Abstract
Immune stimulating complexes (ISCOMs) containing the saponin adjuvant Quil A are vaccine adjuvants that promote a wide range of immune responses in vivo, including delayed-type hypersensitivity (DTH) and the secretion of both T helper 1 (Th1) and Th2 cytokines. However, the antigen-presenting cell (APC) responsible for the induction of these responses has not been characterized. Here we have investigated the role of dendritic cells (DC), macrophages (Mφ) and B cells in the priming of antigen-specific CD4+ T cells in vitro by ISCOMs containing ovalbumin (OVA). OVA ISCOMs pulsed bone marrow (BM)-derived DC but not BM Mφ, nor naïve B cells prime resting antigen-specific CD4+ T cells, and this response is greatly enhanced if DC are activated with lipopolysaccharide (LPS). Of the APC found in the spleen, only DC had the capacity to prime resting antigen specific CD4+ T cells following exposure to OVA ISCOMs in vitro, while Mφ and B cells were ineffective. DC, but not B cells purified from the draining lymph nodes of mice immunized with OVA ISCOMs also primed resting antigen-specific CD4+ T cells in vitro, suggesting that DC are also critical in vivo. Using DC and T cells from interleukin (IL)-12 p40−/− mice, we also identified a crucial role for IL-12 in the priming of optimal CD4+ T cell responses by OVA ISCOMs. We suggest that DC are the principal APC responsible for the priming of CD4+ T cells by ISCOMs in vivo and that directed targeting of these vectors to DC may enhance their efficancy as vaccine adjuvants.
Introduction
An important goal of vaccine research is to develop a single-dose, orally administered, recombinant vaccine that induces long-lasting mucosal and systemic immunity.1 As recombinant proteins are poorly immunogenic, particularly when given by mucosal routes, their use in vaccines requires coadministration of an adjuvant.2 A number of mucosal vaccine adjuvants have been described, including live vectors such as attenuated Salmonella and non-viable agents such as cholera toxin or the heat-labile enterotoxin of Escherichia coli (LT).3 Our approach has been to use immune-stimulating complexes (ISCOMs) containing the saponin adjuvant Quil A. ISCOMs are rigid cage-like structures that form spontaneously when mixing cholesterol, phosphatidylcholine, and Quil A.4 We and others have shown that proteins incorporated in ISCOMs become highly immunogenic in vivo, inducing a wide range of immune effector responses, including delayed-type hypersensitivity (DTH), class I major histocompatibility complex (MHC)-restricted cytotoxic T lymphocyte activity, serum antibody production, T-cell proliferation, and secretion of both T helper 1 (Th1)- and Th2-dependent cytokines.5–10 Of great importance, ISCOMs are effective by most parenteral and mucosal routes and therefore it would be useful to investigate their modes of action, with the aim of exploiting these for improved efficacy.
It is now appreciated that one of the most important properties of an adjuvant is how it interacts with professional antigen-presenting cells (APC), both in the way it is processed and presented and its ability to induce costimulatory molecules and inflammatory cytokines.11 A number of different APC types have been implicated in presentation of antigen to T cells, including dendritic cells (DC), macrophages (Mφ) and B cells. However, DC are recognized to be the most potent APC population for priming both CD4+ and CD8+ T cells in vivo.12 As a result, targeting and activating DC is one of the most promising strategies for developing vaccine related adjuvants.
In previous work, we showed that ISCOMs recruit DC to inflammatory sites in vivo13 and expansion of DC numbers with flt3 ligand (flt3L) enhances antigen specific CD4+ and CD8+ T-cell responses in vivo.14 In addition, ISCOM-associated antigen is presented very efficiently to class I restricted CD8+ T cells in vitro by DC.15 As priming of CD4+ T cells is central to the induction of most immune responses in vivo, here we extended our previous studies by examining the role of DC in presenting ISCOM-associated antigen to CD4+ T cells in vivo and in vitro. In addition, we have compared DC with B cells and Mφ. As ISCOMs induce strong Th1 responses in vivo and their immunogenicity depends partly on an interleukin (IL)-12-dependent cascade of innate immune responses13 we also investigated the role of IL-12 in the presentation of ISCOMs to CD4+ T cells. Our results show that of the APC examined, only DC prime antigen specific CD4+ T cells in vitro in response to ovalbumin (OVA) ISCOMs and suggest that IL-12 is of central importance in this process.
Materials and methods
Mice
Female BALB/c (H-2d) mice were obtained from Harlan Olac (Bicester, UK). DO11.10 mice transgenic for a T-cell receptor specific for OVA 323-339++I-Ad obtained originally from Dr Nils Lycke and IL-12 p40−/− mice on the BALB/c background obtained from Dr J Magram, Roche Pharmaceuticals (Nutley, NJ), were bred and maintained in Central Research Facility (CRF). DO11.10 IL-12 p40−/− mice were generated by back-crossing IL-12 p40−/− and DO11.10 mice. All mice were first used from 8 weeks of age.
Antigens
Native OVA (Sigma Fraction V) or the synthetic OVA peptide 323–339 were obtained from Sigma Genosys (Cambridge, UK).
Preparation of OVA ISCOMs
ISCOMs containing palmitified OVA (Sigma, Poole, UK) were prepared using phosphatidylcholine, cholesterol and Quil A (obtained from Dr K Lövgren-Bengtsson, Department of Virology, Biomedicum, Uppsala, Sweden) as described previously.5 The integrity of the OVA ISCOMs was confirmed by electron microscopy, and the protein content was determined by the Bradford reaction (Bio-Rad, Hemel Hempstead, UK). The ISCOMs used in this study contained OVA and Quil A at a ratio of 10 : 1 and were approximately 40 nm in diameter. ISCOMs preparations were prepared with pyrogen-free reagents wherever possible and as published previously13 quantification of endotoxin using the E-Toxate Reagent Kit (Sigma) showed levels of <0·5 µg/ml. For the majority of experiments in this study this is equivalent to <5 ng/ml endotoxin being present in the in vitro assays. All OVA ISCOMs concentrations mentioned in the text refer to OVA protein content.
Expanding DC numbers in vivo
To expand DC numbers in vivo, BALB/c mice were injected intraperitoneally with 10 µg of flt 3 ligand (flt3L, a kind gift from Immunex Corporation, Seattle, WA) daily for 8 days.
Preparation of spleen and lymph node cells
Spleen and lymph nodes were harvested from DO11.10 or DO11.10 IL-12 p40−/− mice and single cell suspensions prepared in sterile RPMI-1640 medium (Gibco BRL, Paisley, UK) by homogenization through Nitex mesh (Cadisch, London, UK). After centrifugation at 400 g for 5 min, the cells were resuspended and viable cell counts were performed using phase contrast microscopy.
Bone-marrow (BM)-derived DC
BM DC were obtained as described previously.15 Briefly, BM cells were cultured in RPMI-1640 containing 2 mm l-glutamine, 100 µg/ml penicillin, 100 µg/ml streptomycin, 1·25 µg/ml fungizone (all from Gibco BRL) and 10% fetal calf serum (FCS) (Harlan Sera Laboratories, Crawley Down, UK) (complete medium) at 37° in 5% CO2 together with 10% supernatant from the X-63 fibroblast cell line transfected with the murine granulocyte–macrophage colony-stimulating factor (GM-CSF) gene.16
Mφ were obtained by culturing bone marrow cells in complete medium supplemented with 2% sodium pyruvate (Gibco BRL) and 15% FCS at 37° in 5% CO2 together with 20% M-CSF containing supernatant from the L-929 fibroblast cell line (a kind gift from Dr James Brewer from this department).
Purification of APC from lymphoid tissues
Naïve B cells were purified from the spleens or lymph nodes of BALB/c mice by negative selection using magnetic-activated cell sorting (MACS). Briefly, after preparation of single cell suspensions, red blood cells were lysed by addition of 5 ml NH4Cl (0·14 m) for 5 min at room temperature and the remaining cells were washed in MACS medium (phosphate-buffered saline (PBS) + 2% FCS), before being resuspended in MACS medium and counted. Following centrifugation, the cells were resuspended at a concentration of 1 × 107 cells/100 µl MACS medium, and 10 µl/107 cells anti-CD43 coated microbeads (Miltenyi Biotec, Towcester, UK) were added for 15 min at 4°. The labelled cells were then passed over a CS MACS column according to the manufacturers instructions (Miltenyi Biotec). Eluted cells were >96% B220+ as assessed by flow cytometry.
For purification of DC, single cell suspensions of LN cells were prepared in MACS medium and 5 µg biotinylated anti-CD11c (PharMingen, Oxford, UK)/107 cells was added for 30 min at 4°. After washing in MACS buffer, the cells were incubated with 10 µl streptavidin-coated microbeads (Miltenyi Biotec)/107 cells for 15 min at 4°, washed and then passed over a mass spectoscopy (MS) MACS column (Miltenyi Biotec) according to the manufacturer's instructions. Eluted cells were repeatedly passed over fresh columns until the eluent contained >85% CD11c+ cells as assessed by flow cytometry.
To determine the APC activity of individual populations in the spleen, 2 × 108 spleen cells in 20 ml complete medium were pulsed with 20 µg/ml OVA ISCOMs for 45 min at 37°. After washing, red blood cells were removed and CD11c+ and B cells purified as described above. To obtain Mφ-enriched cells, the remaining CD11c negative, B-220 negative cells were plated in 90 cm Petri dishes (Bibby Sterilin, Stone, UK) in 10 ml complete medium for 4 hr at 37°. Non-adherent cells were removed by repeated washing with complete medium and the adherent cells harvested by the addition of cold complete medium and scraping. The adherent cell population was >80% F4-80+.
Assessment of APC activity
APC activity was assessed in vitro as described in detail elsewhere.15 Briefly, 4 × 106 APC were pulsed with OVA ISCOMs, OVA protein or OVA peptide for 2 hr at 37° in a total volume of 1 ml, treated with 50 µg/ml mitomycin C and washed four times in RPMI-1640. In some assays, 10 µg/ml lipopolysaccharide (LPS; Escherichia coli serotype 026:B6, Sigma) or LPS + 80 ng/ml recombinant mouse interferon-γ (IFN-γ; Sigma) was added for 24 hr following the antigen pulse period to induce maturation, before the APC were cultured in triplicate with lymph node cells from DO11.10 or DO11.10 IL-12 p40−/− mice at a 1 : 2 ratio. Proliferation was assessed by thymidine (3H-TdR) uptake.
Measurement of OVA-specific IFN-γ production
Supernatants from cultures of antigen-stimulated T cells were harvested after 48 hr of culture, centrifuged at 13 000 g and stored at −20° until required. As described previously,13 IFN-γ levels were determined using a sandwich enzyme-linked immunosorbent assay with a pair of IFN-γ-specific antibodies (PharMingen) and standardized with known concentrations of recombinant murine IFN-γ (PharMingen).
Two- and three-colour flow cytometry
Aliquots of 1–10 × 105 cells were washed in fluorescence-activated cell sorting (FACS) buffer (PBS/2%FCS/0·05% sodium azide) and incubated for 30 min at 4° in 100 µl anti-CD16/CD32 antibody (diluted 1 : 100) (Fc Block, PharMingen) to block non-specific binding. After washing in FACS buffer, combinations of biotinylated (bio) and fluorochrome-labelled monoclonal antibodies were added in 100 µl FACS buffer and incubated for 30 min at 4° in the dark. After washing twice, the cells were incubated for 30 min at 4° in 100 µl of phycoerythrin (PE), fluoroscein isothiocyanate (FITC) or PerCP conjugated to streptavidin (diluted 1 : 200) (all PharMingen) to visualize the biotinylated antibodies. The monoclonal antibodies used were: PE–anti-CD4, biotinylated anti-KJ1.26, FITC–anti-CD69, FITC–anti-CD25, PE–anti-B220, PE–anti-CD11c and PE–anti-F480 (all PharMingen). Appropriate isotype controls (all PharMingen) were used in all experiments. The cells were analysed on a Becton Dickinson FACS Calibur flow cytometer and analysed using Cellquest software.
Measurement of cytokine mRNA levels by real time polymerase chain reaction (PCR)
Total RNA was prepared using Trizol (Invitrogen, Renfrew, UK) according to the manufacturer's instructions. RNA was treated with DNase 1 (Ambion, Austin, TX) and reverse transcribed to cDNA using Superscript reverse transcriptase (Invitrogen). cDNA levels of murine IL-12 (p40), type-1 IFN (IFN-β), tumour necrosis factor-α (TNF-α) and hypoxanthine phosphoribosyl transferase (HPRT), were quantitated by real-time PCR using an ABI Prism 7700 sequence detector (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Amplification was achieved using an initial cycle of 50° for 2 min and 95° for 10 min, followed by 40 cycles of 95° for 15 s and 50° for 1 min. cDNA levels during the linear phase of amplification were normalized against HPRT controls. Determinations were made in triplicate and mean ± 1 SD was determined.
Statistics
Where appropriate, results are expressed as the mean from triplicate cultures ± 1 SD and were compared using Student's t-test.
Results
DC, but not Mφ or B cells are responsible for uptake and presentation of ISCOM-associated antigen to CD4+ T cells in vivo and in vitro
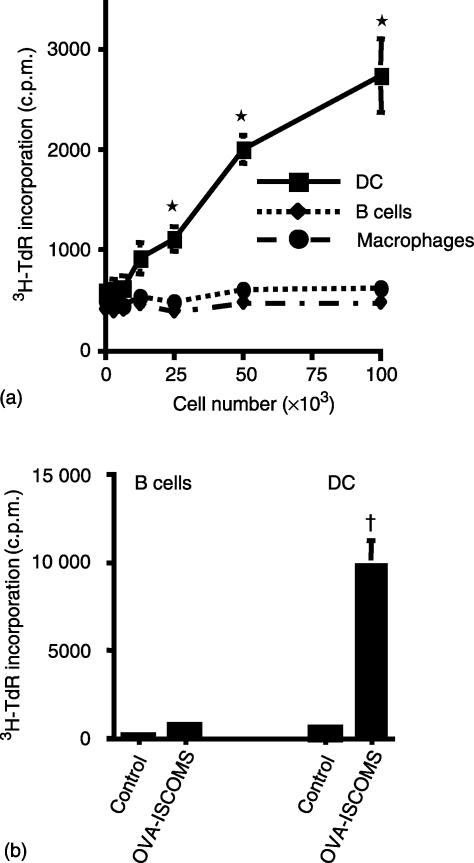
In the first experiments, we investigated which cells in the normal immune system are capable of priming resting antigen-specific CD4+ T cells when pulsed with OVA ISCOMs in vitro. Whole spleen cell preparations were incubated with OVA ISCOMs for 45 min before the DC, B cells and Mφ were purified and cultured with DO11.10 T cells. Of the three APC populations, only CD11c+ DC were capable of stimulating OVA-specific TcR transgenic CD4+ T cells and neither B cells nor Mφ had any ability to prime DO11.10 T cells over the range of APC numbers investigated (Fig. 1a).
Figure 1.
Uptake and presentation of ISCOM-associated antigen to CD4+ T cells in vitro and in vivo. (a) Whole spleen cell preparations were pulsed with 20 µg/ml OVA in ISCOMs for 45 min before CD11c+, B-220+ and F4-80+ cells were purified and cultured with DO11.10 lymphocytes for 72 hr. (b) Flt-3 ligand treated naïve mice were immunized in the rear footpad with 50 µg OVA ISCOMs. Four hr after immunization the draining lymph nodes were harvested and the CD11c+ and B-220+ cells purified by MACS and cultured with DO11.10 lymphocytes for 72 hr. The results shown are mean 3H-TdR incorporation ± 1 SD from triplicate cultures. (*P < 0·01 versus B cells or Mφ, †P < 0·01 versus control DC or B cells).
We next investigated how these findings were representative of what might occur in vivo after immunization with OVA ISCOMs. As DC are rare in normal lymph nodes, we expanded their numbers using flt-3 L, a cytokine that selectively expands DC numbers in vivo.17 Flt-3 L treated mice were immunized subcutaneously with 50 µg of OVA in ISCOMs and 4 hr later, DC and B cells were purified from the draining lymph nodes and cocultured with antigen specific CD4+ T cells in vitro. Under these conditions, in vivo loaded DC had a high capacity to stimulate OVA-specific CD4+ T cells, whereas B cells purified from the same animals had no APC activity (Fig. 1b). Mφ were rare in immunized lymph nodes and we were unable to isolate sufficient Mφ to examine their APC activity in vivo.
Class II MHC restricted presentation of ISCOM-associated antigen by APC in vitro
To confirm the preferential presentation of ISCOMs associated antigen by DC and to determine if other APC might be capable of doing this under any conditions, we generated relatively homozygous populations of DC, Mφ and B cells and pulsed them with OVA ISCOMs directly in vitro. In addition to using resting cells we also activated each cell type with LPS ± IFN-γ before addition to DO11.10 cells.
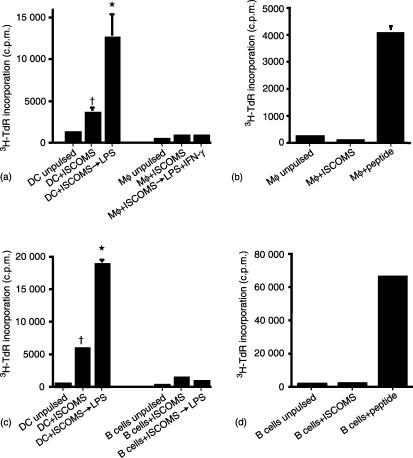
Resting DC derived from BM cultures showed the same high ability to present OVA ISCOMs to CD4+ T cells as their in vivo counterparts and this was greatly enhanced when the DC were treated with LPS after pulsing with antigen (Fig. 2a and c). Neither Mφ derived from BM cultures nor purified naïve B cells could present OVA ISCOMs to CD4+ T cells, irrespective of whether resting cells or cells activated with LPS ± IFN-γ were used (Fig. 2a and c). This is despite the fact that LPS increased the expression of class II MHC and costimulation molecules on both cell types (data not shown) and that the same populations of Mφ and B cells could present the class II-restricted OVA 323-339 peptide to DO11.10 T cells (Fig. 2b and d).
Figure 2.
DC but not Mφ or naïve B cells present ISCOMs OVA to antigen specific CD4+ T cells in vitro and this is enhanced by exposure to LPS. (a and c) BM DC, BM mφ or naïve B cells were pulsed with 10 µg/ml OVA ISCOMs for 2 hr, or pulsed with antigen and then stimulated with LPS ± IFN-γ for 24 hr before washing and culture with DO11.10 lymphocytes for 48 hr. (b) Resting BM mφ or (d) naïve B cells were pulsed with 10 µg/ml OVA ISCOMs or 10 µg/ml OVA peptide for 2 hr, washed and cultured with DO11.10 lymphocytes for 48 hr. The results shown are mean 3H-TdR incorporation ± 1 sd from triplicate cultures. (*P < 0·01 versus DC not treated with LPS, †P < 0·01 versus ISCOMs pulsed B cells or Mφ).
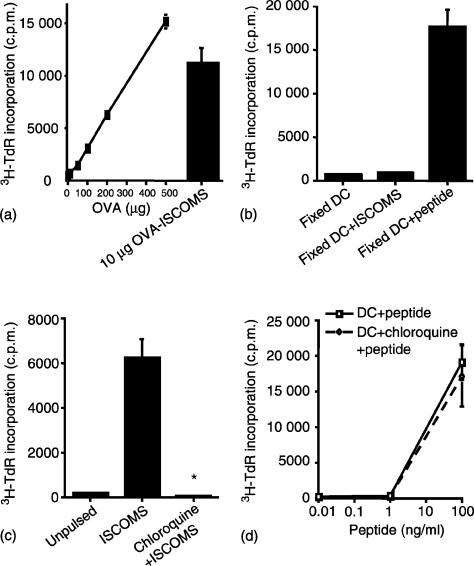
The remarkable efficiency of class II MHC-restricted presentation of OVA ISCOMs by DC was confirmed by the fact that a 30–40-fold greater amount of soluble OVA was required to produce similar levels of proliferation to those obtained using 10 µg OVA in ISCOMs (Fig. 3a). In addition, as little as 0·5 µg OVA in ISCOMs could stimulate T cells when presented by DC (see below). The high response to OVA ISCOMs presented by DC was not caused by contaminating peptides in the preparation, as DC were completely unable to stimulate DO11.10 cells if they were fixed with paraformaldehyde before pulsing with OVA ISCOMs, whereas presentation of peptide was retained under these conditions (Fig. 3b).
Figure 3.
Cellular basis of presentation of ISCOMs associated antigen to CD4+ T cells by DC. (a) BM DC were pulsed with 0–500 µg/ml OVA protein or 10 µg/ml OVA ISCOMs for 2 hr, washed and cocultured with DO11.10 lymphocytes for 48 hr. (b) BM DC were fixed with paraformaldehyde, pulsed with 10 µg/ml OVA ISCOMs or 10 µg/ml OVA peptide for 2 hr, washed and cocultured with DO11.10 lymphocytes for 48 hr. (c) BM DC were pulsed with OVA ISCOMs or (d) with OVA peptide for 5 hr in the presence or absence of 20 µm chloroquine, fixed with paraformaldehyde, washed and cultured with DO11.10 lymphocytes for 48 hr. 3H-TdR incorporation was assessed after 48 hr in culture. The results shown are mean ± 1 sd from triplicate cultures. (*P < 0·01 versus ISCOMs pulse).
To confirm that ISCOMs antigen was entering a classic pathway of MHC class II processing, we next treated DC with chloroquine to prevent acidification of endosomes. When DC were pulsed with OVA ISCOMs in the presence of chloroquine before being fixed and cultured with DO11.10 lymphocytes, the presentation of ISCOMs OVA to DO11.10 lymphocytes was completely abrogated (Fig. 3c). In contrast, chloroquine had no effect on the presentation of OVA peptide (Fig. 3d). These results suggest that OVA ISCOMs enter an endosomal pathway and the processing of ISCOMs OVA for MHC class II presentation requires the acidification of these endosomes.
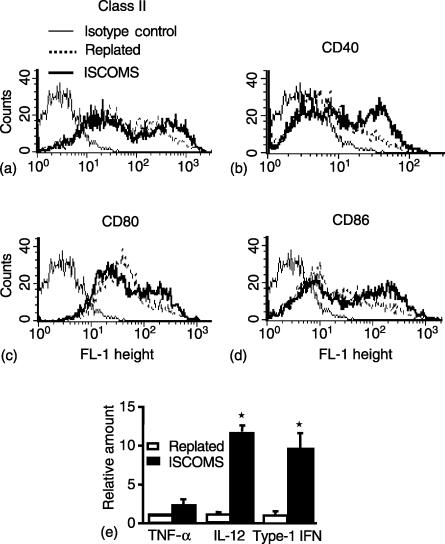
A possible reason for the high efficiency of presentation of OVA in ISCOMs compared to native protein is that ISCOMs may activate DC. BM DC were exposed to OVA ISCOMs for 4 hr in vitro and the expression of class II MHC and costimulatory molecules assessed by flow cytometry. mRNA was also harvested and cytokine gene expression measured by real time PCR. As controls, DC were replated in medium only for 4 hr. Exposure of DC to OVA ISCOMs caused a moderate increase in surface expression of class II MHC, CD40, CD80 and CD86 molecules when compared with replated control DC (Fig. 4a–d). In addition there was significant up-regulation of IL-12 and type-1 IFN mRNA in OVA ISCOMs treated DC (Fig. 4e).
Figure 4.
Activation of DC by ISCOMs. BM DC were plated in the absence or presence of 10 µg/ml OVA ISCOMs for 4 h and the expression of (a) class II MHC (b) CD40 (c) CD80 and (d) CD86 was assessed on CD11c+ DC by flow cytometry. (e) RNA was extracted from the DC, reverse transcribed, and the relative amount of TNF alpha, IL-12 and type-1 IFN (IFN-β) determined by real time PCR and normalized to HPRT levels in each sample. Determinations were made in triplicate and mean ± 1 SD were determined. (*P < 0·01 versus controls).
The inability of B cells and Mφ to present OVA ISCOMs is not a result of toxicity of ISCOMs
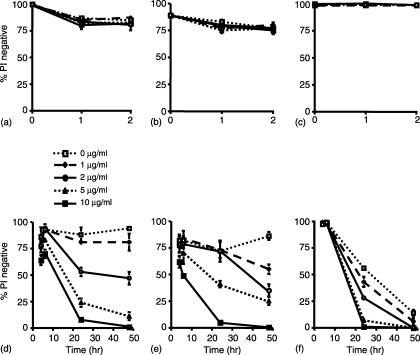
ISCOMs have been reported to be toxic to cells in vivo18 and therefore it was possible that the apparently better presentation of OVA ISCOMs by DC reflected a selective toxic effect on Mφ and/or B cells in our culture system. To assess this, we cultured BM DC, BM Mφ or naïve B cells with OVA ISCOMs for up to 48 hr. When the proportion of viable cells was determined by flow cytometry using propidium iodide (PI) staining, cell death was not observed over the first 2 hr of exposure to OVA ISCOMs in any cell population (Fig. 5a–c). DC and Mφ then showed similar patterns of toxicity after exposure to OVA ISCOMs, with concentration dependent cell death that first became apparent at 6 hr and progressed thereafter (Fig. 5d, e). B cells showed a slightly different pattern of response, as there was no cell death over the first 6 hr of incubation with OVA ISCOMs (Fig. 5c, f). By 24 hr, the B cells showed concentration-dependent cell death in the presence of OVA ISCOMs, but there were also significant amounts of spontaneous cell death at this time, with nearly 50% of untreated B cells staining PI positive. By 48 hr, the majority of B cells were PI positive irrespective of the presence or absence of ISCOMs. Thus, the preferential ability of DC to present OVA ISCOMs in vitro is not caused by their enhanced survival after exposure to ISCOMs. In addition, the results show that the 2 hr incubation period we used to pulse APC with OVA ISCOMs is not associated with cell death during this time.
Figure 5.
Effects of ISCOMs on viability of antigen presenting cells. (a and d) BM DC (b and e) BM Mφ and (c and f) naïve B cells were plated in triplicate at 1 × 105 cells/well in 96-well plates before the addition of 0–10 µg/ml OVA ISCOMs. At the times indicated, the cells were harvested, washed in FACs buffer and viability assessed by flow cytometry after addition of 5 µg/ml PI. The results shown are mean ± 1 SD from triplicate cultures.
Together, these results indicate that DC may be the only cell which can prime OVA-specific CD4+ T cells with ISCOM-associated antigen. To study this process in more detail, we explored the activation of the CD4+ T cells at the cellular level.
Antigen-specific CD4+ T cells primed with OVA ISCOM-loaded DC express cellular activation markers
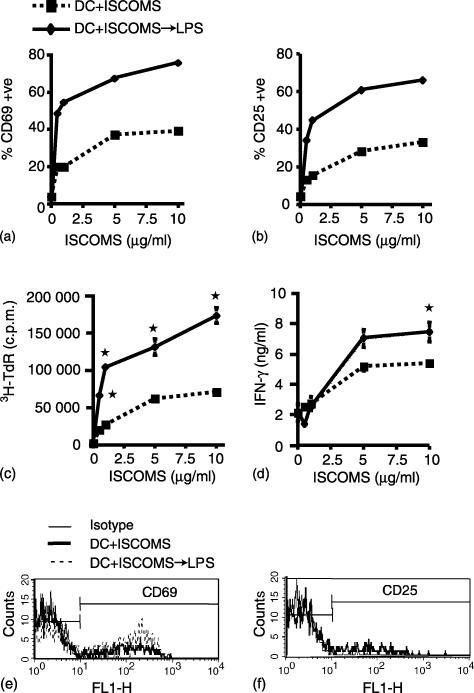
As the DO11.10 cells used as responders in these experiments were heterogeneous in nature, we wished to confirm directly that the OVA ISCOMs pulsed BM DC were indeed stimulating the OVA-specific CD4+ T cells. Therefore we examined the expression of CD69 and CD25 on transgenic CD4+ KJ1.26+ DO11.10 cells by flow cytometry. Twenty-four hr after culture with OVA ISCOMs pulsed DC, the CD4+ KJ1.26+ cells showed a dose-dependent increase in the expression of CD69 and CD25, with as little as 0·5 µg/ml OVA in ISCOMs stimulating responses by the transgenic cells (Fig. 6a, b, e, f). These responses were enhanced when DC had been activated with LPS, when almost 80% and 65%, respectively, of the CD4+ KJ1.26+ T cells expressed both markers in response to LPS activated DC which had been pulsed with 10 µg/ml OVA in ISCOMs (Fig. 6a and b). In parallel to the T cells being activated phenotypically, there was dose-dependent proliferation (Fig. 6c) and production of IFN-γ (Fig. 6d) in the cultures, indicating that the presentation of OVA ISCOMs by DC induced functional differentiation of the CD4+ KJ1.26+ T cells. Again, these responses were enhanced by LPS treatment of DC after uptake of OVA ISCOMs.
Figure 6.
Antigen dose-dependent activation of antigen specific CD4+ T cells by ISCOM-associated antigen presented by DC. BM DC were pulsed with 0–10 µg/ml OVA ISCOMs for 2 hr or with OVA ISCOMs followed by stimulation with LPS for 24 hr, washed and cultured with DO11.10 lymphocytes for 72 hr. (a and b) Expression of CD69 and CD25 on CD4+ KJ1.26+ T cells, assessed by flow cytometry after 24 hr in culture. (c) 3H-TdR incorporation and (d) IFN-γ production were assessed after 72 and 48 hr in culture, respectively. (e and f) FACS plots showing CD69 and CD25 expression on CD4+KJ1·26+ T cells 24 hr after culture with DC pulsed with 0·5 µg/ml OVA ISCOMs. The results shown are mean ± 1 SD from triplicate cultures. (*P < 0·01 versus responses with equivalent concentration of OVA ISCOMs in the absence of LPS).
Optimal presentation of OVA in ISCOMs to antigen-specific CD4+ T cells by DC is IL-12 dependent
One of the important factors produced by DC during presentation of antigen to CD4+ T cells is IL-12 and we showed previously that IL-12−/− mice have defective immune responses to OVA ISCOMs.13 Therefore, we investigated the role of IL-12 in the activation of OVA-specific CD4+ T cells by OVA ISCOMs pulsed BM DC in vitro.
In the first experiments, we compared the ability of OVA ISCOMs pulsed BM DC derived from BALB/c and IL-12 p40−/− mice to prime OVA-specific CD4+ T cells in vitro. Cultures containing the usual number of CD4+ KJ1.26+ DO11.10 cells (4 × 104/well) responded equally, irrespective of whether they were stimulated with wild type or IL-12 p40−/− DC pulsed with either OVA ISCOMs or with OVA protein (Table 1). In addition, both wild type and IL-12 p40−/− DC showed enhanced presentation of OVA ISCOMs when activated with LPS, although the responses to LPS activated IL-12 p40−/− DC loaded with soluble OVA were approximately 50% less than those obtained using LPS activated wild type DC (Table 1). To determine whether the requirement of IL-12 could be influenced by the number of OVA-specific T cells present in the cultures, we carried out identical experiments using cultures containing fivefold fewer OVA-specific CD4+ KJ1.26+ T cells. Under these conditions, CD4+ T cells responded normally to resting IL-12 p40−/− DC pulsed with OVA ISCOMs, but had significantly decreased responses when these DC had been pulsed with OVA protein. In addition, IL-12 p40−/− DC now induced much reduced responses to either soluble OVA or OVA ISCOMs after activation with LPS when compared with LPS-activated wild type DC (Table 1).
Table 1.
The role of IL-12 in the priming of wild type antigen-specific CD4+ T cells by OVA ISCOM-loaded DC
| 4 × 104 OVA-specific T cells/well | 8 × 103 OVA-specific T cells/well | |||
|---|---|---|---|---|
| BALB/c DC | IL-12 p40−/− DC | BALB/c DC | IL-12 p40−/− DC | |
| Unpulsed | 2077 ± 313 | 734 ± 422 | 1784 ± 50 | 795 ± 29 |
| OVA ISCOMs | 81 218 ± 6516 | 85 473 ± 6297 | 18 837 ± 2713 | 16 908 ± 1307 |
| OVA | 69 323 ± 5141 | 61 512 ± 652 | 14 169 ± 1633 | 7365 ± 1029* |
| OVA ISCOMs→LPS | 149 157 ± 14 365 | 128 297 ± 10 136 | 46 631 ± 3558 | 28 095 ± 1972* |
| OVA→LPS | 141 587 ± 6060 | 55 038 ± 8560* | 39 545 ± 1900 | 13 779 ± 1843* |
P < 0·01 versus BALB/c DC.
BM DC derived from BALB/c or IL-12 p40−/− mice were pulsed with 10 µg/ml OVA ISCOMs or 1 mg/ml OVA protein for 2 hr, or with antigen and then stimulated with LPS for 24 hr, before culture with DO11.10 lymphocyte populations containing 4 × 104 or 8 × 103 OVA specific CD4+ T cells/well. The results shown are mean c.p.m. ± 1 SD for 72 hr cultures.
As the responder cell populations used in these experiments were heterogeneous, we wished to exclude a possible role for contaminating IL-12 producing cells in the responding DO11.10 lymph node preparations. Thus we compared the responses of OVA-specific CD4+ T cells from wild type DO11.10 mice with those of DO11.10 mice backcrossed onto the IL-12 p40−/− background. When 4 × 104 CD4+ KJ1.26+ cells/well were used, DO11.10 IL-12 p40−/− cells showed significantly lower responses to both OVA ISCOMs and OVA protein presented by wild type BALB/c DC (Table 2). However, the wild type and IL-12 p40−/− DO11.10 cells responded similarly to OVA ISCOMs or OVA protein when LPS-activated DC were used (Table 2). When the responding lymphocytes contained fivefold fewer OVA-specific CD4+ T cells, the IL-12 p40−/− DO11.10 lymphocytes showed significantly reduced responses to both OVA ISCOMs and OVA protein, irrespective of whether the APC had been activated with LPS or not (Table 2).
Table 2.
The role of IL-12 in the priming of antigen-specific wild type or IL-12 p40−/− CD4+ T cells by OVA ISCOM-loaded DC
| 4 × 104 OVA-specific T cells/well | 8 × 103 OVA-specific T cells/well | |||
|---|---|---|---|---|
| Wild type DO11.10 | DO11.10 IL-12 p40−/− | Wild type DO11.10 | DO11.10 IL-12 p40−/− | |
| Unpulsed | 2077 ± 313 | 3696 ± 633 | 1784 ± 50 | 2775 ± 554 |
| OVA ISCOMs | 81 218 ± 6516 | 53 309 ± 7061* | 18 837 ± 2713 | 12 655 ± 491* |
| OVA | 69 323 ± 5141 | 48 567 ± 5582* | 14 169 ± 1633 | 5775 ± 429* |
| OVA ISCOMs→LPS | 149 157 ± 14 365 | 125 684 ± 8207 | 46 631 ± 3558 | 34 340 ± 784* |
| OVA→LPS | 141 587 ± 6060 | 128 151 ± 344 | 39 545 ± 1900 | 16 843 ± 1762* |
P < 0·01 versus DO11.10 lymphocytes.
BM DC derived from BALB/c mice were pulsed with 10 µg/ml OVA ISCOMs or 1 mg/ml OVA protein for 2 hr, or with antigen and then stimulated with LPS for 24 hr, before culture with DO11.10 or DO11.10 IL-12 p40−/− lymphocyte populations containing 4 × 104 or 8 × 103 OVA specific CD4+ T cells/well. The results shown are mean c.p.m. ± 1 standard deviation for 72 hr cultures.
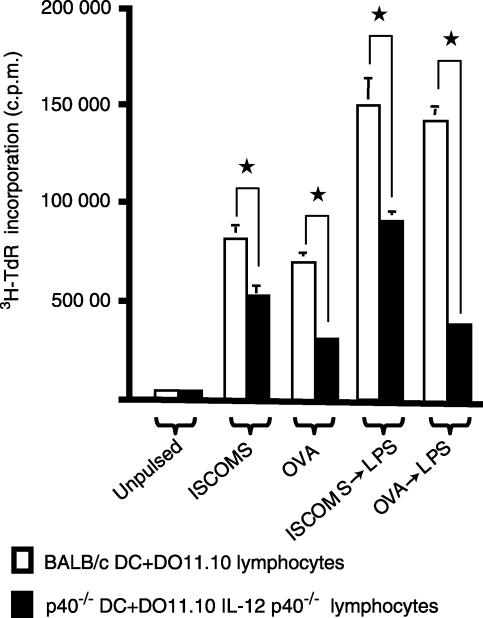
Finally, we examined the response of OVA-specific CD4+ T cells under conditions where IL-12 was entirely absent, using IL-12 p40−/− DC and IL-12 p40−/− DO11.10 lymphocytes. In this case, even the higher number of responding OVA-specific CD4+ responder T cells showed significant decreases in their responses to OVA ISCOMs or OVA protein presented by resting or activated DC (Fig. 7). Thus, IL-12 is required to costimulate the activation of antigen specific CD4+ T cells by OVA ISCOMs presented by DC, especially when the number of specific T cells is limiting.
Figure 7.
The role of IL-12 in the priming of antigen specific CD4+ T cells by OVA ISCOM-loaded DC. BM DC derived from BALB/c or IL-12 p40−/− mice were pulsed with 10 µg/ml OVA ISCOMs or 1 mg/ml OVA protein for 2 hr, or with antigen and then stimulated with LPS for 24 hr, before culture with lymphocyte populations containing 4 × 104 OVA specific CD4+ T cells/well from DO11.10 or DO11.10 IL-12 p40−/− mice, respectively. 3H-TdR incorporation was assessed after 72 hr in culture. The results shown are mean ± 1 SD from triplicate cultures. (*P < 0·01 versus p40−/− DC + DO11.10 IL-12 p40−/− lymphocytes).
Discussion
The current results show that DC pulsed with OVA ISCOMs prime resting OVA-specific CD4+ T cells very efficiently in vitro and indicate that DC are also the principal APC involved in taking up ISCOMs associated antigen for class II MHC restricted processing in vivo. B cells and Mφ appear to play no role in the presentation of ISCOMs in vivo or in vitro, even if activated by LPS and/or IFN-γ, while the presentation by DC is partly dependent on the presence of IL-12.
ISCOMs have a potent ability to prime CD4+ T cell dependent immune responses in vivo8 but the APC responsible for this has not been identified previously. An earlier study reported that influenza virus envelope proteins incorporated into ISCOMs can be taken up and presented to T cells by a variety of APC including unfractionated peritoneal or spleen cells and splenic DC or B cells. However, this work used a heterogeneous mixture of CD4+ and CD8+ T cells which had been primed previously with antigen.19 In contrast, using purified populations of APC to present OVA ISCOMs to naïve, peptide specific CD4+ T cells, we show that DC are the only APC able to present ISCOM-associated antigen. This was the case even when homogeneous populations were exposed to OVA ISCOMs in vitro and when the APC were obtained from whole spleen cells, where B cells and Mφ make up much higher proportions of the initial populations.
To ensure that these results using in vitro pulsed APC derived from single cell suspensions were representative of antigen uptake and processing events in vivo, we also purified APC from the draining lymph nodes of mice immunized subcutaneously with OVA ISCOMs. Again we found that DC, but not B cells, stimulated antigen-specific CD4+ T cells very efficiently in vitro. In addition, when APC purified from lymphoid tissues or BM cultures were pulsed directly with OVA ISCOMs in vitro, we found that B cells and Mφ were unable to present the antigen to antigen specific CD4+ T cells, suggesting that it is very unlikely that these cells play a role as APC in vivo. Mφ have been suggested to take up ISCOMs in vivo20 and have been shown to present antigen to CD4+ T cells in chronic inflammatory conditions21 while B cells have been implicated in the priming of naïve CD4+ T cells in vivo, especially in the differentiation of effector Th2 CD4+ T cells.22 Our finding that DC are so efficient at priming CD4+ T cells with ISCOMs particles are therefore entirely consistent with their central role in initiating T-cell dependent responses and also with their high endocytic and phagocytic ability.23,24 Our earlier findings that CD4+ T-cell responses to OVA ISCOMs in vivo are greatly enhanced by expanding DC numbers with flt-3L14 are further support for the central role of DC in presenting OVA ISCOMs in vivo.
Two features about the presentation of OVA ISCOMs by DC are worthy of note. First is the high efficiency of ISCOMs in gaining access to the class II MHC processing pathway in DC, as the same quantity of soluble OVA induced no antigen-specific CD4+ T-cell responses and 30–40 times as much soluble antigen was required to stimulate equivalent levels of proliferation. The mechanisms of uptake and processing of ISCOM-associated antigen by DC are not fully known, but our studies have implicated a role for acidic endosomes in the presentation to CD4+ T cells, as this was inhibited by chloroquine. Interestingly, our previous work showed that acidic endosomal processing was also critical for the presentation of OVA ISCOMs by DC to CD8+ T cells15 underlining the possibility that ISCOMs are particularly effective in accessing these compartments. Although consistent with previous findings that ISCOMs localize in endosomal compartments in macrophages20 the exact nature of the vesicle is unknown and whether ISCOMs are taken up by phagocytosis is unclear. Quil A is very rich in sugars and therefore endocytosis via mannose receptors, scavenger receptors or other sugar binding receptors is one possible route of entry to these compartments.
The second feature worthy of comment is that although the presentation of OVA ISCOMs by BM DC was greatly enhanced by activation with LPS, the responses obtained using resting DC were also substantial. As the priming of CD4+ T cells requires the expression of costimulation molecules that require maturation of DC12,25 these findings suggest that ISCOMs not only enter the class II MHC processing pathway very efficiently, but that ISCOMs also have some ability to induce DC maturation. This was supported by our findings that ISCOMs activated DC in vitro, as shown by increased expression of MHC class II and the costimulation molecules CD40, CD80 and CD86, as well as by the enhanced expression of mRNA for IL-12 and type-1 IFN. The fact that we could isolate antigen loaded, stimulatory DC from the draining lymph node soon after subcutaneous administration of ISCOMs is further indirect support for this idea, as it is known that the migration of antigen-loaded DC from tissues to the draining lymph node requires activation-induced changes in chemokine receptor expression.26 Thus, we propose that ISCOMs are taken up efficiently by DC in tissues, which then become activated, migrate to the draining lymph node and present antigenic epitopes in the context of class II MHC to CD4+ T cells. This effect on DC could involve the particulate nature of ISCOMs, or the presence of a ‘danger’ signal27 in the ISCOMs structure. The most likely source of this is the Quil A component, which is a complex mixture of triterpenoid containing saponins and is a potent adjuvant in its own right.28 The effects of ISCOMs on DC maturation and migration in vivo and in vitro are currently being investigated in more detail.
Our previous studies identified a critical role for IL-12 in CD4+ T-cell dependent immune responses to OVA ISCOMs in vivo, but we did not identify the IL-12 producing cells or establish the basis of this effect.13 Here we have extended these findings by showing that IL-12 plays an important role in the priming of antigen-specific CD4+ T cells by DC in vitro, especially when the numbers of antigen-specific T cells are limiting. The presentation of soluble OVA was even more dependent on IL-12, perhaps again indicating that ISCOMs can stimulate resting DC to produce other molecules that can substitute for IL-12 that may normally be required for full T-cell priming. These could include cytokines such as IL-1829 and type-1 IFN, or molecules like CD40, CD80 and CD86, which we found to be induced by DC exposed to ISCOMs in vitro. When DC had been activated by LPS, a potent stimulator of IL-12 production30 IL-12 then became necessary for the maximum increase in the priming of limiting numbers of CD4+ T cells in response to both OVA ISCOMs and OVA protein. This indicates that ISCOM-induced activation cannot substitute for IL-12 production by the DC when in a more highly activated state. On the basis of these results, we propose that DC-derived IL-12 is required for the optimal production of and response to IL-2 by ISCOMs stimulated T cells. This may either be because IL-12-dependent signalling is necessary for IL-2R expression31 or because IL-12-dependent IFN-γ production by T cells is necessary to upregulate or maintain the expression of costimulatory molecules on APC which are needed for sustained T-cell clonal expansion.
In summary, our results indicate that DC are extremely potent at processing and presenting ISCOM-associated antigen to CD4+ T cells and we propose that targeting DC may be the principal mechanism underlying the adjuvant activity of ISCOMs on CD4+ T-cell priming. By investigating the cellular basis of these processes, it may be feasible to target ISCOMs better to DC in vivo and hence improve further the usefulness of ISCOMs as potential vaccine vectors for protection against different pathogens.
Acknowledgments
Supported by The Wellcome Trust and by grants BIO4-CT98-0505 and QLK2-CT-1999–0228 from the EC Biotechnology Programme Frameworks 4 and 5. We thank Professor Paul Garside for critical reading of the manuscript.
References
- 1.Bloom BR. Vaccines for the Third World. Nature. 1989;342:115–20. doi: 10.1038/342115a0. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RK, Siber GR. Adjuvants for human vaccines. current status problems and future prospects. Vaccine. 1995;13:1263–76. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 3.Levine MM, Kaper JB, Black RE, Clements ML. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–50. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovgren K, Morein B. The requirement of lipids for the formation of immunostimulating complexes (ISCOMS) Biotechnol Appl Biochem. 1988;10:161–72. [PubMed] [Google Scholar]
- 5.Mowat AM, Donachie AM, Reid G, Jarrett O. Immune stimulating complexes containing Quil A and protein prime class-I MHC-restricted T lymphocytes in vivo and are immunogenic by the oral route. Immunology. 1991;72:317–22. [PMC free article] [PubMed] [Google Scholar]
- 6.Kazanji M, Laurent F, Pery P. Immune-responses and protective effect in mice vaccinated with surface sporozoite protein of Eimeria falciformis in ISCOMS. Vaccine. 1994;12:798–804. doi: 10.1016/0264-410x(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 7.Estrada A, Laarveld B, Li B, Redmond MJ. Induction of systemic and mucosal immune-responses following immunisation with somatostatin avidin complexes incorporated into ISCOMS. Immun Invest. 1995;24:819–28. doi: 10.3109/08820139509060709. [DOI] [PubMed] [Google Scholar]
- 8.Maloy KJ, Donachie AM, Mowat AM. Induction of Th1 and Th2 CD4+ T cell responses by oral and parenteral immunization with ISCOMS. Eur J Immunol. 1995;25:2835–41. doi: 10.1002/eji.1830251019. [DOI] [PubMed] [Google Scholar]
- 9.Morein B. ISCOMS. Vet Microbiol. 1990;23:79–84. doi: 10.1016/0378-1135(90)90138-l. [DOI] [PubMed] [Google Scholar]
- 10.Heeg K, Kuon W, Wagner H. Vaccination of class-I major histocompatibility complex (MHC) -restricted murine CD8+ cytotoxic T-lymphocytes towards soluble-antigens immunostimulating-ovalbumin complexes enter the class-1 MHC-resricted antigen pathway and allow sensitisation against the immunodominant peptide. Eur J Immunol. 1991;21:1523–7. doi: 10.1002/eji.1830210628. [DOI] [PubMed] [Google Scholar]
- 11.Schijns VEJC. Immunological concepts of vaccine adjuvant activity. Curr Opin Immunol. 2000;12:456–63. doi: 10.1016/s0952-7915(00)00120-5. [DOI] [PubMed] [Google Scholar]
- 12.Bell D, Young JW, Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- 13.Smith RE, Donachie AM, Grdic D, Lycke N, Mowat AM. Immune-stimulating complexes induce an IL-12-dependent cascade of innate immune responses. J Immunol. 1999;162:5536–46. [PubMed] [Google Scholar]
- 14.Beacock-Sharp H, Donachie AM, Robson NC, Mowat AM. A role for dendritic cells in the priming of antigen specific CD4+ and CD8+ T lymphocytes by immune stimulating complexes in vivo. Int Immunol. 2003;15:711–20. doi: 10.1093/intimm/dxg067. [DOI] [PubMed] [Google Scholar]
- 15.Robson NC, Beacock-Sharp H, Donachie AM, Mowat AM. Dendritic cell maturation enhances CD8+ T cell responses to exogenous antigen via a proteasome independent mechanism of MHC class I loading. Immunology. 2003;109:374–83. doi: 10.1046/j.1365-2567.2003.01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz MB, Kukutsch N, Ogilvie ALJ, Rofner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 17.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–62. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stieneker F, Kersten G, van Bloois L, Crommelin DJA, Hem SL, Lower J, Kreuter J. Comparison of 24 different adjuvants for inactivated HIV-2 split whole virus as antigen in mice. Induction of titres of binding antibodies and toxicity of the formulations. Vaccine. 1995;13:45–53. doi: 10.1016/0264-410x(95)80010-b. [DOI] [PubMed] [Google Scholar]
- 19.Villacres-Eriksson M. Antigen presentation by naïve macrophages, dendritic cells and B cells to primed T lymphocytes and their cytokine production following exposure to immunostimulating complexes. Clin Exp Immunol. 1995;102:46–52. doi: 10.1111/j.1365-2249.1995.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson DL, Lovgren K, Watson NA, Fossum C, Morein B, Hoglund S. Inflammatory response and antigen localization following immunization with influenza virus ISCOMs. Inflammation. 1989;13:641–9. doi: 10.1007/BF00914308. [DOI] [PubMed] [Google Scholar]
- 21.Tsark EC, Wang W, Teng YC, Arkfeld D, Dodge GR, Kovats S. Differential MHC class II-mediated presentation of rheumatoid arthritis autoantigens by human dendritic cells and macrophages. J Immunol. 2002;169:6625–33. doi: 10.4049/jimmunol.169.11.6625. [DOI] [PubMed] [Google Scholar]
- 22.Constant S, Schweitzer N, Weast J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155:3734–41. [PubMed] [Google Scholar]
- 23.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 24.Iyoda T, Shimoyama S, Lin K, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane P. Role of OX40 signals in coordinating CD4 T cell selection, migration, and cytokine differentiation in T helper (Th) 1 and Th2 cells. J Exp Med. 2000;191:201–6. doi: 10.1084/jem.191.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 28.Kensil CR. Saponins as vaccine adjuvants. Crit Rev Ther Drug Carrier Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 29.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 30.Langenkamp A, Messi M, Lanzavechia A, Sallusto F. Kinetics of dendritic cell activation. impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–6. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen T, Wang R, Russell JH. IL-12 enhances IL-2 function by inducing CD25 expression through a p38 mitogen-activated protein kinase pathway. Eur J Immunol. 2000;30:1445–52. doi: 10.1002/(SICI)1521-4141(200005)30:5<1445::AID-IMMU1445>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]