Abstract
The noradrenergic system in the prefrontal cortex (PFC) is involved in many physiological and psychological processes, including working memory and mood control. To understand the functions of the noradrenergic system, we examined the regulation of NMDA receptors (NMDARs), key players in cognition and emotion, by α1- and α2-adrenergic receptors (α1-ARs, α2-ARs) in PFC pyramidal neurons. Applying norepinephrine or a norepinephrine transporter inhibitor reduced the amplitude but not paired-pulse ratio of NMDAR-mediated excitatory postsynaptic currents (EPSC) in PFC slices. Specific α1-AR or α2-AR agonists also decreased NMDAR-EPSC amplitude and whole-cell NMDAR current amplitude in dissociated PFC neurons. The α1-AR effect depended on the phospholipase C–inositol 1,4,5-trisphosphate–Ca2+ pathway, whereas the α2-AR effect depended on protein kinase A and the microtubule-based transport of NMDARs that is regulated by ERK signaling. Furthermore, two members of the RGS family, RGS2 and RGS4, were found to down-regulate the effect of α1-AR on NMDAR currents, whereas only RGS4 was involved in inhibiting α2-AR regulation of NMDAR currents. The regulating effects of RGS2/4 on α1-AR signaling were lost in mutant mice lacking spinophilin, which binds several RGS members and G protein-coupled receptors, whereas the effect of RGS4 on α2-AR signaling was not altered in spinophilin-knockout mice. Our work suggests that activation of α1-ARs or α2-ARs suppresses NMDAR currents in PFC neurons by distinct mechanisms. The effect of α1-ARs is modified by RGS2/4 that are recruited to the receptor complex by spinophilin, whereas the effect of α2-ARs is modified by RGS4 independent of spinophilin.
Keywords: adrenergic receptor, RGS4, neuropsychiatric diseases, G protein-coupled receptor, microtubule
Adrenergic receptors (ARs) can be divided into three main types: α1 (α1A–D), α2 (α2A–C), and β (1–3), based on sequence information, receptor pharmacology, and signaling mechanisms. Although β-ARs are located mainly in the cardiovascular system, most α1- and α2-AR subtypes (except α1C and α2B) are highly expressed in CNS regions, e.g., prefrontal cortex (PFC), hippocampus, and brainstem. Norepinephrine, through the action of α1-ARs and α2-ARs, has been implicated in many key functions of PFC, including working memory and emotional control (1–3). An aberrant noradrenergic system, complementing altered serotonergic or dopaminergic signaling, contributes significantly to the pathophysiology of a variety of neuropsychiatric diseases associated with PFC dysfunction, such as depression, anxiety, schizophrenia, and attention-deficit hyperactivity disorder (4–7). Therefore, modifying noradrenergic signaling has been considered one of the key therapeutic actions of many antidepressants, anxiolytic drugs, and antipsychotics (8, 9). To understand the functional role of α1- and α2-ARs, we need to know their cellular targets that are important for cognition and emotion. The NMDAR channel has been implicated in normal cognitive processes and mental disorders (10–12), which makes it a potentially important target by which α1- and α2-ARs may regulate PFC functioning.
Results
Activation of α1-ARs or α2-ARs Reduces NMDAR-Mediated Currents in PFC Pyramidal Neurons.
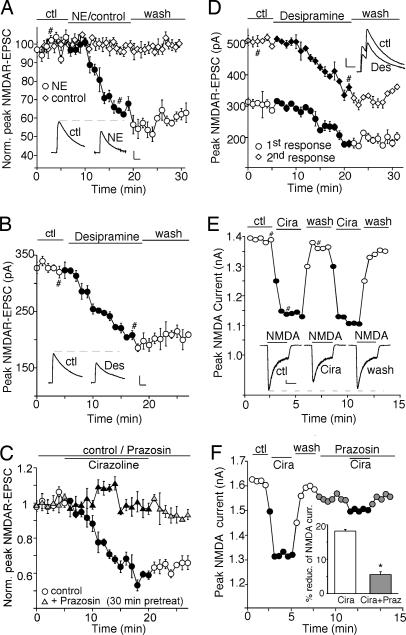
We first examined the effect of the noradrenergic system on NMDAR-mediated excitatory postsynaptic currents (NMDAR-EPSC) evoked by stimulation of synaptic NMDARs in PFC slices. As shown in Fig. 1A, application of 100 μM natural neurotransmitter norepinephrine induced a strong and persistent reduction in the amplitude of NMDAR-EPSC, whereas in parallel control measurements where no norepinephrine was administered, NMDAR-EPSC remained stable throughout the recording. Application of 20 μM desipramine, a norepinephrine transporter inhibitor, to activate endogenous noradrenergic receptors also caused a potent and long-lasting reduction of the NMDAR-EPSC amplitude (Fig. 1B). The specific α1-AR agonist cirazoline (40 μM) produced a similar reducing effect, which was blocked by 40 μM prazosin, a specific α1-AR antagonist (Fig. 1C). In a sample of neurons we tested, norepinephrine, norepinephrine transporter inhibitors, and α1-AR agonist all significantly suppressed synaptic NMDAR-mediated responses (norepinephrine: 31.5 ± 8.5%, n = 4; desipramine: 34.2 ± 2.7%, n = 6; nisoxetine (an norepinephrine transporter inhibitor, 50 μM): 36.3 ± 5.3%, n = 3; cirazoline: 36.2 ± 3.3%, n = 4). We further examined the noradrenergic effect on NMDAR-EPSC evoked by paired pulses, a measure that is sensitive to changes in the probability of transmitter release (13). As shown in Fig. 1D, application of desipramine reduced the amplitudes of NMDAR-EPSC triggered by both pulses, but it did not cause a significant change in the ratio of the paired-pulse facilitation (control: 1.72 ± 0.1; desipramine: 1.73 ± 0.1, n = 5). This observation suggests that activation of noradrenergic receptors in PFC pyramidal neurons is likely to induce a change in postsynaptic NMDARs rather than glutamate release.
Fig. 1.
Activation of α1-ARs reduces the amplitude of NMDAR-EPSC and whole-cell NMDAR currents. (A–C) Plot of peak NMDAR-EPSC in PFC slices showing the effect of 100 μM norepinephrine (A), 20 μM norepinephrine transporter inhibitor desipramine (B), or 40 μM α1-AR agonist cirazoline in the absence or presence of 40 μM α1-AR antagonist prazosin (C). (D) Plot of peak NMDAR-EPSC evoked by double pulses (interstimuli interval, 100 ms) as a function of time and 20 μM desipramine application. (Insets A, B, and D) Representative current traces (average of three trials) at time points denoted by #. (Scale bars: 100 pA, 0.1 s.) (E and F) Plot of peak 100 μM NMDA-evoked currents in dissociated PFC pyramidal neurons showing the effect of 40 μM cirazoline (E) and its blockade by 40 μM prazosin (F). (Inset E) Representative current traces (at time points denoted by #). (Scale bars: 100 pA, 1 s.) (Inset F) Cumulative data (mean ± SEM) summarizing the percentage reduction of NMDAR currents by cirazoline in the absence or presence of prazosin. ∗, P < 0.01, ANOVA.
To verify the potential influence of α1-ARs on NMDARs, we examined the effect of α1-ARs on NMDAR-mediated whole-cell currents in dissociated PFC pyramidal neurons. As shown in Fig. 1E, application of 40 μM cirazoline caused a reversible reduction in the amplitude of currents evoked by 100 μM NMDA (18.2 ± 0.5%, n = 130). This effect was significantly attenuated by 40 μM prazosin (Fig. 1F; 5.5 ± 0.9%, n = 10), confirming that α1-AR activation suppresses NMDAR currents.
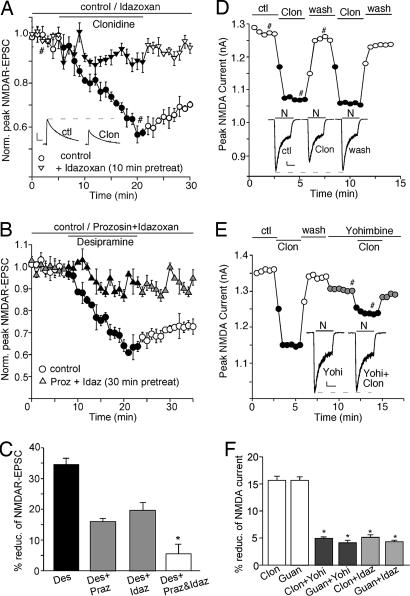
We also examined the effect of α2-ARs on NMDAR-mediated synaptic and whole-cell currents. As shown in Fig. 2A, application of 100 μM specific α2-AR agonist clonidine potently reduced the amplitude of NMDAR-EPSC (38.3 ± 3.2%, n = 5), which was diminished by 40 μM specific α2-AR antagonist idazoxan (9.0 ± 2.3%, n = 5). The effect of the norepinephrine transporter inhibitor desipramine on NMDAR-EPSC was partially reduced by prazosin or idazoxan alone, but it was almost abolished by coapplication of the two antagonists (Fig. 2 B and C), suggesting the involvement of both α1-ARs and α2-ARs. Clonidine also decreased NMDA-evoked currents in dissociated PFC neurons (Fig. 2D; 15.6 ± 0.8%, n = 30), which was abolished by 60 μM yohimbine (4.9 ± 0.3%, n = 7), another specific α2-AR antagonist (Fig. 2E). As summarized in Fig. 2F, the effects of different α2-AR agonists on NMDAR currents were all diminished by various α2-AR antagonists, confirming that α2-AR activation also suppresses NMDAR currents.
Fig. 2.
Activation of α2-ARs reduces the amplitude of NMDAR-EPSC and whole-cell NMDAR currents. (A) Plot of peak NMDAR-EPSC in PFC slices showing the effect of 100 μM α2-AR agonist clonidine in the absence or presence 40 μM idazoxan, a specific α2-AR antagonist. (Inset) Representative current traces (at time points denoted by #). (Scale bars: 100 pA, 0.1 s.) (B) Plot of peak NMDAR-EPSC showing that blocking both α1 and α2 receptors with prazosin and idazoxan prevented the effect of norepinephrine transporter inhibitor desipramine. (C) Cumulative data (mean ± SEM) summarizing the percentage reduction of NMDAR-EPSC by desipramine in the absence or presence of different antagonists. ∗, P < 0.01, ANOVA. (D and E) Plot of peak NMDAR currents showing the effect of 100 μM clonidine (D) and its blockade by 60 μM yohimbine (E), a specific α2-AR antagonist. (Insets D and E) Representative current traces (at time points denoted by #). (Scale bars: 100 pA, 1 s.) (F) Cumulative data (mean ± SEM) summarizing the percentage reduction of NMDAR currents by different α2-AR agonists (clonidine or guanfacine at 100 μM) in the absence or presence of different α2-AR antagonists (yohimbine or idazoxan). ∗, P < 0.01, ANOVA.
Because both α1-AR and α2-AR reduce NMDAR currents, we also examined whether their effects were additive to determine whether these processes had common targets at the NMDAR level. As shown in Fig. 8A, which is published as supporting information on the PNAS web site, in the presence of 100 μM α2-AR agonist clonidine, 40 μM α1-AR agonist cirazoline caused further suppression of NMDAR currents (cirazoline: 18.5 ± 1.2%; clonidine: 15.3 ± 0.6%; cirazoline + clonidine: 32.0 ± 2.7%, n = 6), indicating that the effects of α1-AR and α2-AR are additive. Moreover, the effect of clonidine, but not cirazoline, was blocked by 3 μM selective NMDAR 2B (NR2B) antagonist ifenprodil (Fig. 8 B and C), suggesting that α1-AR and α2-AR target different pools of NMDARs.
We further examined whether α1-AR and α2-AR agonists may have direct effects on NMDARs. As shown in Fig. 9, which is published as supporting information on the PNAS web site, both cirazoline and clonidine produced similar effects on NMDAR currents at different voltages (−60 mV, −40 mV, −20 mV), suggesting that these compounds do not cause a voltage-dependent block of NMDAR channels. Moreover, both cirazoline and clonidine produced similar effects on NMDAR currents in the presence of saturating (20 μM) or subsaturating (1 μM) concentrations of glycine, suggesting that these compounds do not reduce affinity of the NMDARs for glycine.
The α1- and α2-AR of NMDAR Currents Depend on Different Signaling Pathways and Cellular Mechanisms.
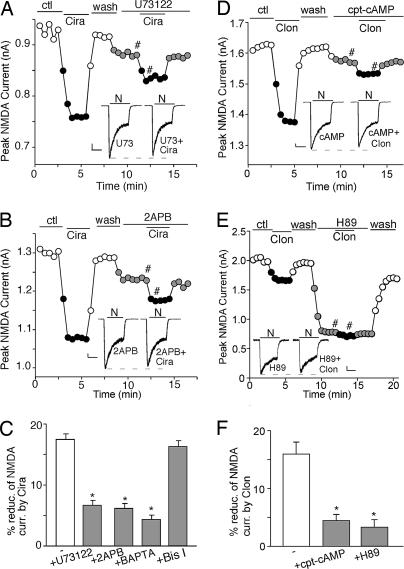
Next, we examined the signaling mechanisms underlying the reduction of NMDAR currents by α1-ARs and α2-ARs, which are coupled to Gq and Gi/o proteins, respectively (14). As shown in Fig. 3A, application of the 1 μM phospholipase C (PLC) inhibitor U73122 significantly blocked the cirazoline-induced decrease of NMDAR currents (6.6 ± 0.9%, n = 7, Fig. 3C), suggesting the involvement of the PLC pathway in α1-AR signaling. PLC activation catalyzes the hydrolysis of membrane phosphoinositol lipids, leading to the release of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. 2-Aminoethoxydiphenyl borane (2APB; 15 μM), a membrane-permeable IP3 receptor antagonist, substantially blocked the reduction of NMDAR currents by cirazoline (Fig. 3B; 6.1 ± 0.8%, n = 7; Fig. 3C). Dialysis with 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA), a Ca2+ chelator, also diminished the effect of cirazoline (4.3 ± 0.7%, n = 6; Fig. 3C), whereas 1 μM selective PKC inhibitor bisindolylmaleimide I failed to do so (16.2 ± 1.0%, n = 6; Fig. 3C). On the other hand, clonidine lost the capability to suppress NMDAR currents in the presence of 50 μM PKA activator cpt-cAMP (Fig. 3D; 4.4 ± 1.1%, n = 7; Fig. 3F), and 10 μM PKA inhibitor H89 reduced NMDAR currents and occluded the effect of clonidine (Fig. 3E; 2.7 ± 1.5%, n = 5; Fig. 3F). These results suggest that the PLC–IP3–Ca2+ pathway is involved in α1-AR regulation of NMDAR currents, whereas inhibition of PKA signaling is required for α2-ARs to regulate NMDAR currents.
Fig. 3.
The PLC–IP3–Ca2+ or PKA pathway is involved in α1- or α2-adrenergic regulation of NMDAR currents, respectively. (A and B) Plot of peak NMDAR currents showing the effect of 40 μM cirazoline in the absence or presence of 1 μM PLC inhibitor U73122 (A) or 15 μM IP3 receptor antagonist 2APB (B). (D and E) Plot of peak NMDAR currents showing the effect of 100 μM clonidine in the absence or presence of 50 μM PKA activator cpt-cAMP (D) or 10 μM PKA inhibitor H89 (E). (Insets A, B, D, and E) Representative current traces (at time points denoted by #). (Scale bars: 100 pA, 1 s.) (C and F) Cumulative data (mean ± SEM) showing the percentage reduction of NMDAR currents by cirazoline (C) or clonidine (F) under various treatments. ∗, P < 0.01, ANOVA.
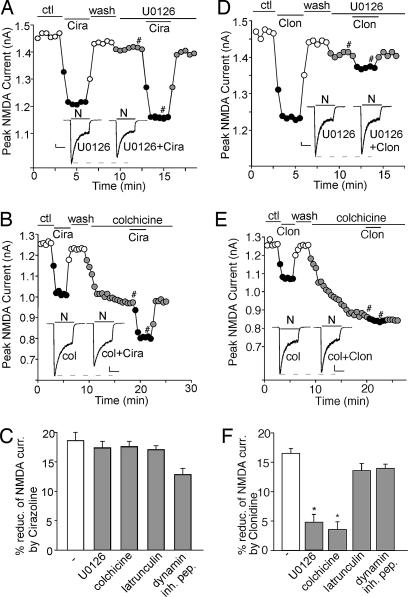
Recent studies have shown that NMDAR function can be regulated through a mechanism dependent on microtubule/motor protein-based dendritic transport of NMDARs that is regulated by ERK signaling (15–17). Therefore, we examined the role of ERK and microtubules in adrenergic regulation of NMDAR currents. As shown in Fig. 4A, in the presence of 20 μM U0126, a specific inhibitor of MEK (the kinase upstream of ERK), the cirazoline-induced decrease of NMDAR currents was not affected (17.3 ± 1.2%, n = 5, Fig. 4C), whereas the effect of clonidine was largely blocked by U0126 (Fig. 4D; 5.7 ± 1.3%, n = 6; Fig. 4F). Application of 30 μM microtubule-depolarizing agent colchicine did not prevent cirazoline from reducing NMDAR currents (Fig. 4B; 17.5 ± 1.0%, n = 5; Fig. 4C), but it occluded the effect of subsequently applied clonidine (Fig. 4E; 3.5 ± 1.3%, n = 6; Fig. 4F). The actin-depolarizing agent latrunculin B (5 μM) or 50 μM dynamin-inhibitory peptide failed to affect either α1- or α2-adrenergic regulation of NMDAR currents (Fig. 4 C and F), suggesting the lack of involvement of actin cytoskeleton or clathrin-mediated endocytosis in the process. Taken together, these results suggest that α2-AR but not α1-AR regulation of NMDAR currents is through a mechanism dependent on microtubule stability.
Fig. 4.
The suppression of NMDAR currents by α2-AR but not α1-AR is through a mechanism involving ERK-regulated microtubule stability. (A, B, D, and E) Plot of peak NMDAR currents showing that the effect of 40 μM cirazoline or 100 μM clonidine on NMDAR currents was affected differentially by 20 μM MEK/ERK inhibitor U0126 (A and D) or 30 μM microtubule-depolymerizing agent colchicine (B and E). (Insets) Representative current traces (at time points denoted by #). (Scale bars: 100 pA, 1 s.) (C and F) Cumulative data (mean ± SEM) showing the percentage reduction of NMDAR currents by cirazoline (C) or clonidine (F) in the absence or presence of various agents. ∗, P < 0.01, ANOVA.
To confirm further that α2-AR affects microtubule-based NMDAR trafficking, we carried out a quantitative surface immunostaining assay in PFC cultures transfected with GFP-NR2B (GFP tag is placed at NR2B extracellular N terminus). The surface distribution of recombinant NR2B was assessed by immunostaining with anti-GFP primary antibody followed by rhodamine-conjugated secondary antibody in nonpermeabilized conditions (16, 17). As shown in Fig. 10, which is published as supporting information on the PNAS web site, in neurons treated with 100 μM α2-AR agonist clonidine for 10 min, the fluorescent GFP-NR2B surface clusters on dendrites were significantly reduced in both the density (control: 38.2 ± 1.6 clusters per 50 μm, n = 14; clonidine-treated neurons: 19.8 ± 0.95 clusters per 50 μm, n = 9; P < 0.01, ANOVA) and the size (control: 0.3 ± 0.03 μm2, n = 14; clonidine-treated neurons: 0.18 ± 0.02 μm2; P < 0.01, ANOVA), which was blocked by 10 μM microtubule stabilizer taxol (cluster density: 37.2 ± 3.1 clusters per 50 μm; cluster size: 0.3 ± 0.02 μm2, n = 6). In contrast, treatment with 100 μM α1-AR agonist cirazoline for 10 min caused little change on surface NR2B clusters. These results suggest the involvement of a microtubule-dependent mechanism in α2-AR regulation of NMDARs.
The α1-AR and α2-AR Effects on NMDAR Currents Are Modulated by Different Regulators of G Protein Signaling (RGS) Proteins in PFC Pyramidal Neurons.
Because RGS proteins play an important role in modulating G protein-coupled receptor (GPCR) signaling (18), we examined their influence on α1-AR and α2-AR regulation of NMDARs. To do so, we infused antibodies against specific RGS family members into neurons through the recording pipette to achieve an effective inhibition of endogenous RGS function (19, 20), and then we tested whether these antibodies could affect α1 or α2 regulation of NMDAR currents. We focused on two RGS family members, RGS2 and RGS4, because RGS2 is dynamically responsive to neuronal activity in a unique fashion (21), whereas RGS4, which exhibits the highest expression in PFC (22), has recently been identified as a schizophrenia-susceptibility gene (23–26).
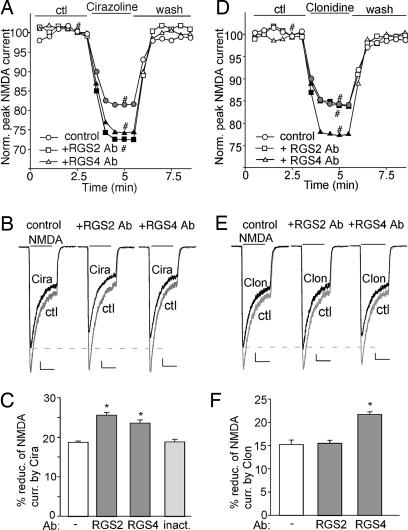
As shown in Fig. 5A and B, bath application of 40 μM cirazoline reduced the amplitude of NMDAR currents, whereas in neurons dialyzed with the anti-RGS2 (19) or anti-RGS4 (20) antibody (4 μg/ml), the reduction of NMDAR currents by cirazoline was strongly augmented. In a sample of neurons we tested (Fig. 5C), both RGS2 and RGS4 antibodies caused a significant potentiation (≈37% increase and ≈26% increase, respectively) in the effect of cirazoline on NMDAR currents [control (−): 18.7 ± 0.4%, n = 14; anti-RGS2: 25.6 ± 0.7%, n = 8; anti-RGS4: 23.6 ± 0.8%, n = 11], whereas the heat-inactivated RGS2 antibody failed to do so (18.8 ± 0.6%, n = 6).
Fig. 5.
Different RGS proteins modulate the effect of α1-ARs or α2-ARs on NMDAR currents. (A and D) Plot of normalized peak NMDAR currents showing the effect of cirazoline (A) or clonidine (D) in neurons dialyzed with 4 μg/ml anti-RGS2 or anti-RGS4 antibody. (B and E) Representative current traces (at time points denoted by #). (Scale bars: 100 pA, 1 s.) (C and F) Cumulative data (mean ± SEM) showing the percentage reduction of NMDAR currents by cirazoline (C) or clonidine (F) in the absence or presence of different RGS antibodies. ∗, P < 0.01, ANOVA.
The role of RGS2 and RGS4 in α2-AR signaling was also examined. As shown in Fig. 5 D and E, the reduction of NMDAR currents by clonidine was strongly potentiated (≈43% increase) by dialysis with the anti-RGS4 antibody [control (−): 15.2 ± 0.9%, n = 9; anti-RGS4: 21.7 ± 0.5%, n = 9; Fig. 5F], but not the anti-RGS2 antibody (15.5 ± 0.6%, n = 8; Fig. 5F). These results suggest that both RGS2 and RGS4 are involved in inhibiting α1-AR signaling, whereas only RGS4 participates in dampening α2-AR regulation of NMDAR currents.
The Scaffold Protein Spinophilin Is Differentially Involved in RGS Modulation of the α1- or α2-AR Effect on NMDAR Currents.
It has been shown that the multidomain scaffolding protein spinophilin binds several GPCRs (27, 28) and RGS proteins (29), suggesting that spinophilin may actively regulate GPCR signaling by recruiting RGS proteins to the receptor complex. To test whether spinophilin is involved in adrenergic regulation of NMDARs, we examined the RGS modulation of α1-AR and α2-AR effects on NMDAR currents in spinophilin-knockout mice (30).
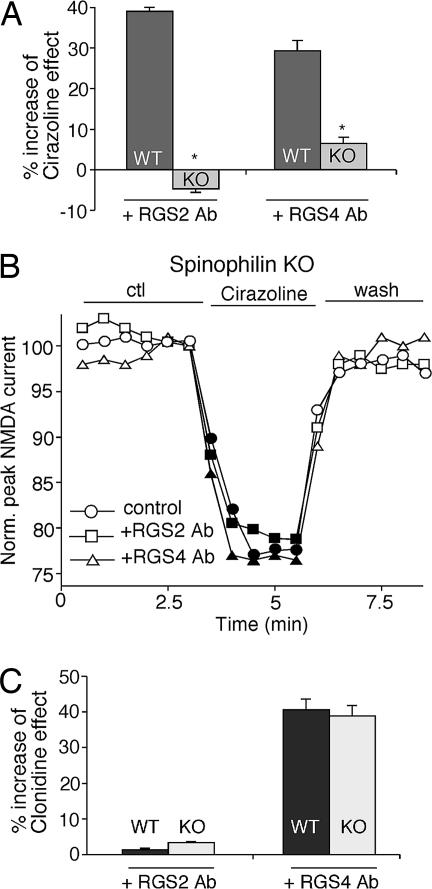
As shown in Fig. 6A, both anti-RGS2 and anti-RGS4 antibodies significantly potentiated the effect of cirazoline on NMDAR currents in neurons from wild-type mice (anti-RGS2: 38.9 ± 1.3% increase, n = 10; anti-RGS4: 28.5 ± 2.5% increase, n = 6). However, in neurons from spinophilin−/− mice, dialysis with the anti-RGS2 or anti-RGS4 antibody failed to augment the effect of cirazoline on NMDAR currents (anti-RGS2: −4.5 ± 1.1% increase, n = 11; anti-RGS4: 6.3 ± 1.5% increase, n = 7). Representative examples from spinophilin−/− mice are shown in Fig. 6B. The loss of RGS2/4-mediated attenuation of α1-AR signaling in spinophilin-deficient cells led to a 25.7 ± 0.5% (n = 11) enhancement in the cirazoline effect on NMDAR currents. In contrast, the effect of anti-RGS2 or anti-RGS4 on clonidine regulation of NMDAR currents remained unaltered in neurons from wild-type vs. spinophilin−/− mice (Fig. 6C; anti-RGS2: wild-type, 1.3 ± 0.5% increase, n = 8; knockout, 3.3 ± 0.3% increase, n = 6; anti-RGS4: wild-type, 40.5 ± 3.1% increase, n = 6; knockout, 38.7 ± 2.9% increase, n = 6). The intact RGS4 modulation of α2-AR signaling in spinophilin-deficient cells led to little change (2.0 ± 0.3%, n = 11) in the clonidine effect on NMDAR currents. These data suggest that the scaffold protein spinophilin functions to facilitate the ability of RGS2/4 to inhibit α1-adrenergic regulation of NMDAR currents, but it is not involved in RGS4 modulation of the α2-AR effect on NMDAR currents.
Fig. 6.
The RGS modulation of the α1-AR but not α2-AR effect on NMDAR currents is lost in spinophilin-knockout mice. (A and C) Cumulative data (mean ± SEM) showing the percentage increase of the effect of cirazoline (A) or clonidine (C) on NMDAR currents by 4 μg/ml anti-RGS2 or anti-RGS4 antibodies in neurons from wild-type (WT) or spinophilin-knockout (KO) mice. ∗, P < 0.01, ANOVA. (B) Plot of normalized peak NMDAR currents showing the effect of cirazoline in neurons dialyzed with an anti-RGS2 or anti-RGS4 antibody in PFC pyramidal neurons from spinophilin-knockout mice.
Discussion
In this work, we found that activating α1-ARs or α2-ARs inhibits NMDAR currents through distinct mechanisms in PFC pyramidal neurons, with the PLC–IP3–Ca2+ pathway involved in the α1-AR effect and PKA as well as the ERK-regulated microtubule-based transport of NMDARs involved in the α2-AR effect. Moreover, the scaffold protein spinophilin selectively facilitates the RGS2/4 modulation of the α1-AR effect on NMDAR currents (Fig. 7). Given the critical role of NMDA signaling in controlling neuronal function, our results provide a potential molecular and cellular mechanism for adrenergic regulation of emotion and cognition subserved by PFC.
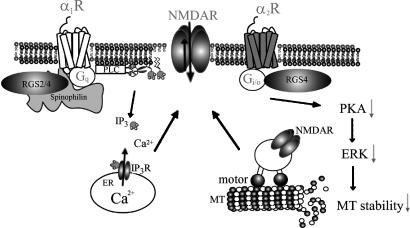
Fig. 7.
Proposed model showing the mechanisms underlying α1- and α2-adrenergic regulation of NMDAR function in PFC pyramidal neurons. α1-AR (Gq-coupled) reduces NMDAR currents via the PLC-IP3 pathway, whereas α2-AR (Gi/o-coupled) decreases NMDAR currents by interfering with the microtubule (MT)/motor protein-based transport of NMDARs that is regulated by the PKA–ERK cascade. RGS2 is specifically involved in dampening the α1-AR signaling, whereas RGS4 is involved in both α1-AR and α2-AR signaling. The scaffold protein spinophilin selectively facilitates the RGS2/4 modulation of the α1-AR effect on NMDAR currents.
Previous studies suggested that NMDAR activity could be regulated by serine/threonine and tyrosine phosphorylation by GPCR-induced signaling pathways (31). Emerging evidence indicates that another key factor in the regulation of NMDAR channel functions is the trafficking of NMDARs, which involves exiting from ER, transporting along dendritic microtubules, delivery to actin-enriched postsynaptic density, internalization, and lateral diffusion at synaptic and extrasynaptic sites (15). Recent studies have revealed the different mechanisms underlying serotonin and dopamine receptor mediated-regulation of NMDAR trafficking and function (16, 32, 33). The present finding on adrenergic regulation of NMDARs has introduced additional players in the process, such as RGS proteins and spinophilin.
RGS proteins are known to regulate the intensity and duration of GPCR signaling by accelerating the GTPase activity of Gα subunits (34, 18). However, the mechanism by which RGS proteins recognize GPCRs to confer signaling specificity remains largely unknown. In vitro studies have found that RGS2 shows preference for Gq class Gα subunits (35), whereas RGS4 attenuates both Gi- and Gq-mediated signaling (36–38). Biochemical evidence suggests that the divergent N-terminal domain of RGS proteins participates in GPCR recognition (39), and signaling specificity in vivo is conferred by interaction of RGS proteins with receptor complexes (40). The differential involvement of endogenous RGS2 and RGS4 in the regulation of NMDAR currents by α1-ARs (Gq-coupled) or α2-ARs (Gi-coupled) suggests that RGS proteins act in a GPCR-specific manner in native neurons.
A recent study has revealed that the scaffolding protein spinophilin, which binds the third intracellular loop of α-ARs and the N-terminal domain of several RGS proteins, enhances the ability of RGS2 to inhibit α1B-AR-evoked Ca2+ signaling (29). Interestingly, we found that the RGS2/4-mediated attenuation of the α1-AR effect on NMDARs was lost in spinophilin-knockout mice, leading to a bigger reduction of NMDAR currents by α1-AR agonists in spinophilin-deficient neurons. In contrast, the RGS4-mediated suppression of the α2-AR effect on NMDARs was intact in spinophilin-knockout mice, despite the potential interaction between activated α2-AR and spinophilin (28, 41). This observation suggests that spinophilin may selectively regulate Gq-coupled receptor signaling by improving access of RGS2/4 to the receptors and facilitating the interaction of RGS2/4 with Gαq.
Materials and Methods
Electrophysiological Recordings in Slices.
To evaluate the regulation of NMDAR-EPSC in pyramidal neurons located in deep layers (V–VI) of PFC, slices from young adult (3–5 weeks postnatal) Sprague–Dawley rats were used for recordings with the whole-cell voltage-clamp technique (16, 32). The brain slice (300 μm) was submerged in oxygenated artificial cerebrospinal fluid (ACSF) containing 20 μM CNQX and 10 μM bicuculline. Cells were visualized with a water-immersion lens (magnification, ×40) and illuminated with near-IR light. A bipolar stimulating electrode was positioned ≈100 μm from the neuron under recording. Before stimulation, cells (clamped at −70 mV) were depolarized to 60 mV for 3 s to relieve fully the voltage-dependent Mg2+ block of NMDAR channels. The Clampfit program (Axon Instruments, Union City, CA) was used to analyze evoked synaptic activity.
Whole-Cell Recordings in Dissociated Neurons.
Acutely dissociated PFC pyramidal neurons were prepared by using procedures described in refs. 16 and 32. Spinophilin-knockout mice were produced as detailed before (30). Recordings of whole-cell ligand-gated ion channel currents used standard voltage-clamp techniques (16). The cell membrane potential was held at −60 mV. NMDA (100 μM) was applied for 2 s every 30 s. Drugs were applied with a gravity-fed sewer-pipe system. The array of application capillaries was positioned near the cell under study. Solution changes were effected by the SF-77B fast-step solution stimulus delivery device (Warner Instrument Co., Hamden, CT).
To test the impact of RGS proteins on GPCR signaling, the antibody raised against a peptide mapping at the highly specific N terminus of RGS2 or RGS4 (Santa Cruz Biotechnology, Santa Cruz, CA) was dialyzed into neurons through the patch electrode for 10 min before electrophysiological recordings started. Data analyses were performed with AxoGraph (Axon Instruments) and Kaleidagraph (Albeck Software, Reading, PA). ANOVA tests were performed to compare the differential degrees of current modulation between groups subjected to different treatments.
Supplementary Material
Acknowledgments
We thank Xiaoqing Chen for technical support. This work was supported by National Institutes of Health Grants MH63128 and NS48911 and a National Alliance for Research on Schizophrenia and Depression Independent Investigator Award (to Z.Y.).
Abbreviations
- 2APB
2-aminoethoxydiphenyl borane
- AR
adrenergic receptor
- EPSC
excitatory postsynaptic currents
- GPCR
G protein-coupled receptor
- IP3
inositol 1,4,5-trisphosphate
- NMDAR
NMDA receptor
- NR2B
NMDAR 2B
- PFC
prefrontal cortex
- PLC
phospholipase C
- RGS
regulators of G protein signaling.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 2.Schramm NL, McDonald MP, Limbird LE. J Neurosci. 2001;21:4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubini A, Bosc M, Polin V. J Psychopharmacol. 1997;11:S17–S23. [PubMed] [Google Scholar]
- 5.Charney DS, Redmond DE., Jr Neuropharmacology. 1983;22:1531–1536. doi: 10.1016/0028-3908(83)90122-3. [DOI] [PubMed] [Google Scholar]
- 6.Arnsten AF. Psychopharmacology. 2004;174:25–31. doi: 10.1007/s00213-003-1724-3. [DOI] [PubMed] [Google Scholar]
- 7.Russell V, Allie S, Wiggins T. Behav Brain Res. 2000;117:69–74. doi: 10.1016/s0166-4328(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 8.Ninan PT. J Clin Psychiatry. 1999;(Suppl 22):12–17. [PubMed] [Google Scholar]
- 9.Hertel P, Fagerquist MV, Svensson TH. Science. 1999;286:105–107. doi: 10.1126/science.286.5437.105. [DOI] [PubMed] [Google Scholar]
- 10.Dingledine R, Borges K, Bowie D, Traynelis SF. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 11.Jentsch JD, Roth RH. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 12.Tsai G, Coyle JT. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 13.Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 14.Harrison JK, Pearson WR, Lynch KR. Trends Pharmacol Sci. 1991;12:62–67. doi: 10.1016/0165-6147(91)90499-i. [DOI] [PubMed] [Google Scholar]
- 15.Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- 16.Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuen EY, Jiang Q, Feng J, Yan Z. J Biol Chem. 2005;280:29420–29427. doi: 10.1074/jbc.M504499200. [DOI] [PubMed] [Google Scholar]
- 18.Vries LD, Zheng B, Fischer T, Elenko E, Farquhar MG. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Hashim S, Anand-Srivastava MB. Cardiovasc Res. 2005;66:503–511. doi: 10.1016/j.cardiores.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Diverse-Pierluissi MA, Fischer T, Jordan JD, Schiff M, Ortiz DF, Farquhar MG, De Vries L. J Biol Chem. 1999;274:14490–14494. doi: 10.1074/jbc.274.20.14490. [DOI] [PubMed] [Google Scholar]
- 21.Ingi T, Krumins AM, Chidiac P, Brothers GM, Chung S, Snow BE, Barnes CA, Lanahan AA, Siderovski DP, Ross EM, et al. J Neurosci. 1998;18:7178–7188. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdely HA, Lahti RA, Lopez MB, Myers CS, Roberts RC, Tamminga CA, Vogel MW. Eur J Neurosci. 2004;19:3125–3128. doi: 10.1111/j.0953-816X.2004.03364.x. [DOI] [PubMed] [Google Scholar]
- 23.Chowdari KV, Mirnics K, Semwal P, Wood J, Lawrence E, Bhatia T, Deshpande SN BK T, Ferrell RE, Middleton FA, et al. Hum Mol Genet. 2002;11:1373–1380. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- 24.Williams NM, Preece A, Spurlock G, Norton N, Williams HJ, McCreadie RG, Buckland P, Sharkey V, Chowdari KV, Zammit S, et al. Biol Psychiatry. 2004;55:192–195. doi: 10.1016/j.biopsych.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Morris DW, Rodgers A, McGhee KA, Schwaiger S, Scully P, Quinn J, Meagher D, Waddington JL, Gill M, Corvin AP. Am J Med Genet Neuropsychiatr Genet. 2004;125B:50–53. doi: 10.1002/ajmg.b.20109. [DOI] [PubMed] [Google Scholar]
- 26.Harrison PJ, Owen MJ. Lancet. 2003;361:417–419. doi: 10.1016/S0140-6736(03)12379-3. [DOI] [PubMed] [Google Scholar]
- 27.Smith FD, Oxford GS, Milgram SL. J Biol Chem. 1999;274:19894–19900. doi: 10.1074/jbc.274.28.19894. [DOI] [PubMed] [Google Scholar]
- 28.Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. J Biol Chem. 2001;276:15003–15008. doi: 10.1074/jbc.M011679200. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, Milgram SL, Penninger JM, Allen PB, Greengard P, et al. Nat Cell Biol. 2005;7:405–411. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- 30.Feng J, Yan Z, Ferreira AB, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Proc Natl Acad Sci USA. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber U. Neuropharmacology. 2002;42:587–592. doi: 10.1016/s0028-3908(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Zhong P, Gu Z, Yan Z. J Neurosci. 2003;23:9852–9861. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. J Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berman DM, Wilkie TM, Gilman AG. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 35.Zheng B, De Vries L, Gist Farquhar M. Trends Biochem Sci. 1999;24:411–414. doi: 10.1016/s0968-0004(99)01474-7. [DOI] [PubMed] [Google Scholar]
- 36.Huang C, Hepler JR, Gilman AG, Mumby SM. Proc Natl Acad Sci USA. 1997;94:6159–6163. doi: 10.1073/pnas.94.12.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hepler JR, Berman DM, Gilman AG, Kozasa T. Proc Natl Acad Sci USA. 1997;94:428–432. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan Y, Chi PP, Bourne HR. J Biol Chem. 1997;272:11924–11927. doi: 10.1074/jbc.272.18.11924. [DOI] [PubMed] [Google Scholar]
- 39.Zeng W, Xu X, Popov S, Mukhopadhyay S, Chidiac P, Swistok J, Danho W, Yagaloff KA, Fisher SL, Ross EM, et al. J Biol Chem. 1998;273:34687–34690. doi: 10.1074/jbc.273.52.34687. [DOI] [PubMed] [Google Scholar]
- 40.Xu X, Zeng W, Popov S, Berman DM, Davignon I, Yu K, Yowe D, Offermanns S, Muallem S, Wilkie TM. J Biol Chem. 1999;274:3549–3556. doi: 10.1074/jbc.274.6.3549. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.