Abstract
The synthesis of 5,6-dimethylbenzimidazole (DMB), the lower ligand of coenzyme B12, has remained elusive. We report in vitro and in vivo evidence that the BluB protein of the photosynthetic bacterium Rhodospirillum rubrum is necessary and sufficient for catalysis of the O2-dependent conversion of FMNH2 to DMB. The product of the reaction (DMB) was isolated by using reverse-phase high-pressure liquid chromatography, and its identity was established by UV-visible spectroscopy and MS. No metals were detected in homogeneous preparations of BluB, and the enzyme did not affect DMB synthesis from 4,5-dimethylphenylenediamine and ribose-5-phosphate. The effect of the lack of bluB function in R. rubrum was reflected by the impaired ability of a ΔbluB strain to convert Mg-protoporphyrin IX monomethyl ester (MPE) into protochlorophylide, a reaction of the bacteriochlorophyll biosynthetic pathway catalyzed by the MPE-cyclase enzyme present in this bacterium (BchE, EC 1.14.13.81), a predicted coenzyme B12-dependent enzyme. The growth defect of the ΔbluB strain observed under anoxic photoheterotrophic conditions was corrected by the addition of DMB or B12 to the culture medium or by introducing into the strain a plasmid encoding the wild-type allele of bluB. The findings reported here close an important gap in our understanding of the enzymology of the assembly of coenzyme B12.
Keywords: B12 biosynthesis, B12 lower ligand synthesis, oxygenases, vitamin metabolism
Adenosylcobalamin (AdoCbl, coenzyme B12) is a structurally complex cobalt-containing cyclic tetrapyrrolidine/pyrroline synthesized by diverse groups of prokaryotes. Consistent with the complexity of its structure, a great deal of genetic information is dedicated to the assembly of AdoCbl (1). Despite the advances in our understanding of the biosynthesis of AdoCbl, there are steps of the pathway that remain unclear.
Complete corrinoids (cobamides; e.g., AdoCbl) consist of a corrin ring and upper and lower axial ligands. The lower axial ligand of AdoCbl is 5,6-dimethylbenzimidazole (DMB) (2). Our current understanding of the biosynthesis of DMB is limited, because the enzymes involved have not been studied. From labeling studies performed in Propionibacterium freundenreichii and Salmonella enterica, it was learned that these bacteria synthesize DMB from FMN (3, 4). This conversion was oxygen-dependent (5), with the C-1 carbon of the ribose moiety of FMN becoming the C-2 carbon of DMB (6, 7). Insights into the possible mechanism for the conversion of FMN to DMB were obtained from studies of the nonenzymatic spontaneous synthesis of DMB from 4,5-dimethylphenylenediamine (DMPDA) and ribose-5-phosphate (8). The conclusion from these studies was that the conversion of FMN to DMB was likely to be catalyzed by a single enzyme responsible for triggering a nonenzymatic oxidative cascade yielding DMB (8).
As mentioned above, the identity of the genes encoding the enzymes of the FMN-to-DMB pathway remains unknown. Previous reports of enzymes involved in DMB synthesis proved misleading (9). The first clue to the identity of the DMB biosynthetic genes in any prokaryote was obtained over a decade ago from studies of the photosynthetic purple bacterium Rhodobacter capsulatus (10) and more recently from studies of the soil bacterium Sinorhizobium meliloti (11). Both studies showed that the function encoded by the bluB gene was involved in coenzyme B12 synthesis, and Campbell et al. (11) showed that B12 synthesis in the S. meliloti bluB strain was restored by exogenously supplied DMB (10). However, neither one of these studies reported biochemical evidence that the BluB protein catalyzed any of the steps of the DMB biosynthetic pathway, nor did they identify the substrates or products of the BluB reaction.
Cobalamin auxotrophs of R. capsulatus accumulate Mg-protoporphyrin IX monomethyl ester (MPE), the substrate of the MPE-cyclase (BchE) enzyme that converts MPE into protochlorophylide, a precursor of bacteriochlorophyll (Bchl) (12). BchE contains a Cbl-binding domain and is believed to require Cbl for catalysis (12). MPE accumulation is readily detectable by its characteristic absorption maximum at 416 nm and can therefore be used to detect a block in Cbl biosynthesis in this bacterium. Purple photosynthetic bacteria (including R. capsulatus and Rhodospirillum rubrum) synthesize Bchl and grow photoheterotrophically in anaerobic or microaerophilic conditions (13). Anaerobic Bchl synthesis depends on BchE activity in these organisms (14). R. rubrum is a well studied bacterium that produces substantial quantities of complete cobamides (15). The genome of this bacterium contains a bluB homolog, and genetic tools for the construction of mutant strains are available. We used R. rubrum to study the effect of the lack of BluB protein on MPE metabolism as a function of DMB in the culture medium. We report here in vivo and in vitro evidence that a R. rubrum bluB strain is defective in DMB synthesis, and that the BluB enzyme from this bacterium is necessary and sufficient for the O2-dependent synthesis of DMB from FMNH2 in vitro.
Results
R. rubrum Contains a BluB Homolog.
ORF Rru_A3536 in R. rubrum is homologous to the bluB genes of R. capsulatus and S. meliloti [supporting information (SI) Fig. 4]. The hypothetical R. rubrum BluB protein was 52% identical/65% similar to the one from R. capsulatus and 44% identical/55% similar to the one from S. meliloti. We used this information to explore the role of the BluB in Cbl biosynthesis in vivo and in vitro.
BluB Catalyzes the O2-Dependent Conversion of FMNH2 to DMB.
The bluB+ gene of R. rubrum was cloned and overexpressed in Escherichia coli. BluBWT protein was purified to homogeneity (SI Fig. 5) and was used to test the hypothesis that it was solely responsible for the conversion of FMNH2 to DMB. Residue R22, a conserved residue in the putative FMN-binding domain of BluB (SI Fig. 4) was mutated to glutamate in the overexpression vector carrying the bluB+ allele. The resulting bluB2 allele encoded the BluBR22E variant protein that was used as negative control for the in vitro experiments described below. H6-tagged BluBWT and BluBR22E proteins were purified by affinity chromatography and detagged by using rTEV protease; a second chromatographic step yielded protein with only three additional N-terminal residues (Gly-Ala-Ser) and ≥97% homogeneity (SI Fig. 5). Results from gel-filtration analysis showed that BluBWT eluted with a molecular mass of 47 kDa, which suggested that BluB is a homodimer in its native state (SI Fig. 6). Inductively coupled plasma emission spectrometry indicated that there are no metal ions associated with BluBWT (data not shown).
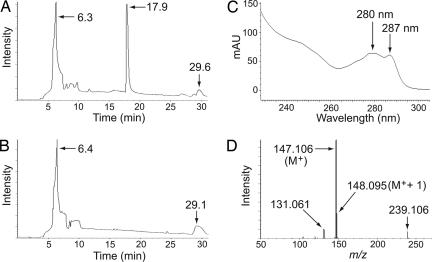
The BluB reaction was performed under reduced O2 levels (see Materials and Methods), and FMNH2 was generated in situ by using E. coli's NADH-dependent FMN reductase (Fre) enzyme (16); reaction products were analyzed by liquid chromatography MS. After incubation, the complete reaction mixture contained a compound that eluted 17.9 min after injection (Fig. 1A). This compound was identified as DMB on the basis of its elution time, its UV-visible spectrum (Fig. 1C), and high-resolution MS data (Fig. 1D), all of which were identical to authentic DMB standards (data not shown). DMB was absent in a control reaction mixture lacking BluBWT (Fig. 1B). The identity of the purified BluB reaction product as DMB was confirmed with a bioassay by using a DMB pseudoauxotrophic S. enterica cobT strain (17). Growth of this strain on minimal medium with cobinamide was permitted by addition of either authentic DMB or HPLC-purified BluB reaction product, neither of which restored growth of an S. enterica cobT cobB strain [a Cbl auxotroph (17, 18); data not shown].
Fig. 1.
BluB converts FMNH2 to DMB. Reactions were prepared with 40 μg of BluBWT enzyme/1 μg of Fre enzyme/500 μM FMN/5 mM NADH/100 mM Hepes (pH 8)/50 mM NaCl and incubated for 18 h at 30°C. Products were analyzed by liquid chromatography MS. A shows the accumulation of a product eluting at 17.9 min, which is not present in the control reaction lacking BluB, shown in B. C and D show the UV-visible absorbance and mass spectra of the product of the BluB reaction, which were identical to authentic DMB standards (not shown).
The rate of FMNH2 conversion to DMB increased as a function of BluBWT concentration. When 20, 30, or 40 μg of BluBWT was present in the reaction mixture, DMB was synthesized at 6, 12, or 25 pmol min−1, respectively. The corresponding calculated specific activities were 285, 410, or 625 pmol DMB min−1·mg−1. DMB was not synthesized in the absence of oxygen. To show dependence on BluBWT, variant BluBR22E protein was substituted for BluBWT in the reaction mixture. BluBR22E failed to catalyze the conversion of FMN to DMB. Fre and NADH were required for DMB synthesis, indicating that FMNH2, not FMN, was the substrate for BluBWT.
BluBWT had no effect on the rate of abiotic DMB synthesis from DMPDA and ribose-5-phosphate (data not shown).
A R. rubrum ΔbluB Mutant Strain Fails to Synthesize Cobalamin.
On the basis of the result described above, we predicted that Cbl synthesis would be blocked in a R. rubrum strain lacking bluB. To test this idea, an in-frame deletion of the R. rubrum bluB gene was constructed (hereafter referred to as ΔbluB). The WT and ΔbluB strains were grown in minimal medium lacking corrinoids under chemoheterotrophic (oxic) or photoheterotrophic (anoxic) conditions. Corrinoids were extracted from these cultures and analyzed by RP-HPLC. WT R. rubrum cells grown in the presence of oxygen contained 1.4 pmol of Cbl per milligram of total protein. The amount of Cbl in aerobically grown ΔbluB cells was below the limit of detection (SI Fig. 7). Extracts of aerobically grown ΔbluB cells contained a complete cobamide that allowed growth of S. enterica strain JE8248 (ΔcobS), which lacks cobalamin (5′-P) synthase and is therefore a Cbl auxotroph (data not shown). The cobamide synthesized by the R. rubrum ΔbluB strain eluted 6.6 min after injection, as compared with 13.6 min for authentic Cbl (SI Fig. 7). Analysis of the MALDI-TOF mass spectrum of this compound identified signals with m/z values of 1,370.5, 1,354.5, and 1,332.5 (data not shown). The concentration of this cobamide was calculated at 0.24 pmol per milligram of total protein, and its identity was not pursued.
Cbl was not detected in WT or ΔbluB R. rubrum strains grown in the absence of oxygen. However, cell-free extracts of these strains contained small amounts of complete cobamides, as detected by bioassay (data not shown). RP-HPLC retention time and MS data identified cobyric acid (Cby) as the most abundant corrinoid present in R. rubrum WT and ΔbluB strains grown anoxically (data not shown). The concentration of Cby in WT R. rubrum grown anoxically was ≈3 pmol per milligram total protein, with similar levels detected in the ΔbluB strain (data not shown).
A ΔbluB Strain of R. rubrum Grows Poorly Under Anoxic Photoheterotrophic Conditions in the Absence of DMB.
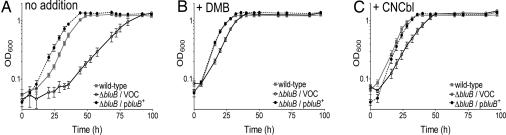
The growth behavior of strains lacking bluB function was assessed under anoxic photoheterotrophic conditions in reduced malate (1 g/liter) minimal medium (rMN); WT R. rubrum was used as positive control. The ΔbluB strain carrying an empty cloning vector grew poorly under these conditions, with an extended lag phase (35 vs. 10 h for the bluB+ strain) and a long doubling time (13 vs. 6 h for the bluB+ strain) (Fig. 2A). The growth behavior of a ΔbluB strain carrying a plasmid encoding bluB+ was similar to that of the bluB+ strain. Addition of DMB to the culture medium shortened the lag phase of the ΔbluB culture and decreased the doubling time of the culture to match those of the bluB+ and ΔbluB/pbluB+ strains (Fig. 2B). Addition of Cbl had a similar effect (Fig. 2C). Together, the above results indicated that Cbl synthesis in R. rubrum required bluB function. The ΔbluB strain did not display any discernable growth defect under oxic growth conditions (data not shown).
Fig. 2.
Effect of the lack of bluB function on growth. R. rubrum strains were grown under anaerobic phototrophic conditions. Optical density at 600 nm was measured for the WT strain (gray lines), the ΔbluB strain carrying an empty cloning vector (VOC, vector-only control) (solid black lines), and the ΔbluB strain carrying the cloning vector encoding a bluB+ allele (dashed black lines). A shows growth in rMN. B and C show growth in rMN supplemented with 1 μM DMB or 100 nM cyanocobalamin.
Impact of the Lack of bluB Function on Chlorophyll Synthesis.
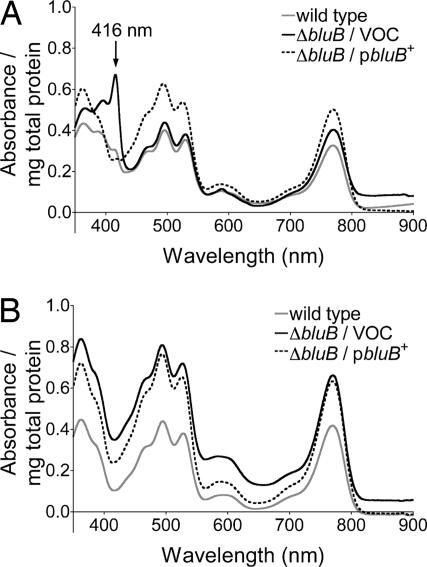
Cbl is required for the formation of the isocyclic ring of Bchl under anoxic conditions (12, 14). We predicted that the ΔbluB strain would accumulate MPE, an intermediate of Bchl biosynthesis with a diagnostic absorption maximum at 416 nm (14). Analysis of the UV-visible spectra of solvent extracts obtained from an early stationary phase culture of the ΔbluB strain grown photoheterotrophically in the absence of oxygen showed the accumulation of a compound with strong absorbance at 416 nm (Fig. 3A). This compound was absent in solvent extracts of the bluB+ or ΔbluB/pbluB+ strains, suggesting its metabolism depended on the availability of Cbl. Consistent with this idea, this compound was absent in solvent extracts of the ΔbluB strain when cells were grown in the presence of DMB (Fig. 3B) or Cbl (data not shown).
Fig. 3.
MPE accumulation in the ΔbluB strain. (A) The absorbance spectra of pigment extracts prepared from early stationary phase anoxic photoheterotrophic cultures of the WT strain (gray lines), the ΔbluB strain carrying an empty cloning vector (VOC, vector-only control) (solid black lines), and the ΔbluB strain carrying the cloning vector encoding a bluB+ allele (dashed black lines). (B) The absorbance spectra of the same strains grown in the presence of 1 μM DMB.
Discussion
The BluB Protein Is Necessary and Sufficient for the O2-Dependent Conversion of FMNH2 to DMB.
Data reported here (Figs. 1 and 2) strongly support the conclusion that the 24-kDa BluB protein of R. rubrum is necessary and sufficient for the O2-dependent conversion of FMNH2 to DMB. Although one report in the literature suggested another protein is responsible for the synthesis of DMB in bacteria, this study did not show biochemical evidence of such an activity (9, 11), and the observed dependence of B12 synthesis on exogenous DMB was later shown to be a pseudoauxotrophy (18).
Our work did not address the mechanism of catalysis of the BluB enzyme. However, previously reported work sheds light onto how the BluB enzyme might work. Elegant studies in P. freundenreichii and S. enterica showed that an O2-dependent system converted FMN to DMB in these bacteria, and that the C-2 carbon of DMB was derived from the C-1 carbon of ribose (4, 5, 7, 19–24). Based on the data reported here, we propose a model in which the BluB protein is the oxygenase enzyme that opens the isoalloxazine ring of FMNH2 to yield a phosphoribitylamine intermediate, which proceeds to DMB by facile nonenzymatic oxidative chemistry (8). Abiotic synthesis of DMB from DMPDA and ribose-5-phosphate was not affected by addition of BluB (data not shown). The details of the mechanism of the BluB-catalyzed conversion of FMNH2 to DMB require further investigation.
Some dioxygenases depend on transition metals or organic cofactors to activate O2. We did not detect any metals in our preparations of BluB protein. A family of cofactor-free dioxygenases involved in the degradation of N-heteroaromatic compounds has been described (25), but these enzymes have no homology to BluB, and their mechanism of catalysis is not well understood.
Rate of the BluB Reaction.
The BluB reaction was slow in vitro (25 pmol·min−1). DMB synthesis is a rate-limiting step in Cbl synthesis, and many other Cbl synthetic enzymes also have low specific activities (1). In vitro DMB-forming reactions were performed under low O2 partial pressures to strike a balance between its role as substrate and its reactivity with FMNH2. Because of the reactivity of O2 and FMNH2, we did not measure exact concentrations of substrates in the reaction, and we explain the slow rate of the reaction as the result of the technical difficulty of providing saturating levels of O2 without depleting FMNH2. Although not optimal, these conditions were efficient enough to demonstrate BluB, FMNH2, and O2 dependence of the reaction.
In vivo, DMB synthesis may occur more efficiently. The BluB reaction could be accelerated if BluB were in close proximity to the cell membrane where the concentration of O2 would be higher. This localization could be afforded by interactions with integral membrane proteins of the Cbl synthesis pathway, like cobalamin (5′-P) synthase and cobinamide-P synthase (ref. 26; C. L. Zayas and J.C.E.-S., unpublished results). Another way of accelerating the BluB reaction would be to facilitate product (DMB) removal. We note that genome sequences of some actinomycetes (e.g., Streptomyces coelicolor, Thermobifida fusca) contain gene fusions of the bluB and cobT genes. The CobT enzyme consumes DMB during the synthesis of α -ribazole-5′-phosphate from nicotinate mononucleotide or nicotinamide adenine dinucleotide (27, 28). It is possible that the BluB-CobT fusion protein catalyzes the synthesis of DMB more efficiently.
In R. rubrum, Lack of bluB Function Impairs Bchl Synthesis.
The genome of R. rubrum encodes all of the genes known to be necessary for the late-cobalt-insertion (aerobic) Cbl biosynthetic pathway (1). This bacterium synthesizes Cbl only aerobically (SI Fig. 7). The ΔbluB strain displayed a growth defect correctable with either DMB or Cbl (Fig. 2). This defect was observed only under conditions that demanded Bchl synthesis, which depends on the activity of BchE, an oxygen-sensitive MPE cyclase enzyme thought to require coenzyme B12 for function (12, 14). R. rubrum lacks a homolog of acsF, the gene encoding the oxygen-tolerant Cbl-independent MPE cyclase enzyme present in other photosynthetic bacteria (14, 29). Our data support the prediction that a block in DMB synthesis in R. rubrum would reduce the amount of Cbl accumulated during aerobic growth of the inoculum. This reduction in the Cbl level would lead to a defect in Bchl synthesis after the shift to anoxic conditions, hence result in poor photosynthetic growth. Growth was not abolished in the ΔbluB strain (Fig. 2A), because this bacterium synthesizes small amounts of an alternative cobamide (SI Fig. 7). Synthesis of an alternative cobamide was not surprising, because the CobT enzyme is known to have low specificity for its base substrate (30), resulting in a variety of naturally occurring cobamides with lower ligands other than DMB (reviewed in refs. 7 and 31).
Effect of the Lack of bluB Function on de Novo Corrin Ring Biosynthesis.
As reported by Campbell et al. (11), the lack of bluB function in S. meliloti causes the accumulation of cobinamide-GDP, the immediate precursor of Cbl. In contrast, the lack of bluB function in R. rubrum leads to the accumulation of Cby, the penultimate intermediate of the de novo corrin biosynthetic branch of the pathway (1). This finding suggests that flux of Cby through the remaining steps of the pathway may be coupled to the activity of the cobalamin (5′-P) synthase enzyme. How this coupling may occur remains an open question. We note that Cby also accumulated in anoxically grown WT cells, consistent with the hypothesis that DMB synthesis occurs in R. rubrum only in the presence of molecular oxygen.
Materials and Methods
Bacterial Strains and Growth Conditions.
All strains and plasmids used in this study are listed in SI Table 1. All R. rubrum strains were derived from WT strain UR2, which is resistant to nalidixic acid and streptomycin (32, 33). R. rubrum was grown at 30°C in supplemented malate minimal medium (SMN) (33), MN (34), or reduced malate MN (rMN) (1 g/liter). Anaerobic growth curves of R. rubrum were performed in rMN to accurately measure optical density. MN and rMN media were supplemented with CoCl2 (1 mg/liter) to ensure efficient Cbl synthesis (15). For growth of R. rubrum in MN or rMN, starter cultures were grown aerobically for 3 days in SMN containing appropriate antibiotics. Cells were rinsed twice with sterile MN and used to inoculate fresh medium (1% vol/vol inoculum). Protocols for preparing anoxic media were as described (35). Aerobic cultures of R. rubrum (500 ml in a 4-liter flask) were grown in the dark with shaking at 200 rpm in an orbital shaker. E. coli was grown at 37°C in lysogenic broth (Difco, Sparks, MD) (36, 37). Salmonella enterica sv. Typhimurium (hereafter S. enterica) was grown at 37°C in nutrient broth (Difco), LB, or no-carbon E minimal medium (38) containing MgSO4 (1 mM) and glucose (11 mM) or glycerol (22 mM). When used, nalidixic acid was at 20 μg/ml, ampicillin was at 100 μg/ml, kanamycin at 10 μg/ml, and tetracycline was at 10 μg/ml (for E. coli or S. enterica; 1 μg/ml for R. rubrum). When added, cyanocorrinoids were present at 100 nM. Chemicals were purchased from Sigma (St. Louis, MO).
Genetic and Molecular Techniques.
DNA manipulations were performed by using described methodology (39). Restriction and modification enzymes were purchased from Fermentas (Ontario, Canada) Promega (Madison, WI) and used according to the manufacturer's instructions. All DNA manipulations were performed in E. coli DH5α. Plasmid DNA was isolated by using the Wizard Plus SV Plasmid Miniprep kit (Promega). PCR products were purified by using the QiaQuick PCR purification kit (Qiagen, Valencia, CA). DNA sequencing reactions were performed by using nonradioactive BigDye protocols (ABI PRISM; Applied Biosystems, Foster City, CA) and resolved at the Biotechnology Center of the University of Wisconsin, Madison. Plasmids derived from plasmids pRK404 (40) and pUX19 (41) were conjugated into R. rubrum as described (42).
Construction of the R. rubrum ΔbluB Mutant.
The sequence of all primers used in this work can be found in SI Table 2. Primers [1] and [2] were used to amplify a 3,021-bp fragment of R. rubrum chromosomal DNA containing bluB and 2,385 bp of flanking sequence (1,185 bp upstream and 1,200 bp downstream). This fragment was cloned into the KpnI and BamHI sites of plasmid pUX19 to yield plasmid pBLUB16.
Primers [3] and [4] were used with plasmid pBLUB16 as template to amplify a 6.3-kbp DNA fragment, which was cut with SacI and ligated to yield plasmid pBLUB17, which contained an in-frame deletion of bluB in which bases 28–564 of bluB were replaced by a 6-bp SacI restriction site. The hypothetical gene product encoded by this ΔbluB (bluB1) allele would be a 32-aa peptide.
Plasmid pBLUB17 was conjugated into WT R. rubrum. Kanamycin-resistant transconjugants were picked, grown for ≈50 generations in SMN broth containing nalidixic acid and screened for kanamycin sensitive variants on SMN agar plates. The presence of the in-frame deletion in bluB was confirmed by amplifying and sequencing the bluB region by using primers [5] and [6].
Construction of bluB+ Plasmid for Complementation Studies.
The bluB+ coding sequence plus 158 bp of 5′ sequence was amplified by using primers [7] and [8], and the resulting product was cloned into the HindIII site of plasmid pRK404 to yield plasmid pBLUB12.
Construction of Plasmids for the Overproduction of BluBWT and BluBR22E Proteins.
The bluB+ coding sequence was amplified with primers [9] and [10] and cloned into the NheI and EcoRI sites of plasmid pTEV5 to yield plasmid pBLUB10. The QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used with primers [11] and [12] and plasmid pBLUB10 to construct plasmid pBLUB13, which carried a bluB allele (bluB2) encoding the variant BluBR22E protein.
Purification of BluB Proteins.
BluBWT or BluBR22E protein fused to an rTEV protease cleavable N-terminal H6 tag was overproduced by using plasmids pBLUB10 (bluB+) or pBLUB13 (bluB2). One milliliter of an overnight culture of the overexpressing strain was inoculated into 2 liters of lysogenic broth containing ampicillin. Cultures were grown at 37°C with shaking to a cell density of OD600 = 0.6, isopropyl-β-d-thiogalactopyranoside added to 0.3 mM, then incubated for 18 h at 15°C with shaking. Cells were harvested by centrifugation for 15 min at 5,000 × g at 4°C, resuspended in 20 ml of 20 mM Tris-HCl buffer (pH 7.9 at 4°C) containing NaCl (1 M) and imidazole (10 mM), and broken with a French press (1.6 × 105 kPa, 4°C). Cell lysate was cleared by centrifugation for 1 h at 14,000 × g at 4°C and filtered through a 0.45-μm syringe filter (Nalge Nunc, Rochester, NY). Tagged BluB proteins were purified by using His-Bind resin (Novagen, San Diego, CA). The H6 tag was removed after 48 h at 4°C with rTEV protease (43) present in the buffer at a 1:100 ratio of BluB:rTEV protease. H6-rTEV protease was resolved from de-tagged BluB proteins by passing the protein mixture over His-Bind resin. Purity was monitored by SDS/PAGE (44) and Coomassie blue staining (45). Fractions containing detagged BluB protein were pooled, dialyzed (Mr cutoff = 10,000 membrane; Pierce, Rockford, IL) at 4°C against 1 liter of Tris·HCl buffer (20 mM, pH 7.9 at 4°C) containing NaCl (50 mM) and glycerol (10% vol/vol), and stored at −80°C after flash freezing in liquid N2. Protein purity was assessed by using the TotalLab software package (Nonlinear Dynamics, Durham, NC).
Metal Analysis of BluB.
BluBWT was analyzed for bound metal ions by inductively coupled plasma emission spectrometry (ICPES; Chemical Analysis Laboratory, University of Georgia, Athens), by using a Thermo Jarrell-Ash 965 (New Port Richey, FL) inductively coupled argon plasma spectrophotometer.
Purification of Fre.
H6-tagged Fre (E. coli flavin reductase) was purified as described (16).
BluB Activity Assays.
DMB synthesis was performed in 200-μl reaction mixtures containing 20–40 μg of BluBWT or BluBR22E protein; 1 μg of Fre protein; 100 mM Hepes buffer, pH 8; 50 mM NaCl; 500 μM FMN; and 5 mM NADH. Reactions were incubated for 0, 2, or 4 h at 30°C in the dark and stopped by boiling for 15 min. Precipitated protein was removed by filtration. Assay mixtures were prepared aerobically in 0.8-ml Eppendorf tubes and overlaid with 100 μl of mineral oil to limit diffusion of oxygen into the reaction. Further reduced O2 conditions were obtained by sparging the buffer containing FMN and NADH with N2 gas for 15 min at 15 ml·s−1. Enzymes were dispensed into 2-ml serum vials (Wheaton, Millville, NJ) on ice and sealed, and the overhead space was flushed with N2 gas for 2 min at 15 ml·s−1. Buffer mixture was added anoxically to each serum vial with a syringe. Anoxic reactions were prepared as above but passing N2 gas over a freshly regenerated copper catalyst, and buffer/FMN/NADH mixtures were sparged for 30 min with O2-free N2 at 15 ml·s−1.
Identification of the Product of the BluB Reaction.
A 200-μl reduced-O2 reaction containing 40 μg of BluBWT was incubated for 18 h at 30°C; a control reaction lacked BluB protein. Removal of proteins by filtration stopped the reaction. Reaction products were identified by liquid chromatography MS (University of Wisconsin Biotechnology Center, Madison, WI) by using an Agilent Technologies (Palo Alto, CA) LC/MS ESI-TOF with a mass accuracy of >3 ppm. Separation was performed with an Agilent 1100 LC with a 2.1 × 100-mm Insertsil C18 column by using a 90-min gradient of 100% solvent A (0.1% formic acid in water) to 100% solvent B (0.1% formic acid in acetonitrile). Reference masses of 121.05087 and 922.0098 atomic mass units from the Agilent API TOF reference mass solution kit were used as a lock mass standard.
Bioassay of BluB Reaction Products.
The identity of the BluB reaction product was confirmed by means of a bioassay. A 6-ml reaction mixture containing 1.2 mg of BluBWT was incubated for 18 h at 30°C; a control reaction lacked BluB protein. Reactions were stopped by boiling for 15 min, and reaction products were purified by RP-HPLC, dried under vacuum by using a Savant concentrator (ThermoElectron, San Jose, CA), desalted by using a 1-ml C18 Sep-Pak cartridge (Waters, Milford, MA), and resuspended in 5 μl of DMSO. S. enterica strains JE1244 (cobT) and JE2501 (cobT cobB) were used as indicator strains in an overlay on glucose minimal medium containing 15 nM cobinamide. A 5-μl sample of purified BluB reaction product (92 nmol; calculated by using the ε = 5,260 M−1·cm−1 for DMB in methanol)/90 nmol of authentic DMB (positive control)/2 pmol of Cbl were spotted onto the agar overlay (positive control). Plates were incubated aerobically at 37°C. S. enterica cobT mutants are DMB pseudoauxotrophs because of the activity of the alternative gene cobB (17). An S. enterica strain lacking both cobT and cobB is a Cbl auxotroph unresponsive to DMB (17, 18).
HPLC Analysis of BluB Reaction Products.
The products of the BluB reaction were resolved by using a Beckman Coulter (Fullerton, CA) System Gold 126 HPLC system equipped with a Beckman Coulter System Gold 508 autosampler and a 150 × 4.6-mm Alltima HP C18 AQ column (Alltech, Jerome, ID). Details of the protocol used have been described (46). Five minutes after injection, the column was developed for 5 min with a linear gradient to a composition of 41.2% solvent A (15% methanol/5 mM ammonium acetate)/58.8% solvent B (100% methanol). A second, 6-min linear gradient developed the column to 23.5% A/76.5% B. A final linear gradient developed the column to 100% B over 5 min. Products were detected with a photodiode array detector. DMB was identified by its absorbance spectrum and by comparison with an authentic DMB standard; the detection limit was 0.5 nmol.
Effect of BluB on Abiotic DMB Synthesis.
DMB synthesis from DMPDA and ribose-5-phosphate was performed in 200-μl reaction mixtures containing 100 mM Hepes buffer (pH 8), 50 mM NaCl, 500 μM DMPDA, and 500 μM ribose-5-phosphate, with and without 40 μg of BluBWT. Assay mixtures were prepared aerobically in 0.8-ml Eppendorf tubes and overlaid with 100 μl of mineral oil. Reactions were incubated at 30°C in the dark and stopped by addition of 20 μl of 75% (wt/vol) trichloroacetic acid. Precipitated protein was removed by filtration. Reaction products were identified by RP-HPLC as described above.
Corrinoid Extraction and Analysis.
The WT and ΔbluB mutant strains were grown in MN broth either anaerobically with light or aerobically in the dark. Corrins were extracted with methanol and purified by RP-HPLC (see SI Methods). Identity of corrins was determined by means of a bioassay and by MALDI-TOF MS (see SI Methods). The detection limit for Cbl was 0.02 pmol of Cbl per milligram of total cell protein.
Pigment Extraction and Analysis.
R. rubrum was grown phototrophically in rMN medium. Samples (100 μl) of each culture taken in early stationary phase were lysed with BugBuster (Novagen, San Diego, CA), and total protein content was determined by using the Bradford assay (Bio-Rad, Hercules, CA) (47). Cells were harvested by centrifugation at 2,000 × g at 4°C for 15 min, pigments were extracted with 2 ml of ice-cold 7:2 (vol/vol) acetone/methanol (14), and cell debris was discarded. Absorption spectra were determined by using a Lambda 45 UV/Vis spectrophotomer (PerkinElmer, Wellesley, MA) and normalized to milligram of total protein.
Supplementary Material
Acknowledgments
We are indebted to Gary Roberts, Ed Pohlmann, and Yaoping Zhang (Department of Bacteriology, University of Wisconsin, Madison, WI) for providing R. rubrum strains, plasmids, and technical assistance. This work was supported by National Institutes of Health Grant R01-GM40313 (to J.C.E.-S.).
Abbreviations
- DMB
5,6-dimethylbenzimidazole
- DMPDA
4,5-dimethylphenylenediamine
- MPE
Mg-protoporphyrin IX monomethyl ester
- Bchl
bacteriochlorophyll
- AdoCbl
adenosylcobalamin
- Cby
cobyric acid
- MN
malate minimal medium
- rMN
reduced MN
- SMN
supplemented MN
- Fre
FMN reductase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission. R.K.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609270104/DC1.
References
- 1.Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC. Nat Prod Rep. 2002;19:390–412. doi: 10.1039/b108967f. [DOI] [PubMed] [Google Scholar]
- 2.Lenhert PG, Hodgkin DC. Nature. 1961;192:937–938. doi: 10.1038/192937a0. [DOI] [PubMed] [Google Scholar]
- 3.Höllriegl V, Lamm L, Rowold J, Hörig J, Renz P. Arch Microbiol. 1982;132:155–158. doi: 10.1007/BF00508722. [DOI] [PubMed] [Google Scholar]
- 4.Keck B, Munder M, Renz P. Arch Microbiol. 1998;171:66–68. doi: 10.1007/s002030050679. [DOI] [PubMed] [Google Scholar]
- 5.Hörig JA, Renz P. Eur J Biochem. 1980;105:587–592. doi: 10.1111/j.1432-1033.1980.tb04536.x. [DOI] [PubMed] [Google Scholar]
- 6.Renz P, Weyhenmeyer R. FEBS Lett. 1972;22:124–126. doi: 10.1016/0014-5793(72)80236-9. [DOI] [PubMed] [Google Scholar]
- 7.Renz P. In: Chemistry and Biochemistry of B12. Banerjee R, editor. New York: Wiley; 1999. pp. 557–575. [Google Scholar]
- 8.Maggio-Hall LA, Dorrestein PC, Escalante-Semerena JC, Begley TP. Org Lett. 2003;5:2211–2213. doi: 10.1021/ol034530m. [DOI] [PubMed] [Google Scholar]
- 9.Chen P, Ailion M, Weyand N, Roth J. J Bacteriol. 1995;177:1461–1419. doi: 10.1128/jb.177.6.1461-1469.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollich M, Klug G. J Bacteriol. 1995;177:4481–4487. doi: 10.1128/jb.177.15.4481-4487.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell GR, Taga ME, Mistry K, Lloret J, Anderson PJ, Roth JR, Walker GC. Proc Natl Acad Sci USA. 2006;103:4634–4369. doi: 10.1073/pnas.0509384103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gough SP, Petersen BO, Duus JO. Proc Natl Acad Sci USA. 2000;97:6908–6913. doi: 10.1073/pnas.97.12.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnheim K, Oelze J. Arch Microbiol. 1983;135:299–304. [Google Scholar]
- 14.Ouchane S, Steunou AS, Picaud M, Astier C. J Biol Chem. 2004;279:6385–6394. doi: 10.1074/jbc.M309851200. [DOI] [PubMed] [Google Scholar]
- 15.Ohmori H, Nakatani K, Shimizu S, Fukui S. Eur J Biochem. 1974;47:207–218. doi: 10.1111/j.1432-1033.1974.tb03684.x. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca MV, Escalante-Semerena JC. J Bacteriol. 2000;182:4304–4309. doi: 10.1128/jb.182.15.4304-4309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trzebiatowski JR, O'Toole GA, Escalante-Semerena JC. J Bacteriol. 1994;176:3568–3575. doi: 10.1128/jb.176.12.3568-3575.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsang AW, Escalante-Semerena JC. J Biol Chem. 1998;273:31788–31794. doi: 10.1074/jbc.273.48.31788. [DOI] [PubMed] [Google Scholar]
- 19.Renz P. FEBS Lett. 1970;6:187–189. doi: 10.1016/0014-5793(70)80053-9. [DOI] [PubMed] [Google Scholar]
- 20.Hörig JA, Renz P, Heckmann G. J Biol Chem. 1978;253:7410–7414. [PubMed] [Google Scholar]
- 21.Kolonko B, Hörig JA, Renz P. Z Naturf Sect C Biosc. 1992;47:171–176. doi: 10.1515/znc-1992-3-401. [DOI] [PubMed] [Google Scholar]
- 22.Lingens B, Schild TA, Volger B, Renz P. Eur J Biochem. 1992;207:981–985. doi: 10.1111/j.1432-1033.1992.tb17133.x. [DOI] [PubMed] [Google Scholar]
- 23.Schulze B, Ruoff D, Volger B, Renz P. Biol Chem Hoppe–Seyler. 1994;375:785–788. [PubMed] [Google Scholar]
- 24.Keck B, Renz P. Arch Microbiol. 2000;173:76–77. doi: 10.1007/s002030050011. [DOI] [PubMed] [Google Scholar]
- 25.Fetzner S. Appl Microbiol Biotechnol. 2002;60:243–257. doi: 10.1007/s00253-002-1123-4. [DOI] [PubMed] [Google Scholar]
- 26.Maggio-Hall LA, Claas KR, Escalante-Semerena JC. Microbiology. 2004;150:1385–1395. doi: 10.1099/mic.0.26952-0. [DOI] [PubMed] [Google Scholar]
- 27.Trzebiatowski JR, Escalante-Semerena JC. J Biol Chem. 1997;272:17662–17667. doi: 10.1074/jbc.272.28.17662. [DOI] [PubMed] [Google Scholar]
- 28.Maggio-Hall LA, Escalante-Semerena JC. Microbiology. 2003;149:983–990. doi: 10.1099/mic.0.26040-0. [DOI] [PubMed] [Google Scholar]
- 29.Pinta V, Picaud M, Reiss-Husson F, Astier C. J Bacteriol. 2002;184:746–753. doi: 10.1128/JB.184.3.746-753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheong CG, Escalante-Semerena JC, Rayment I. J Biol Chem. 2002;277:41120–41127. doi: 10.1074/jbc.M203535200. [DOI] [PubMed] [Google Scholar]
- 31.Rondon MR, Trzebiatowski JR, Escalante-Semerena JC. Prog Nucleic Acid Res Mol Biol. 1997;56:347384. doi: 10.1016/s0079-6603(08)61010-7. [DOI] [PubMed] [Google Scholar]
- 32.Kerby RL, Hong SS, Ensign SA, Coppoc LJ, Ludden PW, Roberts GP. J Bacteriol. 1992;174:5284–5294. doi: 10.1128/jb.174.16.5284-5294.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzmaurice WP, Saari LL, Lowery RG, Ludden PW, Roberts GP. Mol Gen Genet. 1989;218:340–347. doi: 10.1007/BF00331287. [DOI] [PubMed] [Google Scholar]
- 34.Lehman LJ, Roberts GP. J Bacteriol. 1991;173:5705–5711. doi: 10.1128/jb.173.18.5705-5711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balch WE, Wolfe RS. Appl Environ Microbiol. 1976;32:781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertani G. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertani G. J Bacteriol. 2004;186:595–600. doi: 10.1128/JB.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berkowitz D, Hushon JM, Whitfield HJ, Roth J, Ames BN. J Bacteriol. 1968;96:215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Greene; 1989. [Google Scholar]
- 40.Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang XW, Finlay DR, Guiney D, Helinski DR. Plasmid. 1985;13:149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- 41.Lies DL. Madison, WI: University of Wisconsin; 1994. PhD thesis. [Google Scholar]
- 42.Liang JH, Nielsen GM, Lies DP, Burris RH, Roberts GP, Ludden PW. J Bacteriol. 1991;173:6903–6909. doi: 10.1128/jb.173.21.6903-6909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kapust RB, Waugh DS. Protein Expr Purif. 2000;19:312–318. doi: 10.1006/prep.2000.1251. [DOI] [PubMed] [Google Scholar]
- 44.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 45.Sasse J. In: Current Protocols in Molecular Biology. Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Vol 1. New York: Wiley; 1991. pp. 10.6.1–10.6.8. [Google Scholar]
- 46.Aliverti A, Curti B, Vanoni MA. In: Flavoprotein Protocols. Chapman SK, Reid GA, editors. Vol 131. Totowa, NJ: Humana; 1999. pp. 9–23. [Google Scholar]
- 47.Bradford MM. Anal Biochem. 1976;72:248–255. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.