Abstract
Interferon-gamma (IFN-γ) is closely associated with the generation of cell-mediated immunity and resistance to intracellular parasites. Interleukin-18 (IL-18) was known to strongly induce IFN-γ production by T cells and natural killer (NK) cells. In order to determine whether injection with DNA encoding IL-18 can stimulate the resistance to Mycobacterium avium complex (MAC) infection, the mature IL-18 cDNA with κ leader sequence was cloned under control of the cytomegalovirus (CMV) promoter (TcCMVIL-18) and its effect on MAC infection was investigated in genetically susceptible BALB/c mice. Injection with the TcCMVIL-18 DNA during intranasal infection with MAC resulted in a significant decrease in bacterial load of lung during the entire 8-week observation period, while injection with the TcCMV control DNA did not. Lung cells in mice injected with the TcCMVIL-18 DNA showed persistent production of IFN-γ throughout the 8-week period. Furthermore, immunization with the TcCMVIL-18 DNA induced and maintained significantly higher levels of cytotoxic activity and nitric oxide production by lung cells than immunization with the TcCMV control vector. This work suggests that IL-18 DNA vaccination may be useful in the immunotherapeutic or immunoprotection approaches of infections by intracellular parasites such as mycobacteria.
Introduction
Bacteria of the Mycobacterium avium complex (MAC) are facultative intracellular pathogens and the most common cause of disseminated bacterial infection in acquired immune deficiency syndrome (AIDS) patients. Acquisition of a MAC infection significantly shortens the life-span of these patients compared with that of patients with the same T-cell counts.1 Control of MAC infection requires the presence of activated CD4+ T cells that produce an array of cytokines, including interferon-γ (IFN-γ), involved in activating macrophage bactericidal activity. Studies involving IFN-γ gene and IFN-γ receptor gene knockout mice showed that IFN-γ, produced by activated CD4+ T cells and natural killer (NK) cells, played an essential role in protective cellular immunity against mycobacteria.2,3 Interleukin-18 (IL-18), first designated as an IFN-γ-inducing factor, is a newly identified cytokine of T helper 1 (Th1) type, and the cDNAs encoding murine and human IL-18 have recently been cloned.4,5 IL-18 has been known to induce IFN-γ production by both CD4+ T cells and NK cells, and to stimulate naive T cells to promote the development of Th1 (IFN-γ-producing) cells.6 The development of a Th1 response and IFN-γ production are central to eradication of various pathogens including Cryptococcus neoformans,7 Leishmania major,8 and Mycobacterium leprae.9 IL-18 knockout mice were susceptible to the infection of the parasite Leishmania major and Staphylococcus aureus, while the wild-type mice were highly resistant to the infection of the parasites.10 The infected IL-18 knockout mice produced significantly lower levels of IFN-γ and larger amounts of IL-4 compared with similarly infected wild-type mice. IL-18 therefore has been known to play a decisive role in host defence against intracellular infectious micro-organisms. Recombinant cytokines have been clinically used in the treatment of human diseases including cancer and infectious diseases.11,12 However, a single injection is not sufficient for a protective or therapeutic effect13,14 and recombinant cytokines should be highly purified before use. Alternatively the cytokine concentration necessary for a therapeutic effect could be secured by administration of DNA encoding an inserted cytokine gene.15,16 Previous reports showed that direct injection of cytokine genes into muscle resulted in the characteristic biological actions of these cytokines in vivo and could modulate the immune response.17 Immunization with cytokine DNA delayed tumour formation and promoted antitumour immunity.18 Coinjection of plasmids encoding cytokines can have a substantial effect on the immune response to a plasmid-encoded antigen.19 Furthermore, because DNA vaccines are relatively inexpensive and easy to manipulate and use, their immunogenicity and efficacy have been analysed in a large number of systems and results from preclinical studies have supported human clinical studies.20 Clinical trials are currently being conducted for diseases such as cancer,21 human immunodeficiency virus (HIV) infection,22 or malaria.23 In this study we investigated the effects of DNA-based delivery of IL-18 on MAC infection. We demonstrate here that IL-18 DNA vaccination significantly induces in vivo persistent IFN-γ production and bactericidal properties during MAC infection, leading to the reduction of a bacterial load in MAC-infected mice for prolonged periods.
Materials and methods
Reagents, antibodies and animals
Middlebrook 7H10 agar, Batch Middlebrook OADC enrichment solution and Middlebrook 7H9 broth were purchased from Difco Laboratories (Detroit, MI). Anti-murine IFN-γ monoclonal antibodies (mAbs; R46A2 and XMG1.2) were purified from ascitic fluids by ammonium sulphate precipitation followed by diethylaminoethyl (DEAE)–Sephacel chromatography (Sigma, St. Louis, MO), and rabbit polyclonal anti-mIL-18 antibody was obtained from Dr I. Choi (KRIBB, Korea). mAb-secreting hybridomas, BALB/3T3 cells, COS-7 cells or P815 cells were obtained from the ATCC (American Type Culture Collection, Rockville, MD). The cells were maintained at 37° in a humidified 5% CO2 in RPMI-1640 or Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and antibiotics (growth medium). Six- to 8-week-old female BALB/c mice were obtained from the Charles River Laboratories (Wilmington, MA), and maintained in pathogen-limited conditions.
Construction of an expression plasmid carrying murine IL-18 cDNA
A mammalian expression plasmid (donated by Dr M. E. Reff)24 containing a SV40 origin of replication and designed for expression of immunoglobulin genes, was modified to eliminate most of the immunoglobulin coding regions as well as the neomycin resistance gene. The TcCMVIL-18 was constructed by first inserting the mature IL-18 cDNA in frame with the human immunoglobulin kappa leader sequence, to allow for secretion of the translated protein. The IL-18 gene was cloned by polymerase chain reaction (PCR) from previously cloned cDNA constructs (obtained from Dr D. Lim, KRIBB) using primers containing the desired restriction sites; mouse IL-18 sense primer (Dralll-IL-18 5′-TTTCCACGATGTGAAACTTTGGCCGACTT-3′) and mouse IL-18 antisense primer (BglII-IL-18 5′-GAAGATCTCTAACTTTGATGTAAGTT-3′). The plasmids used in this study were, respectively, electroporated into Escherichia coli, and purified from large-scale cultures by alkaline lysis and caesium chloride density gradient centrifugation. The endotoxin level of the purified plasmids was < 20 EU(endotoxin unit)/mg DNA, as detected using the Limulus amoebocyte lysate (LAL) assay kit (BioWhittaker, Walkersville, MD).
Transfection, sodium dodecyl sulphate–polyacrylamide gel elctrophoresis (SDS–PAGE), and Western blotting
COS-7 cells were transfected with the TcCMVIL-18 or TcCMV DNA using DEAE–dextran in a standard protocol.25 Culture supernatants from the transfected cells were harvested after 3 days, and secreted protein was immunoprecipitated using anti-mIL-18 antibody-conjugated CNBr-activated Sepharose 4B resin (Sigma). The resin was washed three times with 0·1% Tween 20 in Tris buffer (pH 8·0), and then the precipitated protein was eluted in SDS–PAGE sample buffer. Following electrophoresis, the protein was transferred into nitrocellulose by electroblotting using a semidry blotter. The blot was then immunostained with anti-mIL-18 antibody and developed by ECL system (Amersham Life Sciences, Arlington Height, IL).
Intramuscular injection of mice with the IL-18-expressing plasmid
Mice were injected i.m. with varying concentrations of DNA in 100 µl of 0·85% normal saline into each quadricep muscle by using a 28-gauge insulin syringe. The quadricep muscles were visualized by making a 1-cm incision in the skin with a microdissecting scissor. The injection depth of the needle was adjusted to 2 mm by using a steel collar. One day after the injection, mice were infected intranasally with 105 MAC organisms inoculated onto the external nares with a micropipette as described in the figure and table legends. The virulent MAC strain 101 was used throughout the study (obtained from Dr P. Gangadharam, Chicago, IL). Single cell suspensions from transparent colonies were obtained and the number of micro-organisms was confirmed by colony-counting techniques as previously described.26
Determination of the number of micro-organisms from lungs of mice infected with MAC
At intervals after infection, five mice per group were killed and the lungs were removed from each mouse aseptically, and homogenized in 5 ml of sterile phosphate-buffered saline (PBS) with a tissue homogenizer (Janke and Kunkle, Breisgau, Germany). Suitable dilutions were placed on Middlebrook 7H11 agar for viable counts, and the plates were incubated in a humidified container at 37° for 7 days before counting.
Preparation of lung cells
At the times indicated during infection, designated mice were killed and lungs were aseptically removed. Single cell suspensions of lung cells were prepared as previously described.27 For cytokine assay, 5 × 105 lung cells were incubated in RPMI-1640 medium containing 1 µg/ml concanavalin A (Con A; Sigma). After 72 hr, supernatants were removed from each culture for measurement of IFN-γ.
Cytokine assays
The quantities of IFN-γ in culture supernatants were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) using mAb specific for IFN-γ as previously described.28 The mAb for coating the plates and the biotinylated second mAb were rat anti-mouse IFN-γ (HB170) and biotinylated rat anti-mouse IFN-γ (XMG1.2), respectively. The lower limit of detection was 125 pg/ml for IFN-γ. The biological activity of IL-18 produced by transfectants was determined by the ability to stimulate IFN-γ production in spleen cells in vitro as previously described.29 2 × 106 spleen cells were cultured in 2 ml of cell culture medium in 12-well plates in the presence of transfectants' supernatants. IFN-γ levels in the supernatants were determined by ELISA.
NO2− assay
Cultures of 2 × 105 lung cells pooled from five mice per group with or without stimulation by 107 live MAC were incubated in 200 µl of DMEM−10% fetal bovine serum in 96-well microtitre plates for 72 hr. The supernatants were then harvested and assayed for NO by the Griess reaction.30 Briefly, culture supernatants (50 µl) were mixed with 100 µl of 1% sulfanilamide (Sigma) and 100 µl of 0·1% N-1-naphthylethylenediamine dihydrochloride in 2·5% polyphosphoric acid at room temperature for 5 min. A540 was measured and NO2− was quantified by comparison to Na(NO2) as a standard.
In vitro cytotoxic assay
The cytotoxicity measurements were performed using a standard 4 hr 51Cr-release assay as previously described.31 The percentage of specific cytolysis was calculated as: [(test c.p.m. − spontaneous c.p.m.)/(maximum c.p.m. − spontaneous c.p.m.)] × 100, where c.p.m. is counts per minute.
In vivo administration of anti-IFN-γ mAb
In some experiments, the course of infection was modulated by i.p. administration of a neutralizing anti-mIFN-γ antibody (XMG1.2, rat immunoglobulin G1; IgG1) or isotype control antibody (rat IgG1). The antibody treatment started one day before MAC infection and continued for 6 days every other day and then one a week until killing of the mice.
Statistical analyses
Student's t-test and one-way anova were used to determine the statistical differences between the various experimental and control groups. P-values < 0·05 were considered significant.
Results
Inhibition of bacterial growth in MAC-infected mice by IL-18 DNA vaccination
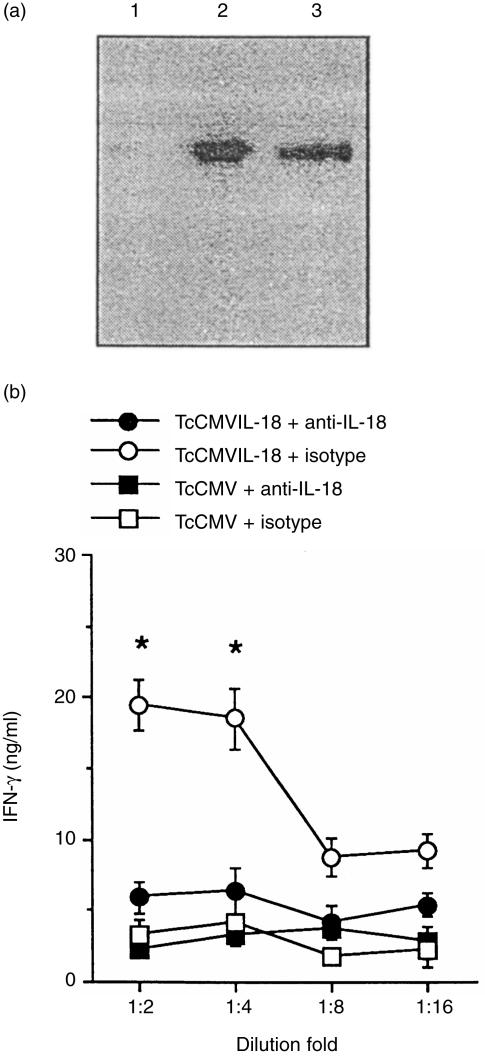
The expression plasmid encoding a mature IL-18 (TcCMVIL-18) was constructed as described in Materials and Methods. It was tested for the ability to produce the proper recombinant IL-18 by transient transfection of COS-7 cells. As shown in Fig. 1(a), culture supernatant contained a protein of the right size recognized by anti-mIL-18 antibody. To further determine whether the COS-7 cell-secreted protein retained bioactivity for the IL-18, IFN-γ induction assay was performed as described in Materials and Methods. As shown in Fig. 1(b), culture supernatants of COS-7 cells transfected with the TcCMVIL-18 DNA enhanced IFN-γ production in spleen cells, which was blocked by anti-IL-18 antibody, indicating that IL-18 in the culture supernatants is functional. In contrast, the culture supernatants from COS-7 cells transfected with the TcCMV control vector did not enhance IFN-γ production in spleen cells. Importantly, following i.m. administration of the TcCMVIL-18 DNA, IL-18 protein was evident in serum in minimal amounts in some animals by day 2 and peaked around 7–10 days postinoculation (38·5 ± 6·7 pg/ml).
Figure 1.
Bioactivity of IL-18 produced by COS-7 cells transfected with the TcCMVIL-18 plasmid. (a) Expression of IL-18 protein from COS-7 cells transfected with the TcCMVIL-18 plasmid. IL-18 protein was immunoprecipitated from culture supernatants of the transfected COS-7 cells, electrophoresed under non-reducing condition, blotted, and detected by anti-mIL-18 antibody. Lanes 1 and 3 represent the culture supernatants of the transfected cells with the TcCMV and TcCMVIL-18 DNA, respectively. Lane 3 represents recombinant IL-18. (b) Bioactivity of IL-18 protein produced by COS-7 cells transfected with the TcCMVIL-18 plasmid. Culture supernatants were titered in the presence of either anti-mIL-18 antibody or isotype control antibody. The values represent the mean±standard deviations of triplicate determinations. ⋆P<0·01, relative to any other groups.
To test the effect of TcCMVIL-18 DNA injection on the resistance to MAC infection, BALB/c mice were injected with the TcCMVIL-18 or TcCMV plasmid as a control, followed by the infection with MAC one day later. At 3 and 8 weeks postinfection, mice were killed and lungs were collected for bacterial analysis. As seen in Table 1, during the first 3 weeks of infection, a significant reduction in infection with MAC was seen in mice injected with the TcCMVIL-18 DNA. The numbers of bacteria in lung culture were approximately 2·5- to 20-fold lower in the TcCMVIL-18 DNA-injected mice than those in the TcCMV DNA-injected mice (P < 0·01). Importantly, by the 8 weeks postinfection, bacterial numbers in TcCMVIL-18 DNA-injected mice sustained at low levels, compared with those in TcCMV DNA-injected mice. The numbers of bacteria were approximately 5·0- to 80-fold lower in the TcCMVIL-18 DNA-injected mice than those in the TcCMV DNA-injected mice at 8 weeks after MAC infection (P < 0·001). Ziehl–Neelsen staining showed a large decrease of infected intracellular bacteria in the lungs of TcCMVIL-18 DNA-injected mice compared with those of TcCMV DNA-injected mice at 8 weeks postinfection (data not shown). The TcCMVIL-18 DNA vaccination stimulated the resistance to MAC in a dose-dependent manner (Table 1). Furthermore, injection with the TcCMVIL-18 DNA prolonged the survival period of MAC-infected mice, compared with that of TcCMV DNA-injected mice.
Table 1.
Effect of IL-18 DNA vaccination on bacterial growth in MAC-infected mice
| No. of bacteria in lung | ||||
|---|---|---|---|---|
| Treatment* | 3 week infected | 8 week infected | Survival days | |
| Uninjected | 5·8 ± 0·2 | 7·6 ± 0·3 | 62 ± 4·7 | |
| TcCMV | (20 µg) | 5·9 ± 0·2 | 7·9 ± 0·4 | ND‡ |
| (100 µg) | 5·7 ± 0·1 | 7·3 ± 0·2 | ND | |
| (300 µg) | 6·0 ± 0·2 | 7·6 ± 0·1 | 62 ± 2·2 | |
| TcCMVIL-18 | (20 µg) | 5·6 ± 0·3 | 7·0 ± 0·2 | ND |
| (100 µg) | 5·0 ± 0·1† | 6·1 ± 0·3† | 73 ± 3·5† | |
| (300 µg) | 5·1 ± 0·1† | 6·0 ± 0·2† | 77 ± 4·7† | |
Mice were i.m. injected with the TcCMVIL-18 or TcCMV DNA, or not injected. One day later, the mice were intranasally infected with 105 MAC. The values (no. of bacteria in lung) represent the mean log bacterial numbers±standard deviations of five mice, and the data (survival days) are the mean±standard deviations of eight mice. The experiment was repeated twice with similar results.
P < 0·01, relative to groups injected with the TcCMV DNA or uninjected.
Not done.
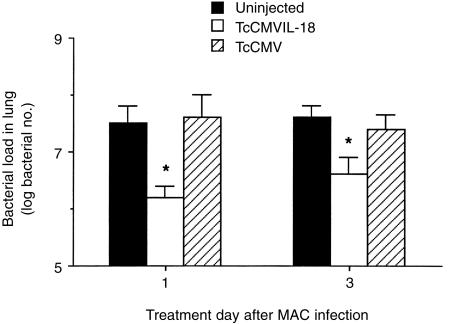
We next investigated the effect of the TcCMVIL-18 DNA in established MAC-infected mice. Mice were first infected with MAC and, 1 or 3 days later, treated with the TcCMVIL-18 DNA, TcCMV DNA, or uninjected. The bacterial numbers of lungs in mice were determined at 8 weeks after MAC infection. As shown in Fig. 2, the bacterial load in lungs was significantly reduced in mice injected with the TcCMVIL-18 DNA, even if the cells were administered into MAC-infected mice at 3 days postinfection (P < 0·05).
Figure 2.
Effect of the TcCMVIL-18 DNA on bacterial growth in mice previously infected with MAC. Mice were intranasally infected with 105 MAC. One and 3 days later, the infected mice were i.m. injected with 300 µg of TcCMVIL-18 DNA or TcCMV DNA, or uninjected. Eight weeks after the infection, the bacterial numbers in lung were determined. Data represent the mean log bacterial numbers±standard deviations of five mice. The experiment was repeated twice with similar results. *P <0·05, relative to the groups injected with the TcCMV DNA or uninjected.
IL-18 DNA vaccination significantly enhanced IFN-γ production by lung cells
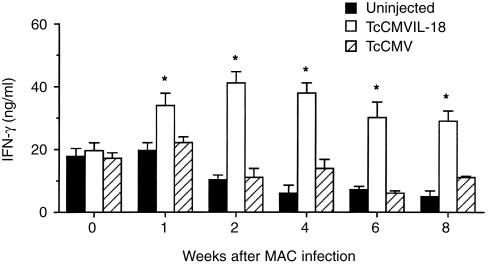
IL-18 is well known to strongly induce IFN-γ production by T and NK cells.6 Experiments were performed to determine whether the TcCMVIL-18 DNA vaccination enhanced IFN-γ production by lung cells, compared with the TcCMV DNA vaccination as a control. At various times after MAC infection, lung cells were cultured in vitro with mitogenic stimulation, and 72-hr culture supernatants were tested for the presence of IFN-γ. As seen in Fig. 3, for Con-A-stimulated lung cells taken from the TcCMVIL-18 DNA-injected mice, IFN-γ production reached maximal levels at 2 weeks. Furthermore, as shown in Fig. 3, lung cells derived from the TcCMVIL-18 DNA-injected mice sustained the high levels of IFN-γ production for the entire 8-week observation period. In contrast, in vitro cultures of lung cells from the TcCMV DNA-injected mice secreted IFN-γ at low levels similar to those of the uninjected mice.
Figure 3.
In vitro production of IFN-γ by lung cells from the TcCMVIL-18 DNA-injected mice following MAC infection. Mice were i.m. injected with the TcCMVIL-18 or TcCMV DNA, or not injected. One day later, the mice were intranasally infected with 105 MAC. Single cell suspensions of lung cells were stimulated in vitro with 1 µg/ml Con A. Culture supernatants were collected after 72 hr and assayed for IFN-γ by sandwich ELISA. The values represent the mean±standard deviations of triplicate determinations from three mice killed at each time point. The data shown are representative of two similar experiments. The asterisk indicates statistically significant differences (P < 0·01) compared with the groups injected with the TcCMV DNA at each time point.
IL-18 DNA vaccination efficiently induced and maintained the bactericidal properties of lung cells during MAC infection
The production of NO is one mechanism used by infected cells to kill invading bacteria.32 To determine the effect of the TcCMVIL-18 DNA vaccination on the NO production, BALB/c mice were i.m. injected with the TcCMVIL-18 or TcCMV DNA, followed by infection with MAC. At 3 and 8 weeks postinfection, mice were killed and lung cells were tested for the NO production. As seen in Table 2, during the first 3 weeks of infection, the levels of NO production by lung cells from the TcCMVIL-18 DNA-injected mice showed a significant increase compared with those of TcCMV DNA-injected mice. Furthermore, by 8 weeks postinfection, the levels of NO production by lung cells in the TcCMVIL-18 DNA-injected mice were significantly higher than those in the TcCMV DNA-injected mice.
Table 2.
Effect of IL-18 DNA vaccination on NO production by lung cell cultures following MAC infection
| NO2− level (μm) | ||
|---|---|---|
| Treatment* | 3 week infected | 8 week infected |
| Uninfected | < 2·0 | < 2·0 |
| Intact infected | 35·2 ± 4·8 | 58·8 ± 1·3 |
| TcCMV (100 µg) | 29·8 ± 6·0 | 60·4 ± 5·2 |
| (300 µg) | 41·3 ± 2·8 | 66·7 ± 4·9 |
| TcCMVIL-18 (100 µg) | 113·7 ± 7·2† | 210·5 ± 13·7† |
| TcCMVIL-18 (300 µg) | 128·3 ± 5·7† | 207·2 ± 18·8† |
Mice were i.m. injected with the TcCMVIL-18 or TcCMV DNA, or not injected. One day later, the mice were intranasally infected with 105 MAC. Cultures of 2 × 105 lung cells were incubated for 72 hr in 200 µl volumes in a 96-well plate. Culture supernatants were harvested and assayed for nitrate levels. Data are the means±standard deviations of triplicate determinations from cultures pooled from five mice infected for 3 or 8 weeks. Experiments were repeated twice with similar results.
P < 0·001, relative to groups injected with the TcCMV DNA.
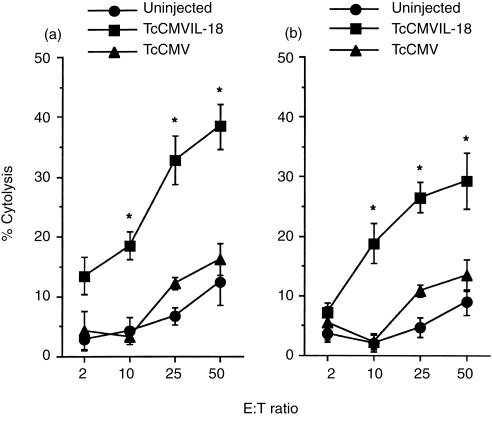
Next, lung cells were used in an in vitro cytotoxicity assay to test their ability to lyse the macrophage-sensitive cell line P815. Lung cells from TcCMVIL-18 DNA-injected mice showed significantly higher levels of cytotoxicity than those in mice injected with the TcCMV DNA at 3 weeks postinfection (Fig. 4). Importantly, the higher levels of cytotoxicity were maintained during the entire 8-week observation period in mice injected with the TcCMVIL-18 DNA.
Figure 4.
Specific lysis of P815 cells by lung cells from the TcCMVIL-18 DNA-injected mice following MAC infection. Mice (five per group) were injected with either TcCMVIL-18 or TcCMV DNA, followed by intranasal infection with 105 MAC 3 (a) or 8 (b) weeks prior to killing. The data are the mean±standard deviations of triplicate determinations. Experiments were repeated three times with similar results. The asterisk indicates statistically significant differences (P <0·01) compared with the groups injected with the TcCMV DNA.
Therefore, the IL-18 DNA vaccination induced in vivo persistent bactericidal properties in MAC-infected mice.
Treatment with a neutralizing anti-IFN-γ mAb abrogated the resistance to MAC infection in mice injected with the TcCMVIL-18 DNA
To further determine whether the increased IFN-γ production resulted in the induction of anti-mycobacterial activity, the mice were injected with the TcCMVIL-18 DNA immediately followed by treatment with anti-IFN-γ antibody or isotype control antibody as described in Materials and Methods. One day later, the mice were infected with MAC, and the anti-mycobacterial activities were determined.
As shown in Table 3, treatment with anti-IFN-γ antibody significantly decreased the anti-mycobacterial activity induced with the TcCMVIL-18 DNA vaccination. The numbers of bacteria in the TcCMVIL-18 DNA-injected mice with anti-IFN-γ antibody treatment were significantly higher than those in the TcCMVIL-18 DNA injected mice with isotype antibody treatment. In addition, the levels of nitric oxide production and cytotoxic activity against P815 cells by lung cells were significantly low in the treated mice with anti-IFN-γ antibody, compared with those in the treated mice with isotype control antibody (Table 3).
Table3.
Effect of anti-mIFN-γ antibody treatment on anti-mycobacterial activity in mice injected with the TcCMVIL-18 DNA
| Treatment* | No. of bacteria in lung | NO2− levels (µm) | % Cytolysis at 50 : 1 (E : T) |
|---|---|---|---|
| TcCMV DNA | 5·9 ± 0·3 | 30·6 ± 4·8 | 13·8 ± 3·2 |
| Anti-IFN-γ antibody | 6·1 ± 0·2 | 22·3 ± 5·7 | 18·1 ± 2·5 |
| Isotype antibody | 5·8 ± 0·2 | 31·8 ± 6·4 | 12·8 ± 1·6 |
| TcCMVIL-18 DNA | 5·1 ± 0·1 | 120·8 ± 5·5 | 29·7 ± 3·3 |
| Anti-IFN-γ antibody | 6·2 ± 0·3† | 36·2 ± 5·1† | 12·6 ± 1·8† |
| Isotype antibody | 5·0 ± 0·2 | 108·7 ± 6·7 | 33·6 ± 4·2 |
Mice were injected with 300 µg of each DNA and either anti-IFN-γ mAb or isotype antibody, as described in Materials and Methods. One day later, the mice were intranasally infected with 105 MAC. The bacterial numbers in lung (the mean log bacterial numbers±standard deviations of three mice), and nitrate levels and cytotoxic activity in lung cells were determined. The values represent the mean±standard deviations of triplicate determinations from cultures pooled from three mice infected for 3 weeks. The values are representative of three experiments.
P < 0·001, relative to a TcCMVIL-18 DNA-injected group treated with isotype antibody.
Discussion
In this study we have demonstrated that IL-18 DNA vaccination induced in vivo persistent IFN-γ production and bactericidal activities, leading to efficiently stimulate the resistance to MAC infection. This was the case if the IL-18 DNA was administered into genetically susceptible BALB/c mice one day before MAC infection, or at 1 or 3 days postinfection. The efficacy was dependent on the injection dose of the TcCMVIL-18 DNA (Table 1).
The reason why the IL-18 DNA vaccination induces anti-mycobacterial activity is not clear. However, the activity may result from the increased levels of IFN-γ production in the immunized mice with the TcCMVIL-18 DNA. This point was supported by several lines of evidence. First, IL-18 DNA vaccination significantly increased the levels of IFN-γ production by lung cells (Fig. 3), and the levels were closely correlated with anti-mycobacterial activity as demonstrated by nitric oxide (NO) production and cytotoxic activity against P815 cells (Table 2 and Fig. 4). In addition, depletion of IFN-γ in the TcCMVIL-18 DNA-injected mice with a neutralizing anti-IFN-γ antibody significantly decreased the nitric oxide production and cytotoxic activity against P815 cells, resulting in the substantial increase of bacterial load in lung (Table 3). The role of NO on MAC infection is likely dependent on the type of MAC strains. MAC was more effectively cleared in mice genetically deficient in the inducible NO synthase (iNOS−/−) gene than in wild-type mice, suggesting that NO is not involved in the anti-mycobacterial mechanisms of MAC-infected macrophages.33 However, others reported that the intracellular growth of NO-sensitive MAC strains was significantly suppressed by NO generated by IFNγ-stimulated macrophages while that of NO-resistant MAC strains was not.34,35
Control of MAC infection requires the presence of activated CD4+ T cells, especially Th1 cells, which produce an array of cytokines, including IFN-γ, involved in activating macrophage bactericidal activity. Studies involving IFN-γ gene and IFN-γ receptor gene knockout mice showed that IFN-γ, produced by activated Th1 cells and perhaps NK cells, played an essential role in protective cellular immunity against mycobacteria.2,3 In this report injection with the TcCMVIL-18 DNA increased and sustained the levels of IFN-γ in lung cells containing NK and Th1 cells, suggesting that the TcCMVIL-18 DNA significantly induced Th1-like immune response for prolong periods.
The persistent effectiveness of the TcCMVIL-18 DNA to induce the resistance to MAC infection may be closely correlated with the IFN-γ production and cytotoxic activity for prolonged periods in lung cells from the immunized mice with the TcCMVIL-18 DNA. During the entire 8 week-observation period, the TcCMVIL-18 DNA-injected mice retained the high levels of IFN-γ production and the activation status of macrophages. Prolonged activation of macrophages by the TcCMVIL-18 DNA vaccination is likely responsible for the more efficient bacterial killing and resistance to MAC infection because cytotoxic activities are increased in activated macrophages, and MAC organisms proliferate less rapidly or may be killed by activated macrophages.36–38 Therefore, the TcCMVIL-18 DNA may function as an effective vehicle to deliver IL-18, resulting in the production of IFN-γ for prolonged periods. Furthermore, immunization with the TcCMVIL-18 DNA did not show any toxicity including high fever, weight loss, etc. Lymphoid hyperplasia or tissue necrosis was also not noted in liver, spleen, or lungs in mice receiving the TcCMVIL-18 or TcCMV DNA (data not shown).
DNA vaccination has been used to elicit protective antibody and cell-mediated immune responses in a wide variety of preclinical animal models for viral, bacterial, and parasitic diseases,39 and now moved to clinical trials.40 Recent study showed that mucosal administration of plasmid DNA led to rapid and widespread distribution around the body, and dissemination likely occurred via the bloodstream because plasmid DNA was present in blood plasma and also in several tissues including draining lymph nodes, spleen and liver.41 Of particular interest, DNA immunization with plasmids encoding cytokines represents a valuable method of modulating the severity of on-going immunoinflammatory diseases.42,43 Furthermore, DNA immunization promised to be a valuable means of achieving immunomodulation by administering DNA for cytokines along with antigen-encoding plasmids,44 and changed the pathological nature of immune responses in allergy, cancer, and autoimmunity.45–47 The precise mechanism by which DNA immunization leads to immune responses is not clear. Specifically, the secretion of intact antigen by resident tissues has not been shown. Our data suggest that this does indeed occur, since the IL-18 used demonstrated biological effects both in vivo and in vitro, suggesting that they were secreted intact. In our study following i.m. administration of IL-18 plasmid DNA, IL-18 protein was evident in serum and the inhibitory effect of the TcCMVIL-18 DNA on MAC infection was systemically induced. In addition, we used cDNA encoding a mature form of IL-18 protein, not a pro-IL-18 because IL-18 is initially synthesized as an inactive precursor molecule (pro-IL-18) lacking a signal peptide, and then cleaved by IL-1β-converting enzyme (ICE) to yield an active molecule.48
In conclusion, IL-18 DNA vaccination seems to serve as an efficient means to deliver IL-18 in MAC model, and may be beneficial in the treatment of diseases caused by undesired Th2-dominated responses including certain parasitic infection and allergic disorders. This would circumvent the short half-life of recombinant IL-18 and the side-effects due to the administration of repetitive, high doses.
Acknowledgments
We would like to thank Drs M. E. Reff, P. Gangadharam, I. Choi, and D. Lim for providing valuable reagents. This work was supported by grants from the Korea Science and Engineering Foundation (HRC 1998G0201) and the Korea Research Foundation (KRF) made in the program year of 1998 (to T. S. Kim).
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- E : T
effector : target
- IFN-γ
interferon-γ
- IL
interleukin
- mAb
monoclonal antibody
- MAC
Mycobacterium avium complex
- NK
natural killer
References
- 1.Chaisson RE, Gallant JE, Keruly JC, Moore RD. Impact of opportunistic disease on survival in patients with HIV infection. AIDS. 1998;12:29–33. doi: 10.1097/00002030-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-γ receptor and susceptibility to mycobacterial infection. J Clin Invest. 1998;101:2364–9. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamura H, Nagata K, Komatsu T, et al. A novel costimulatory factor for γ interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63:3966–72. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 6.Lebel-Binay S, Berger A, Zinzindohoue F, Cugnenc P, Thiounn N, Fridman WH, Pages F. Interleukin-18: biological properties and clinical implications. Eur Cytokine Netw. 2000;11:15–26. [PubMed] [Google Scholar]
- 7.Kawakami K, Koguchi Y, Qureshi MH, et al. Reduced host resistance and Th1 response to Cryptococcus neoformans in interleukin-18 deficient mice. FEMS Microbiol Lett. 2000;186:121–6. doi: 10.1111/j.1574-6968.2000.tb09092.x. 10.1016/s0378-1097(00)00128-2. [DOI] [PubMed] [Google Scholar]
- 8.Ohkusu K, Yoshimoto T, Takeda K, et al. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect Immun. 2000;68:2449–56. doi: 10.1128/iai.68.5.2449-2456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia VE, Uyemura K, Sieling PA, et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–21. [PubMed] [Google Scholar]
- 10.Wei XQ, Leung BP, Niedbala W, et al. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J Immunol. 1999;163:2821–8. [PubMed] [Google Scholar]
- 11.Opal SM, Wherry JC, Grint P. Interleukin-10: potential benefits and possible risks in clinical infectious diseases. Clin Infect Dis. 1998;27:1497–507. doi: 10.1086/515032. [DOI] [PubMed] [Google Scholar]
- 12.Bukowski RM. Cytokine combinations: therapeutic use in patients with advanced renal cell carcinoma. Semin Oncol. 2000;27:204–12. [PubMed] [Google Scholar]
- 13.Qureshi MH, Zhang T, Koguchi Y, Nakashima K, Okamura H, Kurimoto M, Kawakami K. Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur J Immunol. 1999;29:643–9. doi: 10.1002/(SICI)1521-4141(199902)29:02<643::AID-IMMU643>3.0.CO;2-E. 10.1002/(sici)1521-4141(199902)29:02<643::aid-immu643>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Hara I, Nagai H, Miyake H, et al. Effectiveness of cancer vaccine therapy using cells transduced with the interleukin-12 gene combined with systemic interleukin-18 administration. Cancer Gene Ther. 2000;7:83–90. doi: 10.1038/sj.cgt.7700083. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–48. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 16.Cohen AD, Boyer JD, Weiner DB. Modulating the immune response to genetic immunization. FASEB J. 1998;12:1611–26. [PubMed] [Google Scholar]
- 17.Raz E, Watanabe A, Baird SM, et al. Systemic immunological effects of cytokine genes injected into skeletal muscle. Proc Natl Acad Sci USA. 1993;90:4523–7. doi: 10.1073/pnas.90.10.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osaki T, Hashimoto W, Gambotto A, et al. Potent antitumor effects mediated by local expression of the mature form of the interferon-γ inducing factor, interleukin-18 (IL-18) Gene Ther. 1999;6:808–15. doi: 10.1038/sj.gt.3300908. [DOI] [PubMed] [Google Scholar]
- 19.Kim JJ, Trivedi NN, Nottingham LK, et al. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur J Immunol. 1998;28:1089–103. doi: 10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L. 10.1002/(sici)1521-4141(199803)28:03<1089::aid-immu1089>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Mor G, Singla M, Steinberg AD, Hoffman SL, Okuda K, Klinman DM. Do DNA vaccines induce autoimmune disease? Human Gene Ther. 1997;8:293–300. doi: 10.1089/hum.1997.8.3-293. [DOI] [PubMed] [Google Scholar]
- 21.Restifo NP, Rosenberg SA. Developing recombinant and synthetic vaccines for the treatment of melanoma. Curr Opin Oncol. 1999;11:50–7. doi: 10.1097/00001622-199901000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calarota S, Bratt G, Nordlund S, Hinkula J, Leandersson AC, Sandstrom E, Wahren B. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet. 1998;351:1320–5. doi: 10.1016/S0140-6736(97)09440-3. 10.1016/s0140-6736(97)09440-3. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Doolan DL, Le TP, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by malaria DNA vaccines. Science. 1998;282:476–80. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 24.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 25.Coligan JE, Kruisbeck AM, Margulies DH, Shevach EM, Strober W. Current Protocols in Immunology. London: Wiley Press; 1995. [Google Scholar]
- 26.Bertram MA, Inderlied CB, Yadegar S, Kolanoski P, Yamada JK, Young LS. Confirmation of the beige mouse model for study of disseminated infection with Mycobacterium avium complex. J Infect Dis. 1986;154:194–5. doi: 10.1093/infdis/154.1.194. [DOI] [PubMed] [Google Scholar]
- 27.Saunders BM, Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance of mice to Mycobacterium avium complex infection. Infect Immun. 1995;63:4011–5. doi: 10.1128/iai.63.10.4011-4015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TS, Cohen EP. Immunization of mice with allogeneic fibroblasts genetically modified for interleukin-2-secretion and expression of melanoma-associated antigens stimulates predetermined classes of antimelanoma effector cells. J Immunother. 1994;16:24–35. doi: 10.1097/00002371-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Bohn E, Sing A, Zumbihl R, Bielfeldt C, Okamura H, Kurimoto M, Heesemann J, Autenrieth IB. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 30.Fortier AH, Polsinelli T, Green SJ, Nacy CA. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992;60:817–25. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TS, DeKruyff RH, Rupper R, Maecker HT, Levy S, Umetsu DT. An ovalbumin-IL-12 fusion protein is more effective than ovalbumin plus free recombinant IL-12 in inducing a T helper cell type 1-dominated immune response and inhibiting antigen-specific IgE production. J Immunol. 1997;158:4137–44. [PubMed] [Google Scholar]
- 32.Bermudez LE. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993;91:277–81. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes MS, Florido M, Pais TF, Appelberg R. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J Immunol. 1999;162:6734–9. [PubMed] [Google Scholar]
- 34.Doi T, Ando M, Akaike T, Suga M, Sato K, Maeda H. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect Immun. 1993;61:1980–9. doi: 10.1128/iai.61.5.1980-1989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jagannath C, Sepulveda A, Actor JK, Luxem F, Emanuele MR, Hunter RL. Effect of poloxamer of CRL-1072 on drug uptake and nitric-oxide-mediated killing of Mycobacterium avium by macrophages. Immunopharmacology. 2000;48:185–97. doi: 10.1016/s0162-3109(00)00203-4. [DOI] [PubMed] [Google Scholar]
- 36.James SL, Nacy C. Effector functions of activated macrophages against parasites. Curr Opin Immunol. 1993;5:518–23. doi: 10.1016/0952-7915(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 37.Bermudez LE. Immunobiology of Mycobacterium avium infection. Eur J Clin Microbiol Infect Dis. 1994;13:1000–6. doi: 10.1007/BF02111501. [DOI] [PubMed] [Google Scholar]
- 38.James SL. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–47. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 40.Le TP, Coonan KM, Hedstrom RC, et al. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine. 2000;18:1893–901. doi: 10.1016/s0264-410x(99)00407-7. 10.1016/s0264-410x(99)00407-7. [DOI] [PubMed] [Google Scholar]
- 41.Chun S, Daheshia M, Lee S, Eo SK, Rouse BT. Distribution fate and mechanism of immune modulation following muscosal delivery of plasmid DNA encoding IL-10. J Immunol. 1999;163:2393–402. [PubMed] [Google Scholar]
- 42.Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse BT. Suppression of ongoing ocular inflammatory disease by topical administration of plasmid DNA encoding IL-10. J Immunol. 1997;159:1945–52. [PubMed] [Google Scholar]
- 43.Rogy MA, Auffenberg T, Espat NJ, Philip R, Remick D, Wollenberg GK, Copeland EM 3rd, Moldawer LL. Human tumor necrosis factor receptor (p65) and interleukin 10 gene transfer in the mouse reduces mortality to lethal endotoxemia and also attenuates local inflammatory responses. J Exp Med. 1995;181:2289–93. doi: 10.1084/jem.181.6.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramsay AJ, Kent SJ, Strugnell RA, Suhrbier A, Thomson SA, Ramshaw IA. Genetic vaccination strategies for enhanced cellular, humoral and mucosal immunity. Immunol Rev. 1999;171:27–44. doi: 10.1111/j.1600-065x.1999.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 45.Conry RM, LoBuglio AF, Loechel F, Moore SE, Sumerel LA, Barlow DL, Curiel DT. A carcinoembryonic antigen polynucleotide vaccine has in vivo antitumor activity. Gene Ther. 1996;2:59–65. [PubMed] [Google Scholar]
- 46.Raz E, Tighe H, Sata Y, et al. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5. doi: 10.1073/pnas.93.10.5141. 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waisman A, Ruiz PJ, Hirschberg DL, et al. Suppressive vaccination with DNA encoding a variable region gene of the T-cell receptor prevents autoimmune encephalomyelitis and activates Th2 immunity. Nat Med. 1996;2:899–905. doi: 10.1038/nm0896-899. [DOI] [PubMed] [Google Scholar]
- 48.Dinarello CA. Interleukin-1β, interleukin-18, and the interleukin-1β converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]