Abstract
Langerhans cells (LC), members of the dendritic cell family, play a central role in the initiation and regulation of the immune response against the protozoan parasite Leishmania major. LC take up antigens in the skin and transport them to the regional lymph nodes for presentation to T cells. However, it is not known whether LC functions are modulated by parasite antigens. In the present study, we examined the effect of a major parasite surface molecule, L. major lipophosphoglycan (LPG), on the maturation of LC and their migratory properties. The results show that exposure to LPG did not affect the expression of major histocompatibility complex (MHC) class II and B7, but induced an up-regulation of CD25, CD31 and vascular endothelial (VE)-cadherin expression and a down-regulation of Mac-1 expression, by LC. Importantly, LPG treatment inhibited the migratory activity of LC, as it reduced their efflux from skin explants and their migration in transwell cultures. These results suggest that Leishmania LPG impairs LC migration out of the skin and thus may modulate their immunostimulatory functions, which require LC translocation from skin to lymph nodes.
Introduction
Leishmaniasis is initiated by the intradermal inoculation of Leishmania promastigotes during the bite of an infected sandfly. The spectrum of disease patterns seen in humans ranges from visceral leishmaniasis (that may be fatal if left untreated) to self-healing cutaneous leishmaniasis.1 Experimental infection of mice with Leishmania major is a widely used model for studying factors that influence the immune response against this pathogen. Upon host invasion, the parasites are phagocytosed by macrophages (Mφ) and epidermal Langerhans cells (LC), the dendritic cells of the skin.2,3 Mφ lack the ability to induce the primary stimulation of T cells and their antigen-presentation functions are impaired following infection with Leishmania.4–6 LC, on the other hand, have the unique feature of being able to transport the ingested parasites from the site of infection in the skin to the T-cell areas of the draining lymph nodes while differentiating into interdigitating dendritic cells that are very efficient in the stimulation of resting T cells.7 Thus, migration and maturation of LC are critical for initiation of the T-cell immune response to Leishmania, and it is probable that these processes are tightly regulated.
The dendritic cell maturation observed in vivo can be mimicked during a short period of in vitro culture and correlates with an increase in the expression of major histocompatibility complex (MHC) class II and costimulatory molecules.8 Furthermore, the expression of adhesion molecules is modulated. For example, E-cadherin, which promotes the retention of LC within the epidermis, is down-regulated,9 whereas the level of intercellular adhesion molecule-1 (ICAM-1), involved in the interaction with T cells, is enhanced.10
Lipophosphoglycan (LPG) is the most abundant glycoconjugate on the cell surface of Leishmania promastigotes.11,12 As many as 106 copies of this molecule are expressed by a single parasite.12 LPG has a unique structure with a conserved glycosylphosphatidylinositol (GPI) anchor, a conserved diphosphoheptasaccharide core structure and repeating phosphodisaccharide units carrying species-specific side chains;13 it is present on all Leishmania species. LPG has been shown to modulate many host cell functions, including the cytokine release, oxidative burst and nitric oxide (NO) synthesis of Mφ.12,14,15 Importantly, it also reduces monocyte transendothelial migration by modulating the expression of cell adhesion molecules.16 Although these findings document the relevance of this molecule for parasite–host interaction, no information has been published on the effects of LPG on LC functions. Given the important role of LC in the early phase of Leishmania infection, we analysed whether LPG affects the maturation and migratory activity of LC. Herein we report that the exposure of LC to Leishmania LPG modified the expression of cell-surface molecules. Moreover, LPG suppressed the emigration of LC from the skin, suggesting a modulation of the immunostimulatory features of LC by LPG.
Materials and methods
Isolation of epidermal cells (EC)
EC were prepared from the ear skin of BALB/c mice by trypsinization procedures, as described previously.17 A concentration of 1% trypsin was used for processing the ventral thick ear halves (90 min), and 0·6% trypsin was used for the dorsal thin ear halves (45 min). These preparations contained MHC class II-bearing LC as well as MHC class II-negative keratinocytes, a source of granulocyte–macrophage colony-stimulating factor (GM-CSF), which is essential for LC differentiation in culture.
Purification of L. major LPG and preparation of L. major-conditioned medium (LCM)
The cloned virulent line of the L. major isolate MHOM/IL/81/FE/BNI was maintained by passage in BALB/c mice. Promastigotes were grown in vitro in blood agar cultures. LPG was purified from stationary-phase L. major promastigotes by sequential solvent extraction and hydrophobic interaction chromatography on octyl-sepharose columns, as previously described,13,18 and was devoid of contaminating protein. The phosphoglycan (PG) moiety of LPG was obtained by delipidating 20 µg of LPG (3 hr at 37°) with phosphatidylinositol-specific phospholipase C (PI-PLC) (0·5 U/ml; Sigma, Taufkirchen, Germany) in 50 mm HEPES, pH 7·4. PG was recovered in the flow-through of an octyl-sepharose column. Prolonged incubation with PI-PLC did not result in an alteration of the biological activity of PG. LCM was prepared by incubating suspensions of stationary-phase promastigotes (2 × 107/ml) overnight at 37°, filtering the supernatants through low-protein-binding 0·22-µm filters and storage at −70° until required. No significant parasite mortality was observed during LCM preparation.
We previously documented that L. major-infected LC contain up to six parasites per host cell (mean: 1·5).19 To imitate these physiological conditions as closely as possible, the dose of LPG used for the present study was adjusted to this ratio (LPG equivalent of six parasites to one host cell). As the amount of LPG per 106 L. major in our hands is ≈0·150 µg, very similar to that reported by McConville & Bacic,20 3 µg of LPG (equivalent to 2 × 107 parasites) per 3 × 106 host cells was used. For LCM, a concentration of 12·5%, prepared under the conditions described above, was also equivalent to 2 × 107 parasites.
EC culture and flow cytometry analysis of cell-surface molecules
EC (3 × 106) were cultured overnight in the presence or absence of LPG (3 µg), LCM (12·5%) or lipopolysaccharide (LPS) (100 ng/ml; Roth, Karlsruhe, Germany) in 2 ml of RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mm l-glutamine, 10 mm HEPES buffer, 60 µg/ml penicillin, 20 µg/ml gentamycin, 17 mm NaHCO3 and 0·05 mm 2-mercaptoethanol. The non-adherent cell population, containing 30–50% LC, was harvested 24 hr later. LC were identified by the expression of MHC class II molecules.21 For two-colour phenotype analysis, LC were labelled sequentially with biotin-conjugated mouse anti-I-Ad (MHC class II) monoclonal antibodies (mAbs) (PharMingen, Hamburg, Germany) and streptavidin-conjugated fluorescein isothiocyanate (FITC) (PharMingen), followed by rat anti-CD80 (B7.1), anti-CD86 (B7.2), anti-CD25, anti-CD31, anti-Mac-1, anti-vascular endothelial (VE)-cadherin (all purchased from PharMingen), anti-L-selectin (Southern Biotechnology Associates, Birmingham, AL) or anti-E-cadherin (clone ECCD-2; R & D Systems, Wiesbaden, Germany) antibodies and phycoerythrin (PE)-conjugated donkey anti-rat antibodies (Dianova, Hamburg, Germany). Isotype-matched antibodies were used as controls. Two-colour analysis of gated MHC class II-positive LC was performed in a fluorescence-activated cell sorter (FACScalibur; Becton-Dickinson, Heidelberg, Germany) using CellQuest software.
Skin explant cultures
Mouse ear skin was rinsed in 70% ethanol and split with forceps into dorsal and ventral halves. Both halves were floated dermal side down in tissue culture wells containing 0·5 ml of culture medium alone, or in culture medium containing LPG (3 µg per 3 × 106 EC), tumour necrosis factor-α (TNF-α) (100 U/ml; Becton-Dickinson), or LPS (100 ng/ml). Two days later, the cells that had migrated from the skin explant into the culture medium were harvested, enumerated in a haemocytometer and analysed for the expression of MHC class II, as previously described.22
Transwell migration assays
Migration of LC was also examined in transwell chambers (8-µm pore size; Costar, Schiphol-Rijk, the Netherlands). The upper compartments were supplemented with 1 × 106 cells in 0·5 ml of culture medium, and 0·8 ml of culture medium containing LPG (1 µg), PG (1 µg), LCM (12·5%), TNF-α (100 U/ml), LPS (100 ng/ml) or none of these reagents, as a control, was added to the lower compartments. After overnight incubation, cells were harvested from the bottom chambers and enumerated in a haemocytometer, followed by FACS analysis for MHC class II expression.
Statistical analysis
The experiments were performed at least three times, and the data obtained from migration experiments were analysed using the Student's t-test.23
Results
Effect of Leishmania LPG on LC maturation
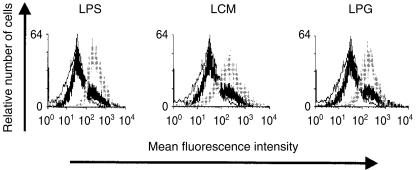
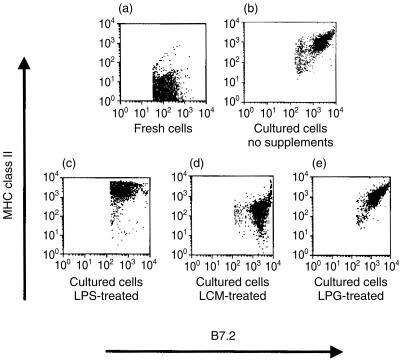
During LC maturation, the expression of MHC class II and costimulatory molecules, such as CD80 (B7.1) and CD86 (B7.2), is strongly up-regulated. Furthermore, mature LC express high levels of the interleukin 2 (IL-2) receptor α-chain (CD25).24 This differentiation is induced during in vitro incubation of LC, and is enhanced by treatment with the bacterial cell wall component LPS.25,26 We examined the ability of L. major LPG to modulate the phenotypic maturation of LC. LCM was used as a control to determine the effect of soluble factors released by the parasites. As expected, overnight incubation of LC with LPS stimulated a significant up-regulation of CD25 expression (Fig. 1). Interestingly, Leishmania LPG and LCM mediated an equivalent effect and strongly enhanced CD25 expression. On the other hand, treatment with LPG, unlike LPS, did not modulate MHC class II and B7 expression of cultured LC (Fig. 2). After exposure to LPG, the levels of MHC class II, and B7.2 (Fig. 2e) and B7.1 (data not shown), were comparable to those detected on LC incubated in the absence of exogenous factors (Fig. 2b). It should be noted that LCM reduced the up-regulation of MHC class II (Fig. 2d), indicating that its effect is distinct from that of LPG. These results suggest that LPG promotes the maturation of LC without affecting molecules related to their antigen-presentation functions.
Figure 1.
Flow cytometric analysis of CD25 expression by gated major histocompatibility complex (MHC) class II-positive Langerhans cells (LC) after overnight incubation in the presence (grey lines) or absence (thick black lines) of lipopolysaccharide (LPS), Leishmania major-conditioned medium (LCM) or lipophosphoglycan (LPG). Isotype-matched antibodies were used as controls (thin black lines).
Figure 2.
Flow cytometric analysis of major histocompatibility complex (MHC) class II and B7.2 expression by Langerhans cells (LC). Freshly isolated cells or cells cultured overnight in the absence of exogenous mediators or in the presence of lipopolysaccharide (LPS), Leishmania major-conditioned medium (LCM) or lipophosphoglycan (LPG), were stained with anti-I-Ad and anti-B7.2 antibodies and subjected to two-colour fluorescence-activated cell sorter (FACS) analysis.
Leishmania LPG modulates the expression of adhesion molecules by LC
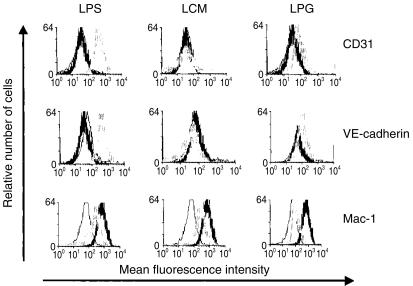
The intercellular adhesion molecules CD31, also termed platelet endothelial cell adhesion molecule 1 (PECAM-1), VE-cadherin, E-cadherin, L-selectin and the integrin Mac-1 are involved in the migration of leucocytes. Therefore, we determined whether LPG affects the expression of these molecules by LC. After culture in the absence of exogenous mediators, LC did not express CD31. Stimulation with LPS strongly enhanced the level of CD31 expression, and LPG treatment resulted in a moderate, but consistent, up-regulation of this molecule, whereas LCM did not have an effect (Fig. 3, upper panels). The expression of VE-cadherin was modulated in a similar manner (Fig. 3, middle panels). In contrast, the expression of Mac-1, which is displayed at high levels by cultured LC, was down-regulated by LC treatment with LPS, LCM and LPG (Fig. 3, lower panels). Importantly, this reduction of Mac-1 expression by LC was most pronounced after exposure to LPG. E-cadherin expression, which is down-regulated upon culture of LC,9 and L-selectin expression were not altered by LC exposure to LPG (data not shown).
Figure 3.
Flow cytometric analysis of CD31, vascular endothelial (VE)-cadherin and Mac-1 expression by gated major histocompatibility complex (MHC) class II-positive Langerhans cells (LC) after overnight incubation in the presence (grey lines) or absence (thick black lines) of lipopolysaccharide (LPS), Leishmania major-conditioned medium (LCM) or lipophosphoglycan (LPG). Isotype-matched antibodies were used as controls (thin black lines).
Leishmania LPG reduces the migratory capacity of LC
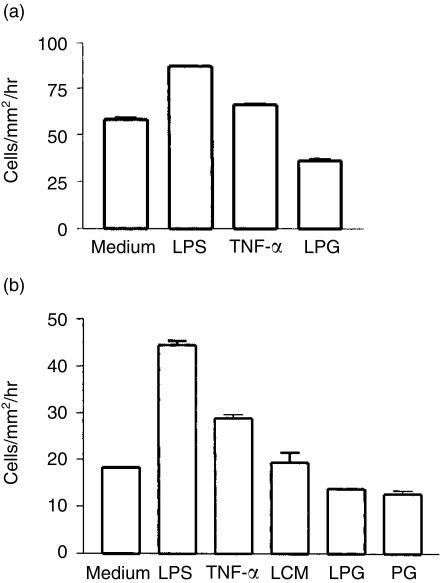
The above findings indicated that Leishmania LPG did not interfere with the expression of molecules involved in the antigen-presentation function of LC, but modulated the levels of several molecules that are known to be relevant to cell migration. Therefore, we next examined whether the LPG-induced changes in surface marker expression correlate with alterations in the migratory properties of LC. For this purpose, we used an in vitro skin culture system.22,27 After 2 days of skin explant culture, the number of MHC class II-positive cells that had migrated out of the skin and into the culture medium was analysed by flow cytometry. In the absence of exogenous mediators, the cells migrated at a basal rate of 58 cells/mm2/hr (Fig. 4a). LPS and TNF-α were used as positive controls and, as expected, enhanced the migration.28 Surprisingly, Leishmania LPG induced the opposite effect and inhibited the emigration of MHC class II-positive cells from the skin by ≈40% (Fig. 4a).
Figure 4.
Lipophosphoglycan (LPG) reduced the migration of Langerhans cells (LC). (a) Efflux of LC from skin explants cultured in the absence of exogenous mediators or in the presence of lipopolysaccharide (LPS), tumour necrosis factor-α (TNF-α) or LPG. Major histocompatibility complex (MHC) class II-positive cells that had migrated from the skin into the medium were detected by fluorescence-activated cell sorter (FACS) analysis (cells in the absence of exogenous mediators versus LPS, P < 0·0001; versus TNF-α, P < 0·002; versus LPG, P < 0·0008). (b) Transwell migration of isolated LC cultured in the absence of exogenous mediators or in the presence of LPS, TNF-α, Leishmania major-conditioned medium (LCM), LPG or phosphoglycan (PG). Cells that had migrated to the bottom chambers were enumerated, and LC were identified by FACS analysis for expression of MHC class II (LC in the absence of exogenous mediators versus LPS, P < 0·0001; versus TNF-α, P < 0·0002; versus LPG, P < 0·001).
In addition to LC, skin explants contain other MHC class II-positive cells. To corroborate the assumption that Leishmania LPG reduces the migratory activity of LC, we used isolated EC (in the epidermis, LC are the only MHC class II-positive cells) and characterized the migration process in a transwell culture system. In addition to LPG, the delipidated PG molecule was examined. LCM was used as a control to estimate the activity of parasite-derived molecules released into the culture medium. The cells were added to the upper chambers of the transwell cultures, and their passage across the microporous membrane was quantified (Fig. 4b). When LPS or TNF-α were present in the lower chambers, an enhancement of cell migration was again observed. In contrast, LPG mediated a significant inhibitory effect on LC migration (25% reduction), confirming the data obtained with skin explant cultures. The PG moiety of Leishmania LPG appears to be responsible for the decreased efflux of LC (Fig. 4b), as its effect was very similar to that induced by the intact molecule (inhibition of LC migration by 31%). LCM treatment, on the other hand, did not modulate LC migration. Taken together, these data suggest that Leishmania LPG inhibits the efflux of LC from the skin.
Discussion
Microbial constituents have been shown to interfere with numerous host cell functions. The major surface glycoconjugates of Leishmania – LPG and glycoinositolphospholipids – inhibit oxidative burst,29 NO synthesis,14,30 and IL-1 and IL-12 production by Mφ,15,29 and reduce monocyte transendothelial migration.16 However, their effect on dendritic cells, the key initiators of the Leishmania-specific T-cell response,31 had not been analysed prior to this study. The data presented here demonstrate that treatment with L. major LPG alters the expression of adhesion molecules by LC. Furthermore, it decreases the migratory activity of LC, suggesting that the parasites have evolved mechanisms to affect the immunostimulatory functions of LC via an impairment of their efflux from the skin.
Previous studies implicated pathogen-associated molecules, such as bacterial LPS, in the activation of dendritic cells.25,26 Our findings show that the effects of L. major LPG are distinct from those of LPS. Both molecules promoted the maturation of LC, as reflected by the enhancement of CD25 expression. In contrast to LPS, however, L. major LPG did not induce an up-regulation of molecules related to the antigen presentation and accessory functions of LC, namely MHC class II and B7. Importantly, whereas LPS is known to enhance dendritic cell migration,32 L. major LPG reduced the migratory activity of LC. This inhibitory effect appears to depend on the PG moiety of LPG, not the lipid anchor. On the other hand, secreted PG chains present in LCM, which lack both the lipid and the phosphosaccharide core of the LPG anchor,33 did not alter the rate of LC migration. Therefore, it is possible that the core region is critical for the PG effect on LC migration. However, it cannot be excluded that the amount of excreted PG in LCM was insufficient to affect the migratory activity of LC, even though it was well able to promote LC maturation.
In addition to the inhibitory effect on LC migration, LPG treatment modulated the expression of the adhesion molecules VE-cadherin and CD31, as well as the integrin Mac-1, by LC. Interestingly, the course of E-cadherin expression, which has been shown to mediate LC adherence to keratinocytes and is down-regulated during LC maturation and mobilization from skin,9 was not affected by LC exposure to LPG. Similarly to E-cadherin, the expression of Mac-1 is down-regulated upon maturation of LC in culture,34 and this decrease was enhanced by treatment with LPG. CD31 regulates the passage of leucocytes across the endothelial lining and has been shown to support the egress of immature dendritic cells from the bloodstream into their target tissues.35 However, the migration of dendritic cells from the tissue into lymphatic vessels may not be as tightly regulated by adhesion molecules, as CD31 expression is also down-regulated upon dendritic cell maturation.36 This decrease of CD31 levels corresponds well with the high T-cell-stimulatory potential of mature dendritic cells, because CD31 is involved in the inhibition of T helper cell responses.37 In this context, it may be of interest that L. major LPG treatment slightly enhanced CD31 expression by LC, but the more pronounced up-regulation induced by LPS and the contrasting effects of LPG and LPS on LC migration corroborate the suggestion that the levels of adhesion molecule expression and LC migratory activities do not correlate rigidly.36 These findings suggest an additional mechanism by which L. major LPG suppresses the migration process of LC. LPG may not act directly on LC, but, as in other experimental models,32 its effects may be mediated by cytokines. It has previously been shown by us22 and others28,38–40 that LC migration is regulated by a spectrum of different mediators, in particular TNF-α, IL-1β, IL-10, macrophage inflammatory protein-1α (MIP-1α) and secondary lymphoid-tissue chemokine, and it is well established that LPG modulates the production of cytokines.15,29,41
An unexpected finding of the present study was the up-regulation of VE-cadherin expression by LC after treatment with microbial antigens. VE-cadherin plays an important role in endothelial biology and is required for the control of vascular permeability. To the best of our knowledge, no information is available on the function of VE-cadherin expressed by dendritic cells. It is tempting, however, to speculate that it may be relevant to the dendritic cell-mediated host response to pathogen invasion.
In the skin, the first target organ during infection with Leishmania, the parasites are phagocytosed by Mφ and LC. Although LC engulf much lower numbers of parasites than Mφ, only LC are able to transport parasites from the site of cutaneous infection to the T-cell regions of the draining lymph nodes and to provide the principal sensitizing signal for stimulation of the primary Leishmania-specific T-cell response.3,7 During their migration, LC differentiate into highly potent antigen-presenting cells, a process that is associated with an up-regulated expression of MHC class II and costimulatory molecules. Both signals – the interaction of MHC–antigen complexes with T-cell receptors and the costimulation – are crucial for T-cell activation. The findings presented here demonstrate that exposure of LC to L. major LPG does not affect their expression of MHC class II and B7 molecules, suggesting that the antigen presentation and accessory functions are not impaired. However, LPG reduced the migration of LC in transwell cultures and their efflux from the skin. As the mobilization of LC plays a pivotal role in the induction and regulation of T-cell-mediated immune responses, the down-regulation of this activity by LPG may result in a modulation of the immunostimulatory functions of dendritic cells in permissive hosts.
In conclusion, our data show that the L. major surface glycoconjugate LPG inhibits LC emigration from the skin. Thus, Leishmania parasites have evolved strategies to interfere with important host defence pathways at different levels, impairing not only Mφ but also dendritic cell functions. This novel finding may help to elucidate the mechanisms by which defined parasite molecules contribute to survival of the pathogen in the host.
Acknowledgments
This work was supported by the Venezuelan grant, CDCH-UCV 09.33. 4580.2001, and the German Bundesministerium für Forschung und Technologie grant KI8906/0. We thank Christine Hambrecht and Christina Rhode for technical assistance. A. Ponte-Sucre spent a sabbatical year in Dr Heidrun Moll's laboratory and received fellowships from CDCH, Venezuela, and the Humboldt Foundation, Germany.
References
- 1.Liew FY, O'Donnell CA. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, Russell DG. The interaction of Leishmania species with macrophages. Adv Parasitol. 1992;31:175–254. doi: 10.1016/s0065-308x(08)60022-6. [DOI] [PubMed] [Google Scholar]
- 3.Moll H. Epidermal Langerhans cells are critical for immunoregulation of cutaneous leishmaniasis. Immunol Today. 1993;14:383–7. doi: 10.1016/0167-5699(93)90138-B. [DOI] [PubMed] [Google Scholar]
- 4.Fruth U, Solioz N, Louis JA. Leishmania major interferes with antigen presentation by infected macrophages. J Immunol. 1993;150:1857–64. [PubMed] [Google Scholar]
- 5.Kaye PM, Rogers NJ, Curry AJ, Scott JC. Deficient expression of co-stimulatory molecules on Leishmania-infected macrophages. Eur J Immunol. 1994;24:2850–4. doi: 10.1002/eji.1830241140. [DOI] [PubMed] [Google Scholar]
- 6.De Souza-Lão S, Lang T, Prina E, Hellio R, Antoine JC. Intracellular Leishmania amazonensis amastigotes internalize and degrade MHC class II molecules of their host cells. J Cell Sci. 1995;108:3219–31. doi: 10.1242/jcs.108.10.3219. [DOI] [PubMed] [Google Scholar]
- 7.Moll H, Fuchs H, Blank C, Röllinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 9.Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–5. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- 10.Cumberbatch M, Peters SW, Gould SJ, Kimber I. Intercellular adhesion molecule-1 (ICAM-1) expression by lymph node dendritic cells: comparison with epidermal Langerhans cells. Immunol Lett. 1992;32:105–10. doi: 10.1016/0165-2478(92)90101-s. [DOI] [PubMed] [Google Scholar]
- 11.Handman E, Goding JW. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985;4:329–36. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 13.McConville MJ, Ferguson MAJ. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305–24. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proudfoot L, Nikolaev AV, Feng GJ, Wei XQ, Ferguson MAJ, Brimacombe JS, Liew FY. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc Natl Acad Sci USA. 1996;93:10984–9. doi: 10.1073/pnas.93.20.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piedrafita D, Proudfoot L, Nikolaev AV, et al. Regulation of macrophage IL-12 synthesis by Leishmania phosphoglycans. Eur J Immunol. 1999;29:235–44. doi: 10.1002/(SICI)1521-4141(199901)29:01<235::AID-IMMU235>3.0.CO;2-S. 10.1002/(sici)1521-4141(199901)29:01<235::aid-immu235>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Lo SK, Bovis L, Matura R, Zhu B, He S, Lum H, Turco SJ, Ho JL. Leishmania lipophosphoglycan reduces monocyte transendothelial migration: modulation of cell adhesion molecules, intercellular junctional proteins, and chemoattractants. J Immunol. 1998;160:1857–65. [PubMed] [Google Scholar]
- 17.Schuler G, Koch F. Enrichment of epidermal Langerhans cells. In: Schuler G, editor. Epidermal Langerhans Cells. Boca Raton, FL: CRC Press; 1990. pp. 139–57. [Google Scholar]
- 18.McConville MJ, Bacic A, Mitchell GF, Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerolphosphoinositol lipid anchor. Proc Natl Acad Sci USA. 1987;84:8941–5. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blank C, Fuchs H, Rappersberger K, Röllinghoff M, Moll H. Parasitism of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J Infect Dis. 1993;167:418–25. doi: 10.1093/infdis/167.2.418. [DOI] [PubMed] [Google Scholar]
- 20.McConville MJ, Bacic A. The glycoinositolphospholipid profiles of two Leishmania major strains that differ in lipophosphoglycan expression. Mol Biochem Parasitol. 1990;38:57–68. doi: 10.1016/0166-6851(90)90205-z. [DOI] [PubMed] [Google Scholar]
- 21.Witmer-Pack MD, Valinsky J, Olivier W, Steinman RM. Quantitation of surface antigens on cultured murine epidermal Langerhans cells: rapid and selective increase in the level of surface MHC products. J Invest Dermatol. 1988;90:387–94. doi: 10.1111/1523-1747.ep12456460. [DOI] [PubMed] [Google Scholar]
- 22.Arnoldi J, Moll H. Langerhans cell migration in murine cutaneous leishmaniasis: regulation by tumor necrosis factor-α, interleukin-1β and macrophage inflammatory protein-1α. Dev Immunol. 1998;6:3–11. doi: 10.1155/1998/21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schefler W. Análisis de varianza. In: Schefler W, editor. Bioestadística. México: Fondo Educativo Interamericano; 1981. pp. 122–57. [Google Scholar]
- 24.Steiner G, Tschachler E, Tani M, et al. Interleukin 2 receptors on cultured murine epidermal Langerhans cells. J Immunol. 1986;137:155–9. [PubMed] [Google Scholar]
- 25.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Smedt T, Pajak B, Muraille E, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–24. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–93. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor-α and interleukin 1β for migration. Immunology. 1997;92:388–95. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frankenburg S, Leibovici V, Mansbach N, Turco SJ, Rosen G. Effect of glycolipids of Leishmania parasites on human monocyte activity. Inhibition by lipophosphoglycan. J Immunol. 1990;145:4284–9. [PubMed] [Google Scholar]
- 30.Proudfoot L, O'Donnell CA, Liew FY. Glycoinositolphospholipids of Leishmania major inhibit nitric oxide synthesis and reduce leishmanicidal activity in murine macrophages. Eur J Immunol. 1995;25:745–50. doi: 10.1002/eji.1830250318. [DOI] [PubMed] [Google Scholar]
- 31.Moll H. The role of dendritic cells at the early stages of Leishmania infection. Adv Exp Med Biol. 2000;479:383–7. doi: 10.1007/0-306-46831-X_14. [DOI] [PubMed] [Google Scholar]
- 32.Roake JA, Rao AS, Morris PJ, Larsen CP, Hankins DF, Austyn JM. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–47. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greis KD, Turco SJ, Thomas JR, McConville MJ, Homans SW, Ferguson MA. Purification and characterization of an extracellular phosphoglycan from Leishmania donovani. J Biol Chem. 1992;267:5876–81. [PubMed] [Google Scholar]
- 34.Anjuère F, Martin P, Ferrero I, López Fraga M, Martinez del Hoyo G, Wright N, Ardavín C. Definition of dendritic cell subpopulations present in spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood. 2000;93:590–8. [PubMed] [Google Scholar]
- 35.Ferrero E, Bondanza A, Leone BE, Manici S, Poggi A, Zocchi MR. CD14+ CD34+ peripheral blood mononuclear cells migrate across endothelium and give rise to immunostimulatory dendritic cells. J Immunol. 1998;160:2675–83. [PubMed] [Google Scholar]
- 36.Ebner S, Lenz A, Reider D, Fritsch P, Schuler G, Romani N. Expression of maturation-/migration-related molecules on human dendritic cells from blood and skin. Immunobiology. 1998;198:568–87. doi: 10.1016/S0171-2985(98)80079-X. [DOI] [PubMed] [Google Scholar]
- 37.Prager E, Sunder-Plassmann R, Hansmann C, Koch C, Holter W, Knapp W, Stockinger H. Interaction of CD31 with a heterophilic counterreceptor involved in downregulation of human T cell responses. J Exp Med. 1996;184:41–50. doi: 10.1084/jem.184.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeki H, Moore AM, Brown MJ, Hwang ST. Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from skin to regional lymph nodes. J Immunol. 1999;162:2472–5. [PubMed] [Google Scholar]
- 39.Wang B, Zhuang L, Fujisawa H, et al. Enhanced epidermal Langerhans cell migration in IL-10 knockout mice. J Immunol. 1999;162:277–83. [PubMed] [Google Scholar]
- 40.Cumberbatch M, Dearman RJ, Antonopoulos C, Groves RW, Kimber I. Interleukin (IL)-18 induces Langerhans cell migration by a tumour necrosis factor-α, IL-1β-dependent mechanism. Immunology. 2001;102:323–30. doi: 10.1046/j.1365-2567.2001.01187.x. 10.1046/j.1365-2567.2001.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatzigeorgiou DE, Geng JY, Zhang Y, et al. Lipophosphoglycan from Leishmania donovani suppresses IL-1β gene expression in human monocytes via an unique promoter sequence. Proc Natl Acad Sci USA. 1996;93:14708–13. doi: 10.1073/pnas.93.25.14708. [DOI] [PMC free article] [PubMed] [Google Scholar]