Abstract
The expression of allograft inflammatory factor-1 (AIF-1) in 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis, a model for T helper 1 (Th1) type disease, was investigated in BALB/c mice. The AIF-1 expression was significantly increased in the colitis lesion compared to that in the normal colon. We then prepared AIF-1 transgenic mice (Tgm) with the BALB/c background that express high levels of AIF-1 in lymphoid tissues and the colon. When AIF-1 Tgm were administrated TNBS, the TNBS-induced colitis was ameliorated compared with that in non-transgenic littermates. The amelioration of colitis was associated with the low expression of interleukin-1β in the colon. The present findings suggest that AIF-1 regulates Th1-type inflammatory responses.
Introduction
Inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn's disease, are chronic inflammatory disorders of the intestinal tract. Although the precise aetiology of IBD is still unclear, it is generally considered that complex interactions among genetic and environmental factors associate with the onset and perpetuation of IBD.1 Recently various animal models for IBD have been developed, and it has been revealed that the pathogenesis of IBD involves immunological abnormality, especially dysfunction of T cells.2–4
Dextran sulphate sodium (DSS)-induced colitis and 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis are murine colitis models that are morphologically similar to human IBD.2,5,6 Lesions of DSS-induced colitis mainly reside in the mucosa and lamina propria.5 Although it was shown that changes of the intestinal microflora population, direct toxicity for epithelial cells, and activation of macrophages were related to the DSS-induced colitis,5,7,8 the exact mechanism remains unclear. In TNBS-induced colitis, a transmural colitis model, it has been reported that T helper type 1 (Th1) responses are involved in induction of the colitis.2,6 Indeed, Th1 cells are dominant infiltrating cells in the lamina propria.
Allograft inflammatory factor-1 (AIF-1) is a novel and hydrophilic polypeptide functioning across species barriers. AIF-1 contains a 12-amino acid region similar to an EF-hand (calcium-binding structure) domain.9–12 AIF-1 was originally identified as a gene product expressed in infiltrating macrophages in cardiac allografts of a rat model with chronic rejection.9 The expression of AIF-1 is increased in macrophages activated by interferon-γ (IFN-γ). Within local inflammatory lesions of chronic autoimmune animal models such as experimental allergic encephalomyelitis (EAE), experimental autoimmune uveoretinitis (EAU) and experimental allergic neuritis (EAN), infiltrating macrophages predominantly express AIF-1.13 Using immunohistochemical analyses it was reported that AIF-1 was expressed in dendritic cells of the intestine and Kupffer cells of the liver in rats. It was also shown in a human system that mRNA of AIF-1 was expressed in the liver, colon and small intestine.10,14 However, immunological functions of AIF-1 in the gastrointestinal tract have been totally unclear, although Mentschel et al.15 reported that AIF-1 expression was elevated in the gut mucosa of malnourished pigs compared to optimally fed controls.
In the present study using TNBS-induced colitis we investigated the expression of AIF-1 in the lesions. We show herein that the AIF-1 expression increases in the colitis lesions. Furthermore, the TNBS-induced colitis is ameliorated in AIF-1 transgenic mice (Tgm) compared with that in non-Tgm littermates. The present findings suggest that AIF-1 regulates TNBS-induced colitis.
Materials and methods
Transgene construction
Murine AIF-1 cDNA was obtained as previously described.12 A 444-bp BamHI fragment of the cDNA was cloned into an expression vector driven by human CD11b promoter with human growth hormone polyadenylation signal, which was provided by Dr Daniel Tenen, Harvard Medical School. It has been reported that the insert under the human CD11b is expressed at highest levels late during differentiation of myeloid cells such as macrophages.16 The expression vector was linearized and 4·4 kb XhoI/SacII fragment was gel-purified and employed for the microinjection into the fertilized eggs.
Generation of Tgm
The linearized DNA containing above described was microinjected into 235 fertilized eggs obtained from (B6 × DBA/2)F1 and transplanted into ICR recipients at Japan SLC, Inc. Department of Bioresource Development (Hamamatsu, Japan). Seventy-one pups were weaned and analysed for the integration of transgene by genomic polymerase chain reaction (PCR). Finally, three lines of Tgm were established and used for further characterization. AIF-1 protein was highly expressed in the CD11b-positive fraction as expected when tested by Western blot analysis. The expression of transgene was obviously detected in lymphoid organs such as spleen, lymph nodes and bone marrow (data not shown). The intensity of AIF-1 expression as tested by Northern blot analysis was high in line #22 and #39 in almost same amount, and weak in line #12. The #39 Tgm were backcrossed for four generations to BALB/c mice as described elsewhere,17 because the experimental system for TNBS-induced colitis was suitable with BALB/c background. BALB/c mice were also obtained from the Japan SLC. These mice were maintained under specific pathogen-free conditions in the animal facility at Institute for Genetic Medicine, Hokkaido University. Six-week-old female mice were used throughout the study. The animal care and experimental procedures conformed to the regulation of Hokkaido University Animal Care and Use Committee.
Induction of colitis
Mice were anaesthetized by intraperitoneal injection of xylazine (50 mg/kg) and ketamine (50 mg/kg). One mg of TNBS (Sigma Chemical Co., St Louis, MO) for normal BALB/c mice or 1·5 mg of TNBS for AIF-1 Tgm and non-transgenic littermates (non-Tgm) in 50% ethanol was administered intrarectally via a vinyl catheter inserted 3·5 cm into the colon. Animals were kept in a vertical position for 30 s. Control mice were administered phosphate-buffered saline (PBS) using the same procedure. Animals were daily observed and weighed. For further analyses animals were killed 3 days after TNBS administration. Distal colon (2·5 cm long) was removed, and 0·5 cm of distal side and 2 cm of proximal side were used for the histological analysis and reverse transcription (RT)–PCR, respectively.
Pathologic studies
Samples were removed 5 mm apart from the anus, embedded in OCT. compounds (Sakura Finetechnical Co. Ltd, Tokyo, Japan) and snap-frozen in liquid nitrogen as described elsewhere.16 The embedded samples were sectioned at 5 µm with a cryostat, fixed with 10% formalin/PBS, and then stained with haematoxylin and eosin. To evaluate the severity of inflammation, six cross-sections that were at intervals of 0·5 mm were graded according to the Ameho criteria by two histologists blinded to the experimental group.18 The mean grade was calculated for each section and expressed as histological score.
For immunohistochemical analysis frozen sections of 5 µm thickness were fixed with acetone and stained with anti-mouse AIF-1 antibody12 as described elsewhere.19
RT–PCR
Total RNA was prepared according to a standard procedure from the colons.20 Complementary DNA (cDNA) was synthesized from 10 µg RNA using random hexamer and Moloney murine leukemia virus reverse transcriptase (SuperScriptTM, Gibco/BRL, Gaithersburg, MD) at 37° for 1 hr in the presence of dNTPs and RNase inhibitor, RNasin (Promega, Madison, WI). One-twentieth of reverse transcripts were used as templates for the amplification reaction. Primers for AIF-1, IFN-γ, interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), IL-10, IL-4, IL-12 p40, IL-13, hypoxanthine phosphoribosyl transferase (HPRT) and β-actin have been described previously.12,21–27 Reaction mixture (50 µl) contained 200 nm of each primer, 0·4 mm dinitrotriphosphate, 20 mm Tris-HCl (pH 8·4), 50 mm KCl, 1·5 mm MgCl2 and 2 units Taq DNA polymerase (Life Technologies, Gaithersburg, MD). PCR amplification was performed with 30 cycles for HPRT, β-actin, IL-1β and TNF-α, or 34 cycles for IL-13, or 35 cycles for IL-4, IL-12 p40, IFN-γ and IL-10 or 38 cycles for AIF-1. An annealing temperature of 58° was used for AIF-1, IL-1β and β-actin, 60° for HPRT, 65° for IL-4, 63° for IL-13 and 55° for IFN-γ, TNF-α, IL-12 p40 and IL-10. Thermal cycling was performed as heat denaturation at 94° for 1 min, annealing temperature for 1 min and 72° for 2 min, and a final extension step at 72° for 10 min For β-actin, IL-1β, TNF-α, IFN-γ, IL-10 and AIF-1 analyses the amplified PCR products were electrophoresed on 1·5% agarose gel containing ethidium bromide. Quantitative analysis of the amplified products was performed by an image analyser with NIH Image software. The result was evaluated as a relative unit determined by normalization of the density of each band to that of the β-actin, which was added as an internal control. For IL-4, IL-12, IL-13 and HPRT analyses the amplified products were electrophoresed on 2% agarose gel and then stained with SYBR Green I (Molecular Probes, Eugene, OR). The fluorescence intensities of the specific bands were visualized using FLA-3000 (Fuji Film, Tokyo, Japan) and were analysed with Science Laboratory 99 Image Gauge Ver. 3·4 software (Fuji Film). The result was evaluated as a relative unit determined by normalization of the density of each band to that of the HPRT, which was presented as percentage HPRT.
Results
AIF-1 mRNA expression in TNBS-induced colitis in BALB/c mice
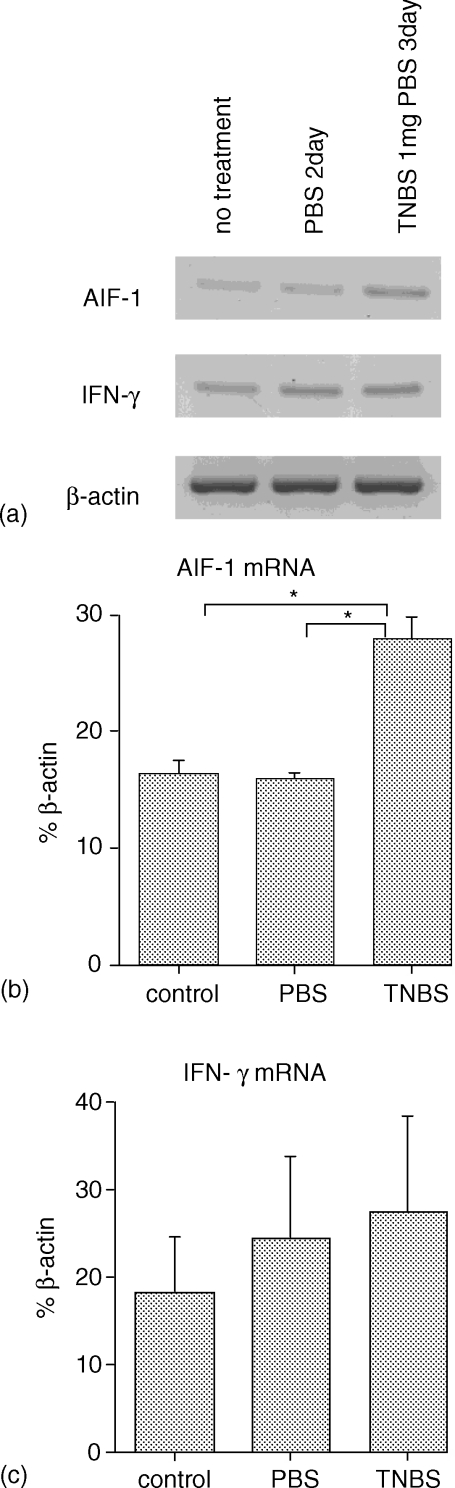
Amounts of AIF-1 and IFN-γ messages in RNA extracted from colons of TNBS- or PBS-treated or non-treated BALB/c mice were semiquantitatively analysed. Figure 1(a) shows a representative result of expression bands of AIF-1, IFN-γ and β-actin (control). An AIF-1 band in TNBS-treated (colitis) group shows higher intensity than that in no treatment or PBS-treated group. This difference is more clearly shown in Fig. 1(b) in which mean of a relative unit determined by normalization of the density of each AIF-1 band relative to that of the β-actin band is illustrated (three to four mice/each group). The colons from TNBS-treated mice expressed significantly larger amounts of AIF-1 than those in control mice. On the other hand no significant differences in the IFN-γ message were detected among these three groups (Fig. 1c).
Figure 1.
Change of AIF-1 mRNA expression in TNBS induced colitis. (a) RT–PCR analysis. Equal amounts of RNA extracted from colons of TNBS- or PBS-treated mice or no treated mice were reverse transcribed with random hexamer, and RT products were amplified with AIF-1 or IFN-γ primer pairs. The same RT products were amplified with β-actin primer pairs for a control. (b, c) Quantitative analysis of the RT products. A relative unit was determined by normalization of the density of each AIF-1 (b) or IFN-γ (c) band relative to that of the β-actin band as described in Materials and methods. The data represents means ± SEM of three or four mice. Statistical analysis was performed by anova with a Bonferroni/Dunn correction. *P < 0·001.
Severity of TNBS-induced colitis in AIF-1 Tgm
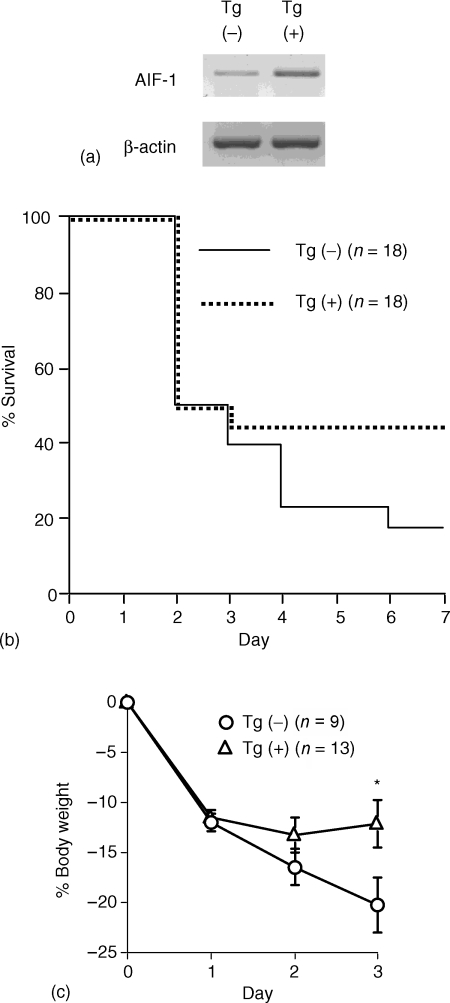
We then analysed TNBS-induced colitis in AIF-1 Tgm and compared with that in non-Tgm littermates. A large amount of AIF-1 message was detected in AIF-1 Tgm colon compared to non-Tgm (Fig. 2a). Five to 7 days after intrarectal administration of TNBS, these AIF-1 Tgm showed higher survival rate than non-Tgm (Fig. 2b). The high survival rate in AIF-1 Tgm appeared to be related to the slight loss of body weight compared to the control (Fig. 2c). The body weight loss in AIF-1 Tgm surviving at 3 days after TNBS treatment was significantly milder than that in non-Tgm. Thus, these findings demonstrate that the TNBS-induced colitis in AIF-1 Tgm is ameliorated compared with that in non-Tgm.
Figure 2.
Severity of TNBS-induced colitis of AIF-1 Tgm ameliorated compared with non-Tgm. (a) RT–PCR analysis. Equal amounts of RNA extracted from colons of AIF-1 Tgm or non-Tgm were reverse transcribed with random hexamer, and RT products were amplified with AIF-1 primer pairs. The same RT products were amplified with β-actin primer pairs for a control. (b) Survival rate of AIF-1 Tgm or non-Tgm with TNBS-induced colitis. Non-Tgm, solid line; Tgm, dotted line. (c) Sequential change of percentage body weight (BW) of AIF-1 Tgm or non-Tgm with TNBS induced colitis which survived more than 3 days after administration of TNBS. The data indicate means ± SEM of nine non-Tgm or 13 Tgm. Non-Tgm, open circle; Tgm, open triangle. Statistical analysis was performed by Student's t-test. *P < 0·05.
Histological analysis of TNBS-induced colitis in AIF-1 Tgm
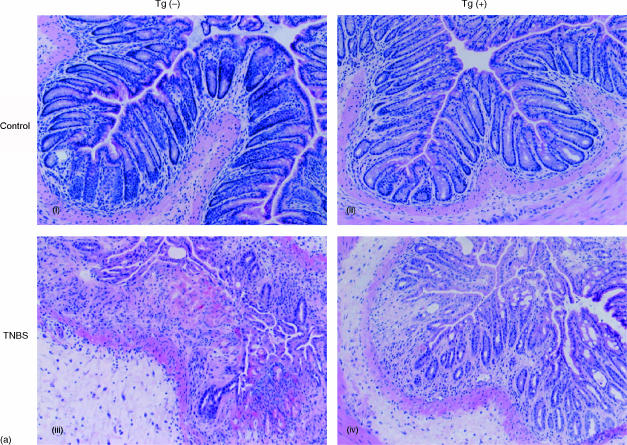
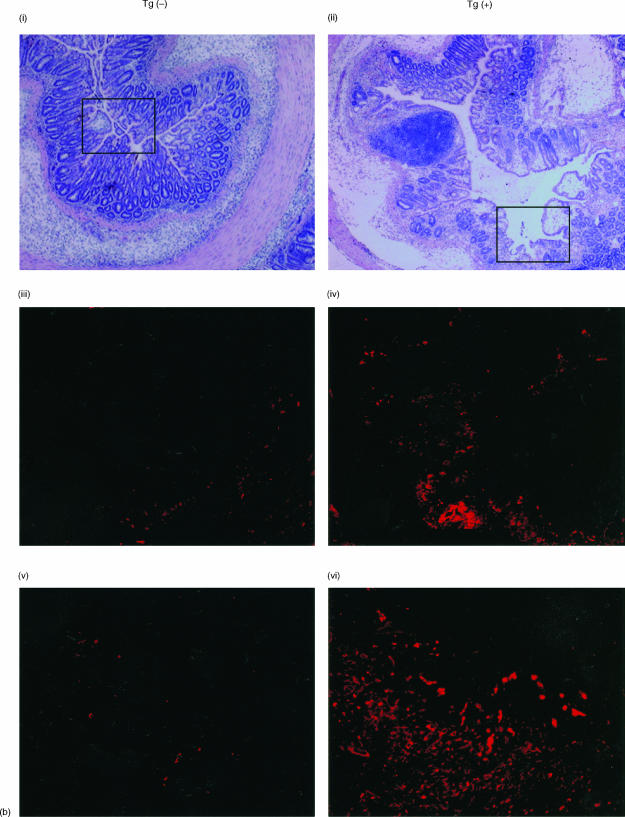
AIF-1 Tgm or non-Tgm were administered TNBS and 3 days later the colons of these mice were removed and histologically analysed. Representative colon sections stained with haematoxylin and eosin are shown in Fig. 3(a). It is demonstrated in this figure that colitis in AIF-1 Tgm is milder than that in non-Tgm (compare Fig. 3a(i) and (ii)). In the non-Tgm group, the damage comprised broad mucosal ulcers with necrosis and haemorrhage, whereas the tissue destruction was mild in the Tgm group. We then analysed immunohistochemically these colitic regions using anti-mouse AIF-1 antibody.12 Fig. 3(b) shows that both epithelial and interstitial cells in the lesion from Tgm are heavily positive for AIF-1. Although only a small number of cells are AIF-1 positive in the lesion of non-Tgm compared to Tgm (Fig. 3b), this population was undetectable in control colons (data not shown).
Figure 3.
Histological analysis of colons from AIF-1 Tgm or non-Tgm. Tgm or non-Tgm that had no treatment or were administered TNBS were killed 3 days later. (a) Representative colon sections stained with haematoxylin and eosin (×100) are shown. (i) Control non-Tgm; (ii) control Tgm; (iii) non-Tgm with TNBS-induced colitis; (iv) Tgm with TNBS-induced colitis. (b) Immunohistochemical analysis of colitic regions. i, iii, v, non-Tgm; ii, iv, vi, Tgm. i, ii, stained with haematoxylin and eosin (×40); iii, iv, boxed areas in (i, ii) stained with anti-AIF-1 (×40); v, vi, stained with anti-AIF-1 (×200).
The histological scores of six to seven mice per group are summarized in Table 1. The mean score in AIF-1 Tgm group was significantly lower than that in non-Tgm group. These findings again demonstrate that the TNBS-induced colitis in AIF-1 Tgm is ameliorated.
Table 1.
Histological score of colons from TNBS-treated AIF-1 Tgm and non-Tgm
| Group | Histological score |
|---|---|
| AIF-1 Tgm (n = 7) | 2·797 ± 0·377* |
| Non-Tgm (n = 6) | 3·958 ± 0·132 |
The data represent mean ± SEM of six or seven mice/group. Statistical analysis was performed by Mann–Whitney U-test.
Significantly lower than that in non-Tgm group (P < 0·05).
Cytokine expression in colitis of AIF-1 Tgm or non-Tgm
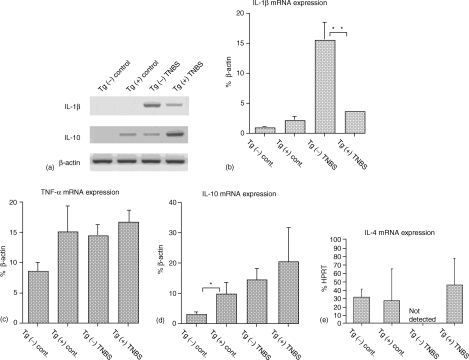
Using RT–PCR analysis expressions of several cytokine mRNA were analysed in TNBS-induced colitis and compared between AIF-1 Tgm and non-Tgm. Mice were administered TNBS or PBS (control) and 3 days later equal amounts of RNA were extracted from these colons and reverse-transcribed with random hexamer. These RT products were then amplified with IL-1β, IL-10, TNF-α or β-actin primer pairs.
Figure 4(a) shows a representative expression pattern of IL-1β and IL-10. A slight band of IL-10 was detected in the colon of control AIF-1 Tgm but not that of control non-Tgm. Both IL-1β and IL-10 bands were observed in colitis of Tgm and non-Tgm. Notably an IL-1β band in colitis of non-Tgm appeared to be more intense than that in colitis of Tgm. A relative unit determined by normalization of the density of each IL-1β, TNF-α or IL-10 band relative to that of the β-actin band is illustrated in Fig. 4(b–d), respectively. It should be noted in Fig. 4(b) that colons from TNBS-treated non-Tgm showed significantly larger amounts of IL-1β message than those from TNBS-treated Tgm. Almost same amounts of TNF-α messages were shown in colons of control Tgm, TNBS-induced colitis of non-Tgm and Tgm (Fig. 4c). However, these amounts were slightly large compared to those in control colons of non-Tgm. Messages of IL-10 were significantly and slightly higher in colons of control Tgm and TNBS-treated Tgm than those in colons of control non-Tgm and TNBS-treated non-Tgm, respectively (Fig. 4d). Colons of TNBS-treated Tgm also showed large amounts of IL-4 mRNA, whereas no IL-4 message was detected in colons of TNBS-treated non-Tgm (Fig. 4e). No differences in amounts of IL-12 p40 and IL-13 messages were seen between Tgm and non-Tgm of either control or TNBS-treated groups (data not shown).
Figure 4.
Cytokine expression of colons from AIF-1 Tgm or non-Tgm. (a) RT–PCR analysis. Equal amounts of RNA extracted from colons of Tgm or non-Tgm that had no treatment or were administered TNBS 3 days previously were reverse transcribed with random hexamer, and RT products were amplified with IL-1β or IL-10 primer pairs. The same RT products were amplified with β-actin primer pairs for a control. (b, c, d, e) Quantitative analysis of the RT products. A relative unit was determined by normalization of the density of each IL-1β (b), TNF-α (c) or IL-10 (d) band relative to that of the β-actin band as described in Materials and Methods. (e) A relative unit of IL-4 was determined by normalization to that of HPRT as described in Materials and Methods. The data represents means ± SEM of seven control or three TNBS administered mice. Statistical analysis was performed by Student's t-test. *P < 0·05. **P < 0·005.
Discussion
AIF-1 was originally identified in infiltrating macrophages in rat cardiac allografts with the chronic cardiac rejection.9 Thereafter it has been reported that AIF-1 is expressed in the local inflammatory lesions of EAE, EAU and EAN.13 Thus, it has been postulated that AIF-1 is involved in enhancement of these inflammatory responses or regulation of the inflammation. However, no exact mechanisms underlying these apparently opposite roles, enhancement or inhibitory influences on the inflammatory responses, of AIF-1 have been demonstrated. It has been reported by immunohistochemical study that AIF-1 is expressed in dendritic cells of the intestine and Kupffer cells of the liver in rats. AIF-1 mRNA is also expressed in the human liver, colon and small intestine.14 However, again functional roles of AIF-1 in the gastrointestinal tract have been obscure.
In the present study, we first demonstrated that the expression of AIF-1 mRNA and protein (data not shown) were increased in acute inflammation sites of TNBS-induced colitis in BALB/c mice. The TNBS-induced colitis as well as EAE, EAU and EAN represent the Th1 type inflammation.28–31 Thus, it seems to us that AIF-1 plays some roles in inflammatory lesions where Th1 dominant immune responses are induced. Indeed, we have reported that the over-expression of AIF-1 in a macrophage cell line results in augmentation of cytokine production of Th2 type.12 Thus, it seemed that the augmented expression of AIF-1 in the colitis lesion reflected some feedback mechanisms leading to suppression of Th1 responses.
To elucidate functional roles of AIF-1, we then analysed TNBS-induced colitis using AIF-1 Tgm. Because a CD11b promoter drives AIF-1 transgenes, it was anticipated that the augmented expression of AIF-1 was detected in cells of myeloid origin including monocyte/macrophage lineage.16 Indeed, these Tgm expressed large amounts of AIF-1 message in various lymphoid tissues and colons (data not shown). We observed ameliorated colitis in the AIF-1 Tgm compared to non-Tgm. In the ameliorated lesions of Tgm markedly augmented expressions of AIF-1 were observed compared to lesions of non-Tgm.
Considerable expressions of AIF-1 were seen not only in myeloid cells but also the colon epithelium of TNBS-treated AIF-1 Tgm. Because the AIF-1 construct is under the control of CD11b promoter, our findings suggest that the CD11b promoter operates even in cells of non-myeloid lineage under certain conditions.32 Alternatively it appears that upon stimulation with TNBS both transgenic and intrinsic AIF-1 products are expressed in AIF-1 Tgm. The augmented expression of transgenic AIF-1 may somehow lead to the substantial expression of intrinsic AIF-1 in the epithelium. It has been reported that AIF-1 is expressed in various types of cells.10,12,19 These points should be elucidated in further studies. At any rate the augmented expression of AIF-1 may be associated with low levels of IL-1β and high levels of IL-10 and IL-4 in the lesion of Tgm treated with TNBS, although differences in the expression pattern were seen between IL-10 and IL-4.
On the basis of these findings and previous one12 we would like to postulate that the Th2 shift following stimulation with antigens is one of the mechanisms underlying the ameliorated TNBS-induced colitis in AIF-1 Tgm. Thus, AIF-1 appears to suppress the Th1-type responses via the enhanced production of Th2 cytokines. Recently we detected an increased AIF-1 expression in uteri at the preimplantation period in allogeneic pregnancy.19 This finding may be related to the regulatory role of AIF-1 in Th1 responses of the maternal immune system to semiallogeneic fetal antigens. We are now investigating various immune responses in AIF-1 Tgm to see whether the immune system of these Tgm is really biased to the Th2 type.
Acknowledgments
We thank Dr Daniel G. Tenen for providing us his expression vector, Dr Toshihiko Iwanaga for his valuable advice on colitis models. This study was supported in part by a Grant-in-Aid for Scientific Research (S, C) by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This study was also supported in part by Japanese Research Group for Behçet's Disease (H14-Tokushitsu-06) from the Ministry of Welfare, Labor and Health, Japan and the Tomakomai East Hospital Foundation.
References
- 1.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 3.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–82. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 4.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–4. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 5.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 6.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 8.Ni J, Chen SF, Hollander D. Effects of dextran sulfate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut. 1996;39:234–41. doi: 10.1136/gut.39.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utans U, Arceci RJ, Yamashita Y, Russell ME. Cloning and characterization of allograft inflammatory factor-1: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. J Clin Invest. 1995;95:2954–62. doi: 10.1172/JCI118003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Autieri MV. cDNA cloning of human allograft inflammatory factor-1. tissue distribution, cytokine induction, and mRNA expression in injured rat carotid arteries. Biochem Biophys Res Commun. 1996;228:29–37. doi: 10.1006/bbrc.1996.1612. [DOI] [PubMed] [Google Scholar]
- 11.Utans U, Quist WC, McManus BM, Wilson JE, Arceci RJ, Wallace AF, Russell ME. Allograft inflammatory factor-1. A cytokine-responsive macrophage molecule expressed in transplanted human hearts. Transplantation. 1996;61:1387–92. doi: 10.1097/00007890-199605150-00018. [DOI] [PubMed] [Google Scholar]
- 12.Watano K, Iwabuchi K, Fujii S, Ishimori N, Mitsuhashi S, Ato M, Kitabatake A, Onoé K. Allograft inflammatory factor-1 augments production of interleukin-6, -10 and -12 by a mouse macrophage line. Immunology. 2001;104:307–16. doi: 10.1046/j.1365-2567.2001.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluesener HJ, Seid K, Kretzschmar J, Meyermann R. Allograft-inflammatory factor-1 in rat experimental autoimmune encephalomyelitis, neuritis, and uveitis: expression by activated macrophages and microglial cells. Glia. 1998;24:244–51. doi: 10.1002/(sici)1098-1136(199810)24:2<244::aid-glia9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZW, Ahren B, Östenson CG, et al. Identification, isolation, and characterization of daintain (allograft inflammatory factor 1), a macrophage polypeptide with effects on insulin secretion and abundantly present in the pancreas of prediabetic BB rats. Proc Natl Acad Sci U S A. 1997;94:13879–84. doi: 10.1073/pnas.94.25.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mentschel J, Deininger MH, Schluesener HJ, Claus R. Effects of malnutrition on the expression of daintain/AIF-1 in the gut mucosa of pigs. J Vet Med A Physiol Pathol Clin Med. 2002;49:184–8. doi: 10.1046/j.1439-0442.2002.00426.x. [DOI] [PubMed] [Google Scholar]
- 16.Pahl HL, Rosmar AG, Tenen DG. Characterization of the myeloid specific CD11b promoter. Blood. 1992;79:865–70. [PubMed] [Google Scholar]
- 17.Ato M, Iwabuchi K, Shimada S, Mukaida N, Onoé K. Augmented expression of tumour necrosis factor-α induced by lipopolysaccharide in spleen of human monocyte chemoattractant protein-1 transgenic mouse enhances the lipopolysaccharide sensitivity of the marginal zone macrophages. Immunology. 2002;106:554–63. doi: 10.1046/j.1365-2567.2002.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ameho CK, Adjei AA, Harrison EK, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor-α production in trinitrobenzene sulfonic acid induced colitis. Gut. 1997;41:487–93. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada S, Iwabuchi K, Watano K, Shimizu H, Yamada H, Minakami H, Onoé K. Expression of allograft inflammatory factor-1 in mouse uterus and poly (I:C)-induced fetal resorption. Am J Reprod Immunol. 2003;50:104–12. doi: 10.1034/j.1600-0897.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Mori K, Kobayashi S, Inobe M, Jia WY, Tamakoshi M, Miyazaki T, Uede T. In vivo cytokine gene expression in various T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Autoimmunity. 1994;17:49–57. doi: 10.3109/08916939409014658. [DOI] [PubMed] [Google Scholar]
- 22.Murray LJ, Lee R, Martens C. In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur J Immunol. 1990;20:163–70. doi: 10.1002/eji.1830200124. [DOI] [PubMed] [Google Scholar]
- 23.Allen RD, Staley TA, Sidman CL. Differential cytokine expression in acute and chronic murine graft-versus-host-disease. Eur J Immunol. 1993;23:333–7. doi: 10.1002/eji.1830230205. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara I, Yamada H, Mizuno S, Li CY, Nakayama T, Taniguchi M. Mycobacterial infection in natural killer T cell knockout mice. Tuberculosis. 2002;82:97–104. doi: 10.1054/tube.2002.0331. [DOI] [PubMed] [Google Scholar]
- 25.Lee T-S, Yen H-C, Pan C-C, Chau L-Y. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–42. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 26.Masuda A, Yoshikai Y, Aiba K, Matsuguchi T. Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distinctly regulated by c-Jun N-terminal kinase and p38 pathways. J Immunol. 2002;169:3801–10. doi: 10.4049/jimmunol.169.7.3801. [DOI] [PubMed] [Google Scholar]
- 27.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–41. [PubMed] [Google Scholar]
- 28.Saoudi A, Kuhn J, Huygen K, de Kozak Y, Velu T, Goldman M, Druet P, Bellon B. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur J Immunol. 1993;23:3096–103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]
- 29.Namba K, Ogasawara K, Kitaichi N, et al. Amelioration of experimental autoimmune uveoretinitis by pretreatment with a pathogenic peptide in liposome and anti-CD40 ligand monoclonal antibody. J Immunol. 2000;165:2962–9. doi: 10.4049/jimmunol.165.6.2962. [DOI] [PubMed] [Google Scholar]
- 30.Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–43. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Link H, Mix E, Olsson T, Huang WX. Th1-like cell responses to peripheral nerve myelin components over the course of experimental allergic neuritis in Lewis rats. Acta Neurol Scand. 1994;90:19–25. doi: 10.1111/j.1600-0404.1994.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 32.Tenen DG, Hromas R, Licht JD, Zhang D-E. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. [PubMed] [Google Scholar]