring resistance to erythromycin in BM2570 were carried by a 150-kilobase self-transferable plasmid, pIP1527, and were cloned separately in E. coli. A single polypeptide with an Mr of 27,000 was encoded by the gene erxA and conferred high-level resistance to macrolide, lincosamide, and streptogramin B-type antibiotics by a mechanism other than drug inactivation. This resistance phenotype, not previously reported for a clinical isolate of enterobacteria, was probably due to modification of the ribosomes. The gene ereB encoded an enzyme with an Mr of 51,000 which inactivated erythromycin and oleandomycin. The two different mechanisms specified by erxA and ereB contributed in more than an additive fashion to the high level of resistance to erythromycin conferred by plasmid pIP1527 to E. coli BM2570.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andremont A., Gerbaud G., Courvalin P. Plasmid-mediated high-level resistance to erythromycin in Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):515–518. doi: 10.1128/aac.29.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andremont A., Raibaud P., Tancrède C. Effect of erythromycin on microbial antagonisms: a study in gnotobiotic mice associated with a human fecal flora. J Infect Dis. 1983 Sep;148(3):579–587. doi: 10.1093/infdis/148.3.579. [DOI] [PubMed] [Google Scholar]
- Andremont A., Sancho-Garnier H., Tancrede C. Epidemiology of intestinal colonization by members of the family Enterobacteriaceae highly resistant to erythromycin in a hematology-oncology unit. Antimicrob Agents Chemother. 1986 Jun;29(6):1104–1107. doi: 10.1128/aac.29.6.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andremont A., Tancrede C. Reduction of the aerobic Gram negative bacterial flora of the gastro-intestinal tract and prevention of traveller's diarrhea using oral erythromycin. Ann Microbiol (Paris) 1981 Nov-Dec;132 B(3):419–427. [PubMed] [Google Scholar]
- Arthur M., Andremont A., Courvalin P. Heterogeneity of genes conferring high-level resistance to erythromycin by inactivation in enterobacteria. Ann Inst Pasteur Microbiol. 1986 Mar-Apr;137A(2):125–134. doi: 10.1016/s0769-2609(86)80017-5. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy P., Autissier D., Gerbaud G., Courvalin P. Enzymic hydrolysis of erythromycin by a strain of Escherichia coli. A new mechanism of resistance. J Antibiot (Tokyo) 1984 Dec;37(12):1692–1696. doi: 10.7164/antibiotics.37.1692. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- CHABBERT Y., BOULINGRE H. Modifications pratiques concernant le dosage des antibiotiques en clinique. Rev Fr Etud Clin Biol. 1957 Jun;2(6):636–640. [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Croissant O., Blangy D. Identification of two plasmids determining resistance to tetracycline and to erythromycin in group D streptococcus. Mol Gen Genet. 1974;132(3):181–192. doi: 10.1007/BF00269391. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Becker D., Davies J. Plasmid-determined fusidic acid resistance in the Enterobacteriaceae. J Gen Microbiol. 1974 Jul;83(0):191–196. doi: 10.1099/00221287-83-1-191. [DOI] [PubMed] [Google Scholar]
- Gots J. S. THE DETECTION OF PENICILLINASE-PRODUCING PROPERTIES OF MICROORGANISMS. Science. 1945 Sep 21;102(2647):309–309. doi: 10.1126/science.102.2647.309. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Gerbaud G., Courvalin P. Translocation of sequences encoding antibiotic resistance from the chromosome to a receptor plasmid in Salmonella ordonez. Mol Gen Genet. 1981;182(3):390–408. doi: 10.1007/BF00293927. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Trieu-Cuot P., Courvalin P. Structural relationship between the genes encoding 3'-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):135–150. doi: 10.1016/s0769-2609(85)80040-5. [DOI] [PubMed] [Google Scholar]
- Malke H., Holm S. E. Expression of streptococcal plasmid-determined resistance to erythromycin and lincomycin in Escherichia coli. Mol Gen Genet. 1981;184(2):283–285. doi: 10.1007/BF00272918. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounissi H., Courvalin P. Nucleotide sequence of the gene ereA encoding the erythromycin esterase in Escherichia coli. Gene. 1985;35(3):271–278. doi: 10.1016/0378-1119(85)90005-8. [DOI] [PubMed] [Google Scholar]
- Rambach A., Hogness D. S. Translation of Drosophila melanogaster sequences in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5041–5045. doi: 10.1073/pnas.74.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Lorian V., Gerstein D., Loder P. B., Finland M. Enhancing effect on alkalinization of the medium on the activity of erythromycin against gram-negative bacteria. Appl Microbiol. 1968 Sep;16(9):1288–1292. doi: 10.1128/am.16.9.1288-1292.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Schmitt R., Bernhard E., Mattes R. Characterisation of Tn1721, a new transposon containing tetracycline resistance genes capable of amplification. Mol Gen Genet. 1979 Apr 17;172(1):53–65. doi: 10.1007/BF00276215. [DOI] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUBENECK U. Susceptibility of Proteus mirabilis and its stable L-forms to erythromycin and other macrolides. Nature. 1962 Oct 13;196:195–196. doi: 10.1038/196195b0. [DOI] [PubMed] [Google Scholar]
- Thakker-Varia S., Ranzini A. C., Dubin D. T. Ribosomal RNA methylation in Staphylococcus aureus and Escherichia coli: effect of the "MLS" (erythromycin resistance) methylase. Plasmid. 1985 Sep;14(2):152–161. doi: 10.1016/0147-619x(85)90075-7. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Gerbaud G., Lambert T., Courvalin P. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985 Dec 16;4(13A):3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. R plasmid gene dosage effects in Escherichia coli K-12: copy mutants of the R plasmic R1drd-19. Plasmid. 1977 Nov;1(1):1–7. doi: 10.1016/0147-619x(77)90003-8. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression--a review. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]