Abstract
Cytotoxic T cell (CTL) memory was analyzed after infection with lymphocytic choriomeningitis virus (LCMV) and recombinant Listeria monocytogenes (rLM) expressing the complete nucleoprotein of LCMV (rLM-NPactA) or only the immunodominant epitope of H-2d mice (rLM-NP118–126). Immunization with LCMV and rLM induced a long-lived increased CTL precursor (CTLp) frequency specific for the viral (NP118–126) and for the bacterial (LLO91–99) epitope, respectively. However, after infection with rLM memory, CTLs were less protective against an intravenous LCMV challenge infection than a comparable number of LCMV-induced memory T cells. LCMV, but not recombinant Listeria-induced memory T cells were able to protect against lethal choriomeningitis by LCMV or a subsequent peripheral infection with recombinant vaccinia virus expressing LCMV-NP. The protective memory after viral and after rLM immunization was paralleled by evidence of LCMV but not rLM antigen persistence on day 15 and 30 after vaccination. These results document a striking difference in protective T cell memory between viral and bacterial vaccines and indicate that rapid T cell-dependent immune protection correlates with antigen persistence.
Although immunological memory is a hallmark of immunity, its nature and evolutionary role are unclear. Virus-specific cytotoxic T cell (CTL) memory has been correlated with clonal burst size or with CTL precursor (CTLp) frequency (1). Adoptive transfer experiments have shown that maintenance of CD8+ T cells with a memory phenotype (CD44hi) and protection against intravenous reinfection was independent of the persistence of the antigen (2, 3). However, in apparent contrast, by using a variety of in vivo readouts, other studies (4–7) have shown that the maintenance of protective antiviral CTL memory is probably antigen-dependent.
Recovery from and protection against acute infections with lymphocytic choriomeningitis virus (LCMV) are almost exclusively dependent on CD8+ T cells (8, 9), and antibodies initially play virtually no role (9, 10). However, the initial CD8+ T cell response apparently controls virus only incompletely; long-term viral control depends on the presence of neutralizing antibodies (11, 12). In general, protection against an intravenous reinfection with virus or a reinfection via mucosal surfaces is mainly mediated by neutralizing antibodies. In contrast, activated CTLs that can emigrate immediately into infected tissues (13) appear crucial for protection against a direct secondary infection. Therefore, in the periphery, CTLs are considerably more efficient than antibodies (14).
Listeria monocytogenes has been used to study immunity against facultative intracellular bacteria. Resistance against a primary infection depends on early innate mechanisms including macrophages and neutrophils with subsequent T cell activation (15), recruitment, and activation of macrophages (16–19) via cytokines, such as tumor necrosis factor α (20) and IFN-γ (21–23). Specific CD8+ T cells play a role in late and complete clearance of the infection (17) and in protection against a reinfection (24–28) in a perforin-dependent manner (29).
The intracytoplasmic life cycle of Listeria provides a means for introducing antigens into the class I pathway of antigen presentation. Listeriolysin O (LLO) (amino acids 91–99) was recently characterized as the immunodominant CTL epitope in the H-2d haplotype (30, 31), and T cells specific for this epitope have been shown to be protective in vivo (32). Earlier studies by North (25) and Jungi (33) had shown that protective T cell memory against Listeria was rather short-lived; the contribution of CTL memory to protective memory has not yet been analyzed in detail.
Recombinant Listeria monocytogenes (rLM) has recently been developed as vaccines to protect against viral infections such as HIV (34) or LCMV (35, 36) and against tumors (37, 38). rLM expressing the nucleoprotein (NP) of LCMV (rLM-NPactA) or the immunodominant epitope in the H-2d haplotyope (rLM-NP118–126) offered the possibility to compare virus- and bacteria-induced CTL memory. The present study shows that mice immunized with LCMV maintained memory T cells that were able to rapidly clear a reinfection, whereas rLM induced a pool of memory T cells that first required reactivation before they were able to protect. Furthermore, the rapid protective capacity of the memory T cells correlated with in vivo persistence of the antigen.
MATERIALS AND METHODS
Mice.
BALB/c (H-2d) mice and IFN-α/β/γ receptor−/− (AG129, extremely susceptible to LCMV and Listeria infection) (39) were purchased from the Institute for Laboratory Animals (Veterinarian Hospital, Zurich, Switzerland). Mice were between 8 and 12 weeks old at the beginning of the experiment and were kept in a conventional mouse house facility.
Viruses and Bacteria.
The LCMV-WE and LCMV-Armstrong isolates were originally obtained from F. Lehmann-Grube (40) (Heinrich-Pette-Institut, Hamburg, Germany) and M. B. A. Oldstone (41) (Scripps Clinic and Research Foundation, La Jolla, California), respectively. Recombinant vaccinia virus for the listeriolysin (amino acids 91–99) (VaccLLO91–99) (42) and LCMV-NP recombinant virus (VaccNP) (43) have been described in detail.
The production of the Listeria recombinants has been described in detail (35). Bacterial titers in organs were determined at the indicated time points by homogenizing the whole spleen or one lobe of the liver, and organ suspensions were plated out in four serial 10-fold dilutions on brain heart infusion agar plates.
Cr-Release Assay and Limiting Dilution.
51Cr-release assays and the limiting-dilution analysis were done as described (44).
RESULTS
Elevated CTLp Frequencies Are Maintained After Immunization with LCMV and Listeria monocytogenes.
BALB/c mice were immunized with 200 plaque-forming units (pfu) of LCMV-WE, 2 × 103 colony-forming units (cfu) of wild-type (wt) Listeria, rLM expressing the complete NP of LCMV (rLM-NPactA), or rLM expressing the immunodominant epitope in the H-2d haplotype (rLM-NP118–126). Spleen cells were restimulated in vitro 8, 60, and 300 days later for 7 days with irradiated BALB/c spleen cells pulsed either with the viral epitope (NP118–126) or the bacterial epitope (LLO91–99) (Table 1). CTLp frequencies were assessed by a limiting-dilution assay (45) because in our hands, tetramer staining yielded no significant increase in stainable cells above controls in the memory phase [day 30–60 (46)]. In addition, the relationship between stainable T cells and potential effector T cells protective in vivo remains unclear.
Table 1.
CTL precursor frequency after immunization with LCMV and Listeria
| Group | Day 8
|
Day 60
|
Day 300
|
|||
|---|---|---|---|---|---|---|
| NP118–126 | LLO91–99 | NP118–126 | LLO91–99 | NP118–126 | LLO91–99 | |
| LCMV | 1/1.0 × 103 | <1/5 × 105 | 1/9.5 × 103 | <1/5 × 105 | 1/6.8 × 103 | <1/5 × 105 |
| (0.8–5.0) | (6.0–20.0) | (4–15) | ||||
| wt-LM | <1/5 × 105 | 1/1.8 × 104 | <1/5 × 105 | 1/8.6 × 104 | <5 × 105 | 1/6.0 × 104 |
| (0.8–5.0) | (6.0–12.0) | (5–7) | ||||
| rLM-NPactA | 1/5.0 × 104 | 1/1.3 × 104 | 1/9.5 × 104 | 1/6.0 × 104 | 1/5.3 × 104 | 1/2.9 × 104 |
| (4.0–7.0) | (0.9–5.0) | (5.0–10.0) | (5.0–10.0) | (5–6) | (1.5–5) | |
| rLM-NP118–126 | 1/8.0 × 103 | 1/8.6 × 103 | 1/5.7 × 104 | 1/5.0 × 104 | 1/8.2 × 104 | 1/3.2 × 104 |
| (6.0–9.0) | (8.0–9.0) | (5.0–8.0) | (3.0–6.0) | (5–15) | (2–10) | |
Three BALB/c mice per group and time point were immunized with 200 pfu of LCMV or 2 × 103 cfu of Listeria (wt-LM; rLM-NPactA; rLM-NP118–126). The number of NP118–126- and LLO91–99-specific CTL precursors was determined 8, 60, and 300 days after immunization by limiting-dilution analysis for the two relevant epitopes, NP118–126 and LLO91–99. The mean values and (in parentheses) the range of the values of the specific CTL frequency of three mice per group and time point is given. For each group and each time point, CTL-precursor frequency was assessed in two independent experiments, with similar results.
The NP118–126-specific CTL precursor frequency 8 days after LCMV infection reached a maximum of 1 specific CTL in about 103 spleen cells. By day 60, the CTLp frequency declined by a factor of 10, to 1 in 104. Thereafter, CTLp frequencies remained constant until day 300. Vaccination with rLM induced and maintained CTLp-frequencies at approximately 10 times lower levels compared with LCMV-immune mice. Infection with wt-LM and with rLM induced LLO91–99-specific CTLps that were in the same range as the NP118–126-specifc CTLp frequency induced by immunization with rLM. Bacteria-specific (LLO91–99) and virus-specific (NP118–126) CTLp frequencies were also maintained over 300 days.
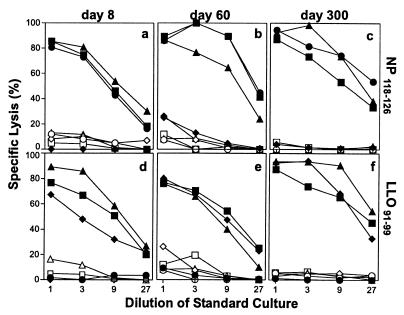
LCMV-primed CD8+ T cells as well as rLM-primed CTLs showed a high cytotoxic activity after in vitro restimulation with the relevant viral or bacterial peptide (Fig. 1). In addition, Listeria-primed and restimulated specific CTLs lysed LLO91–99-labeled target cells very efficiently, even at day 300 after vaccination. Therefore, LCMV, as well as rLM, can induce and maintain long-lived elevated frequencies of CTLps that can differentiate into efficient effector CTLs on in vitro restimulation.
Figure 1.
Memory CTL after immunization with LCMV and rLM efficiently lyse peptide-pulsed target cells for up to 300 days after restimulation in vitro. BALB/c mice were immunized with 200 pfu of LCMV (●) or 2 × 103 cfu of Listeria (wt-LM, ♦; rLM-NPactA, ▴; rLM-NP118–126, ■). At the indicated time points, spleen cells were restimulated for 5 days in vitro with peptide-pulsed spleen cells [NP118–126 (a–c); LLO91–99 (d–f)] and tested in a conventional 51Cr-release assay on P815 target cells pulsed with NP118–126 (a–c) or LLO91–99 (d–f). Filled symbols represent lysis of target cells pulsed with the relevant peptide, open symbols show lysis of unlabeled target cells. Each symbol represents the mean of three different mice tested. The spontaneous 51Cr-release was <20%. One of three similar experiments is shown.
Rapid Protection in Vivo Is Long-Lived After Immunization with LCMV but Short-Lived After Vaccination with rLM.
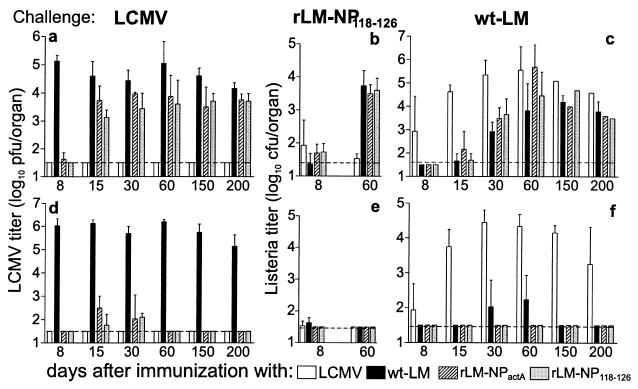
BALB/c mice were immunized either with 200 pfu of LCMV-WE or with 2 × 103 cfu of wt-LM or rLM (rLM-NPactA or rLM-NP118–126). At different time points after immunization, mice were challenged i.v. with 200 pfu of LCMV (Fig. 2). To assess rapid protection by CTL, LCMV titers were determined 36 hours after a challenge infection (Fig. 2a). A second time point 5 days after challenge infection was chosen to assess the protective capacity at a time when reactivation of T cells by the challenge inoculum should have occurred (Fig. 2d).
Figure 2.
In vivo protection against a challenge infection with LCMV, rLM, and wt-LM after vaccination with recombinant Listeria. BALB/c mice were immunized with 200 pfu of LCMV or 2 × 103 cfu of Listeria (wt-LM; rLM-NPactA; rLM-NP118–126). Memory mice were challenged after the time points indicated with 200 pfu of LCMV (a and d), 104 cfu of rLM-NP118–126 (b and e), or104 cfu of wt-LM (c and f) intravenously. The viral and bacterial titers in the spleen and the liver were determined 36 hours (a–c) and 5 days (d–f) later. The horizontal dashed line indicates the detection limit. The mean ± SD of three mice per group is given. Naive mice challenged with 104cfu of Listeria had bacterial titers in the spleen of 6.5 ± 0.1 cfu after 36 hours, 4.7 ± 0.9 cfu at day 5. Only bacterial titers in the spleen are shown in the graph; Listeria titers in the liver were comparable.
Mice immunized with LCMV 8–200 days earlier cleared a challenge LCMV infection within 36 hours. In contrast, mice vaccinated with rLM lost the capacity to be rapidly protected against a LCMV challenge soon after priming (Fig. 2a). However, rLM-immunized mice were able to clear the virus within 5 days of reinfection until day 200 after priming, indicating that increased CTLps were still present at this time point.
We also tested LCMV- and Listeria-immune mice for protection against reinfection with wt-LM. Similarly to the short-lived immediate protection against LCMV, Listeria-immunized mice challenged with wt-LM 30–60 days later did not exhibit protection in the spleen (Fig. 2c) or in the liver (data not shown) assessed 36 hours after the challenge infection. Again, Listeria-immune mice could control reinfection within 5 days (Fig. 2f). The partial protection against Listeria on day 8 after priming with LCMV is probably caused by macrophage activation because of increased cytokine production, including IFN-γ (39, 47), at the peak of the LCMV-specific immune response. The observation that LCMV-primed mice are protected from a challenge infection with rLM-NP118–126 indicates that LCMV-specific CD4+ T cells or neutralizing antibodies do not contribute to this protective response. LCMV-immune mice maintained immediate protection against the rLM 60 days after immunization, whereas Listeria-immune mice were not protected at this time point (Fig. 2b).
These results show that LCMV-induced memory CTLs mediated very rapid clearance of a reinfection with either LCMV or rLM (within 36 hours) for more than 200 days, whereas rLM or wt-LM-induced memory T cells lost the capacity to protect in this assay within 15–30 days. After a certain minimal reactivation time in vivo or in vitro, rLM and wt-LM-vaccinated mice exhibited antiviral or antibacterial protection in vivo, and their T cells efficiently killed target cells in vitro.
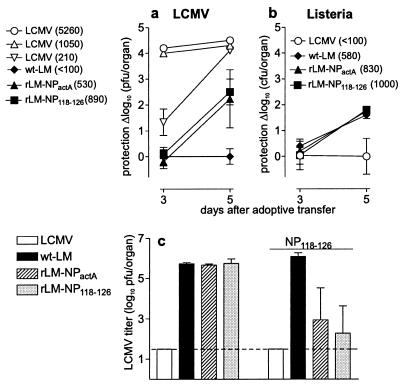
Fewer LCMV-Specific Memory CTLs than Listeria-Specific Memory CTLs Are Needed to Protect Against an Infection.
To exclude the possibility that soluble factors such as neutralizing antibodies or cytokines play a major role in the protection against challenge infections with LCMV or L. monocytogenes and also to compensate for differences in the CTLp frequencies, antiviral and antibacterial protection was studied after adoptive transfer of spleen cells. Antiviral protection was assessed by infection of mice with 200 pfu of LCMV, and 10 hours later, adoptive transfer of spleen cells from immunized mice (Fig. 3a). To assess differences in the elimination kinetics of the challenge infection in correlation with the memory state of the T cell, viral titers were determined 3 and 5 days after infection. The number of transferred specific memory T cells was calculated from the CTLp frequency (Table 1) and the number of adoptively transferred splenocytes. Dilution of memory spleen cells (1:3) was performed (details of several of the titrations not shown), and the dilution that yielded comparable specific CTL numbers is shown in Fig. 3a. Interestingly, a clear difference was found between LCMV-primed memory cells and rLM-primed memory T cells: 890 recombinant Listeria-induced memory cells protected 10,000 times less efficiently than did 1,050 LCMV memory cells (Fig. 3a). This result indicates that besides the quantitative differences in CTLp frequencies induced by LCMV or rLM, there also existed qualitative differences between the memory CTLs.
Figure 3.
Protective capacity of adoptively transferred memory spleen cells. BALB/c mice were immunized with 200 pfu of LCMV (open symbols) or 2 × 103 cfu of Listeria (wt-LM, ♦; rLM-NPactA, ▴; rLM-NP118–126, ■). Sixty days after immunization, NP118–126- or LLO91–99-specific CTLs were adoptively transferred (the number transferred is indicated in parentheses in legend), and protection against an LCMV infection initiated 10 hours before (a) or against a wt Listeria infection initiated 2 hours before (b) was assessed. LCMV and Listeria titers were measured 3 and 5 days after adoptive transfer. The protective capacity of memory cells is given as the difference between the organ titers after adoptive transfer of memory cells of the control group (wt Listeria-immunized mice for LCMV-challenge and LCMV-immunized mice for wt-LM challenge) and after adoptive transfer of memory spleen cells of the four groups tested. Each symbol represents the mean of three mice ± SD. (c) Sixty days after infection, memory mice were immunized with 500 μg of NP118–126 peptide i.v. (four bars at the right side) or left untreated (4 bars on the left side). Twenty-four hours later, all groups were challenged with 200 pfu of LCMV and organ titers were assessed on day 2 after challenge infection. The mean ± SD of three mice per group is given. Experiments were repeated twice, with comparable results.
To characterize Listeria-specific CTL memory in adoptive transfer experiments, recipient BALB/c mice were infected with 104 cfu of wt-LM; 2 hours later, spleen cells from immunized mice were adoptively transferred (Fig. 3b). Comparable to the results obtained from challenge infections directly into the immunized host, adoptively transferred day-60 Listeria memory T cells could not protect within 3 days against a challenge infection. Thus, after vaccination with rLM, the protective capacity of memory T cells against Listeria (Fig. 3b) faded as rapidly as protection against LCMV (Fig. 3a).
Early protection after infection suggested the presence of memory T cells in a rapidly inducible effector state, whereas late protection, after a period that included time for in vivo restimulation and activation, seemed to correlate with elevated CTLp frequencies of resting memory T cells. To test the hypothesis that differences in the effector state and not only different CTLp frequencies were responsible for the observed striking differences in in vivo protection, Listeria memory mice were boosted with 500 μg of NP118–126 peptide i.v. 24 hours before challenge infection with 200 pfu of LCMV (Fig. 3c). As shown previously (44), this protocol cannot prime naive mice and has no measurable impact on the number of LCMV-specific antipeptide CTL (NP118–126) but may allow reactivation of so-called resting memory cells. As shown previously, 60 days after immunization, only LCMV-immune mice were able to clear the virus within 2 days after the challenge infection, and rLM-immune mice revealed high LCMV titers in the spleen (Fig. 3c). After boosting with 500 μg of NP118–126 peptide, but not with 500 μg of the irrelevant peptide GP33–41 (data not shown), rLM-immune mice exhibited potent anti-LCMV protection (2–3.5 log10 pfu reduction) within 2 days.
Only Activated T Cells Can Protect Against a Peripheral Infection.
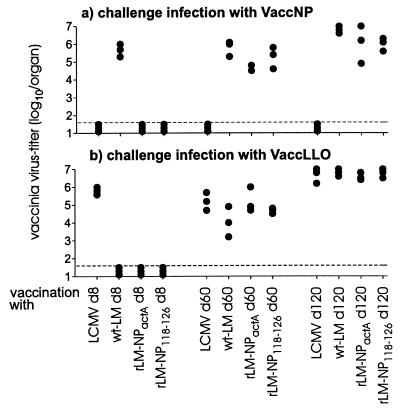
The above experiments indicate a different effector state of memory cells induced after vaccination with LCMV as compared with rLM. To analyze the significance of this in more detail, protection by CTLs specific for the viral (NP118–126) or bacterial (LL091–99) epitope was examined against infection of a peripheral solid organ with the relevant recombinant vaccinia variants. Mice were primed with LCMV or rLM and challenged with recombinant vaccinia viruses that preferentially replicate in the ovaries. This experiment tested CTLs exclusively, because recombinant vaccinia virus does not express the product of the recombinantly expressed protein in the envelope and, therefore, neutralizing antibodies do not interfere with CTLs in this assay. Day 8, 60, and 120 LCMV-and Listeria-immune mice were infected intraperitoneally with 107 pfu of recombinant vaccinia virus expressing the nucleoprotein of LCMV (VaccNP) or expressing VaccLLO91–99 (Fig. 4).
Figure 4.
Protection against a peripheral infection with recombinant vaccinia viruses after immunization with LCMV or recombinant Listeria. BALB/c mice were immunized with 200 pfu of LCMV or 2 × 103 cfu of Listeria (wt-LM; rLM-NPactA; rLM-NP118–126). Eight, sixty, and one hundred and twenty days after primary immunization, mice were challenged with 107 pfu of VaccNP (a) or VaccLL0 (b) i.p., Five days after the challenge infection, vaccinia titers in the ovaries were determined by a plaque-forming assay, and the results of three mice per group are shown. The horizontal dashed line represents the detection limit. One of two similar experiments is shown.
Day 8 LCMV-immune mice, as well as day 60 and day 120 memory mice, were efficiently protected against a peripheral VaccNP infection but not against a control VaccLLO91–99 infection. After immunization with rLM, protection against both VaccNP and VaccLLO91–99 was effective 8 days, but not 60 or 120 days, after the initial vaccination. Listeria-immune mice were only protected against a challenge with VaccLLO91–99 on day 8 but not day 60 or day 120 after vaccination.
To extend these observations, we tested the capacity of rLM-NP118–126 to protect against the CD8+ T cell-mediated lethal immunopathology caused by an intracerebral LCMV infection. Only mice immunized with rLM 5 days, but not 60 or 120 days previously, were protected against an intracerebral challenge infection with 30 pfu of of the neurotropic LCMV-Armstrong virus (data not shown). Thus, only Listeria-specific T cells assessed during the phase of primary activation (that could be called an early memory phase) protected against a peripheral infection. In contrast, LCMV-primed memory mice possess T cells capable of protecting against an intracerebral infection with LCMV or against a challenge infection with recombinant vaccinia virus expressing LCMV-NP up to day 60–120 (Fig. 4).
Different Antigen Persistence After Immunization with LCMV and rLM.
Persistence of the two pathogens in vivo was assessed by injecting the homogenized spleen of LCMV and rLMactA memory mice into IFN-α/β/γ receptor−/− (AG129) mice (Table 2). AG129 mice exhibit uninhibited growth of both LCMV and Listeria: 0.1 pfu of LCMV and 1 cfu of rLMactA grew to high titers in these mice (data not shown). Four days postinjection, LCMV and Listeria titers in the spleen and the liver were assessed. In this very sensitive assay, LCMV could be detected in spleens (other organs were not tested in this assay) 15 days after immunization in all animals tested and 30 days postimmunization in 2 of 4 animals. In the same assay, Listeria had been completely cleared within 8–10 days after infection. Differences in the in vivo persistence of LCMV and Listeria were confirmed indirectly by using a nested reverse transcription–PCR for detection of RNA of the nucleoprotein of LCMV at different time points (48). Nested reverse transcription–PCR specific for LCMV-NP was positive on day 1 after infection with recombinant Listeria but was negative on day 15 or 30 after infection. In contrast, after infection with LCMV, a NP-specific PCR product was detectable 30 days after infection (data not shown). Although the stability of RNA in eukaryotic and prokaryotic cells may not be compared readily (49), together with the results of antigen transfer into AG129 mice, our findings indicate that after an infection with LCMV, antigen production is present over an extended period in vivo, whereas L. monocytogenes is cleared rapidly.
Table 2.
Persistence of the antigen
| Group | Days after immunization
|
|||||
|---|---|---|---|---|---|---|
| 2 | 5 | 8 | 10 | 15 | 30 | |
| LCMV | 5.1 ± 0.3 | 4.9 ± 0.6 | 3.6 ± 0.2 | 2.7 ± 0.6 | <1.5 | <1.5 |
| rLM-NPactA | 4.3 ± 0.2 | 2.7 ± 0.8 | <1.5 | <1.5 | <1.5 | <1.5 |
| LCMV → AG129 | n.d. | n.d. | 4/4 | 4/4 | 4/4 | 2/4 |
| rLM-NPactA → AG129 | n.d. | 4/4 | 2/4 | 0/4 | 0/4 | 0/4 |
Four BALB/c mice per group and time point were immunized with 200 pfu of LCMV or 2 × 103 cfu of rLM-NPactA. Homogenates of the entire spleen of LCMV- and rLM-NPactA-infected animals were injected intraperitoneally into AG129 mice. Values given are bacterial and viral titers in the spleen (log10 or no. positive/no. tested). The organ titer in the spleen was assessed at the indicated time points as described in Material and Methods. The titer is given as log10 of the mean ± SD of four mice tested after direct plaque-forming assay or as mice positive for LCMV or rLM per mice tested after transfer into AG129 mice.
DISCUSSION
LCMV and recombinant Listeria were able to induce and maintain increased CTLp frequencies specific for the immunodominant epitope (NP118–126 as viral epitope, LLO91–99 as bacterial epitope) for up to 300 days. These elevated frequencies of specific CTLs were able to efficiently clear an intravenous LCMV challenge infection within 4–5 days. These findings are consistent with many studies that have shown long-lived increased CD8+ T cell precursors independent of in vivo antigen persistence (2, 3, 50).
Nevertheless, the effectiveness of memory T cells early in a challenge infection differs between viral and bacterial vaccines and also depends on the time after vaccination and when during the challenge infection protection was assessed. After a direct challenge infection in the immunized host or after adoptive transfer of a standardized number of memory CTLs, our study showed that at early time points after challenge, LCMV-induced memory CTLs were more efficiently protective compared with recombinant Listeria-induced memory CTLs. As shown in Fig. 3a, about 1,000 LCMV-specific memory cells (day 60) conferred 104 times greater protection than about 900 LCMV-NP-specific rLM immune T cells. These findings do not necessarily contradict similar studies with the same recombinant Listeria strains to study CTL memory against an i.v. challenge with high-dose LCMV (36), because this high dose and the i.v. challenge route are optimal for very early and efficient activation or reactivation of LCMV-specific CTLp, particularly in the spleen. Therefore, this assay cannot readily reveal differences in the effector state of memory CTLs.
What is the explanation for the obvious differences in effector state of bacterial versus viral memory T cell populations? The underlying mechanisms of T cell memory, and especially its dependence on frequency and/or antigen, are still the subject of an ongoing debate (13, 51). In the present study, we standardized memory CTLp and used adoptive transfer experiments to reveal a clear difference of efficiency of memory CTL isolated from LCMV- versus rLM-immune donors. We therefore conclude that the differences in absolute numbers of CTLp cannot explain the observed differences in protection. The potential role of antigen was substantiated in the following two experiments. First, we showed that rLM-immune mice that were not protected against a LCMV challenge infection could be restimulated with the relevant CTL peptide within 24 hours to express protection at a level comparable to that in LCMV-immune mice (Fig. 3c). Because this short period of restimulation is too short to expand T cells but is sufficient to reactivate primed T cells to become effector T cells (44), this result is compatible with the notion that the presence of stimulatory antigen is necessary and sufficient for the maintenance of protection. Second, persistence of antigen was assessed in spleen homogenates that were injected into IFN-α/β/γ receptor−/− mice. This assay revealed a marked difference in the in vivo persistence of infectious viral and bacterial agents. rLM were detectable only until day 8 after infection. In contrast, LCMV was detected at least until day 30, and with more sensitive assays, LCMV was found for even longer periods (up to 80 days; A.C., P.K., E. Horvath, B. Odermatt, A.F.O., L. Hunziker, H.H., and R.M.Z., unpublished data). The short in vivo persistence of rLM of only 8 days correlated with the short-lived presence of immediately protective memory T cells, whereas the long-term protective capacity in LCMV immune mice correlated with a much longer persistence of LCMV in spleen and potentially in other organs. Memory T cells protective in the short-term antiviral assay or in the assay on protection against LCMV-induced choriomeningitis were still present 60 and even 200 days after LCMV infection. This protection correlates well with the finding of low-level persistence of LCMV. Alternatively, or in addition, LCMV information may persist in DNA form and contribute to long-term protective memory (48).
Taken together, the accumulated data indicate that a quantitative aspect of T cell memory, i.e., CTLp frequency, is long-lived and independent of antigen persistence. The quality of memory T cells, i.e., rapid versus delayed effector state of memory CTLs, correlates with persistence of the antigen. This concept of sustained antigen-driven activation of effector T cells to control low levels of persistent infections, e.g., by Mycobacterium tuberculosis in granulomas, has been called “infection or concomitant immunity” by Mackaness (52).
The present experiments explain and extend earlier studies showing that protective immunity against Listeria is rather short-lived (25, 33) and that repetitive injections with viable Listeria (53) or stimulation of the memory T cells with ConA before adoptive transfer (54) prolonged protective antilisterial T cell memory, whereas immunization with avirulent Listeria with an in vivo persistence of less than 48 hours induced only low levels of protection (55). On the other hand, viral infections that apparently persist at varying levels, such as LCMV, conferred long-lived protection against recombinant Listeria, as shown in the present and an earlier study (56).
The ability of Listeria to gain direct access to the cytosol, its natural adjuvant properties, its susceptibility to antibiotics, and its capacity to induce strong cell-mediated responses led to the development of recombinant Listeria strains as vaccines against LCMV (35, 36), HIV (34), influenza type A (57), and tumors (37, 58). We have shown in the present study that Listeria maintains a pool of memory T cells that cannot act quickly, i.e., cannot rapidly extravasate and protect against a peripheral viral infection. Therefore, the efficacy of rLM to protect against an acute viral challenge infection in peripheral tissues may be limited. In this context, it is worth noting that intravenous challenge infections by most viruses are generally rare (except arthropod-borne viruses) and infection of peripheral cells and solid tissues is usually controlled by activated (and not by quiescent) T cells that are capable of emigrating into infected solid tissues (5, 6, 59). In contrast, intravenously spreading viruses are usually controlled by neutralizing antibodies (60). Even protection against peripheral tumors (nonlymphohemopoietic in origin and location) may be mediated by resting memory T cells only with difficulty if they are not restimulated periodically (61).
Collectively, these and earlier findings indicate that the balance between T cell activation and/or effector state, the time during which activated memory T cells are available, and their frequency, as well as the replication kinetics of the pathogen (virus, bacterium, or tumor) are crucial for protection and for the potential efficiencies of vaccines.
Acknowledgments
This work was supported by the Swiss National Sience Foundation (Grant 31-50900.97 to R.M.Z), the Kanton Zürich, and National Institutes of Health Grant AI-27028 to J.L.W.
ABBREVIATIONS
- rLM
recombinant Listeria monocytogenes
- wt-LM
Listeria strain 10403S
- rLM-NPactA
recombinant Listeria expressing the nucleoprotein of LCMV
- rLM-NP118–126
recombinant Listeria expressing the nucleoprotein (amino acids 118–126) of LCMV
- LCMV
lymphocytic choriomeningitis virus
- CTL
cytotoxic T cell
- CTLp
cytotoxic T cell precursor
- VaccNP
vaccinia virus recombinant for the nucleoprotein of LCMV
- VaccLLO91–99
vaccinia virus recombinant for listeriolysin O (amino acids 91–99)
- NP
nucleoprotein of LCMV
- LLO
liseriolysin O
- wt
wild type
- pfu
plaque-forming unit
- cfu
colony-forming unit
References
- 1.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Nature (London) 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson B D, Ahmed R. J Exp Med. 1989;169:1993–2005. doi: 10.1084/jem.169.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Nature (London) 1994;369:648–652. [Google Scholar]
- 4.Gray D, Matzinger P. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann M F, Kündig T M, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1997;94:640–645. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kündig T M, Bachmann M F, Oehen S, Hoffmann U W, Simard J J L, Kalberer C P, Pircher H, Ohashi P S, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oehen S, Waldner H, Kündig T M, Hengartner H, Zinkernagel R M. J Exp Med. 1992;176:1273–1281. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinkernagel R M, Welsh R M. J Immunol. 1976;117:1495–1502. [PubMed] [Google Scholar]
- 9.Byrne J A, Ahmed R, Oldstone M B. J Immunol. 1984;133:433–439. [PubMed] [Google Scholar]
- 10.Hotchin J. Cold Spring Harbor Symp Quant Biol. 1962;27:479–499. doi: 10.1101/sqb.1962.027.001.046. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen A R, Johansen J, Marker O, Christensen J P. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 12.Planz O, Ehl S, Furrer E, Horvath E, Brundler M A, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kündig T M, Bachmann M F, Ohashi P S, Pircher H, Hengartner H, Zinkernagel R M. Immunol Rev. 1996;150:63–90. doi: 10.1111/j.1600-065x.1996.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 14.Zinkernagel R M, Bachmann M F, Kündig T M, Oehen S, Pircher H, Hengartner H. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 15.Ladel C H, Hess J, Daugelat S, Mombaerts P, Tonegawa S, Kaufmann S H. Eur J Immunol. 1995;25:838–846. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- 16.Rosen H, Gordon S, North R J. J Exp Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bancroft G J, Schreiber R D, Unanue E R. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 18.Conlan J W, North R J. J Exp Med. 1991;174:741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conlan J W, North R J. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R M, Steinmetz M, Bluethmann H. Nature (London) 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 21.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 23.Harty J T, Bevan M J. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 24.Lane F C, Unanue E R. J Exp Med. 1972;135:1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.North R J. J Exp Med. 1973;138:342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop D K, Hinrichs D J. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 27.Mielke M E, Niedobitek G, Stein H, Hahn H. J Exp Med. 1989;170:589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki T, Mieno M, Udono H, Yamaguchi K, Usui T, Hara K, Shiku H, Nakayama E. J Exp Med. 1990;171:1141–1154. doi: 10.1084/jem.171.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kägi D, Ledermann B, Bürki K, Hengartner H, Zinkernagel R M. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- 30.Pamer E G, Harty J T, Bevan M J. Nature (London) 1991;353:852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villanueva M S, Sijts A J, Pamer E G. J Immunol. 1995;155:5227–5233. [PubMed] [Google Scholar]
- 32.Harty J T, Bevan M J. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jungi T W. J Reticuloendothel Soc. 1980;28:405–417. [PubMed] [Google Scholar]
- 34.Frankel F R, Hegde S, Lieberman J, Paterson Y. J Immunol. 1995;155:4775–4782. [PubMed] [Google Scholar]
- 35.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R, Miller J F. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slifka M K, Shen H, Matloubian M, Jensen E R, Miller J F, Ahmed R. J Virol. 1996;70:2902–2910. doi: 10.1128/jvi.70.5.2902-2910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan Z K, Ikonomidis G, Pardoll D, Paterson Y. Cancer Res. 1995;55:4776–4779. [PubMed] [Google Scholar]
- 38.Paterson Y, Ikonomidis G. Curr Opin Immunol. 1996;8:664–669. doi: 10.1016/s0952-7915(96)80083-5. [DOI] [PubMed] [Google Scholar]
- 39.van den Broek M, Muller U, Huang S, Aguet M, Zinkernagel R M. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann-Grube F. Virol Monogr. 1971;10:1–173. [Google Scholar]
- 41.Buchmeier M J, Welsh R M, Dutko F J, Oldstone M B A. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 42.An L L, Pamer E, Whitton J L. Infect Immun. 1996;64:1685–1693. doi: 10.1128/iai.64.5.1685-1693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hany M, Oehen S, Schulz M, Hengartner H, Mackett M, Bishop D H, Overton H, Zinkernagel R M. Eur J Immunol. 1989;19:417–424. doi: 10.1002/eji.1830190302. [DOI] [PubMed] [Google Scholar]
- 44.Ehl S, Klenerman P, Aichele P, Hengartner H, Zinkernagel R M. Eur J Immunol. 1997;27:3404–3413. doi: 10.1002/eji.1830271240. [DOI] [PubMed] [Google Scholar]
- 45.Moskophidis D, Assmann Wischer U, Simon M M, Lehmann-Grube F. Eur J Immunol. 1987;17:937–942. doi: 10.1002/eji.1830170707. [DOI] [PubMed] [Google Scholar]
- 46.Gallimore A, Glithero A, Godkin A, Tissot A C, Plückthun A, Elliott T, Hengartner H, Zinkernagel R M. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kündig T M, Hengartner H, Zinkernagel R M. J Immunol. 1993;150:2316–2321. [PubMed] [Google Scholar]
- 48.Klenerman P, Hengartner H, Zinkernagel R M. Nature (London) 1997;390:298–301. doi: 10.1038/36876. [DOI] [PubMed] [Google Scholar]
- 49.Darnell J, Lodish H, Baltimore D. In: Molecular Cell Biology. Darnell J, Lodish H, Baltimore D, editors. New York: Sci. Am. Books; 1986. pp. 269–302. [Google Scholar]
- 50.Müllbacher A. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sprent J. Curr Opin Immunol. 1997;9:371–379. doi: 10.1016/s0952-7915(97)80084-2. [DOI] [PubMed] [Google Scholar]
- 52.Mackaness G B. Infect Immun. 1964;9:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willers J M, Hofhuis F M, Van-der M C. Immunology. 1982;46:787–792. [PMC free article] [PubMed] [Google Scholar]
- 54.Barry R A, Hinrichs D J. Infect Immun. 1982;35:560–565. doi: 10.1128/iai.35.2.560-565.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldridge J R, Thomashow M F, Hinrichs D J. Infect Immun. 1988;56:2109–2113. doi: 10.1128/iai.56.8.2109-2113.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen H, Miller J F, Fan X, Kolwyck D, Ahmed R, Harty J T. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 57.Ikonomidis G, Portnoy D A, Gerhard W, Paterson Y. Vaccine. 1997;15:433–440. doi: 10.1016/s0264-410x(96)00188-0. [DOI] [PubMed] [Google Scholar]
- 58.Pan Z K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 59.Mackay C R. Immunol Today. 1991;12:189–192. doi: 10.1016/0167-5699(91)90051-T. [DOI] [PubMed] [Google Scholar]
- 60.Zinkernagel R M. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 61.Ochsenbein A F, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]