Abstract
An altered β-tubulin gene that confers resistance to the fungicide benomyl was isolated from a genomic library of a UV-induced mutant of Cercospora kikuchii and used as a selectable marker for transformation. The level of benomyl resistance conferred to the transformants was at least 150-fold greater than the intrinsic resistance of the C. kikuchii recipient protoplasts. In the majority of cases, the tubulin fragment was integrated at the native β-tubulin locus, apparently by gene replacement or gene conversion. The frequency of transformation ranged from 0.2 to 6 transformants per μg of DNA, depending on the recipient strain. Transformation with linearized plasmid resulted in a higher frequency, without changing the type of integration event. Transformants were phenotypically stable after eight consecutive transfers on medium without benomyl. This is the first report of a genetic transformation system for a Cercospora species.
Full text
PDF


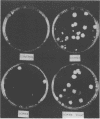
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch D. K., Kinsey J. A. Relationship of vector insert size to homologous integration during transformation of Neurospora crassa with the cloned am (GDH) gene. Mol Gen Genet. 1990 Mar;221(1):37–43. doi: 10.1007/BF00280365. [DOI] [PubMed] [Google Scholar]
- Dunne P. W., Oakley B. R. Mitotic gene conversion, reciprocal recombination and gene replacement at the benA, beta-tubulin, locus of Aspergillus nidulans. Mol Gen Genet. 1988 Aug;213(2-3):339–345. doi: 10.1007/BF00339600. [DOI] [PubMed] [Google Scholar]
- Ehrenshaft M., Upchurch R. G. Isolation of Light-Enhanced cDNAs of Cercospora kikuchii. Appl Environ Microbiol. 1991 Sep;57(9):2671–2676. doi: 10.1128/aem.57.9.2671-2676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fincham J. R. Transformation in fungi. Microbiol Rev. 1989 Mar;53(1):148–170. doi: 10.1128/mr.53.1.148-170.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber R. C., Yoder O. C. Isolation of DNA from filamentous fungi and separation into nuclear, mitochondrial, ribosomal, and plasmid components. Anal Biochem. 1983 Dec;135(2):416–422. doi: 10.1016/0003-2697(83)90704-2. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Marzluf G. A. Transformation of Neurospora crassa with the trp-1 gene and the effect of host strain upon the fate of the transforming DNA. Curr Genet. 1988;13(1):65–70. doi: 10.1007/BF00365758. [DOI] [PubMed] [Google Scholar]
- Orbach M. J., Porro E. B., Yanofsky C. Cloning and characterization of the gene for beta-tubulin from a benomyl-resistant mutant of Neurospora crassa and its use as a dominant selectable marker. Mol Cell Biol. 1986 Jul;6(7):2452–2461. doi: 10.1128/mcb.6.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip E. R., Woloshuk C. P., Payne G. A., Curtis S. E. Isolation and sequence analysis of a beta-tubulin gene from Aspergillus flavus and its use as a selectable marker. Appl Environ Microbiol. 1990 Dec;56(12):3686–3692. doi: 10.1128/aem.56.12.3686-3692.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch R. G., Walker D. C., Rollins J. A., Ehrenshaft M., Daub M. E. Mutants of Cercospora kikuchii Altered in Cercosporin Synthesis and Pathogenicity. Appl Environ Microbiol. 1991 Oct;57(10):2940–2945. doi: 10.1128/aem.57.10.2940-2945.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer S. J., Yanofsky C. Efficient cloning of genes of Neurospora crassa. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4869–4873. doi: 10.1073/pnas.83.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshuk C. P., Seip E. R., Payne G. A., Adkins C. R. Genetic transformation system for the aflatoxin-producing fungus Aspergillus flavus. Appl Environ Microbiol. 1989 Jan;55(1):86–90. doi: 10.1128/aem.55.1.86-90.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hartingsveldt W., Mattern I. E., van Zeijl C. M., Pouwels P. H., van den Hondel C. A. Development of a homologous transformation system for Aspergillus niger based on the pyrG gene. Mol Gen Genet. 1987 Jan;206(1):71–75. doi: 10.1007/BF00326538. [DOI] [PubMed] [Google Scholar]