Abstract
Aims: To investigate the presence and distribution of the protein maspin in carcinoma ex pleomorphic adenoma (CXPA).
Methods: Maspin expression was studied by means of immunohistochemistry in 16 cases of CXPA, using the labelled polymer method.
Results: According to the extent of invasion, the tumours were subdivided into: intracapsular (five cases), minimally invasive (four cases), and invasive (seven cases). Twelve patients had carcinoma with only epithelial differentiation, whereas four had a malignant myoepithelial component. Non-luminal cells in the duct-like structures of the remnant pleomorphic adenoma were strongly positive for maspin, whereas only a few luminal cells were immunopositive. A few positive cells were seen in the frequent hypocellular and hyalinised areas. Maspin was abundantly expressed, mainly in non-luminal cells, in transitional areas of CXPA with only epithelial differentiation. In frankly carcinomatous areas there was a gradual decrease in maspin expression. Almost all cells were maspin positive in CXPA with a myoepithelial component. When present, luminal cells were in general negative for maspin.
Conclusions: When only epithelial cells undergo malignant transformation, maspin expression is gradually lost. In cases with a myoepithelial component, maspin expression is high, and this might be related to the tumour suppressor activity attributed to this cell.
Keywords: carcinoma ex pleomorphic adenoma, maspin, myoepithelial cell, immunohistochemistry
Pleomorphic adenoma (PA), the most frequent tumour in salivary glands, can undergo malignant transformation. Although rare, this condition has been reported to be responsible for 4.5–15% of salivary gland malignancies.1–3
Carcinoma ex pleomorphic adenoma (CXPA) has no distinctive sex predilection,4 and is more frequent in the 5–6th decades of life.3,5 These tumours are highly aggressive, with high rates of metastasis,6 and carry a poor overall prognosis.4 It has been suggested that a carcinoma may develop within a recurrent PA or when it remains untreated over an extended period of time.7,8 Despite the recognised clinical importance of CXPA, little is known about its biology. The detection of early transformation in PA is a diagnostic challenge in pathology. Using the interpretation of cellular atypia, increased mitotic index, and lack of encapsulation as signs of malignant transformation is controversial, because these signs can also be seen in ordinary PA.3,6
“The detection of early transformation in pleomorphic adenoma is a diagnostic challenge in pathology”
Maspin (mammary serine protease inhibitor) is a member of the serpin superfamily of protease inhibitors, which has a peculiar role as a tumour suppressor.9 Its expression has been inversely correlated with malignant behaviour. It is expressed in normal cells, downregulated in neoplastic cells, and absent in metastatic cells.10–12 Evidence suggests that maspin suppresses tumour growth and metastasis by inhibiting tumour cell invasion and motility.10,13–15
We recently detected maspin in a series of salivary gland tumours.16 Maspin expression correlated with tumour malignancy—more aggressive histological types showed lower immunostaining for maspin. Hence, maspin was abundant in PA.
MATERIALS AND METHODS
For our study, 16 cases of CXPA were retrieved from the files of the department of pathology of the University of Campinas and department of oral pathology, School of Dentistry, University of São Paulo, Brazil.
For morphological analysis, 5 µm sections were obtained from formalin fixed, paraffin wax embedded samples and routinely stained with haematoxylin and eosin.
In each selected case, a malignancy was seen in association with PA. However, for the purposes of our study the histological diagnosis of the malignant component was not important; it was the nature of the neoplastic cells that had undergone malignant transformation—epithelial cells only or epithelial and myoepithelial cells—confirmed morphologically and by a panel of antibodies17 that was of interest. The tumours were also classified according to the extension of invasion related to the capsule of the previous PA: intracapsular, minimally invasive (⩽ 15 mm of invasion), and frankly invasive.
For immunohistochemistry, 3 μm sections were obtained from formalin fixed, paraffin wax embedded specimens. After dewaxing and rehydration, antigen retrieval was carried out in 10mM citrate solution (pH 6.0) at 95°C for 30 minutes in a water bath. Sections were then incubated for 30 minutes in a 6% hydrogen peroxide/methanol (1/1) solution to quench endogenous peroxidase activity.
Immunohistochemical staining was performed on a Dako Autostainer (Dako, Carpinteria, California, USA). Briefly, anti-maspin antibody (BD Pharmingen, San Diego, California, USA) was incubated for 30 minutes at a dilution of 1/100. Goat antimouse immunoglobulin conjugated to peroxidase labelled dextran polymer was incubated by a one step technique using the EnVision system (Dako) for 30 minutes. This method avoids non-specific staining as a result of endogenous biotin, which makes it useful for studying salivary gland sections. Diaminobenzidine was used as the chromogen, followed by counterstaining with Mayer’s haematoxylin.
Fragments of lining epithelium and non-neoplastic salivary gland tissue served as internal positive controls. The primary antibody was omitted as a negative control.
For each tissue specimen, the extent and intensity of staining was evaluated visually, using a light microscope.
RESULTS
According to the extent of invasion, the tumours were subdivided into the following categories: intracapsular (five cases), minimally invasive (four cases), and invasive (seven cases). Twelve cases had carcinoma with only epithelial differentiation (five non-invasive, three minimally invasive, and four frankly invasive), whereas four cases had a malignant myoepithelial component (one minimally invasive, and three of the invasive type).
Although we saw both cytoplasmic and nuclear staining in all specimens, positivity in the cytoplasm disappeared first as malignancy progressed. Areas with faint immunostaining showed nuclear staining only.
Remnant PA
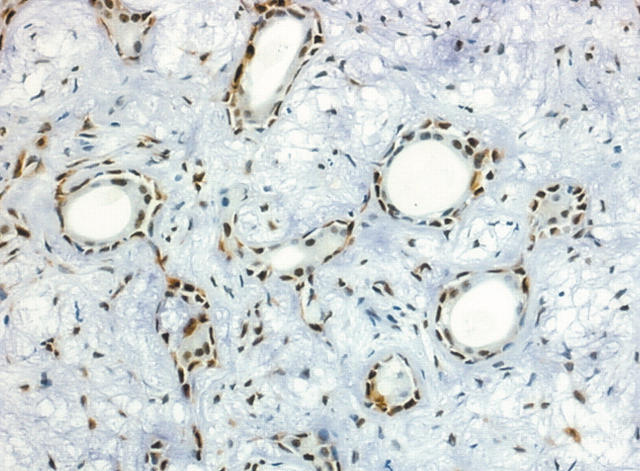
In all cases studied, the area of PA showed the same pattern of staining for maspin. Non-luminal cells in the duct-like structures were strongly positive for maspin, whereas only a few luminal cells were immunostained (fig 1). Maspin was abundant in the rare highly cellular areas, whereas more loosely arranged parts showed decreased expression. Cells in the hyalinised zones were occasionally positive.
Figure 1.
Area of remnant pleomorphic adenoma showing non-luminal cells and occasional luminal cells positive for maspin (original magnification, ×100).
CXPA with only epithelial differentiation
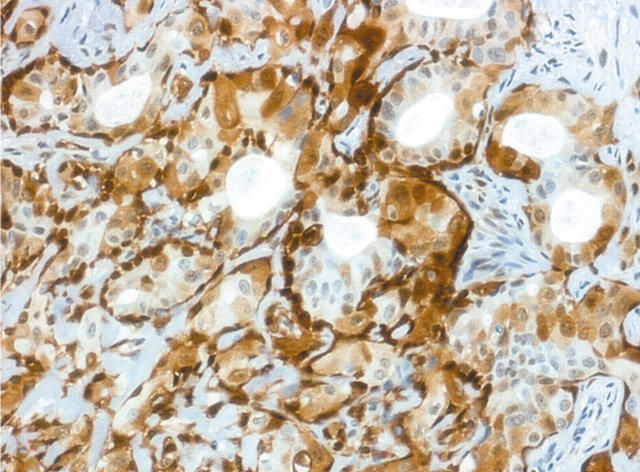
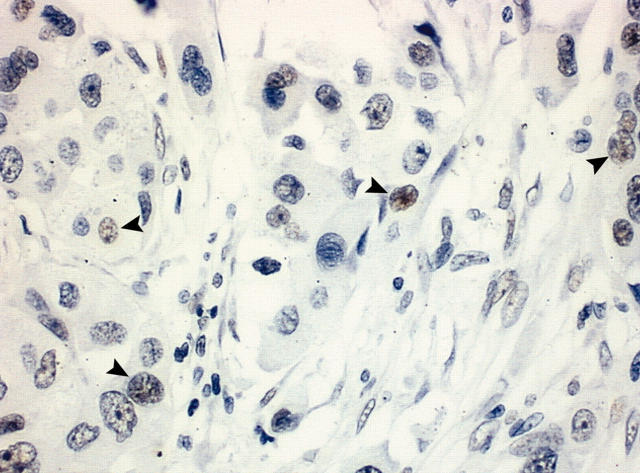
In the transitional areas, duct-like structures were still present, with circumjacent benign myoepithelial cells, which were oval or spindle shaped. Strong staining for maspin was seen in these benign myoepithelial cells and rarely in luminal cells, which were large and polygonal. In two cases—both intracapsular—focal areas revealed strong staining of the luminal cells (fig 2). In the frankly carcinomatous areas there was a gradual decrease in maspin expression until it was completely absent in the zones where carcinomatous islets were small. When visible, the periphery of the invasive lesions was negative or weakly positive (fig 3).
Figure 2.
Transitional area in a carcinoma ex pleomorphic adenoma with epithelial differentiation only. Strongly positive non-luminal and luminal cells are seen in this specimen (original magnification, ×100).
Figure 3.
Frankly invasive area showing only weak staining in occasional cells, restricted to the nuclei (arrowheads) (original magnification, ×200).
CXPA with a myoepithelial component
In these cases, maspin was abundant. In transitional areas, where remnants of duct-like structures were seen, luminal cells were sometimes negative. In frankly malignant areas, where cells with myoepithelial differentiation predominated, all cells were strongly positive for maspin. One case showed areas of squamous metaplasia and these cells were also positive for maspin (fig 4).
Figure 4.
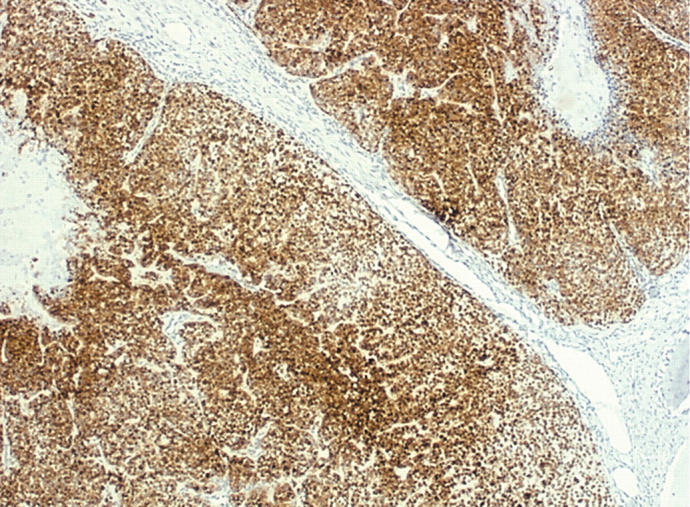
Carcinoma ex pleomorphic adenoma with only myoepithelial component showed strong staining for maspin (original magnification, ×25).
DISCUSSION
CXPA is a malignant tumour that develops from the epithelial component of PA.18 Tumour progression results in frankly carcinomatous islets that gradually invade adjacent tissue.19 We analysed maspin expression in a series of 16 cases of CXPA presenting intracapsular, minimally invasive, and invasive patterns.
Maspin is a member of the serpin family of proteinase inhibitors. It has been associated with decreased cell motility, invasion capability, and metastasis formation, conferring a tumour suppressor role on this protein.9,13,20
In the 12 cases where luminal cells had undergone malignant transformation, maspin was abundant in the initial phases (most often in benign myoepithelial cells and rarely in malignant epithelial cells in contact with those cells), and it was gradually lost concomitant with myoepithelial cell disappearance. Cases with myoepithelial differentiation showed high maspin expression in all phases.
“During tumour progression, the disappearance of benign myoepithelial cells results in reduced maspin expression by epithelial cells until it completely disappears”
Maspin has been consistently associated with the myoepithelial cell. Several studies have found it to be present predominantly or even exclusively in these cells,21–23 and its use as a myoepithelial cell marker has already been suggested.23 We reported previously that in normal salivary glands maspin is expressed by myoepithelial cells almost exclusively.16 Similarly, ordinary PA shows positivity in cells with myoepithelial differentiation, whereas only occasional epithelial (luminal) cells are immunopositive for this protein.16 In transitional phases of CXPA, when benign myoepithelial cells are still present, this profile is exacerbated, whereas in some areas epithelial cells also highly express maspin. These findings suggest that maspin production is coordinated by myoepithelial cells and that epithelial cells are influenced by them. During tumour progression, the disappearance of benign myoepithelial cells results in reduced maspin expression by epithelial cells until it completely disappears. It has been postulated by Sternlicht and Barsky24 that myoepithelial cells exert an influence over epithelial cells in a paracrine or contact mediated manner.
Our results are in agreement with the theory that the myoepithelial cell is a natural tumour suppressor,25 and that even when transformed, this cell continues to show low grade behaviour and to have the characteristics of a normal cell (for example, high maspin expression).
Barsky et al have shown that transformed myoepithelial cells retain or even augment the synthesis of basement membrane molecules, an important feature for tumour suppressor activity.26 Our results suggest that in malignant transformation of luminal cells, remnant myoepithelial cells are stimulated to exhibit their complete phenotype, and exert tumour suppressor activity.
Although its interpretation is subjective, high expression of maspin in the early phases of CXPA might be diagnostically useful. Despite showing the same pattern of maspin expression seen in ordinary PA,16 areas of PA present in association with CXPA characteristically show conspicuous hypocellularity and hyalinisation, a feature reported in several series.3,6,27 These areas show low maspin expression, contrasting greatly with the strong positivity seen in transitional areas. Unfortunately, this use is restricted to CXPA with a malignant epithelial component only.
Maspin was first described as a cytoplasmic protein, but it was later reported in the cell nucleus.21,23,28 Although all known maspin activities depend on a cytoplasmic distribution, there is probably a biological reason for its presence in the nucleus. Recently, Mohsin et al studied nuclear maspin expression in invasive breast cancer, and found that 96% of samples showed nuclear staining, and this was related to hormone receptor expression.29 The authors found both cytoplasmic and nuclear staining in myoepithelial cells, but predominantly nuclear staining in luminal cells. In our study, we detected a difference in distribution between both compartments in the frankly invasive areas where staining decreased. Maspin disappeared first in the cytoplasm, whereas faint staining was seen in the nuclei for longer.
Take home messages.
We investigated the expression of the tumour suppressor protein maspin in carcinoma ex pleomorphic adenoma by means of immunohistochemistry
When only epithelial cells had undergone malignant transformation, maspin expression was downregulated during malignant progression, as would be expected
However, when myoepithelial cells were also transformed, high maspin expression was seen in all phases, perhaps as a result of the tumour suppressor activity attributed to this cell type
In conclusion, when only epithelial cells undergo malignant transformation in PA, maspin expression is downregulated during malignant progression, as would be expected—although expression is higher in the early stages compared with normal salivary glands and benign PA. In contrast, when myoepithelial cells are also transformed, high maspin expression is seen in all phases, and this might be related to the tumour suppressor activity attributed to this cell type.
Acknowledgments
We thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for supporting this study (grant number 04/07960-0).
Abbreviations
CXPA, carcinoma ex pleomorphic adenoma
PA, pleomorphic adenoma
REFERENCES
- 1.LiVolsi VA, Perzin KH. Malignant mixed tumors arising in salivary glands. I. Carcinomas arising in benign mixed tumors: a clinicopathologic study, Cancer 1977;39:2209–30. [DOI] [PubMed] [Google Scholar]
- 2.Gnepp DR. Malignant mixed tumors of the salivary glands: a review. Pathol Annu 1993;28:279–328. [PubMed] [Google Scholar]
- 3.Ellis GL, Auclair PL. Malignant epithelial tumors. In: Atlas of tumor pathology, Series 3, Section 5, Fascicle 17. Washington, DC: Armed Forces Institute of Pathology, 1996:155–373.
- 4.Yoshihara T, Tanaka M, Itoh M, et al. Carcinoma ex pleomorphic adenoma of the soft palate. J Laryngol Otol 1995;109:240–3. [DOI] [PubMed] [Google Scholar]
- 5.Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck 2001;23:705–12. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol 2001;32:596–604. [DOI] [PubMed] [Google Scholar]
- 7.Eneroth CM, Zetterberg A. Malignancy in pleomorphic adenoma. A clinical and microspectrophotometric study. Acta Otolaryngol 1974;77:426–32. [DOI] [PubMed] [Google Scholar]
- 8.Thackray AC, Lucas RB. Carcinoma in pleomorphic adenoma. In: Atlas of tumor pathology, Series 2, Fascicle 10. Washington, DC: Armed Forces Institute of Pathology, 1974:107–17.
- 9.Sheng S, Pemberton PA, Sager R. Production, purification, and characterization of recombinant maspin proteins. J Biol Chem 1994;269:30988–93. [PubMed] [Google Scholar]
- 10.Zou Z, Anisowicz A, Hendrix MJ, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 1994;263:526–9. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Magit D, Sager R. Expression of maspin in prostate cells is regulated by a positive ets element and a negative hormonal responsive element site recognized by androgen receptor. Proc Natl Acad Sci U S A 1997;94:5673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maass N, Teffner M, Rosel F, et al. Decline in the expression of the serine proteinase inhibitor maspin is associated with tumour progression in ductal carcinomas of the breast. J Pathol 2001;195:321–6. [DOI] [PubMed] [Google Scholar]
- 13.Sheng S, Carey J, Seftor EA, et al. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci U S A 1996;93:11669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sager R, Sheng S, Pemberton P, et al. Maspin: a tumor suppressing serpin. Curr Top Microbiol Immunol 1996;213:51–64. [DOI] [PubMed] [Google Scholar]
- 15.Seftor RE, Seftor EA, Sheng S, et al. Maspin suppresses the invasive phenotype of human breast carcinoma. Cancer Res 1998;58:5681–5. [PubMed] [Google Scholar]
- 16.Navarro RL, Martins MT, Araujo VC. Maspin expression in normal and neoplastic salivary gland. J Oral Pathol Med 2004;33:435–40. [DOI] [PubMed] [Google Scholar]
- 17.Altemani A, Martins MT, Freitas L, et al. Carcinoma ex pleomorphic adenoma (CXPA): immunoprofile of the cells involved in the carcinomatous transformation. Histopathology 2005;46:635–41. [DOI] [PubMed] [Google Scholar]
- 18.Ellis GL, Aulclair PL, Gnepp DR. Surgical pathology of the salivary glands. Philadelphia: WB Saunders, 1991.
- 19.Clairmont AA, Conley JJ. Malignant mixed tumor of the soft palate. J Oral Surg 1978;36:394–6. [PubMed] [Google Scholar]
- 20.Sheng S, Truong B, Fredrickson D, et al. Tissue-type plasminogen activator is a target of the tumor suppressor gene maspin. Proc Natl Acad Sci U S A 1998;95:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lele SM, Graves K, Gatalica Z. Immunohistochemical detection of maspin is a useful adjunct in distinguishing radial sclerosing lesion from tubular carcinoma of the breast. Appl Immunohistochem Mol Morphol 2000;8:32–6. [DOI] [PubMed] [Google Scholar]
- 22.Hojo T, Akiyama Y, Nagasaki K, et al. Association of maspin expression with the malignancy grade and tumor vascularization in breast cancer tissues. Cancer Lett 2001;171:103–10. [DOI] [PubMed] [Google Scholar]
- 23.Reis-Filho JS, Milanezi F, Silva P, et al. Maspin expression in myoepithelial tumors of the breast. Pathol Res Pract 2001;197:817–21. [DOI] [PubMed] [Google Scholar]
- 24.Sternlicht MD, Barsky SH. The myoepithelial defense: a host defense against cancer. Med Hypotheses 1997;48:37–46. [DOI] [PubMed] [Google Scholar]
- 25.Sternlicht MD, Kedeshian P, Shao ZM, et al. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res 1997;3:1949–58. [PubMed] [Google Scholar]
- 26.Barsky SH, Layfield L, Varki N, et al. Two human tumors with high basement-membrane-producing potential. Cancer 1988;61:1798–806. [DOI] [PubMed] [Google Scholar]
- 27.Brandwein M, Huvos AG, Dardick I, et al. Noninvasive and minimally invasive carcinoma ex mixed tumor: a clinicopathologic and ploidy study of 12 patients with major salivary tumors of low (or no?) malignant potential. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:655–64. [DOI] [PubMed] [Google Scholar]
- 28.Reis-Filho JS, Milanezi F, Schmitt FC. Maspin is expressed in the nuclei of breast myoepithelial cells. J Pathol. 2002;197: 272–3; author reply 273–4,. [DOI] [PubMed]
- 29.Mohsin SK, Zhang M, Clark GM, et al. Maspin expression in invasive breast cancer: association with other prognostic factors. J Pathol 2003;199:432–5. [DOI] [PubMed] [Google Scholar]