Abstract
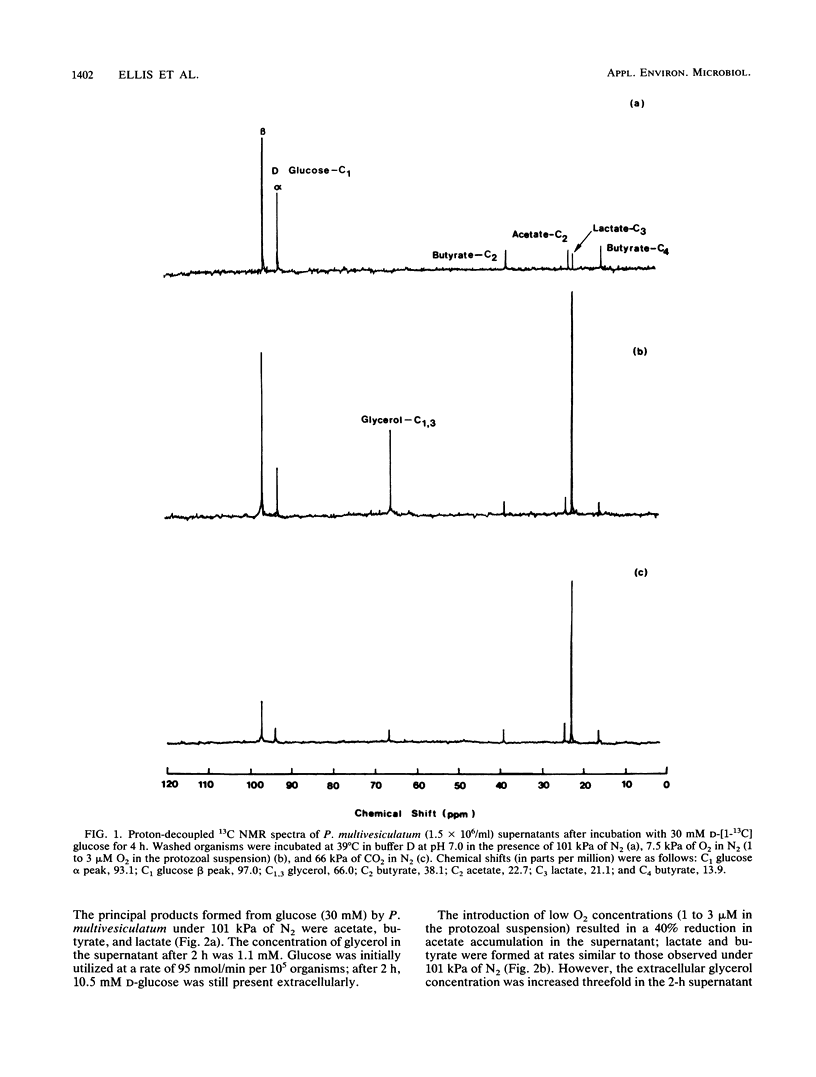
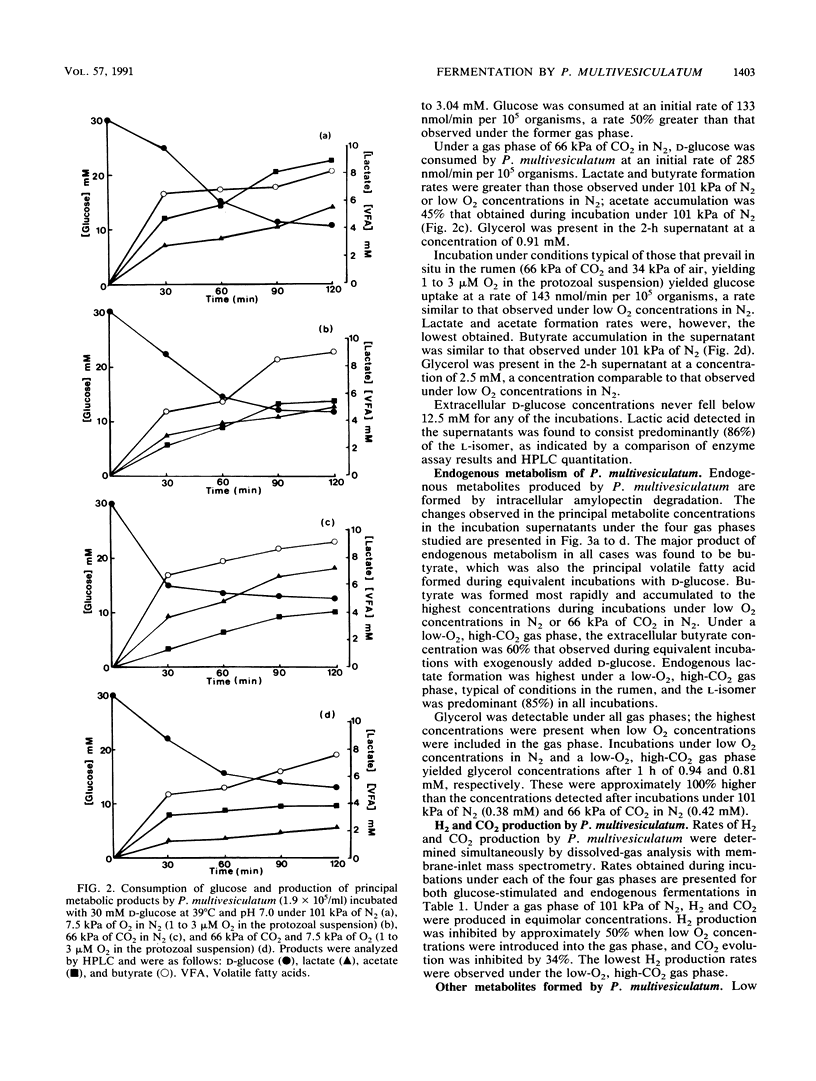
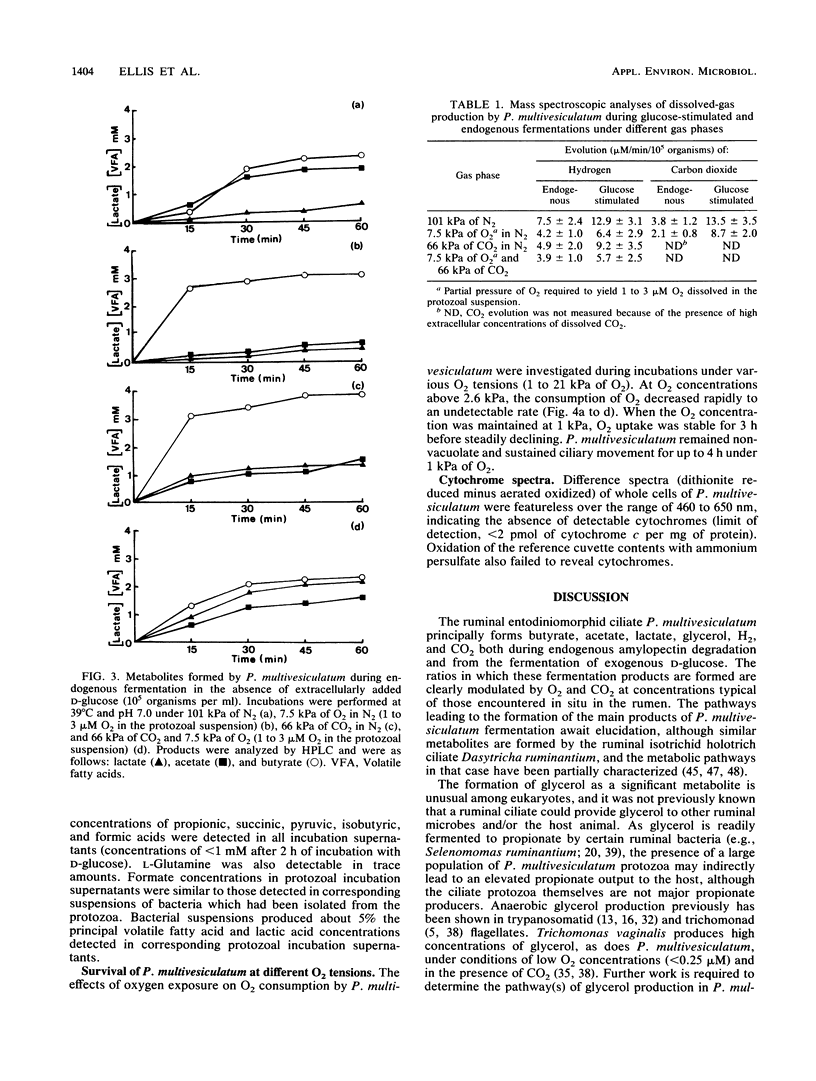
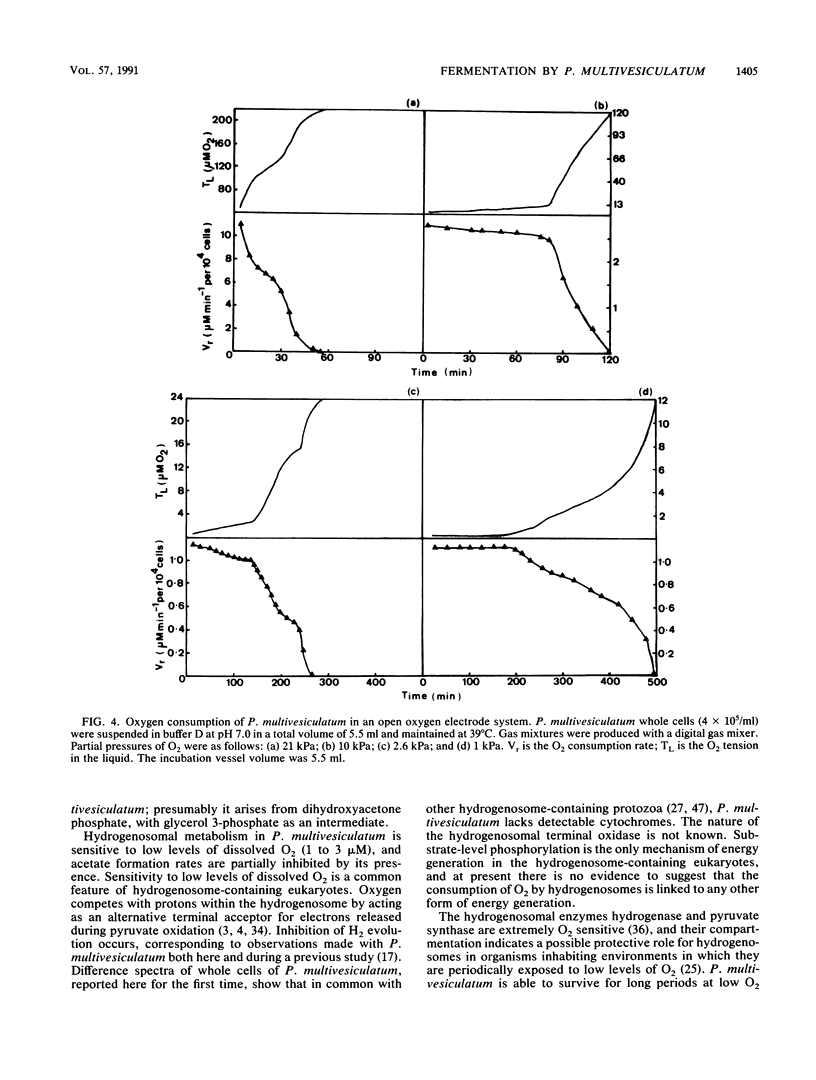
The effects of ruminal concentrations of CO2 and oxygen on the end products of endogenous metabolism and fermentation of D-glucose by the ruminal entodiniomorphid ciliate Polyplastron multivesiculatum were investigated. The principal metabolic products were butyric, acetic, and lactic acids, H2, and CO2. 13C nuclear magnetic resonance spectroscopy identified glycerol as a previously unknown major product of D-[1-13C]glucose fermentation by this protozoan. Metabolite formation rates were clearly influenced by the headspace gas composition. In the presence of 1 to 3 microM O2, acetate, H2, and CO2 formation was partially depressed. A gas headspace with a high CO2 content (66 kPa) was found to suppress hydrogenosomal pathways and to favor butyrate accumulation. Cytochromes were not detected (less than 2 pmol/mg of protein) in P. multivesiculatum; protozoal suspensions, however, consumed O2 for up to 3 h at 1 kPa of O2. Under gas phases of greater than 2.6 kPa of O2, the organisms rapidly became vacuolate and the cilia became inactive. The results suggest that fermentative pathways in P. multivesiculatum are influenced by the O2 and CO2 concentrations that prevail in situ in the rumen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKKADA A. R., EADIE J. M., HOWARD B. H. THE BIOCHEMISTRY OF RUMEN PROTOZOA. 7. THE CARBOHYDRASES OF POLYPLASTRON MULTIVESICULATUM (DOGIEL & FEDOROWA). Biochem J. 1963 Nov;89:268–272. doi: 10.1042/bj0890268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerkasov J., Cerkasovová A., Kulda J., Vilhelmová D. Respiration of hydrogenosomes of Tritrichomonas foetus. I. ADP-dependent oxidation of malate and pyruvate. J Biol Chem. 1978 Feb 25;253(4):1207–1214. [PubMed] [Google Scholar]
- Chapman A., Cammack R., Linstead D. J., Lloyd D. Respiration of Trichomonas vaginalis. Components detected by electron paramagnetic resonance spectroscopy. Eur J Biochem. 1986 Apr 1;156(1):193–198. doi: 10.1111/j.1432-1033.1986.tb09567.x. [DOI] [PubMed] [Google Scholar]
- Chapman A., Linstead D. J., Lloyd D., Williams J. 13C-NMR reveals glycerol as an unexpected major metabolite of the protozoan parasite Trichomonas vaginalis. FEBS Lett. 1985 Oct 28;191(2):287–292. doi: 10.1016/0014-5793(85)80026-0. [DOI] [PubMed] [Google Scholar]
- Coleman G. S., Davies J. I., Cash M. A. The cultivation of the rumen ciliates Epidinium ecaudatum caudatum and Polyplastron multivesiculatum in vitro. J Gen Microbiol. 1972 Dec;73(3):509–521. doi: 10.1099/00221287-73-3-509. [DOI] [PubMed] [Google Scholar]
- Darling T. N., Davis D. G., London R. E., Blum J. J. Products of Leishmania braziliensis glucose catabolism: release of D-lactate and, under anaerobic conditions, glycerol. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7129–7133. doi: 10.1073/pnas.84.20.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degn H., Cox R. P., Lloyd D. Continuous measurement of dissolved gases in biochemical systems with the quadrupole mass spectrometer. Methods Biochem Anal. 1985;31:165–194. doi: 10.1002/9780470110522.ch3. [DOI] [PubMed] [Google Scholar]
- Degn H., Lundsgaard J. S. Dynamic gas mixing techniques. J Biochem Biophys Methods. 1980 Oct;3(4):233–242. doi: 10.1016/0165-022x(80)90062-7. [DOI] [PubMed] [Google Scholar]
- Ellis J. E., Williams A. G., Lloyd D. Oxygen consumption by ruminal microorganisms: protozoal and bacterial contributions. Appl Environ Microbiol. 1989 Oct;55(10):2583–2587. doi: 10.1128/aem.55.10.2583-2587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON P. N., MANN S. O. The isolation of glycerol-fermenting and lipolytic bacteria from the rumen of the sheep. J Gen Microbiol. 1961 Jun;25:227–240. doi: 10.1099/00221287-25-2-227. [DOI] [PubMed] [Google Scholar]
- HOWARD B. H. The biochemistry of rumen protozoa. 1. Carbohydrate fermentation by Dasytricha and Isotricha. Biochem J. 1959 Apr;71(4):671–675. doi: 10.1042/bj0710671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J Biol Chem. 1973 Nov 25;248(22):7724–7728. [PubMed] [Google Scholar]
- Linstead D. J., Bradley S. The purification and properties of two soluble reduced nicotinamide: acceptor oxidoreductases from Trichomonas vaginalis. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):125–133. doi: 10.1016/0166-6851(88)90032-1. [DOI] [PubMed] [Google Scholar]
- Lloyd D., Williams J., Yarlett N., Williams A. G. Oxygen affinities of the hydrogenosome-containing protozoa Tritrichomonas foetus and Dasytricha ruminantium, and two aerobic protozoa, determined by bacterial bioluminescence. J Gen Microbiol. 1982 May;128(5):1019–1022. doi: 10.1099/00221287-128-5-1019. [DOI] [PubMed] [Google Scholar]
- Mackenzie N. E., Hall J. E., Flynn I. W., Scott A. I. 13C nuclear magnetic resonance studies of anaerobic glycolysis in Trypanosoma brucei spp. Biosci Rep. 1983 Feb;3(2):141–151. doi: 10.1007/BF01121945. [DOI] [PubMed] [Google Scholar]
- Müller M., Lindmark D. G. Respiration of hydrogenosomes of Tritrichomonas foetus. II. Effect of CoA on pyruvate oxidation. J Biol Chem. 1978 Feb 25;253(4):1215–1218. [PubMed] [Google Scholar]
- Paget T. A., Lloyd D. Trichomonas vaginalis requires traces of oxygen and high concentrations of carbon dioxide for optimal growth. Mol Biochem Parasitol. 1990 Jun;41(1):65–72. doi: 10.1016/0166-6851(90)90097-6. [DOI] [PubMed] [Google Scholar]
- Paul R. G., Williams A. G., Butler R. D. Hydrogenosomes in the rumen entodiniomorphid ciliate Polyplastron multivesiculatum. J Gen Microbiol. 1990 Oct;136(10):1981–1989. doi: 10.1099/00221287-136-10-1981. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A., Müller M. Glycerol, a metabolic end product of Trichomonas vaginalis and Tritrichomonas foetus. Mol Biochem Parasitol. 1986 Jul;20(1):45–55. doi: 10.1016/0166-6851(86)90141-6. [DOI] [PubMed] [Google Scholar]
- Williams A. G. Rumen holotrich ciliate protozoa. Microbiol Rev. 1986 Mar;50(1):25–49. doi: 10.1128/mr.50.1.25-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. G. The selectivity of carbohydrate assimilation by the anaerobic rumen ciliate Dasytricha ruminantium. J Appl Bacteriol. 1979 Dec;47(3):511–520. doi: 10.1111/j.1365-2672.1979.tb01212.x. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Hann A. C., Lloyd D., Williams A. G. Hydrogenosomes in a mixed isolate of Isotricha prostoma and Isotricha intestinalis from ovine rumen contents. Comp Biochem Physiol B. 1983;74(2):357–364. doi: 10.1016/0305-0491(83)90025-1. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Hann A. C., Lloyd D., Williams A. Hydrogenosomes in the rumen protozoon Dasytricha ruminantium Schuberg. Biochem J. 1981 Nov 15;200(2):365–372. doi: 10.1042/bj2000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N., Lloyd D., Williams A. G. Butyrate formation from glucose by the rumen protozoon Dasytricha ruminantium. Biochem J. 1985 May 15;228(1):187–192. doi: 10.1042/bj2280187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N., Lloyd D., Williams A. G. Respiration of the rumen ciliate Dasytricha ruminantium Schuberg. Biochem J. 1982 Aug 15;206(2):259–266. doi: 10.1042/bj2060259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N., Orpin C. G., Munn E. A., Yarlett N. C., Greenwood C. A. Hydrogenosomes in the rumen fungus Neocallimastix patriciarum. Biochem J. 1986 Jun 15;236(3):729–739. doi: 10.1042/bj2360729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Santos C., Buldain G., Frydman B., Cannata J. J., Cazzulo J. J. Carbon-13 nuclear magnetic resonance analysis of [1-13C]glucose metabolism in Crithidia fasciculata. Evidence of CO2 fixation by phosphoenolpyruvate carboxykinase. Eur J Biochem. 1985 Jun 3;149(2):421–429. doi: 10.1111/j.1432-1033.1985.tb08942.x. [DOI] [PubMed] [Google Scholar]


