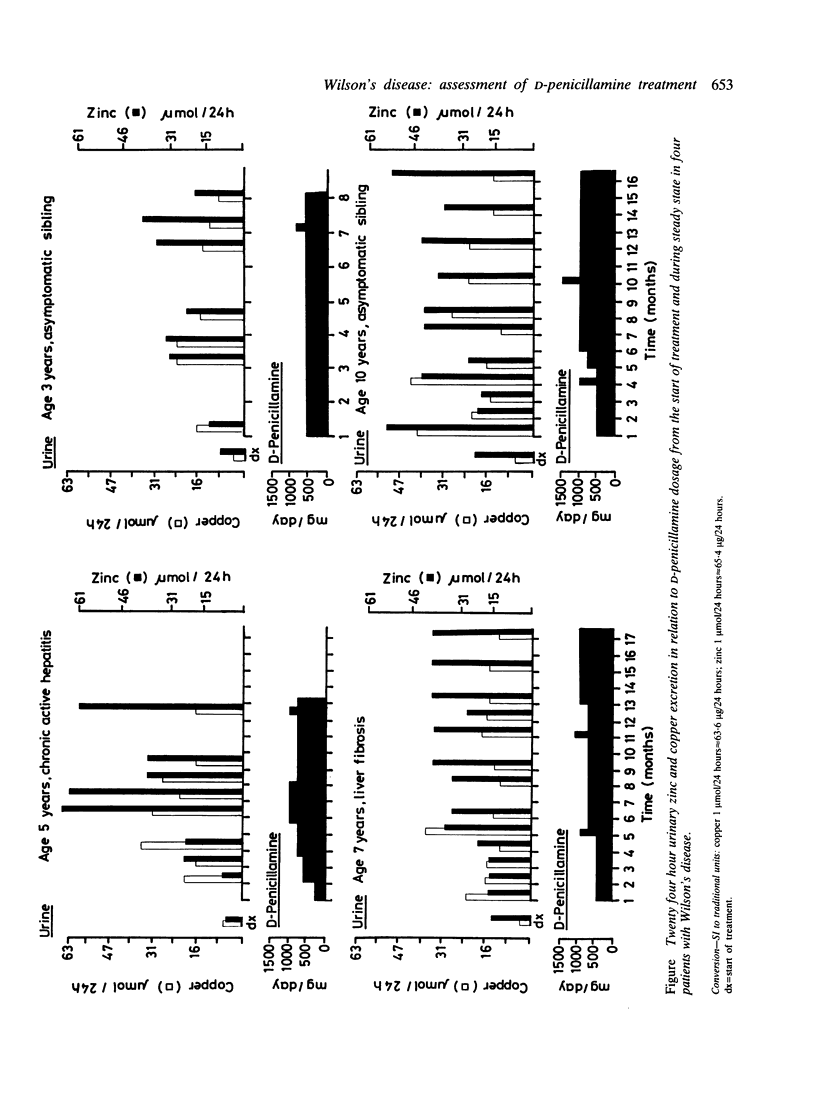
Abstract
Serum copper and zinc concentrations and 24 hour urinary copper and zinc excretion were determined serially from the beginning of treatment with D-penicillamine in four children with Wilson's disease. The data show a progressive decrease in both serum copper and zinc concentrations in all. Urinary copper excretion gradually levelled off to approximately 50% of initial values, but zinc excretion increased. Urinary zinc:copper ratios therefore increased with the duration of treatment. Copper elimination was considered adequate as soon as challenge with a test dose of D-penicillamine did not result in an increase in copper excretion. Urinary zinc excretion was increased further by the test dose. Zinc depletion was suspected clinically in one patient on D-penicillamine maintenance treatment. Lowering the dose alleviated the symptoms, urinary zinc loss decreased from 64 to 34 mumol/24 hours, and copper excretion remained largely unchanged. Data obtained indicate that D-penicillamine alters the metabolism of both copper and zinc. The extent of this is not only dose dependent but is also related to the efficacy of copper elimination. Both copper and zinc concentrations must by monitored to assess the benefits of treatment and the risks of inducing manifest or subclinical zinc deficiency.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima M., Takeshita K., Yoshino K., Kitahara T., Suzuki Y. Prognosis of Wilson's disease in childhood. Eur J Pediatr. 1977 Oct 12;126(3):147–154. doi: 10.1007/BF00442196. [DOI] [PubMed] [Google Scholar]
- Crawhall J. C. Penicillamine: twenty-five years later. Ann Intern Med. 1980 Aug;93(2):367–368. doi: 10.7326/0003-4819-93-2-367. [DOI] [PubMed] [Google Scholar]
- Greer K. E., Askew F. C., Richardson D. R. Skin lesions induced by penicillamine. Occurrence in a patient with hepatolenticular degeneration (Wilson Disease). Arch Dermatol. 1976 Sep;112(9):1267–1269. doi: 10.1001/archderm.112.9.1267. [DOI] [PubMed] [Google Scholar]
- Halverson P. B., Kozin F., Bernhard G. C., Goldman A. L. Toxicity of penicillamine. A serious limitation to therapy in rheumatoid arthritis. JAMA. 1978 Oct 20;240(17):1870–1872. doi: 10.1001/jama.240.17.1870. [DOI] [PubMed] [Google Scholar]
- Landrieu P., Choulot J. J. Le traitement de la maladie de Wilson chez l'enfant. A propose de cinq observations. Arch Fr Pediatr. 1976 Aug-Sep;33(7):665–675. [PubMed] [Google Scholar]
- Liberman U. A., Barzel U., De Vries A., Ellis H. Myositis ossificans traumatica with unusual course. Effect of EDTA on calcium, phosphorus and manganese excretion. Am J Med Sci. 1967 Jul;254(1):35–47. doi: 10.1097/00000441-196707000-00004. [DOI] [PubMed] [Google Scholar]
- Sternlieb I., Scheinberg I. H. Prevention of Wilson's disease in asymptomatic patients. N Engl J Med. 1968 Feb 15;278(7):352–359. doi: 10.1056/NEJM196802152780702. [DOI] [PubMed] [Google Scholar]
- Strickland G. T., Frommer D., Leu M. L., Pollard R., Sherlock S., Cumings J. N. Wilson's disease in the United Kingdom and Taiwan. I. General characteristics of 142 cases and prognosis. II. A genetic analysis of 88 cases. Q J Med. 1973 Jul;42(167):619–638. [PubMed] [Google Scholar]


