Abstract
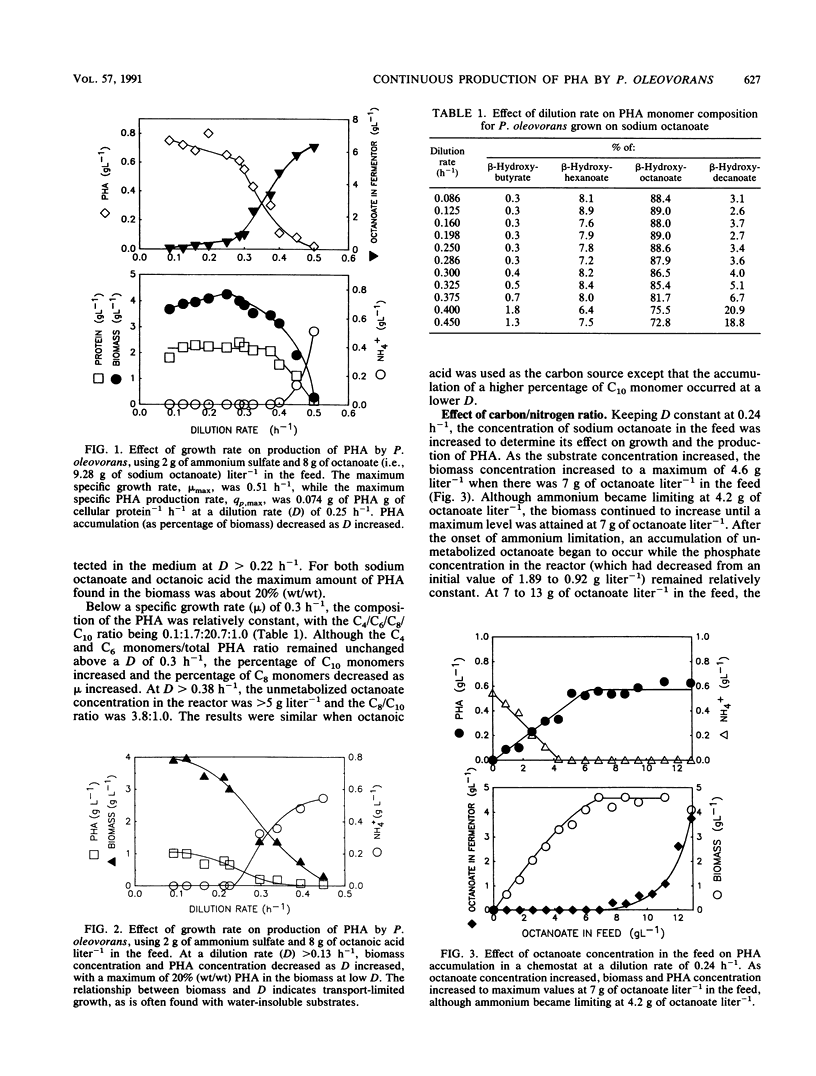
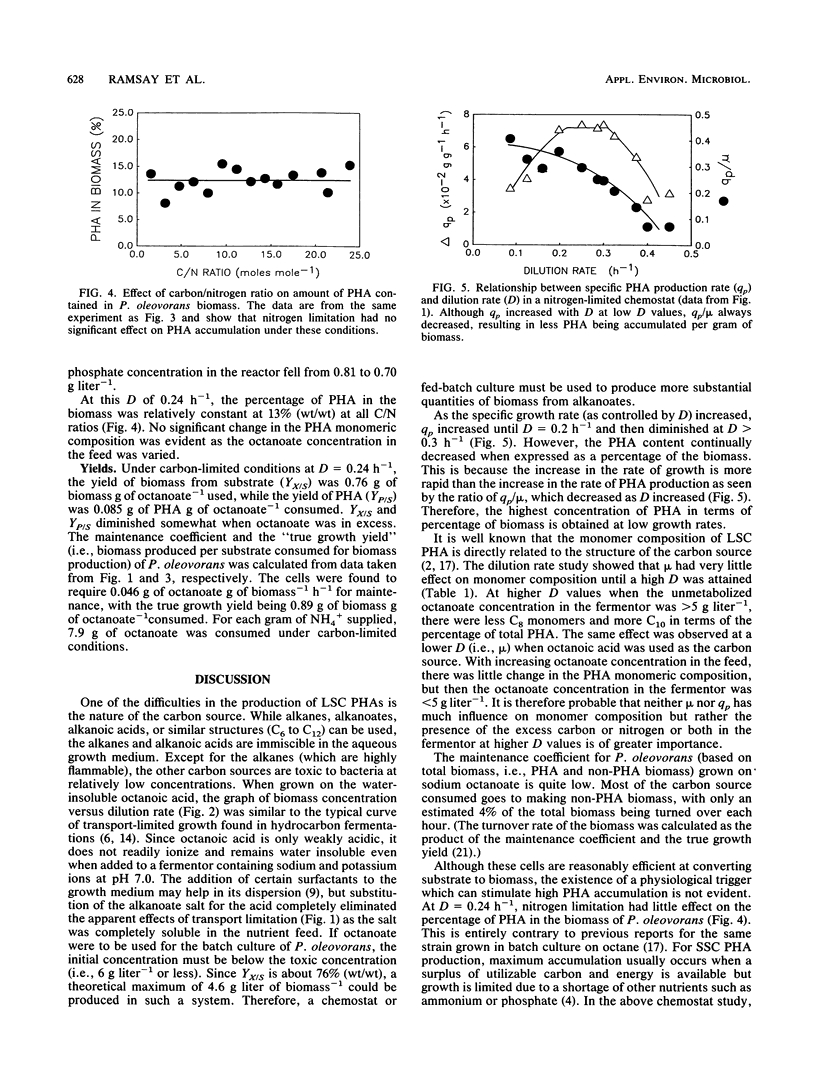
Shake flask experiments showed that Pseudomonas oleovorans began to be growth inhibited at 4.65 g of sodium octanoate liter-1, with total inhibition at 6 g liter-1. In chemostat studies with 2 g of ammonium sulfate and 8 g of octanoate liter-1 in the feed, the maximum specific growth rate was 0.51 h-1, and the maximum specific rate of poly-β-hydroxyalkanoate (PHA) production was 0.074 g of PHA g of cellular protein-1 h-1 at a dilution rate (D) of 0.25 h-1. When the specific growth rate (μ) was <0.3 h-1, the PHA composition was relatively constant with a C4/C6/C8/C10 ratio of 0.1:1.7:20.7:1.0. At μ > 0.3 h-1, a decrease in the percentage of C8 with a concomitant increase in C10 monomers as μ increased was probably due to the effects of higher concentrations of unmetabolized octanoate in the fermentor. At D = 0.24 h-1 and an increasing carbon/nitrogen ratio, the percentage of PHA in the biomass was constant at 13% (wt/wt), indicating that nitrogen limitation did not affect PHA accumulation. Under carbon-limited conditions, the yield of biomass from substrate was 0.76 g of biomass g of octanoate-1 consumed, the yield of PHA was 0.085 g of PHA g of octanoate-1 used, and 7.9 g of octanoate was consumed for each gram of NH4+ supplied. The maintenance coefficient was 0.046 g of octanoate g of biomass-1 h-1. Replacement of sodium octanoate with octanoic acid appeared to result in transport-limited growth due to the water insolubility of the acid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Haywood G. W., Dawes E. A. Biosynthesis and composition of bacterial poly(hydroxyalkanoates). Int J Biol Macromol. 1990 Apr;12(2):102–105. doi: 10.1016/0141-8130(90)90060-n. [DOI] [PubMed] [Google Scholar]
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Fritzsche K., Lenz R. W., Fuller R. C. Bacterial polyesters containing branched poly(beta-hydroxyalkanoate) units. Int J Biol Macromol. 1990 Apr;12(2):92–101. doi: 10.1016/0141-8130(90)90059-j. [DOI] [PubMed] [Google Scholar]
- Fritzsche K., Lenz R. W., Fuller R. C. Production of unsaturated polyesters by Pseudomonas oleovorans. Int J Biol Macromol. 1990 Apr;12(2):85–91. doi: 10.1016/0141-8130(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Gerson D. F., Cooper D. G., Ramsay B., Zajic J. E. The effect of substrate on inhibition of Corynebacterium lepus by isonicotinic acid hydrazide (Isoniazid). Can J Microbiol. 1980 Dec;26(12):1498–1500. doi: 10.1139/m80-247. [DOI] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Huisman G. W., de Leeuw O., Eggink G., Witholt B. Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol. 1989 Aug;55(8):1949–1954. doi: 10.1128/aem.55.8.1949-1954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lageveen R. G., Huisman G. W., Preusting H., Ketelaar P., Eggink G., Witholt B. Formation of Polyesters by Pseudomonas oleovorans: Effect of Substrates on Formation and Composition of Poly-(R)-3-Hydroxyalkanoates and Poly-(R)-3-Hydroxyalkenoates. Appl Environ Microbiol. 1988 Dec;54(12):2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchessault R. H., Monasterios C. J., Morin F. G., Sundararajan P. R. Chiral poly(beta-hydroxyalkanoates): an adaptable helix influenced by the alkane side-chain. Int J Biol Macromol. 1990 Apr;12(2):158–165. doi: 10.1016/0141-8130(90)90068-l. [DOI] [PubMed] [Google Scholar]
- Matin A., Veldhuis C., Stegeman V., Veenhuis M. Selective advantage of a Spirillum sp. in a carbon-limited environment. Accumulation of poly-beta-hydroxybutyric acid and its role in starvation. J Gen Microbiol. 1979 Jun;112(2):349–355. doi: 10.1099/00221287-112-2-349. [DOI] [PubMed] [Google Scholar]
- Oeding V., Schlegel H. G. Beta-ketothiolase from Hydrogenomonas eutropha H16 and its significance in the regulation of poly-beta-hydroxybutyrate metabolism. Biochem J. 1973 May;134(1):239–248. doi: 10.1042/bj1340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay B. A., Lomaliza K., Chavarie C., Dubé B., Bataille P., Ramsay J. A. Production of poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acids. Appl Environ Microbiol. 1990 Jul;56(7):2093–2098. doi: 10.1128/aem.56.7.2093-2098.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay B. A., Ramsay J. A., Cooper D. G. Production of Poly-beta-Hydroxyalkanoic Acid by Pseudomonas cepacia. Appl Environ Microbiol. 1989 Mar;55(3):584–589. doi: 10.1128/aem.55.3.584-589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet M. J., Eggink G., Witholt B., Kingma J., Wynberg H. Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J Bacteriol. 1983 May;154(2):870–878. doi: 10.1128/jb.154.2.870-878.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


