Abstract
B-lymphocyte maturation is considered to be independent of the thymus. However, there is circumstantial evidence suggesting that it may be impaired in nude animals that lack the thymus. Our study shows that the proportion of immature B-lymphocyte subsets (CD90high IgMhigh and CD90high IgMlow) was significantly increased, whereas that of mature B-lymphocyte subsets (CD90– IgMlow and CD90– IgMhigh) was decreased in the blood and lymph nodes of nude rats. In addition, the expression of major histocompatibility complex class II, intercellular adhesion molecule-1, CD44 and l-selectin was significantly down-regulated both on immature and mature B-lymphocyte subsets. After implantation of thymic tissue under the kidney capsule of nude rats the block in B-lymphocyte maturation was alleviated and the expression of surface molecules was normalized. Comparable effects were seen after the adoptive transfer of T lymphocytes. Thus, we show that in nude rats B cells do not mature properly because of the lack of T-cell help and that T lymphocytes are required for the peripheral phase of B-lymphocyte maturation, as well as for the appropriate expression of surface molecules. This should be considered when treating patients with T-cell deficiencies.
Keywords: B-cell maturation, B-cell/T-cell interactions, B-cell surface molecules
Introduction
It is now generally accepted that in mammals, after the central phase of development within the bone marrow, B lymphocytes pass through an additional peripheral phase of maturation within the spleen, where the final shaping of the repertoire occurs.1,2 After leaving the bone marrow, B lymphocytes pass through several successive developmental stages. Kroese et al.3 performed a detailed phenotypic characterization and clarified the developmental relations of these stages in the rats: namely, using Thy-1 glycoprotein (CD90) in combination with immunoglobulin M (IgM), two subsets of immature CD90high and two subsets of mature CD90– B lymphocytes can be clearly discerned in the peripheral immune system of rats. In brief, the immature, bone marrow-generated newly formed B cells (NF; CD90high IgMhigh) enter the circulation and migrate into the spleen, where they may develop to the second immature subset – early recirculating follicular (ERF; CD90high IgMlow). ERF-B lymphocytes may ultimately differentiate to mature recirculating follicular (RF; CD90– IgMlow) B cells. Marginal zone phenotype B cells (MZ; CD90– IgMhigh), which are believed to develop from both ERF and RF-B lymphocytes, represent the final stage of differentiation.3,4 The corresponding stages of B-lymphocyte differentiation have been described in mice as well.5
Generally, the B-lymphocyte development has been considered independent of thymus and T lymphocytes, primarily because the numbers of splenic and peripheral B cells in adult thymectomized, αβ-T-cell receptor (TCR)-deficient mice and nude animals are normal.6,7 The nude mice and rats show the mutations in the Whn (winged helix nude) transcription factor, the product of nude locus. In normal animals the Whn transcription factor is required for the differentiation of thymic epithelial precursor cells and the proper keratinization of the hair shaft.8 Because of mutations in the Whn gene, nude animals are hairless and lack a functional thymus with the consequent absence of T cells from the peripheral lymphocyte pool.9 The early works of Sprent10 and Sprent and Basten11 have shown that the numbers of B lymphocytes in the thoracic duct lymph, as well as the homing characteristics of these cells are the same in normal and nude mice. Since then, the latter were considered to have an essentially normal B-cell system and were used as an ideal material in studies on B-lymphocyte physiology.7,12 The fact that mature B cells poorly develop in the absence of thymus (in nude mice with X-linked immune deficiency) was largely overlooked.13,14 However, recently it has been shown that the bone marrow phase of B-lymphocyte maturation was impaired in nude mice: the numbers of bone marrow pre-B cells were decreased and fewer pre-B cells expressed recombination-activating gene-1 mRNA.15 This opened a question of whether the peripheral phase of B-lymphocyte maturation is also abnormal in nude animals.
Therefore, we studied in greater detail this phase of B-lymphocyte maturation in athymic, nude rats with the specific aim to shed light on the role of thymus and T lymphocytes in this process. We examined the proportions and surface molecule expression on B-lymphocyte subsets in bone marrow, peripheral blood and lymph nodes of nude rats and either engrafted these animals with thymic tissue or reconstituted them with congeneic T lymphocytes. We revealed a disturbance in proportions of peripheral B cells in nude rats consistent with a block in maturation. Nude B lymphocytes also displayed a progressive impairment of surface molecule expression in the bone marrow, peripheral blood and lymph nodes. These defects were amendable by thymus grafting or T-lymphocyte reconstitution, which disclosed a role of T cells in the peripheral phase of B-cell maturation.
Materials and methods
Animals
Normal LEW/Ztm (Ptprca or RT7a) and congeneic athymic nude LEW-Whnrnu/Ztm rats, 4 months old, were used. There were eight animals (four male and four female) in each group. These rats were obtained from the Central Animal Facility of the Hannover Medical School. In the experiment with thymus implantation normal WAG/RijCrl and congeneic athymic nude WAG-Whnrnu/Ztm rats were used. The former were obtained from Charles River Laboratories (Schweinfurt, Germany) and the latter from the Central Animal Facility of the Hannover Medical School. All rats were kept under specified pathogen-free conditions. Clinically they appeared healthy and their health was monitored as described elsewhere.16
Collection of lymphocytes from blood, lymph nodes and bone marrow
Blood was collected from the abdominal aorta under ether anaesthesia. Next, mesenteric lymph nodes and both femora were removed and cell suspensions were prepared. The volume and the total number of leukocytes (Coulter Counter, Luton, UK) were determined for different cell suspensions. The B- and T-lymphocyte subsets were analysed by flow cytometry (FACScan and FACScalibur flow cytometer, Becton Dickinson, Mountain View, CA), as described below. To compare the surface molecule expression, the blood, lymph node and bone marrow samples of LEW and the congeneic nude animals were always analysed on the same day, whereby the different suspensions were treated identically with respect to the applied suspension medium, erythrocyte lysis, and setting of fluorescence-activated cell sorting (FACS) parameters.
Identification of B- and T-lymphocyte subsets
The blood, lymph node and bone marrow cell suspensions were washed and adjusted to 1 × 106 cells/well. To differentiate B and T cells, the cell suspensions were stained with the biotinylated monoclonal antibody against immunoglobulin M (IgM; MARM-4; Serotec, Oxford, UK or G53-238; Becton Dickinson, San Jose, CA), which was revealed with streptavidin conjugated Red 670 (Gibco, Gaithersburg, MD) in combination with FITC-conjugated R73 antibody (Serotec) against the TCR.
The different B-cell subsets were identified using the biotinylated monoclonal antibody against IgM, which was revealed with streptavidin, conjugated Red 670 (FL3), in combination with FITC-conjugated monoclonal antibody Ox-7 against CD90 (FL1; PharMingen, San Diego, CA). In this manner, four B-cell populations were identified: NF (CD90high IgMhigh), ERF (CD90high IgMlow), RF (CD90– IgMlow) and MZ phenotype B cells (CD90– IgMhigh), as characterized earlier in the rat.3,4
The incubation period was 30 min at 4° for each antibody. Using FACScan, PC-LYSYS and CELLQuest Pro (Becton Dickinson Biosciences) or WinList (Verity Software House, Inc., Topsham, USA) software 1 × 105 viable cells were analysed and the percentage of B and T lymphocytes, as well as of B-lymphocyte subsets, was determined.
Surface molecule expression on B-lymphocyte subsets
Cell suspensions were treated as described above. Two colours were used to define the different B-cell subpopulations (FL1 and FL3) and the third colour was employed to determine their surface molecule expression. In brief, cells were incubated with antibodies against major histocompatibility complex (MHC) class II RT1B (Ox-6; Serotec, Oxford, UK), as well as for MHC class II RT1D (Ox-17; Serotec), MHC class I (Ox-18; Serotec), interleukin-2 receptor α chain (Ox-39; PharMingen), intercellular adhesion molecule-1 (ICAM-1; 1A29; PharMingen), leucocyte function-associated antigen-1 (LFA-1; WT.1; PharMingen), CD44 (Ox-49; PharMingen), α4-integrin (HP2/1; PharMingen) and l-selectin (Ox-85; Serotec). These antibodies were revealed with a phycoerythrin (PE)-conjugated anti-mouse immunoglobulin secondary antibody (FL2; Serotec). Than, the cells were incubated with a fluoroscein isothiocyanate (FITC)-conjugated Ox-7 monoclonal antibody against CD90 (FL1) and a biotinylated antibody against IgM to identify the B-cell subsets. Finally, the anti-IgM antibody was revealed with streptavidin-Red 670 (FL3). To reveal the unconjugated antibodies, the goat-anti-mouse immunoglobulin-antigen-presenting cell polyclonal antibody (Becton Dickinson) was also used – in this case we used FL1 and FL2 to separate B-lymphocyte subpopulations and FL4 to determine surface molecule expression. All incubations were performed for 30 min at 4°. An irrelevant, matched isotype mouse antibody was used as a control. The surface molecule expression was analysed by gating on the respective population.
Implantation of thymic tissue in nude rats
In this experiment WAG/RijCrl and congeneic nude WAG-Whnrnu/Ztm rats were used. Three experimental groups were formed: normal WAG (three females and two males), nude sham-operated (two females and one male) and nude thymus-implanted (three females and two males). At the age of 2 months the nude animals were either sham-operated or implanted with pieces of thymic tissue under the kidney capsule.17 All rats were killed 36 weeks after thymus implantation. Samples of blood and lymph node tissue were collected and prepared as described above.
Reconstitution of nude rats with T lymphocytes
Three normal LEW and two athymic, nude LEW rats (RT7a) were used in this experiment. T lymphocytes were obtained from blood of LEW rats (RT7b) congeneic for RT7 alloantigenic determinant. This enabled the subsequent distinction between the donor RT7·2 and host RT7·1 lymphocytes. For differentiation of T and B cells of host and donor origin the monoclonal antibody His-41 (PharMingen) was used, which binds to leucocytes of rat strains carrying the RT7.2 but not the RT7.1 allotype.18 For reconstitution the donor blood T lymphocytes were separated from B lymphocytes by immunomagnetic sorting on MACS (Miltenyi Biotech, Bergish Gladbach, Germany) using Ox-12 coupled with magnetic beads (Miltenyi Biotech). Two nude rats were reconstituted with 200 million donor T lymphocytes suspended in PBS, which were injected into the tail vein. Two control LEW rats were injected with the same quantity of physiological saline. The blood samples were taken for analyses by the retro-orbital puncture from normal and nude rats immediately before the injection of donor T cells, as well as 20 days after the reconstitution. Cell suspensions were stained as described with the biotinylated antibody against IgM, which was revealed with streptavidin-conjugated Red 670 (FL1), in combination with FITC-conjugated R73 antibody against the TCR (FL3). Monoclonal antibody His-41 was revealed with a PE-conjugated antimouse Ig secondary antibody (FL2). Using PC-LYSYS software the lymphocytes were gated on forward vs. side scatter plots and host (His-41–) and donor (His-41+) cells were determined. Finally, the percentage of host and donor B (IgM+) and T (R73+) lymphocytes were determined gating on the respective populations. The expression of surface molecules on B lymphocytes was determined as describes above.
In all experiments no differences were registered regarding the gender of normal and nude animals.
Statistical analysis
The statistical package SPSS for Windows 10.0 (SPSS Inc., Chicago, IL) was used to calculate the means and standard deviations, as well as to indicate significant differences (Mann–Whitney U-test, Wilcoxon matched-pairs signed-ranks test at P < 0·05 and one-way anova analysis of variance at P < 0·05).
Results
Proportions of immature B-lymphocyte subsets are increased in the peripheral blood and lymph nodes of nude rats
First, we checked the number of lymphocytes in the peripheral blood of normal and nude rats. Compared with normal rats, the nude animals showed a decreased number of lymphocytes per µl of peripheral blood, which was caused by an almost complete absence of T cells. In contrast, the number of B lymphocytes in the blood of nude rats was comparable to that of normal rats (Table 1).
Table 1.
The number of B lymphocytes in the blood of nude rats is normal
| Blood cells (per µl) | Normal | Nude |
|---|---|---|
| Lymphocytes | 8813 ± 948 | 2512 ± 355* |
| T lymphocytes | 6295 ± 861 | 178 ± 34* |
| B lymphocytes | 1976 ± 243 | 2300 ± 354 |
The values are expressed as mean ± SD (n = 8 for each group). Asterisks represent significant difference between normal and nude rats (Mann–Whitney U-test)
P < 0·01.
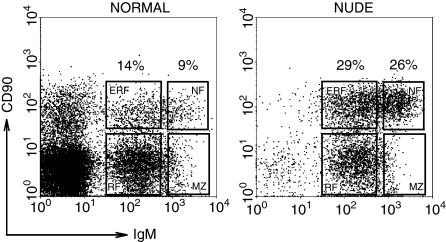
All subpopulations of B lymphocytes were readily demonstrated in the peripheral blood of normal and nude rats (Fig. 1). The immature cells (NF and ERF) represented less than one quarter (23%), whereas mature (RF and MZ) cells comprised more than three quarters (77%) of B lymphocytes in the blood of normal rats (Fig. 1). On the contrary, a prominent imbalance between immature and mature B-lymphocyte subsets was registered in the blood of nude rats: the immature cells prevailed (55%), whereas the mature cells represented less than half (45%) of blood B lymphocytes (Fig. 1). Thus, the ratio between immature CD90+ and mature CD90– cells was significantly increased in nude rats (1·2), in comparison with normal rats (0·3).
Figure 1.
The proportions of B lymphocyte subsets are disturbed in the blood of nude rats. The percentages of immature NF (CD90highIgMhigh) and ERF (CD90high IgMlow) B cells are increased, whereas those of mature RF (CD90– IgMlow), and MZ phenotype (CD90– IgMhigh) B cells are decreased in nude rats, compared with normal rats (each subset is framed, flow cytometry was used to perform the analysis, as described in Materials and methods).
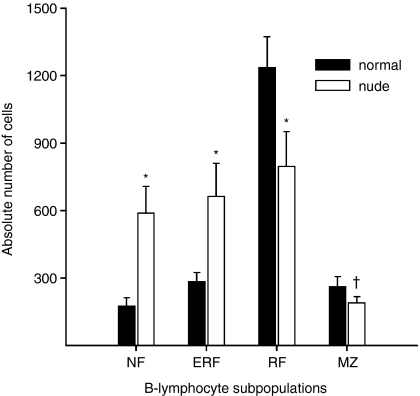
The numbers of B-lymphocyte subsets per µl of peripheral blood in normal and nude rats further strengthened these findings. The numbers of NF and ERF were significantly higher, whereas the numbers of RF and MZ phenotype B cells were significantly lower in nude rats, compared with normal rats (Fig. 2).
Figure 2.
The number of B cells of different subsets per µl of blood in normal and nude rats. In the blood of nude rats immature B lymphocyte numbers are increased, whereas those of mature B lymphocytes are decreased. Bars represent the mean ± SD (n = 8 for each group). * and † indicate a significant difference between normal and nude rats (Mann–Whitney U-test); *P < 0·05; †P < 0·01.
To exclude the possibility that the observed defects were restricted only to blood of nude rats, we also investigated the percentages of B-cell subsets in lymph nodes. The NF-B lymphocytes are normally almost absent from lymph nodes and the MZ phenotype B cells are present in low numbers.3–5 Therefore, we focused our attention on immature ERF and mature RF-B cells, which are present in sufficient numbers within lymph nodes. Similarly as in blood, the percentage of ERF-B cells was significantly increased in lymph nodes of nude rats (18 ± 3; n = 8) when compared with normal animals (11 ± 1; n = 8).
The surface molecule expression is decreased on B-lymphocyte subsets in the peripheral blood and lymph nodes of nude rats
Next, we investigated the expression of a palette of surface molecules involved in antigen presentation, cell interactions and migration of B lymphocytes. Among the molecules tested, a significant decrease in surface expression of MHC class II, ICAM-1, CD44 and l-selectin on B lymphocytes in the peripheral blood of nude rats was revealed when compared with normal animals.
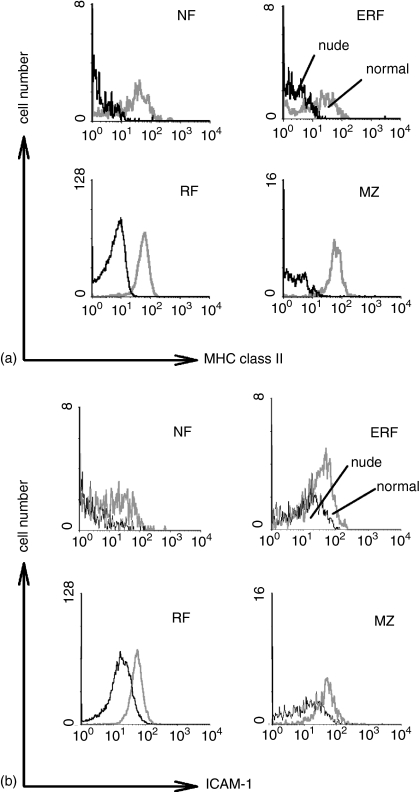
In normal rats we revealed a characteristic pattern of MHC class II expression. This molecule was increasingly expressed in a stepwise fashion according to the stage of B-cells maturity: from NF to the RF/MZ phenotype B cells. The same pattern of MHC class II expression was registered on B-lymphocyte subpopulations in nude rats (Table 2). However, the surface expression of MHC class II molecules was significantly decreased on all subsets of blood B cells of nude animals, in comparison with normal rats (Fig. 3; Table 2). The identical results were obtained for RT1B and RT1D haplotypes of MHC class II molecules (expression of RT1B is shown in Table 2).
Table 2.
Surface expression of MHC class II, ICAM-1, CD44 and l-selectin is decreased on B lymphocyte subpopulations in the blood of nude rats
| MHC class II | ICAM-1 | CD44 | l-selectin | LFA-1 | α4-integrin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells | Normal | Nude | Normal | Nude | Normal | Nude | Normal | Nude | Normal | Nude | Normal | Nude |
| NF | 307 ± 69 | 129 ± 75* | 362 ± 52 | 255 ± 55† | 189 ± 93 | 101 ± 58 | 70 ± 6 | 63 ± 10 | 284 ± 81 | 196 ± 51 | 174 ± 99 | 161 ± 85 |
| ERF | 387 ± 25 | 168 ± 77* | 405 ± 26 | 347 ± 31† | 147 ± 45 | 115 ± 40 | 191 ± 16 | 121 ± 52† | 292 ± 49 | 305 ± 35 | 210 ± 35 | 161 ± 58 |
| RF | 450 ± 16 | 281 ± 50* | 449 ± 12 | 355 ± 25* | 262 ± 33 | 168 ± 22* | 379 ± 8 | 292 ± 37† | 378 ± 52 | 386 ± 24 | 288 ± 25 | 241 ± 35 |
| MZ | 423 ± 33 | 224 ± 87* | 458 ± 33 | 336 ± 45* | 289 ± 90 | 181 ± 59† | 239 ± 36 | 154 ± 33† | 347 ± 93 | 349 ± 35 | 277 ± 72 | 199 ± 71 |
The mean fluorescence intensities are presented as mean ± SD and were determined by flow cytometry (n = 8 for each group). Asterisks and crosses represent significant differences between normal and nude rats (Mann–Whitney U-test)
P < 0·005
P < 0·05.
Figure 3.
Decreased expression of MHC class II and ICAM-1 on all blood B cell subsets of nude rats. (a) Representative histograms of the MHC class II expression. (b) Representative histograms of the ICAM-1 expression on NF (CD90high IgMhigh), ERF (CD90high IgMlow), RF (CD90– IgMlow), and MZ phenotype (CD90– IgMhigh) blood B lymphocytes in normal (grey line) and nude (black line) animals.
A stepwise increase of ICAM-1 surface expression from NF to RF/MZ-B cells was also observed in normal and nude rats (Table 2). However, the expression of ICAM-1 was significantly reduced on all subpopulations of B lymphocytes in the peripheral blood of nude rats, when compared with normal rats (Fig. 3; Table 2).
When the surface expression of CD44 on subsets of blood B lymphocytes of normal rats was compared with that of nude animals a reduction was observed on all B-cell subsets, but it was significant only on RF and MZ phenotype B cells (Table 2).
A distinctive pattern of l-selectin expression was registered on normal blood B cells. A very low level of l-selectin expression is a characteristic feature of normal NF-B cells5 (Table 2). However, the expression of this molecule was also decreased on MZ phenotype blood B cells of normal rats (Table 2). l-selectin expression was also low on NF-B lymphocytes from the peripheral blood of nude rats, but a significant decrease was observed on other subsets of nude B cells as well, when compared with the corresponding normal B lymphocytes (Table 2).
There was no difference in surface expression of LFA-1, α4-integrin (Table 2), MHC class I, and interleukin-2 receptor α chain (not shown) between normal and nude rats.
We also explored whether the expression of surface molecules was deranged not only on blood B lymphocytes, but on lymph node B cells of nude animals as well. Considering that NF-B and MZ phenotype B cells are found in very low numbers within the lymph nodes, we focused our attention on ERF and RF-B lymphocytes. In comparison with normal animals, a significant decrease of MHC class II, ICAM-1 and CD44 was registered on lymph node ERF and RF-B cells in nude rats (not shown).
Similarly as in the blood, no difference was registered in surface expression of LFA-1, α4-integrin, MHC class I, and interleukin-2 receptor α chain between normal and nude rats (not shown).
Proportions of B-lymphocyte subsets are normal in the bone marrow of nude rats
Next, we tried to gain more insight in the central phase of B-lymphocyte maturation in nude rats and to investigate if it was disturbed as well. Therefore we studied the proportions of B-cell subsets in the bone marrow of nude rats in the same manner as in the blood and lymph nodes. However, no difference was revealed in percentages of B-lymphocyte subsets within the bone marrow between normal and nude rats.
Further, we wished to define a developmental level at which the defect in surface molecule expression on nude B lymphocytes becomes evident. Therefore, we compared the surface molecule expression on B-cell subsets in the bone marrow of normal and nude rats.
Although the proportions of B-cell subsets were found to be normal in the bone marrow of nude rats, some impairment of surface molecule expression on different subsets was seen. Only the expression of MHC class II was significantly decreased on nude NF and ERF-B lymphocytes in the bone marrow (Table 3). On the other hand, the mature bone marrow RF-B and MZ phenotype B cells (which probably do not represent the local, resident cells, but the lymphocytes that re-entered the bone marrow from elsewhere in the body) showed a decreased expression of MHC class II, ICAM-1 and CD44 in nude versus normal rats (Table 3).
Table 3.
The differential expression of surface molecules on B lymphocyte subpopulations in the bone marrow of normal and nude rats
| MHC class II | ICAM-1 | CD44 | ||||
|---|---|---|---|---|---|---|
| Cells | Normal | Nude | Normal | Nude | Normal | Nude |
| NF | 189 ± 48 | 76 ± 30† | 307 ± 52 | 313 ± 43 | 185 ± 46 | 149 ± 25 |
| ERF | 179 ± 18 | 101 ± 12† | 351 ± 22 | 339 ± 7 | 187 ± 32 | 146 ± 27 |
| RF | 377 ± 4 | 207 ± 39† | 438 ± 3 | 347 ± 5* | 308 ± 21 | 211 ± 28* |
| MZ | 355 ± 67 | 202 ± 125† | 422 ± 32 | 327 ± 44* | 363 ± 52 | 221 ± 68* |
The mean fluorescence intensities are presented as mean ± SD and were determined by flow cytometry (n = 8 for each group). Crosses and asterisks represent significant differences between normal and nude rats (Mann–Whitney U-test)
P < 0·05
P < 0·005.
LFA-1, α4-integrin, MHC class I, and interleukin-2 receptor α chain were expressed at similar levels on bone marrow B lymphocytes of normal and nude rats (not shown).
Thymus implantation abolishes the block in maturation and defective expression of surface molecules on B lymphocytes in the blood and lymph nodes of nude rats
Thymic tissue grafts restored the number of T cells within the blood of nude rats (nude: 6 ± 3%, nude thymus-implanted: 25 ± 7%, control: 40 ± 8%) There was no significant difference between control and nude thymus-implanted rats.
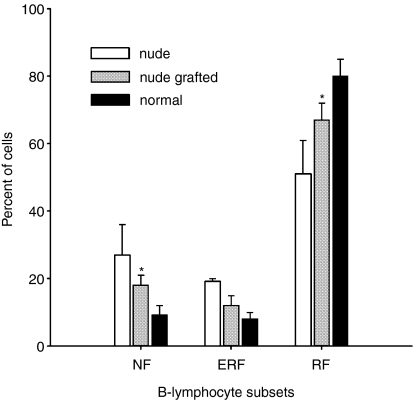
Thymic grafts strongly improved the peripheral maturation of B cells in nude rats. A decrease in number of immature NF and ERF-B lymphocytes was registered in blood of thymus-implanted animals (Fig. 4). Very notably, the percentage of ERF cells was decreased to a greater extent than the percent of NF cells. Thus, in the case of ERF cells there was no significant difference between normal and nude thymus-implanted animals. At the same time, thymus grafts induced an increase in number of mature RF (Fig. 4) and MZ-B cells in nude rats. Likewise, a significant decrease of immature ERF-B lymphocytes in lymph nodes, with a concomitant increase of mature RF-B cells was observed after thymus implantation to nude rats (not shown). Thus, no significant difference could be seen between thymus-implanted and control animals.
Figure 4.
The percentages of NF, ERF, and RF-B cells in the blood of nude, nude thymus-grafted and normal rats. The imbalance between immature and mature B lymphocytes in the blood of nude rats is alleviated by thymus engraftment (n = 3–5 for each group). Asterisk indicates a significant difference between normal and nude thymus-grafted rats (one-way anova analysis of variance); *P < 0·05.
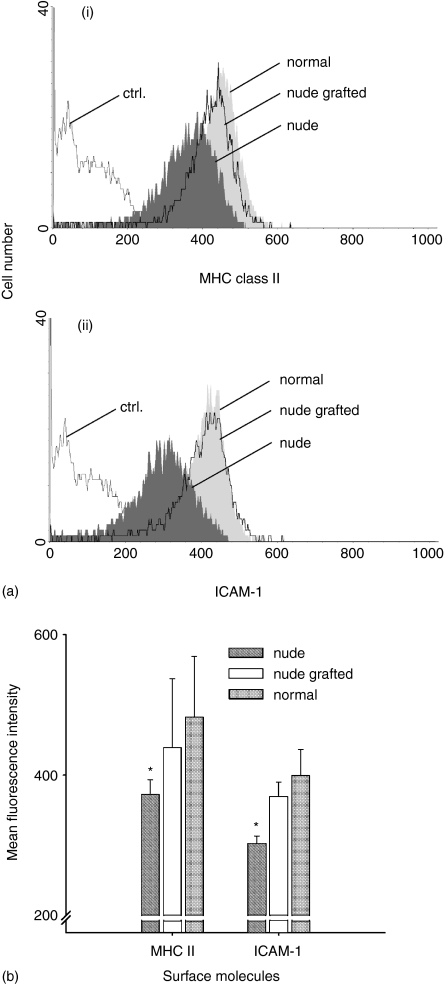
Moreover, thymus grafts alleviated the defective expression of surface molecules on B-cell subpopulations in the blood of nude animals. An increase in surface expression of MHC class II was observed in all subpopulations of B cells (Fig. 5a(I), b), whereas the expression of ICAM-1 was increased on ERF, RF, and MZ phenotype B lymphocytes (Fig. 5a(ii), b). In the lymph nodes only ERF and RF were studied, because of paucity of NF and MZ phenotype B lymphocytes. Changes corresponding to those observed in the blood were also registered in the lymph nodes (not shown).
Figure 5.
Thymus implantation normalizes the decreased expression of MHC class II and ICAM-1 on recirculating follicular B cells in the blood of nude rats. (a) Representative histograms of the MHC class II (i), and ICAM-1 (ii) expression on RF-B lymphocytes (CD90– IgMlow) in the blood of normal (light grey), nude (dark grey), and nude thymus-grafted (transparent) animals are demonstrated. Ctrl., the background staining of cells in the absence of primary antibody. (b) The MHC class II and ICAM-1 expression on blood RF-B cells in nude, nude thymus-implanted and normal rats are shown. Bars represent the mean of the channel of the mean fluorescence intensity ± SD (n = 3–5 for each group). There is no significant difference between normal and nude thymus-grafted animals. Asterisk indicates a significant difference between normal and nude rats; *P < 0·05.
Defective surface expression of MHC class II on B lymphocytes of nude rats is abolished by reconstitution with T lymphocytes
Finally, we investigated whether the increased expression of MHC class II on B lymphocytes of nude rats after thymus engraftment was due to the presence of thymic tissue per se or to the resulting occurrence of T lymphocytes. Therefore, we reconstituted the nude rats with congeneic T lymphocytes. In this manner we restored their peripheral T-lymphocyte pool, but evaded the presence of thymic tissue.
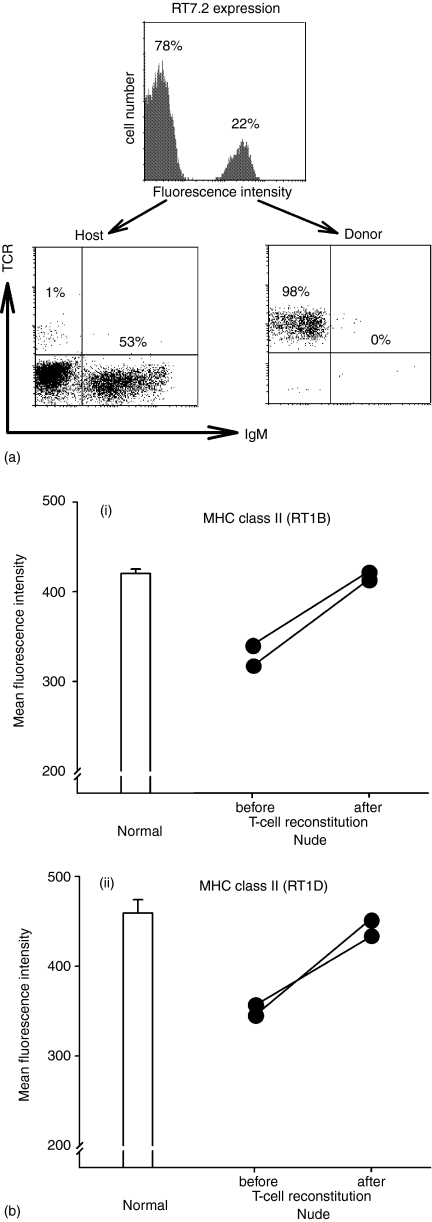
The reconstitution of nude rats with T lymphocytes from blood of normal, congeneic donors was successful. In the reconstitution system used the host and donor cells could be easily distinguished (Fig. 6a). It was also clear that the separation of donor T and B lymphocytes before the injection was successful and that the inoculum consisted of donor T lymphocytes only. More than 20% of donor T lymphocytes were found 20 days after reconstitution in the blood of nude rats and only very few donor B lymphocytes were present (Fig. 6a). Similar numbers of T lymphocytes were found in lymph nodes and spleen of the nude rats.
Figure 6.
Successful reconstitution with congeneic T cells improves the defective MHC class II expression on blood B lymphocytes of nude rats. (a) The lymphocytes of host and donor origin are clearly distinguished in the blood of nude recipients after reconstitution with congeneic T cells. A substantial proportion of blood cells represent RT7.2+ lymphocytes of donor origin, exclusively composed of T cells. The majority of blood cells are RT7.2– lymphocytes of host origin containing very few T cells. (b) Twenty days after reconstitution of nude rats with congeneic T lymphocytes the expression of MHC class II RT1B (i), and RT1D (ii) on blood B lymphocytes is increased. The values of blood B-cell MHC class II expression for two nude rats before and 20 days after reconstitution with congeneic T lymphocytes are shown as black circles connected by a line.
Before reconstitution, the expression of MHC class II (both RT1B and RT1D) on blood B lymphocytes of nude animals was remarkably low. However, 20 days after reconstitution with congeneic T lymphocytes, the expression of MHC class II (both RT1B and RT1D) (Fig. 6b(i), (ii)) on blood B lymphocytes of nude rats was greatly increased and corresponded well to that of normal animals.
Discussion
It is well known that nude animals are thymus aplastic and show a generalized lack of T lymphocytes. However, their B-lymphocyte system was considered to be normal, primarily because the numbers of splenic and peripheral B cells in nude animals are normal,7,10,11 as in αβ-TCR-deficient or adult thymectomized mice.6,7 However, our study shows that, despite normal number, the B-lymphocyte population in nude rats is abnormal.
The proportions of B-lymphocyte subsets are greatly disturbed in the peripheral lymphoid system of nude animals. The numbers of immature B cells, i.e. NF and ERF, are significantly increased, whereas the proportion of mature RF-B lymphocytes is significantly decreased, which points towards a block in the peripheral, post-bone marrow phase of B-lymphocyte maturation in nude rats because of the lack of T-cell help. A similar arrest in B-lymphocyte maturation, at the transition between immature and mature peripheral B-cell subsets, also occurs in animals having a defective B-cell receptor signalling pathway5 or lacking the invariant chain Ii.19 In these animals the proportion of immature B cells is also increased, whereas the mature B-cell compartment is markedly reduced. In double mutant mice lacking the invariant chain and DM molecules20 or having different defects in molecular signalling pathways21,22 this block is also characterized by a similar disturbance of B-cell subsets in the blood.
We also show that in nude animals the block in B-cell maturation is accompanied by a decreased expression of a selected set of surface molecules. The molecules that we showed in this study to be affected in nude B cells are of the utmost functional significance. Therefore, the B-lymphocyte system of nude animals cannot be regarded as normal. Some early studies documented the impaired functional capacity of the nude B-lymphocyte system. For example, after injection of endotoxin the response in the draining lymph nodes of nude mice is fundamentally different to that in normal animals.23 Poor B-cell proliferation is also seen in the nude mouse spleen after injection of lipopolysaccharide.24 Repeatedly, the reduced immunoglobulin level was reported in nude animals25,26 and a reduction in the serum IgA and IgG classes, but not in IgM level, was observed.27,28 Of course, this can be attributed to the lack of T-cell help, but also may reflect the immature status of peripheral nude B lymphocytes and a defective expression of surface molecules. In this context, our results acquire an additional meaning. The fact the T cells co-operate with B cells during the immune responses is, of course, very well known. Our study extends this knowledge: we show that T cells not only participate in co-operation, but also induce on B lymphocytes the expression of surface molecules which are necessary for successful co-operation. This finding has its bearing on our insight in pathogenesis of T-cell directed diseases such as, for example, human immunodeficiency virus, as well on the strategy of vaccine design.
Our initial hypothesis, that the defect of B-lymphocyte maturation in nude rats is related to the absence of T lymphocytes, was confirmed by engraftment of thymus tissue in nude rats. We demonstrated that the block in the peripheral phase of B-lymphocyte maturation in nude rats can be largely overcome by thymus engraftment: not only the imbalance between immature and mature B-cell subpopulations was alleviated, but also the anomalous surface molecule expression was normalized. Interestingly, the most prominent effect was observed on ERF-B lymphocytes. Thymus implants decreased the percentage of these cells to a level not statistically different from that of control rats (Fig. 5). This in vivo finding corresponds well with the in vitro results of Chung et al.29,30 They demonstrated that T-cell help to precisely this subset of B cells promotes their survival and proliferation compared with the developmentally earlier transitional B-cell stage. Although the proportion of NF-B cells was substantially decreased in nude rats after thymus engraftment, the proportion of ERF-B lymphocytes was reduced to an even greater extent. This was accompanied by a proportional increase of mature RF-B cells. These data show that thymus graft rescued the maturation of B lymphocytes and confirm that signalling to ERF cells is critical in the development of the peripheral B-cell pool.31 Very notably, we found that the rescue of maturation was accompanied by the restoration of normal surface expression of several functionally significant molecules, among which is ICAM-1. This is very interesting, as the addition of the ligand molecule for ICAM-1, i.e. LFA-1, to the transitional B-cell/T-cell coculture strongly decreases the supportive effect of T lymphocytes.32 The fact that the decrease in number of NF-B lymphocytes after thymus implantation was not as large as that of ERF cells confirms that the former are subject to different selection processes which may be partly thymus-independent.30 Finally, by reconstitution of nude rats with blood T lymphocytes we demonstrated that the defective surface molecule expression on B lymphocytes was rectified under the direct influence of T cells and not by the soluble products released by thymic tissue itself.
The decreased expression of several functionally significant surface molecules on the mature RF-B-lymphocyte subset of nude animals and subsequent up-regulation by thymus implants show that the maintenance of mature B-lymphocyte viability (at least as reflected by the expression of surface molecules) also depends on their in vivo interactions with normal T lymphocytes.
We believe that, as in many other immunological reactions, both direct cell contacts between T and B cells and cytokines produced by T cells, probably acting in a paracrine manner, are required for the peripheral phase of B lymphocyte maturation. The molecules involved in direct cell contacts may be those influencing the decision-making by B cells, as for example, CD40 ligand.33 On the other hand, interferon-γ is well known to increase the surface MHC class II expression on different cells,34 which makes it a suitable soluble factor to participate in the peripheral phase of B-cell maturation. Such issues deserve further attention and in vitro investigation.
An interesting question is whether the spleen or lymph nodes are required for the peripheral phase of B cell maturation. l-selectin is not expressed on NF-B lymphocytes and therefore these cells are absent from lymph nodes [3–5; this work]. In l-selectin deficient animals all lymphocytes are directed into the spleen.35 Considering these data, it seems likely that at least the first stage of peripheral phase of B-cell maturation from NF- to ERF-B cells takes place only in the spleen. Further steps of maturation may involve lymph nodes as well.
A remarkable point is that in nude rats B cells attain normal overall frequency without maturing. This shows that homeostasis of B-lymphocyte number is very tightly regulated by factors which balance the cell death and proliferation: as in nude rats (this work), the balance between various functional B-cell subsets is disturbed in aged mice, still the overall number of these cells remains unaltered.36
Our study shows that in nude rats: (a) the peripheral phase of B-lymphocyte maturation is defective; (b) the block is located at the transition between the immature and mature stages; (c) the expression of a set of surface molecules on B lymphocytes is decreased; (d) at least a part of defects in peripheral phase of B-lymphocyte maturation and surface molecule expression is abrogated by thymus engraftment; (e) this amelioration is not induced by thymic stroma, but by direct influence of T lymphocytes; and (f) the defects are not caused by a genetic mutation. Significantly, the described anomalies were verified in two strains of nude rats. These data greatly extend the earlier reports that demonstrated the dependence of B-lymphocyte development in xid mice on the presence of thymus/T lymphocytes.13,14,37 Mutual cellular interactions lie at the base of all control and effector immune reactions. Close cell–cell interactions also govern the maturation of lymphocytes, for example, in the thymus.38 In addition to thymic epithelial and dendritic cells, B lymphocytes emerge as cell type with a significant role in thymocyte maturation.39 Thus, it would not be surprising that opposite could also hold true and T lymphocytes might be involved in B-cell maturation. This view is congruent with a well-known role T cells play in a special phase of B-lymphocyte maturation, which occurs in germinal centres of lymphoid follicles.40 The new ways of interfering with T-cell function, such as transfer of bacterial artificial chromosome clones are now much on the agenda. Our study, for instance, the influence of T-cell reconstitution on B-cell maturation, provides a new approach to test these functions in vivo.
Acknowledgments
Thanks are due to Kerstin Bankes, Ingeborg Dressendörfer, Frauke Weidner, Marie-Luise Leppin, Karola von Lingelsheim and Lidija Gutjahr for their technical assistance and Professor Jelena Marinković for one-way anova analysis of variance. This work was supported by Deutsche Forschungsgemeinschaft (We 1175/4-3) and the grant of the Ministry for Science and Protection of Natural Environment of Republic of Serbia (no. 1472). N.M.M. was a fellow of the Alexander von Humboldt-Foundation, Bonn, Germany.
Abbreviations
- ERF
early recirculating follicular
- MZ
marginal zone
- NF
newly formed
- RF
recirculating follicular
References
- 1.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 2.Cancro MP. Peripheral B-cell maturation. the intersection of selection and homeostasis. Immunol Rev. 2004;197:89–101. doi: 10.1111/j.0105-2896.2004.0099.x. [DOI] [PubMed] [Google Scholar]
- 3.Kroese FGM, de Boer NK, de Boer T, Nieuwenhuis P, Kantor AB, Deenen GJ. Identification and kinetics of two recently bone marrow-derived B cell populations in peripheral lymphoid tissues. Cell Immunol. 1995;162:185–93. doi: 10.1006/cimm.1995.1068. [DOI] [PubMed] [Google Scholar]
- 4.de Boer NK. B cell lineages in the rat: proefschrift. Groningen: Rijksuniversiteit Groningen; 1994. [Google Scholar]
- 5.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mombaerts P, Mizoguchi E, Ljunggren H-G, et al. Peripheral lymphoid development and function in TCR mutant mice. Int Immunol. 1994;6:1061–70. doi: 10.1093/intimm/6.7.1061. [DOI] [PubMed] [Google Scholar]
- 7.Kindred B. Nude mice in immunology. Prog Allergy. 1979;26:137–238. [PubMed] [Google Scholar]
- 8.Nehls M, Kyewski B, Messerle M, Waldschutz R, Schuddekopf K, Smith AJ, Boehm T. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996;272:886–9. doi: 10.1126/science.272.5263.886. [DOI] [PubMed] [Google Scholar]
- 9.Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–7. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 10.Sprent J. Circulating T and B lymphocytes of the mouse. I. Migratory properties. Cell Immunol. 1973;7:10–39. doi: 10.1016/0008-8749(73)90180-9. [DOI] [PubMed] [Google Scholar]
- 11.Sprent J, Basten A. Circulating T and B lymphocytes of the mouse. II. Lifespan. Cell Immunol. 1973;7:40–59. doi: 10.1016/0008-8749(73)90181-0. [DOI] [PubMed] [Google Scholar]
- 12.Croy BA, Osoba D. Nude mice-A model system for studying the cellular basis of the humoral immune response. Cell Immunol. 1973;9:306–18. doi: 10.1016/0008-8749(73)90082-8. [DOI] [PubMed] [Google Scholar]
- 13.Mond JJ, Scher I, Cossman J, Kessler S, Mongini PK, Hansen C, Finkelman FD, Paul WE. Role of the thymus in directing the development of a subset of B lymphocytes. J Exp Med. 1982;155:924–36. doi: 10.1084/jem.155.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wortis HH, Burkly L, Hughes D, Roschelle S, Waneck G. Lack of mature B cells in nude mice with X-linked immune deficiency. J Exp Med. 1982;155:903–13. doi: 10.1084/jem.155.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabo P, Zhao K, Kirman I, Le Maoult J, Dyall R, Cruikshank W, Weksler ME. Maturation of B cell precursors is impaired in thymic-deprived nude and old mice. J Immunol. 1998;161:2248–53. [PubMed] [Google Scholar]
- 16.Milićević NM, Luettig B, Trautwein C, Wüstefeld T, Mähler M, Jecker P, Wonigeit K, Westermann J. Splenectomy of rats selectively reduces lymphocyte function-associated antigen 1 and intercellular adhesion molecule 1 expression on B-cell subsets in blood and lymph nodes. Blood. 2001;98:3035–41. doi: 10.1182/blood.v98.10.3035. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson A. Implantation of tissue under the kidney capsule. In: Weir DM, editor. Applications of Immunological Methods in Biomedical Sciences, 4th edn. Handbook of experimental immunology, Vol. 4. Vol. 133. Oxford: Blackwell Scientific Publishing; 1986. pp. 15–7. [Google Scholar]
- 18.Kampinga J, Kroese FG, Pol GH, et al. RT7-defined alloantigens in rats are part of the leucocyte common antigen family. Scand J Immunol. 1990;31:699–710. doi: 10.1111/j.1365-3083.1990.tb02821.x. [DOI] [PubMed] [Google Scholar]
- 19.Shachar I, Flavell RA. Requirement for invariant chain in B cell maturation and function. Science. 1996;274:106–8. doi: 10.1126/science.274.5284.106. [DOI] [PubMed] [Google Scholar]
- 20.Kenty G, Martin WD, Van Kaer L, Bikoff EK. MHC class II expression in double mutant mice lacking invariant chain and DM functions. J Immunol. 1998;160:606–14. [PubMed] [Google Scholar]
- 21.Kim U, Gunther CS, Roeder RG. Genetic analyses of NFKB1 and OCA-B function: defects in B cells, serum IgM level, and antibody responses in Nfkb1–/–Oca-b–/– mice. J Immunol. 2000;165:6825–32. doi: 10.4049/jimmunol.165.12.6825. [DOI] [PubMed] [Google Scholar]
- 22.Yun TJ, Tallquist MD, Aicher A, et al. Osteoprotegerin, a crucial regulator of bone metabolism, also regulates B cell development and function. J Immunol. 2001;166:1482–91. doi: 10.4049/jimmunol.166.3.1482. [DOI] [PubMed] [Google Scholar]
- 23.Parrot DMV, de Sousa MAB. Proceedings of the 1st International Workshop on Nude Mice. Stuttgart: Fischer Verlag; 1974. B cell stimulation in nude (nu/nu) mice; p. 61. [Google Scholar]
- 24.Hanaoka M, Takigawa M. Proceedings of the 2nd International Workshop on Nude Mice. Tokyo: University of Tokyo Press; 1977. Proliferation of B lymphocytes in the spleen of nude mice; p. 185. [Google Scholar]
- 25.Pantelouris EM. Observations on the immunobiology of ‘nude’ mice. Immunology. 1971;20:247–52. [PMC free article] [PubMed] [Google Scholar]
- 26.Wortis HH. Immunological responses of ‘nude’ mice. Clin Exp Immunol. 1971;8:305–17. [PMC free article] [PubMed] [Google Scholar]
- 27.Crewther P, Warner NL. Serum immunoglobulins and antibodies in congenitally athymic (nude) mice. Aust J Exp Biol Med Sci. 1972;50:625–35. doi: 10.1038/icb.1972.55. [DOI] [PubMed] [Google Scholar]
- 28.Luzzati AL, Jacobson EB. Serum immunoglobulin levels in nude mice. Eur J Immunol. 1972;2:473–4. doi: 10.1002/eji.1830020518. [DOI] [PubMed] [Google Scholar]
- 29.Chung JB, Sater RA, Fields ML, Erikson J, Monroe JG. CD23 defines two distinct subsets of immature B cells which differ in their responses to T cell help signals. Int Immunol. 2002;14:157–66. doi: 10.1093/intimm/14.2.157. [DOI] [PubMed] [Google Scholar]
- 30.Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends Immunol. 2003;24:343–9. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 31.Su TT, Guo B, Wei B, Braun J, Rawlings DJ. Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunol Rev. 2004;197:161–78. doi: 10.1111/j.0105-2896.2004.0102.x. [DOI] [PubMed] [Google Scholar]
- 32.Chung JB, Wells AD, Adler S, Jacob A, Turka LA, Monroe JG. Incomplete activation of CD4 T cells by antigen-presenting transitional immature B cells: implications for peripheral B and T cell responsiveness. J Immunol. 2003;171:1758–67. doi: 10.4049/jimmunol.171.4.1758. [DOI] [PubMed] [Google Scholar]
- 33.Laman JD, Claassen E, Noelle RJ. Functions of CD40 and its ligand, gp39 (CD40L) Crit Rev Immunol. 1996;16:59–108. doi: 10.1615/critrevimmunol.v16.i1.40. [DOI] [PubMed] [Google Scholar]
- 34.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 35.Tang MLK, Steeber DA, Zhang X-Q, Tedder TF. Intrinsic differences in 1-selectin expression levels affect T and B lymphocyte subset-specific recirculation pathways. J Immunol. 1998;160:5113–21. [PubMed] [Google Scholar]
- 36.Klinman NR, Kline GH. The B-cell biology of aging. Immunol Rev. 1997;160:103–14. doi: 10.1111/j.1600-065x.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 37.Sprent J, Bruce J. Physiology of B cells in mice with X-linked immunodeficiency, II. Influence of the thymus and mature T cells on B cell differentiation. J Exp Med. 1984;160:335–40. doi: 10.1084/jem.160.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milićević NM, Milićević Ž. Thymus cell–cell interactions. Int Rev Cytol. 2004;235:1–52. doi: 10.1016/S0074-7696(04)35001-1. [DOI] [PubMed] [Google Scholar]
- 39.Joao C, Ogle BM, Gay-Rabinstein C, Platt JL, Cascalho M. B cell-dependent TCR diversification. J Immunol. 2004;172:4709–16. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 40.Tarlinton D. Germinal centers: a second childhood for lymphocytes. Curr Biol. 1997;7:R155–9. doi: 10.1016/s0960-9822(97)70077-0. [DOI] [PubMed] [Google Scholar]