Abstract
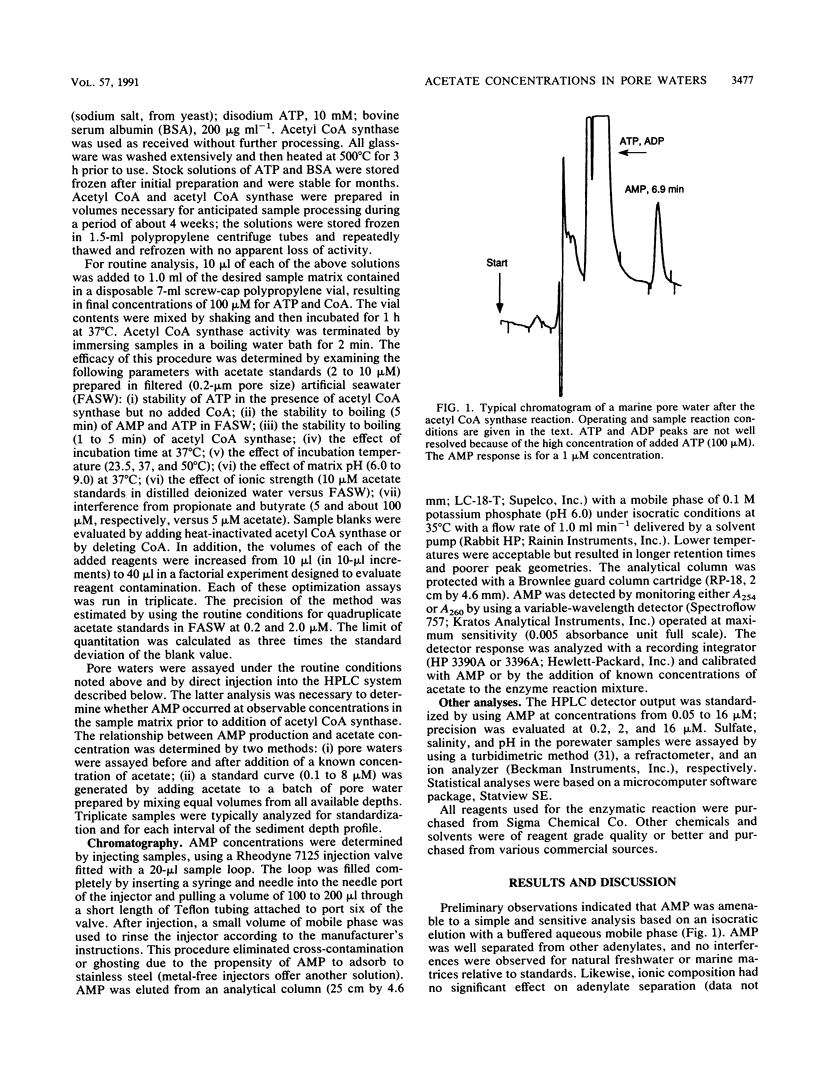
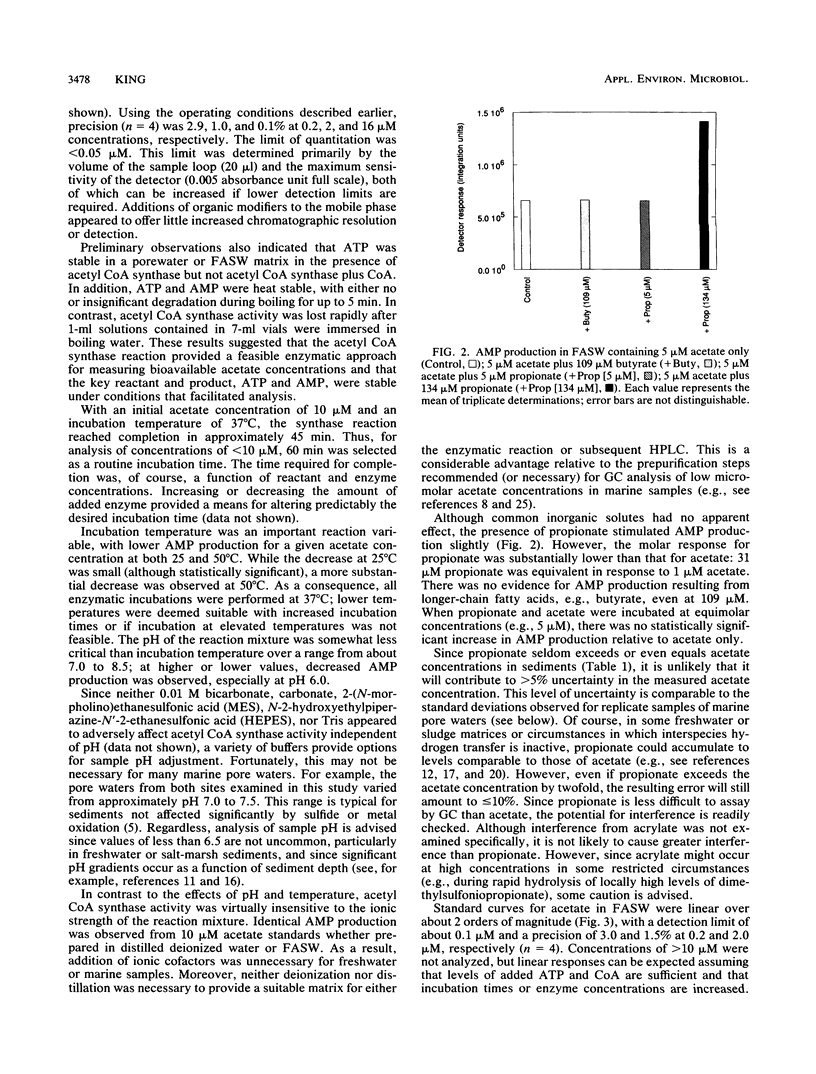
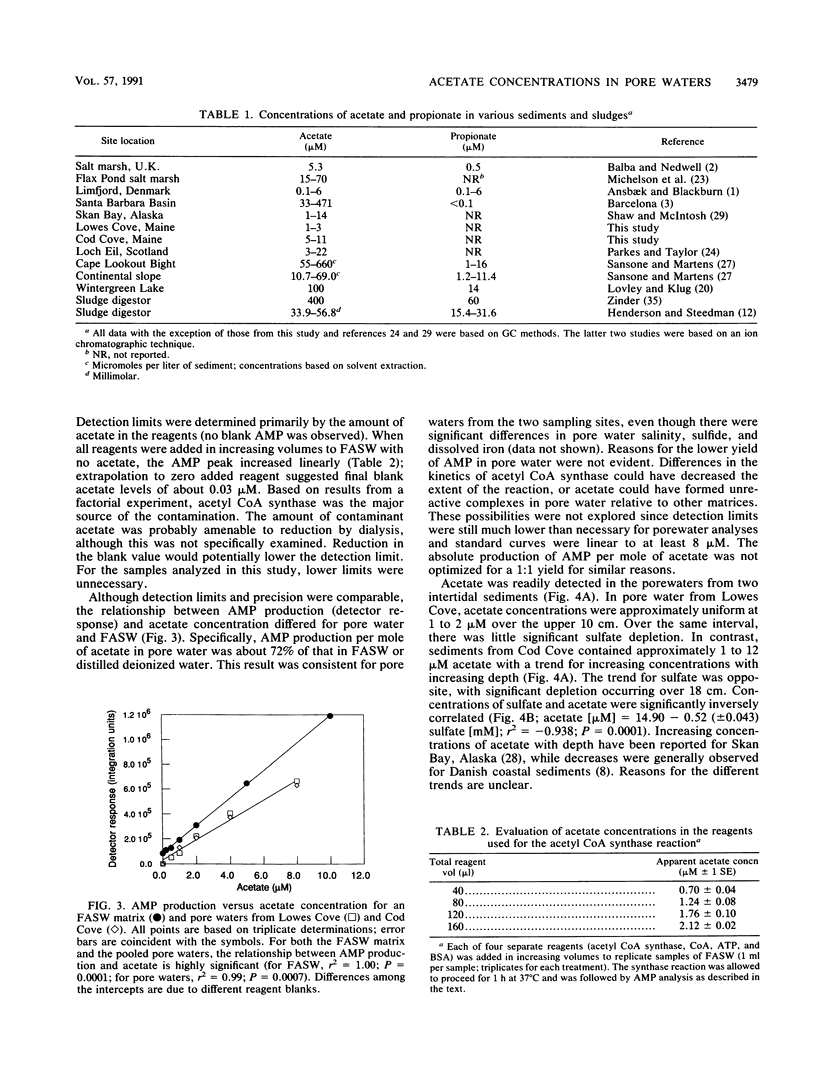
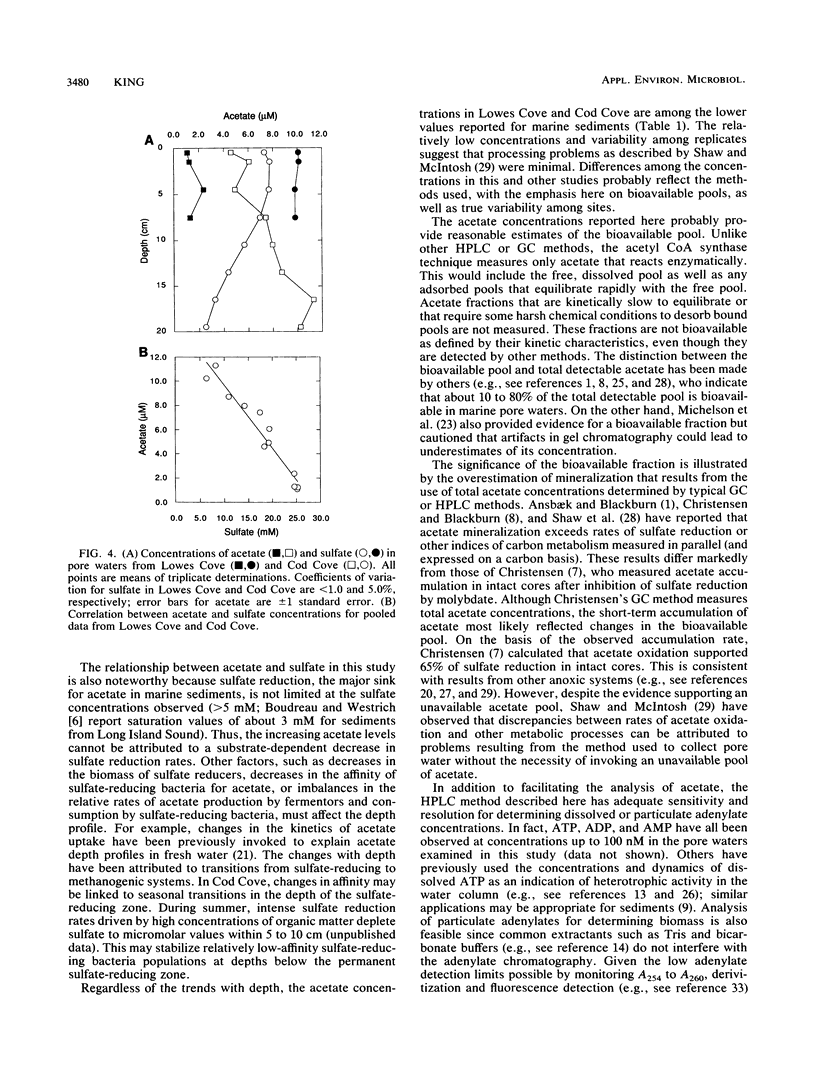
Acetate concentrations in marine and freshwater matrices were measured by an enzymatic technique which coupled the synthesis of acetyl coenzyme A to AMP production. The resulting AMP was assayed by a sensitive and relatively rapid high-pressure liquid chromatography method, using an aqueous, isocratic mobile phase for elution. The method was insensitive to the presence of seawater salts and required no sample prepurification or distillation. Propionate caused a minor, but statistically insignificant, interference when equimolar with acetate; butyrate caused no interference, even at relatively high concentrations. Detection limits for acetate were approximately 100 nM with a precision of about 5%. Pore waters from two intertidal sediments contained approximately 1 to 12 μM acetate; the concentrations were linearly but inversely correlated with porewater sulfate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collin D. P., McCormick P. G., Schmitt M. G., Jr Quantitative gas-chromatographic determination of short-chain fatty acids in aqueous samples. Clin Chem. 1974 Sep;20(9):1235–1237. [PubMed] [Google Scholar]
- Goodwin S., Zeikus J. G. Ecophysiological adaptations of anaerobic bacteria to low pH: analysis of anaerobic digestion in acidic bog sediments. Appl Environ Microbiol. 1987 Jan;53(1):57–64. doi: 10.1128/aem.53.1.57-64.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M. Characterization of beta-Glucosidase Activity in Intertidal Marine Sediments. Appl Environ Microbiol. 1986 Feb;51(2):373–380. doi: 10.1128/aem.51.2.373-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Klug M. J. Glucose metabolism in sediments of a eutrophic lake: tracer analysis of uptake and product formation. Appl Environ Microbiol. 1982 Dec;44(6):1308–1317. doi: 10.1128/aem.44.6.1308-1317.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Klug M. J., Lovley D. R. Metabolism of acetate, methanol, and methylated amines in intertidal sediments of lowes cove, maine. Appl Environ Microbiol. 1983 Jun;45(6):1848–1853. doi: 10.1128/aem.45.6.1848-1853.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanbroek H. J., Pfennig N. Oxidation of short-chain fatty acids by sulfate-reducing bacteria in freshwater and in marine sediments. Arch Microbiol. 1981 Jan;128(3):330–335. doi: 10.1007/BF00422540. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J., Christensen D., Jørgensen B. B. Volatile Fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981 Jul;42(1):5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viarengo A., Secondini A., Scoppa P., Orunesu M. A rapid HPLC method for determination of adenylate energy charge. Experientia. 1986 Dec 1;42(11-12):1234–1235. doi: 10.1007/BF01946400. [DOI] [PubMed] [Google Scholar]


