Abstract
Monocyte chemoattractant protein-1 (MCP-1) is a protective cytokine in murine endotoxaemia induced by lipopolysaccharide (LPS). In this study, LPS-induced pathophysiology in the human (h) MCP-1 transgenic mouse (Tgm) line was investigated. The hMCP-1 Tgm showed a marked increase in the mortality and weight loss following LPS administration. In the Tgm spleens, disappearance of marginal zone macrophages (MZMφ) and dendritic cells (DC) was induced by a smaller amount of LPS than that required for the disappearance in non-transgenic littermates. A significant number of apoptotic cells were seen in these areas. Furthermore, expressions of tumour necrosis factor-α (TNF-α), interleukin-1α (IL-1α), and IL-6 mRNA were enhanced and sustained in the LPS-treated Tgm. Neutralization of TNF-α considerably depressed the LPS-sensitivity of Tgm. These findings demonstrate that the continuous and systemic presence of MCP-1 is no more protective toward endotoxaemia and suggest that the high sensitivity of the MZMφ and DC to LPS is attributed to the enhanced TNF-α production in the hMCP-1 Tgm.
Introduction
Lipopolysaccharide (LPS, endotoxin) is a major component of the outer membrane of Gram-negative bacteria. LPS causes an endotoxin shock, which is associated with coagulopathy and multiorgan dysfunction.1 It is generally considered that the endotoxin shock is induced by numerous pro-inflammatory cytokines including tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1 that are mainly released by macrophages (Mφ).2–5 LPS also induces activation and migration of dendritic cells (DC) and Mφ in the mouse spleen.6–8 Following LPS administration, marginal metallophilic Mφ (MMMφ) and DC migrate from the marginal zone (MZ) into the inside of the white pulp, and marginal zone macrophages (MZMφ) disappear. It has been considered that these MZMφ first encounter and uptake LPS from blood amongst various kinds of spleen cells.9
The migration of DC and Mφ is also influenced by chemokines. It has been reported that MCP-1, a member of the C-C chemokine subfamily, is a protective chemokine expressed in a murine model of endotoxaemia induced by LPS administration.10 However, the mechanism underlying the protective effect of MCP-1 remains elusive.
We have established a human (h) MCP-1 transgenic mouse (Tgm) line that constitutively produces a large amount of hMCP-1 in the serum.11 Although the expression of transgenes was detected in various tissues by reverse transcriptase–polymerase chain reaction (RT–PCR), an accumulation of Mφ was detected only in lymphoid organs. Peritoneal Mφ of the hMCP-1 Tgm possessed depressed phagocytic activities, but showed up-regulated kinase activities of the src-family as compared with those in normal mice. On the other hand, Tgm showed increased migration of Langerhans' cells into the regional lymph nodes and augmented contact hypersensitivity responses, when soluble hapten antigens were applied to the skin.12 These findings suggest that MCP-1 exerts different functional influences on subpopulations of haematopoietic cells, especially Mφ and DC lineages in vivo.
In the present study using the hMCP-1 Tgm, we examined the role of MCP-1 in LPS-induced endotoxin shock. Unexpectedly, these hMCP-1 Tgm showed a high sensitivity to LPS as compared with normal mice. Thus, it was suggested that the constitutive expression of MCP-1 results in the opposite outcome to the beneficial effect of transiently induced MCP-1 in the endotoxin shock model reported earlier.10 The mechanisms underlying the enhanced sensitivity to LPS in our hMCP-1 Tgm model are discussed.
Materials and methods
Mice
C57BL/6 (B6)-hMCP-1 Tgm were produced by backcrossing B6 mice into hMCP-1 Tgm as described.11 B6 mice were purchased from Japan SLC (Hamamatsu, Japan). All experiments were performed on 6-week-old male mice. Mice were maintained under specific pathogen-free conditions. The animal care and experimental procedures conformed to the regulations of Hokkaido University Animal Care and Use Committee.
LPS administration and pretreatment with monoclonal antibodies (mAb)
LPS (Difco Laboratories, Detroit, MI; Escherichia (E. coli) 055:B5) were intraperitoneally injected to mice.13 Body weight (BW) and survival ratio were evaluated subsequently. Three hundred µg of endotoxin-free anti-TNF-α mAb (XT22: Endogen, Woburn, MA), anti-IL-10 mAb (2A5: Endogen) or isotype control rat immunoglobulin G (IgG; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were intravenously injected to mice 30 min before the LPS administration. The dose of mAb was determined with preliminary experiments where a 300 µg/mouse mAb showed the maximum effect.
Immunohistochemistry and flow cytometry
The following anti-mouse mAb were used for immunohistochemistry and flow cytometry: MOMA-1 (anti-sialoadhesin, Serotec, Oxford, UK), ER-TR9 (anti-MZMφ, BMA Biomedicals Ltd, Switzerland), RA3-6B2 (anti-B220, PharMingen, San Diego, CA), G7 (anti-Thy1, PharMingen), and N418 (anti-CD11c, PharMingen). Immunohistochemistry was performed as previously described.14 Briefly, cryosections (5 µm thick) of the spleen were fixed in acetone for 10 min, washed with phosphate-buffered saline (PBS), and incubated with the first antibodies overnight at 4°. After washing with PBS, the sections were incubated with horseradish peroxidase-conjugated rabbit anti-rat immunoglobulin antibody (DAKO, Kyoto, Japan) for 60 min at room temperature. The final staining was performed with Stable DAB™ (KPL, Gaithersburg, MD) according to the manufacturer's protocol. Nuclear staining was performed using haematoxylin. The areas of MZ and MZMφ were analysed using an NIH-image software. Flow cytometry was performed as described elsewhere.15
RT–PCR
Total RNA extracted from the spleen was prepared according to a standard procedure.16 cDNA was synthesized from 10 µg RNA using random hexamer and Moloney murine leukaemia virus reverse transcriptase (SuperScript™, Gibco BRL, Gaithersburg, MD). The pairs of primers of chemokines and cytokines were used with PCR and the sequences were described elsewhere.17–19
Detection of apoptotic cells
Apoptotic cells in the frozen section were detected with ApopTagTM Peroxidase In Situ Oligo Ligation Kit (Intergen Co., Purchase, NY) according to the manufacturer's protocol. Double strand break with short-3′ overhang that is more selective for apoptosis than other types of cell death was labelled with biotinylated oligonuclotides by T4 DNA ligase.20 Labelled DNA was reacted with streptavidin–horseradish peroxidase and detected with diaminobenzidine substrate provided within the kit. Nuclei were stained with methyl green.
Enzyme-linked immunosorbent assay (ELISA)
Serum concentrations of hMCP-1 and murine MCP-1 (mMCP-1) were quantitated by ELISA as previously described elsewhere.11
Statistical analysis
Student's t-test or two-factor factorial analysis of variance (ANOVA) was performed and P-values less than 0·05 were regarded as significant.
Results
HMCP-1 Tgm show high susceptibility to LPS
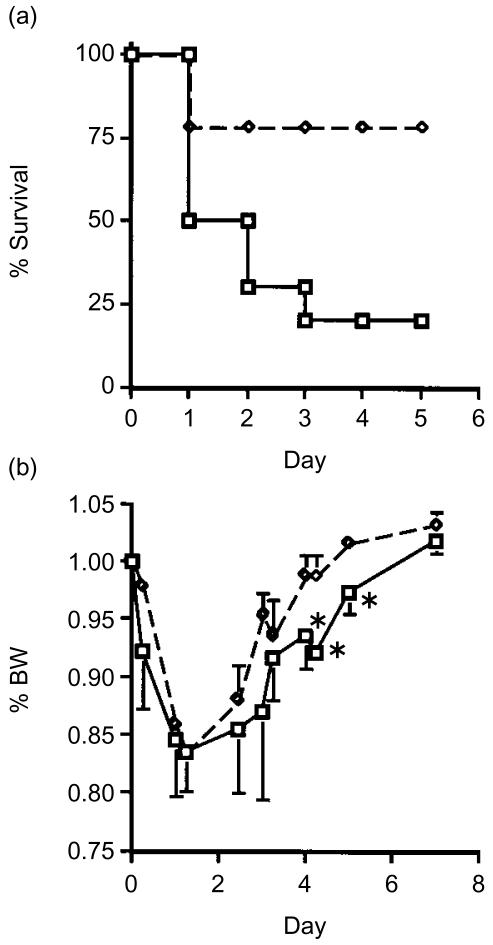
To examine how the constitutive presence of MCP-1 influences the protective role in an endotoxin shock model, we administrated LPS (25 mg/kg) to hMCP-1 Tgm and non-Tgm control mice, and compared the susceptibility to the LPS. As shown in Fig. 1(a), Tgm showed high mortality within 72 hr as compared to control mice.
Figure 1.
Time course of survival ratio and BW change in hMCP-1 Tgm and control mice administrated LPS. LPS, 25 mg/kg (n = 8 per each group) (a) or 5 mg/kg (n = 9 per each group) (b), were administrated intraperitoneally. (a) Survival ratio and (b) BW loss after LPS administration were evaluated in Tgm (square) and non-Tgm (diamond) (*P < 0·05).
We then compared the influence of a low dose (5 mg/kg) LPS on the BW between Tgm and control mice.21 The mean BW of the Tgm group was significantly lower than that of control group from 96 to 120 hr after LPS administration (Fig. 1b). These findings demonstrate that hMCP-1 Tgm show high sensitivity to LPS compared with normal mice.
MZMφ in Tgm spleen show high sensitivity to LPS
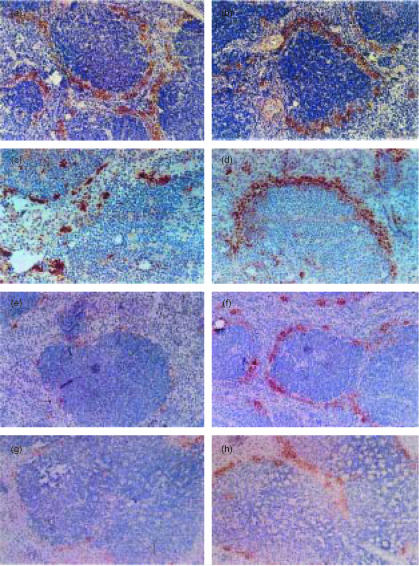
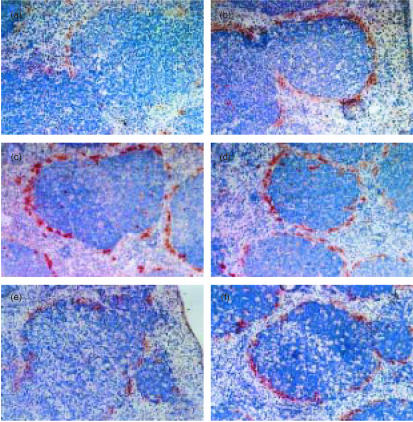
We then examined the distribution of Mφ in the spleen of either Tgm or control mice treated with LPS.22 The frozen sections of Tgm and control spleens were stained with ER-TR9 mAb at different periods after intraperitoneal administration of 5 mg/kg LPS. At 0 hr ER-TR9-positive MZMφ were clearly observed in the MZ of both Tgm and control spleens (Fig. 2a, b). Almost no change was detected at 6 h (data not shown). At 24 hr, a marked disappearance of MZMφ was seen in the Tgm spleen, whereas the distribution of MZMφ showed minimal changes in control mice (Fig. 2c, d). At 48 hr, almost all MZMφ and a significant number of MZMφ disappeared in Tgm and control mice, respectively (Fig. 2e, f). No considerable changes were detected in the distribution pattern of T cells, B cells, and red pulp (Rp) Mφ by immunohistochemistry and flow cytometry (data not shown). When 25 mg/kg LPS was injected to Tgm and littermates, the disappearance of MZMφ was seen in both Tgm and control groups after 24 hr, although a small number of MZMφ remained undeleted in the MZ of control spleens compared to Tgm spleens (Fig. 2g, h). In these spleens it was also noted that Mφ in the white pulp (Wp) underwent marked degeneration.
Figure 2.
Distribution of MZMφ in spleen at different stages after LPS administration. LPS (5 mg/kg (a–f) or 25 mg/kg (g, h)) were administrated intraperitoneally to Tgm and control mice, and these mice were sequentially sacrificed at the following time points, 0 hr (a, b), 24 hr (c, d, g, and h), or 48 hr (e, f). Frozen sections of Tgm (a, c, e, and g) or littermates (b, d, f, and h) were stained with ER-TR9 mAb. The micrographs shown are representative of multiple sections analysed from at least three mice per group. Original magnification is ×100.
These histological findings are morphometrically summarized in Table 1. No significant difference was seen in the area occupied by MZMφ in the splenic MZ between Tgm and control mice before LPS administration (0 hr). The MZMφ area was reduced after LPS administration (5 mg/kg) in both Tgm and non-Tgm. However, the MZMφ areas were significantly smaller in Tgm spleens than those in non-Tgm spleens at 24 and 48 hr. When a high dose (25 mg/kg) of LPS was administered, the reduction rate of MZMφ area in Tgm spleens was again significantly greater than that of control spleens after 24 hr.
Table 1. Proportion of MZMφ-area in the MZ of spleen.
| 5 mg/kg | 25 mg/kg | |||
|---|---|---|---|---|
| LPS Time | 0 hr | 24 hr | 48 hr | 24 hr |
| Tgm (%) | 89·8 ± 4·8 | 52·1 ± 1·9*† | 23·9 ± 2·0*† | 35·2 ± 0·9*† |
| Non-Tgm (%) | 94·4 ± 3·6 | 92·6 ± 1·9 | 63·4 ± 7·8† | 73·6 ± 6·4† |
ER-TR9 positive area was measured from at least 10 sections of the spleen per mouse using NIH Image software. Data represent the mean±SEM for at least three mice per group.
Significantly smaller than non-Tgm (P<0·05).″
Significantly smaller than the group before LPS administration (0 hr) (P<0·05).
MMMφ and DC in Tgm spleens also show high sensitivity to LPS
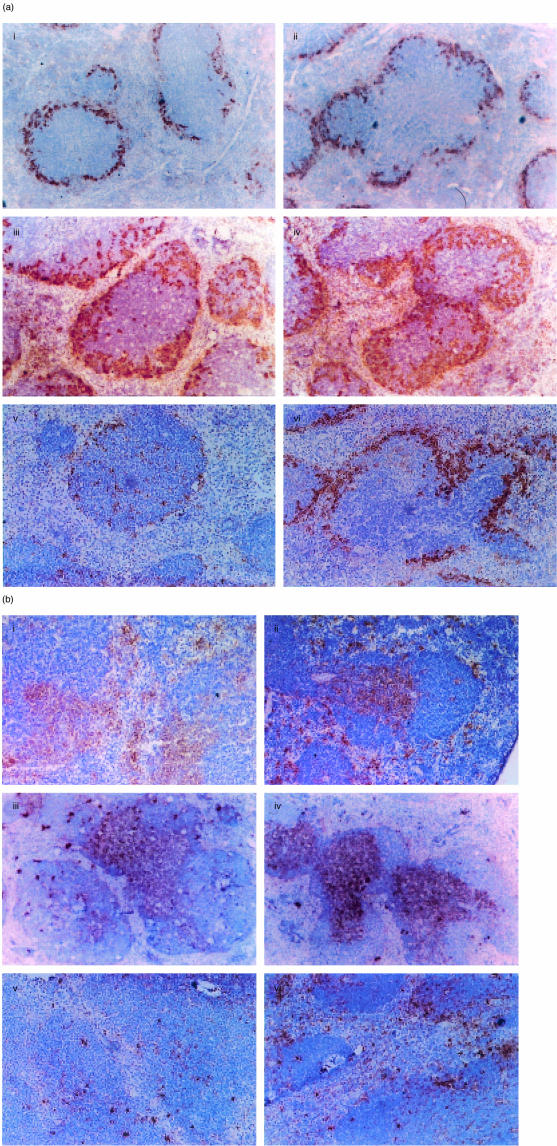
Next, we examined the distribution pattern of MMMφ in spleens of Tgm and control mice. MMMφ were linearly stained with MOMA-1 mAb at the inner surface of the MZ before LPS administration in both Tgm and control spleens (Fig. 3a(i, ii)). These MMMφ started the migration from MZ to follicles 6 hr after LPS administration in Tgm and control mice (data not shown). At 24 hr, a marked migration of MMMφ into the follicles was seen in both Tgm and control mice (Fig. 3a(iii, iv)). However, at 48 hr MMMφ were scarcely seen in the MZ and follicles of Tgm spleen, whereas a considerable number of MMMφ were still present in the control spleen (Fig. 3a(v, vi)).
Figure 3.
Distribution of MMMφ and DC after LPS administration. LPS (5 mg/kg) were administrated, and mice were sequentially killed at the following time points, 0 hr (i, ii), 24 hr (iii, iv), or 48 hr (v, vi). Frozen sections of Tgm (i, iii, and v) or control mice (ii, iv, and vi) were stained with MOMA-1 mAb for MMMφ (a) and N418 for DC (b). The micrographs shown are representative of multiple sections analysed from at least three mice per group. Original magnification is ×100.
We then examined the distribution of DC in spleens of Tgm and control mice (Fig. 3b). CD11c+ DC were present in the MZ and periarteriolar lymphoid sheath (PALS) of both Tgm and control spleens before LPS administration (Fig. 3b(i, ii)). Twenty-four hr after LPS administration, DC could be seen only in the PALS of both Tgm and control spleens (Fig. 3b(iii, iv)). However, at 48 hr DC were scarcely seen in the PALS of Tgm spleen, whereas a small but substantial number of DC were still observed in the control spleen (Fig. 3b(v, vi)). These findings suggest that MMMφ and DC of Tgm are also more sensitive to LPS than those of control mice.
Disappearance of MZMφ from the MZ is attributed to the apoptosis in Tgm spleen
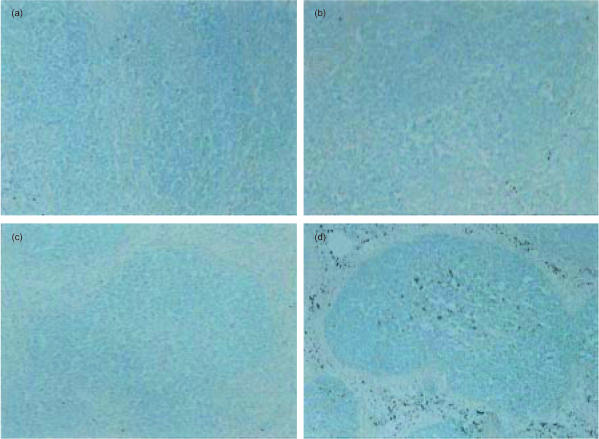
We then examined whether a disappearance of MZMφ of Tgm is attributed to apoptosis or other mechanisms such as a migration of MZMφ or down modulation of MZMφ markers using an in situ oligo labelling technique.20 Apoptotic cells were scarcely seen in spleens of non-Tgm 24 hr after PBS injection (Fig. 4a) or LPS (5 mg/kg) administration (Fig. 4c). The number of signal-positive cells was slightly larger in the spleen of the MCP-1 Tgm injected with PBS (Fig. 4b) than that in the spleen of non-Tgm (Fig. 4a). It should be noted that a large proportion of labelled cells were observed in the spleen of Tgm 24 hr after administration with LPS (Fig. 4d). The signal-positive cells were detected in the MZ and also inside the Wp, which appeared to correspond to MZMφ and MMMφ (and DC), respectively (Fig. 4d).
Figure 4.
Distribution of apoptotic cells in spleens of Tgm and control mice after LPS administration. Twenty-four h after PBS (a, b) or LPS administration (c, d), frozen sections of the spleens of non-Tgm (a, c) and MCP-1 Tgm (b, d) were used for detection of apoptotic cells by an in situ oligo labelling technique. Apoptotic cells were seen as grey to black colour in the representative sections. Nuclei were counter-stained with 0·5% methyl green. Note massive apoptotic cells showing grey to black colour in Tgm spleen (d). Original magnification is ×40.
When mice were intravenously administered 0·5% Indian ink 24 hr after LPS injection, a large number of MZMφ incorporating the carbon particles were detected in the spleen of non-Tgm but not in that of Tgm (data not shown). These findings on the whole suggest that the disappearance of MZMφ in Tgm spleens is attributed to the apoptosis and probably not to the migration.
MRNA expressions of pro-inflammatory cytokines and chemokines are augmented in LPS-administrated Tgm
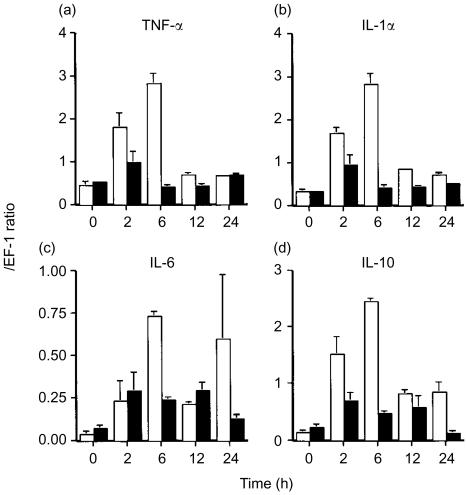
We then analysed sequentially the mRNA expressions of various cytokines in spleens of Tgm and control mice administrated a low dose of LPS (5 mg/kg). In control mice, the expressions of TNF-α and IL-1α messages showed the peaks at 2 hr and were promptly reduced to the baseline levels before LPS were injected (Fig. 5a, b). The expression of IL-6 mRNA lasted for a slightly longer time (Fig. 5c). On the other hand, in Tgm the peak expressions of TNF-α, IL-1α, and IL-6 messages were detected at 6 hr, and these expression levels were considerably larger than those of normal mice. Interestingly, the mRNA expression of IL-10, a suppressive cytokine to the endotoxin shock, was also enhanced in Tgm compared with that in control mice (Fig. 5d). No difference was observed in expressions of other cytokines, IL-2, 4, 5, and 12, interferon-γ, and transforming growth factor-β between Tgm and control mice (data not shown).
Figure 5.
mRNA expressions of various cytokines in spleens of Tgm and control mice following LPS administration. Time course of mRNA expressions of cytokines in spleens of Tgm (open column) and control mice (closed column) that received 5 mg/kg LPS is shown. Expression of TNF-α (a), IL-1α (b), IL-6 (c), and IL-10 (d) mRNA were semiquantitatively measured by RT–PCR and results are expressed in arbitrary densitometry units that were normalized for levels of EF-1 expression. Data represent the mean±SEM for four mice per group.
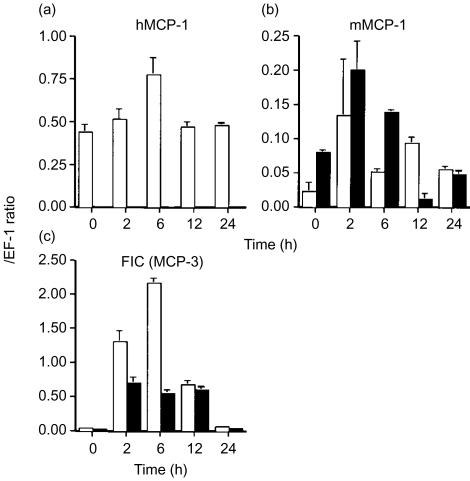
Next we examined mRNA expressions of various chemokines. In Tgm spleens constitutive expressions of hMCP-1 mRNA were observed during the entire period with a peak at 6 hr after LPS administration (Fig. 6a). No hMCP-1 mRNA was detected in control mice. On the other hand, in either Tgm or control mice mRNA expressions of intrinsic mMCP-1 were observed and these expressions showed the peaks at 2 hr after LPS administration (Fig. 6b). The expression levels of mMCP-1 were generally higher in control mice than those in Tgm. Serum concentrations of both hMCP-1 and mMCP-1 showed similar time course when measured by ELISA (data not shown). The expression of FIC (MCP-3) increased from 2 to 12 hr after LPS administration in both Tgm and control mice (Fig. 6c). However, the peak expression of FIC mRNA was enhanced in Tgm as compared with that in control mice. No difference was observed in expressions of other chemokines examined, MIP-1α and RANTES, between Tgm and control mice (data not shown).
Figure 6.
mRNA expressions of various chemokines in spleens of Tgm and control mice following LPS administration. Tgm and control mice were administrated 5 mg/kg LPS. Time course of expressions of hMCP-1 (a), mMCP-1 (b), and FIC/MCP-3 (c) mRNA that were measured by RT–PCR in spleens of Tgm (open column) and control mice (closed column) is shown. Results are expressed in arbitrary densitometry units that were normalized for levels of EF-1 expression. Data represent the mean±SEM for four mice per group.
Neutralization of TNF-α inhibits MZMφ disappearance in Tgm spleen
To directly examine the role of cytokines in LPS-induced disappearance of Mφ-subsets in the spleen, anti-TNF-α or anti-IL-10 mAb was administrated to Tgm and control mice before LPS administration (25 mg/kg). Neutralization of TNF-α resulted in a marked improvement of survival rate and clinical symptoms induced by LPS administration (three out of three mice (100%) survived at 24 hr in anti-TNF-α-treated Tgm, and three out of six mice (50%) survived in isotype-matched immunoglobulin-treated Tgm 24 hr after LPS administration).
Twenty-four hr after administration of a low dose of LPS (5 mg/kg) to mice that had been given control antibodies, a marked disappearance of MZMφ was detected in Tgm spleens as compared to that in normal mice (Fig. 7a, b). This result is consistent with that shown in Fig. 2(c, d). On the other hand, the neutralization of TNF-α resulted in a considerable prevention of the disappearance of MZMφ in the Tgm spleens (Fig. 7c). The same treatment showed minimal effects in LPS-treated control mice (Fig. 7d).
Figure 7.
Influence of anti-cytokine mAb administration on distribution of MZMφ in spleens after LPS administration. LPS (5 mg/kg) were administrated intraperitoneally 30 min after intravenous injection of 300 µg of following Ab: rat control IgG1 (a, b), anti-mouse TNF-α mAb (c, d), and anti-mouse IL-10 mAb (e, f). Mice were killed 24 hr after LPS administration. Frozen sections of Tgm (a, c, and e) or control mice (b, d, and f) were stained with ER-TR9 mAb. The micrographs shown are representative of multiple sections analysed from at least three mice per group. Original magnification is ×100.
When the neutralizing antibody against IL-10 was administrated to LPS (5 mg/kg)-treated mice, a considerable degeneration of Mφ was detected in the Wp of both Tgm and control spleens (Fig. 7e, f). This marked degeneration of the Wp Mφ seemed quite similar to that seen in mice administrated a high dose of LPS (see Fig. 2g, h). By contrast, the distribution of MZMφ was not significantly influenced by anti-IL-10 mAb (Fig. 7e). Administration of either anti-TNF-α mAb or anti-IL-10 mAb exerted no significant influences on the distribution patterns of MMMφ and DC (data not shown).
These histological findings are summarized in Table 2. As already demonstrated in Fig. 7, a marked reduction of the MZMφ area was observed 24 hr after LPS administration in Tgm treated with isotype control antibody compared to that in non-Tgm. By contrast, when Tgm were pretreated with anti-TNF-α mAb, the reduction rate was significantly decreased and no difference was detected between these Tgm and control mice administrated anti-TNF-α mAb (Table 2). Anti-IL-10 mAb showed no effect on the reduction rate of MZMφ area.
Table 2. Proportion of MZMφ-area in the splenic MZ of mice pretreated with various antibodies.
| 5 mg/kg 24hr | |||
|---|---|---|---|
| LPS Time Antibody | Isotype | anti-TNF-α | anti-IL-10 |
| Tgm (%) | 36·1 ± 12·9* | 70·7 ± 2·9† | 36·8 ± 1·1* |
| Non-Tgm (%) | 82·5 ± 1·4 | 76·1 ± 3·0 | 71·7 ± 5·9 |
ER-TR9 positive area was measured from at least 10 sections of the spleen per mouse using NIH Image Software. Data represent the mean±SEM for three mice per group.
Significantly smaller than non-Tgm (P<0·05).
Significantly larger than isotype-control Tgm group (P<0·05).
Discussion
When a body is exposed to release of LPS in the circulation, tissue Mφ secrete various cytokines and chemokines. It has been proposed that TNF-α secretion by these cells is a primary event, followed by production of IL-6, IL-8, and IL-1β, which lead to subsequent pathophysiology by the LPS.23 The cytotoxic effects of TNF-α are represented by inhibition of RNA or protein synthesis in most cell types.24 TNF-α also induces DNA fragmentation and cell death by apoptosis. Recently, it has been reported that the LPS-induced production of serum TNF-α results in apoptosis of a Mφ population, and the subsequent severe liver injury in mice.25,26
In the present study, we demonstrated that hMCP-1 Tgm showed a high sensitivity to LPS as compared to control mice. Furthermore, these LPS-treated Tgm showed larger numbers of granulocytes and macrophages in the liver than that in normal mice (data not shown). We also found that splenic MZMφ in Tgm underwent apoptosis by a lower dose of LPS than that required in normal mice. In these LPS-treated Tgm spleens, significantly augmented expressions of TNF-α mRNA were detected. Pretreatment of Tgm with anti-TNF-α mAb improved the survival rate with a concomitant prevention of the apoptosis of MZMφ. On the other hand, no difference was observed in the production of nitric oxide (NO) from splenic and peritoneal Mφ between LPS-administrated Tgm and control mice (data not shown). Thus, we consider that TNF-α is mainly responsible for the high sensitivity of our hMCP-1 Tgm to LPS. The high sensitivity of the Tgm Mφ to LPS may be related to our previous report that Mφ of hMCP-1 Tgm showed up-regulated kinase activities of the src-family.11 This relationship between the high LPS sensitivity and enhanced activities of the src-family appears to be consistent with a report of Lowell et al.27
The MZ surrounding Wp of the spleen forms an intriguing area in which a variety of cell types are present.28 MZMφ are highly phagocytotic cells distributed throughout the MZ, and uptake neutral polysaccharides such as LPS.29 We showed here that MZMφ were primarily influenced by LPS treatment and underwent apoptosis in hMCP-1 Tgm with a lower dose LPS than that inducing disappearance of MZMφ in normal mice. The disappearance of MZMφ was also evidenced by the lack of cells phagocytosing Indian ink in the MZ. In hMCP-1 Tgm, an accumulation of Mφ was seen in Wp of the spleen.11 However, we found a similar distribution pattern of MMMφ, MZMφ, and DC in Tgm and control spleens in a resting condition. Treatment of these mice with LPS led to a prompt migration of DC to the PALS in both Tgm and control spleens. MMMφ also showed a prompt migration to the follicle following LPS administration. These migration patterns were not significantly altered in the presence of anti-TNF-α mAb (data not shown). At 48 hr after LPS treatment, however, almost complete disappearance of DC and MMMφ was seen only in Tgm spleens but not in control spleens. These findings suggest that TNF-α plays a limited role in the migration of DC and MMMφ compared to that of MZMφ. Nevertheless, the augmented expression of TNF-α in hMCP-1 Tgm spleen appeared to induce certain levels of activation-induced cell death in DC and MMMφ as demonstrated in MZMφ. As for the direct influence of MCP-1, it has been reported that MCP-1 induces migration of monocytes but not that of mature Mφ.30 It seems to us that the influence of a large amount of hMCP-1 in Tgm is minimal as far as migrations of MMMφ and DC are concerned.
Members of the chemokine family show potent pro-inflammatory functions in several murine inflammation models, including endotoxaemia.12,31 However, Zisman et al.10 demonstrated that MCP-1 played a protective role in mice receiving endotoxin challenge by enhancing the expression of IL-10 but inhibiting those of IL-12 and TNF-α.10 In our hMCP-1 Tgm spleen, however, larger amounts of both IL-10 and TNF-α mRNA than those in control spleens were induced after LPS administration. Nevertheless, the hMCP-1 Tgm showed a high sensitivity to LPS, suggesting that the function of TNF-α was dominant over that of IL-10 in our LPS-administrated Tgm system. We consider that the transient but not constitutive expression of MCP-1 after the LPS-administration seems critical for the MCP-1 to function as an anti-inflammatory chemokine.10 The mechanism underlying the opposite functions of the transiently expressed MCP-1 and constitutively expressed MCP-1 in the endotoxin shock, especially in induction or suppression of these cytokines, should be elucidated in further studies.
A mammalian Toll-like receptor-4 (TLR-4) primary functions in the recognition of LPS and transduces the LPS signal in mice.32 In addition, it was shown that LPS treatment led to strong and transient suppression of TLR-4 mRNA in RAW264.7 cells33 but not in P338D1 cells.34 It remains to be examined whether TLR-4 expression is increased or TLR-4 related signals are enhanced in our hMCP-1 Tgm in the context of LPS stimulation.
Acknowledgments
We wish to thank Ms Kaori Kohno for her secretarial assistance in preparation of this manuscript. This study was supported in part by a Grant-in Aid for Scientific Research (B, C) by the Ministry of Education, Science, Sports and Culture, Japan. This study was also supported in parts by Grants from The Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Hokkaido Foundation for the Promotion of Scientific and Industrial Technology, The Tomakomai East Hospital Foundation, and The Nishimura Aging Fund.
Glossary
Abbreviation
- BW
body weight
- DC
dendritic cell(s)
- IL
interleukin
- LPS
lipopolysaccharide
- mAb
monoclonal antibody(s)
- MCP
monocyte chemoattractant protein
- Mφ
macrophage(s)
- MM
marginal metallophile
- MZ
marginal zone
- NO
nitric oxide
- PALS
periarteriolar lymphoid sheath
- Rp
red pulp
- RT
reverse transcription
- Tgm
transgenic mouse (mice)
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
- Wp
white pulp
References
- 1.Astiz ME, Rackow EC. Septic shock. Lancet. 1998;351:1501–5. doi: 10.1016/S0140-6736(98)01134-9. [DOI] [PubMed] [Google Scholar]
- 2.Remick DG, Strieter RM, Eskandari MK, Nguyen DT, Genord MA, Raiford CL, Kunkel SL. Role of tumor necrosis factor-α in lipopolysaccharide-induced pathologic alterations. Am J Pathol. 1990;136:49–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Okusawa S, Gelfand JA, Ikejima T, Connolly RJ, Dinarello CA. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988;81:1162–72. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Peterson JW, Niesel DW, Klimpel GR. Bacterial lipoprotein and lipopolysaccharide act synergistically to induce lethal shock and proinflammatory cytokine production. J Immunol. 1997;159:4868–78. [PubMed] [Google Scholar]
- 5.Salkowski CA, Neta R, Wynn TA, Strassmann G, van Rooijen N, Vogel SN. Effect of liposome-mediated macrophage depletion on LPS-induced cytokine gene expression and radioprotection. J Immunol. 1995;155:3168–79. [PubMed] [Google Scholar]
- 6.De Smedt T, Pajak B, Muraille E, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharidein vivo. J Exp Med. 1996;184:1413–24. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sousa CR, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–29. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groeneveld PH, van Rooijen N, Eikelenboom P. In vivo effects of lipopolysaccharide on lymphoid and non-lymphoid cells in the mouse spleen. Migration of marginal metallophils towards the follicle centres. Cell Tissue Res. 1983;234:201–8. doi: 10.1007/BF00217413. [DOI] [PubMed] [Google Scholar]
- 9.Groeneveld PH, Erich T, Kraal G. The differential effects of bacterial lipopolysaccharide (LPS) on splenic non-lymphoid cells demonstrated by monoclonal antibodies. Immunology. 1986;58:285–90. [PMC free article] [PubMed] [Google Scholar]
- 10.Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J, Standiford TJ. MCP-1 protects mice in lethal endotoxemia. J Clin Invest. 1997;99:2832–6. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ato M, Iwabuchi K, Matsuki N, et al. Delayed clearance of zymosan-induced granuloma and depressed phagocytosis of macrophages with concomitant up-regulated kinase activities of Src-family in a human monocyte chemoattractant protein-1 transgenic mouse. Immunobiology. 2000;201:432–49. doi: 10.1016/s0171-2985(00)80096-0. [DOI] [PubMed] [Google Scholar]
- 12.Mizumoto N, Iwabuchi K, Nakamura H, et al. Enhanced contact hypersensitivity in human monocyte chemoattractant protein-1 transgenic mouse. Immunobiology. 2001;204:409–25. doi: 10.1078/0171-2985-00057. [DOI] [PubMed] [Google Scholar]
- 13.Chvatchko Y, Hoogewerf AJ, Meyer A, et al. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–64. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatakeyama S, Iwabuchi K, Ato M, et al. Fgr expression restricted to subpopulation of monocyte/macrophage lineage in resting conditions is induced in various hematopoietic cells after activation or transformation. Microbiol Immunol. 1996;40:223–31. doi: 10.1111/j.1348-0421.1996.tb03338.x. [DOI] [PubMed] [Google Scholar]
- 15.Iwabuchi C, Iwabuchi K, Nakagawa K, et al. Intrathymic selection of NK1.1+α/β T cell antigen receptor (TCR)+ cells in transgenic mice bearing TCR specific for chicken ovalbumin and restricted to I-Ad. Proc Natl Acad Sci USA. 1998;95:8199–204. doi: 10.1073/pnas.95.14.8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwabuchi K, Hatakeyama S, Takahashi A, et al. Csk overexpression reduces several monokines and nitric oxide productions but enhances prostaglandin E2 production in response to lipopolysaccharide in the macrophage cell line J774A. 1. Eur J Immunol. 1997;27:742–9. doi: 10.1002/eji.1830270324. [DOI] [PubMed] [Google Scholar]
- 17.Murray LJ, Lee R, Martens C. In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur J Immunol. 1990;20:163–70. doi: 10.1002/eji.1830200124. [DOI] [PubMed] [Google Scholar]
- 18.Mori K, Kobayashi S, Inobe M, Jia WY, Tamakoshi M, Miyazaki T, Uede T. In vivo cytokine gene expression in various T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Autoimmunity. 1994;17:49–57. doi: 10.3109/08916939409014658. [DOI] [PubMed] [Google Scholar]
- 19.Stafford S, Li H, Forsythe PA, Ryan M, Bravo R, Alam R. Monocyte chemotactic protein-3 (MCP-3)/fibroblast-induced cytokine (FIC) in eosinophilic inflammation of the airways and the inhibitory effects of an anti-MCP-3/FIC antibody. J Immunol. 1997;158:4953–60. [PubMed] [Google Scholar]
- 20.Smith S, Cartwright M. Spatial visualization of apoptosis using a whole-mountin situ DNA labeling techniques. Biotechniques. 1997;22:832–4. doi: 10.2144/97225bm08. [DOI] [PubMed] [Google Scholar]
- 21.Leon LR, Kozak W, Peschon J, Kluger MJ. Exacerbated febrile responses to LPS, but not turpentine, in TNF double receptor-knockout mice. Am J Physiol. 1997;272:R563–9. doi: 10.1152/ajpregu.1997.272.2.R563. [DOI] [PubMed] [Google Scholar]
- 22.Groeneveld PH, van Rooijen N. Localization of intravenously injected lipopolysaccharide (LPS) in the spleen of the mouse. An immunoperoxidase and histochemical study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;48:237–45. doi: 10.1007/BF02890132. [DOI] [PubMed] [Google Scholar]
- 23.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–7. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 24.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–4. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 25.Xaus J, Comalada M, Valledor AF, Lloberas J, Lopez-Soriano F, Argiles JM, Bogdan C, Celada A. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood. 2000;95:3823–31. [PubMed] [Google Scholar]
- 26.Ogata M, Yoshida S, Kamochi M, Shigematsu A, Mizuguchi Y. Enhancement of lipopolysaccharide-induced tumor necrosis factor production in mice by carrageenan pretreatment. Infect Immun. 1991;59:679–83. doi: 10.1128/iai.59.2.679-683.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowell CA, Berton G. Resistance to endotoxic shock and reduced neutrophil migration in mice deficient for the Src-family kinases Hck and Fgr. Proc Natl Acad Sci USA. 1998;95:7580–4. doi: 10.1073/pnas.95.13.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:331–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 29.Humphrey JH, Grennan D. Different macrophage populations distinguished by means of fluorescent polysaccharides. Recognition and properties of marginal-zone macrophages. Eur J Immunol. 1981;11:221–8. doi: 10.1002/eji.1830110311. [DOI] [PubMed] [Google Scholar]
- 30.Audran R, Lesimple T, Delamaire M, Picot C, Van Damme J, Toujas L. Adhesion molecule expression and response to chemotactic agents of human monocyte-derived macrophages. Clin Exp Immunol. 1996;103:155–60. doi: 10.1046/j.1365-2249.1996.d01-4.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanOtteren GM, Strieter RM, Kunkel SL, Paine R, 3rd, Greenberger MJ, Danforth JM, Burdick MD, Standiford TJ. Compartmentalized expression of RANTES in a murine model of endotoxemia. J Immunol. 1995;154:1900–8. [PubMed] [Google Scholar]
- 32.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–6. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 33.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 34.Fujihara M, Wakamoto S, Ito T, Muroi M, Suzuki T, Ikeda H, Ikebuchi K. Lipopolysaccharide-triggered desensitization of TNF-α mRNA expression involves lack of phosphorylation of IκBα in a murine macrophage-like cell line, P388D1. J Leukoc Biol. 2000;68:267–76. [PubMed] [Google Scholar]