Abstract
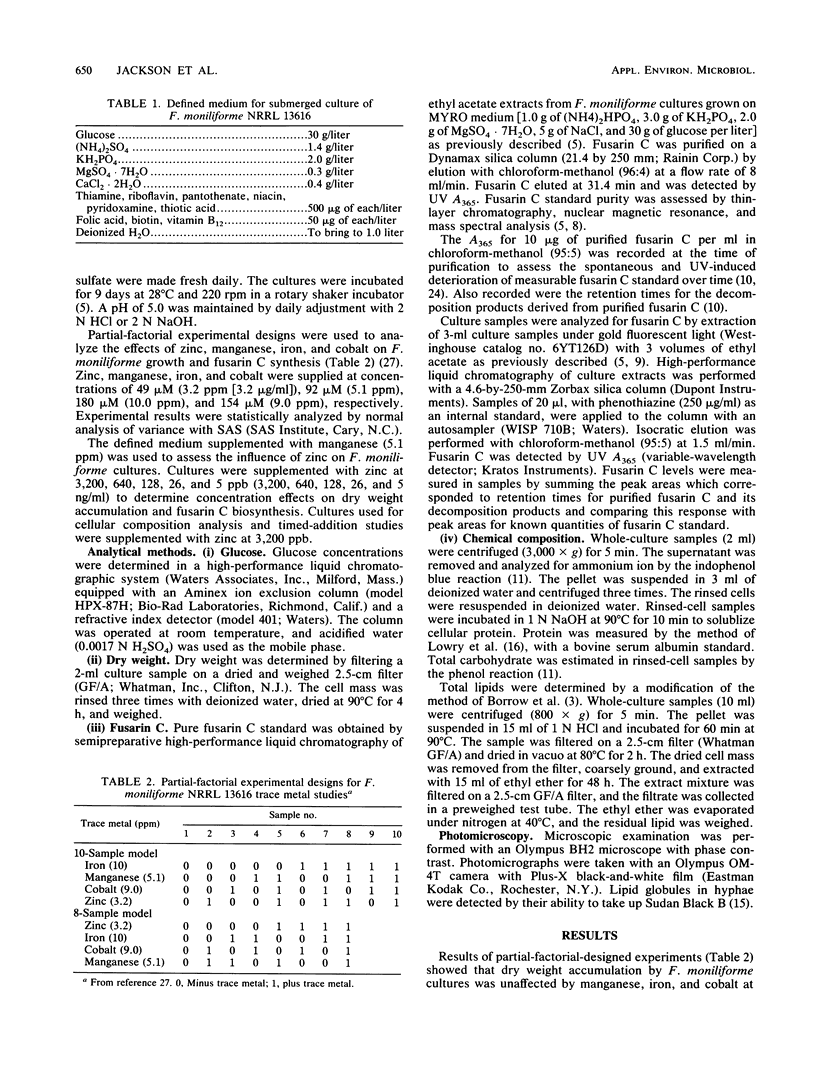
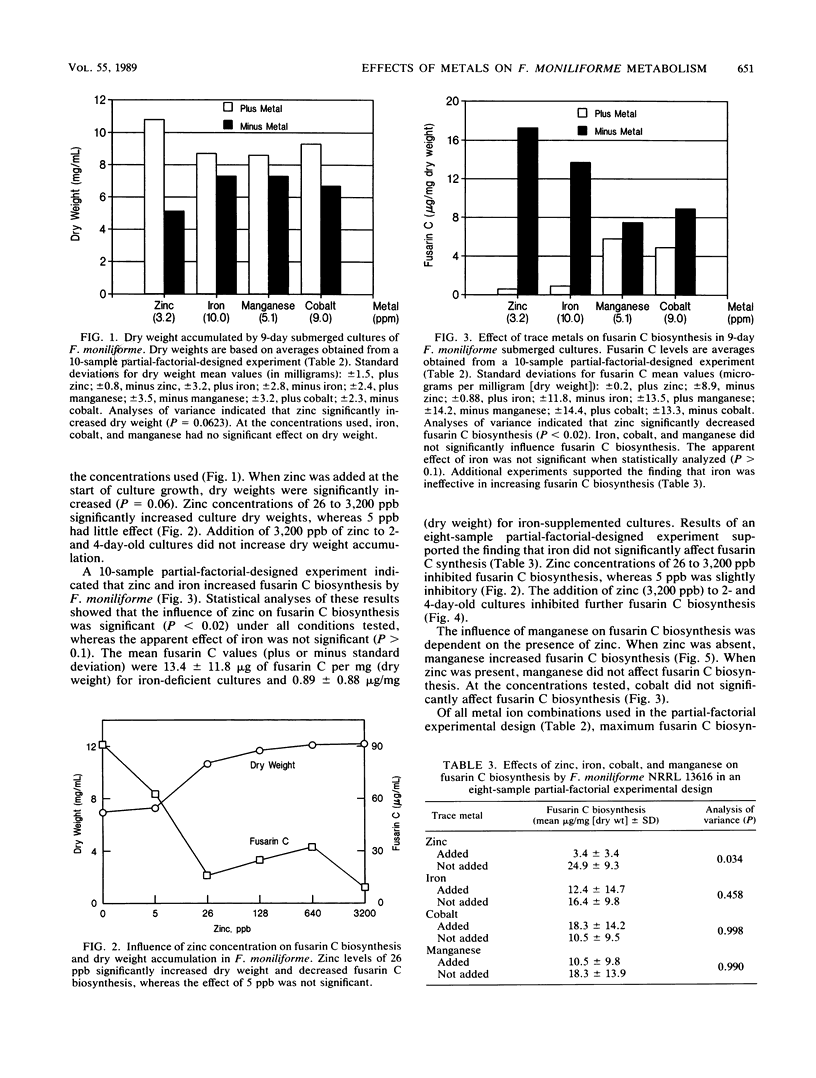
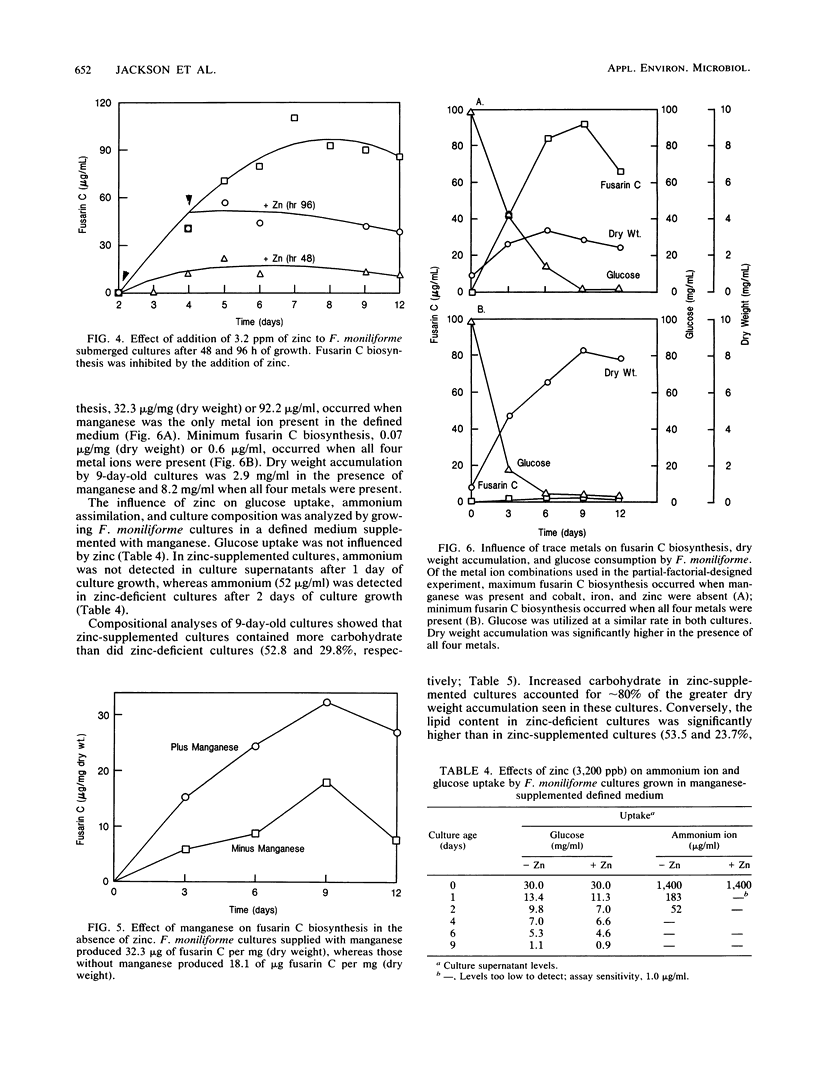
The influence of zinc, iron, cobalt, and manganese on submerged cultures of Fusarium moniliforme NRRL 13616 was assessed by measuring dry weight accumulation, fusarin C biosynthesis, and ammonia assimilation. Shake flask cultures were grown in a nitrogen-limited defined medium supplemented with various combinations of metal ions according to partial-factorial experimental designs. Zinc (26 to 3,200 ppb [26 to 3,200 ng/ml]) inhibited fusarin C biosynthesis, increased dry weight accumulation, and increased ammonia assimilation. Carbohydrate was found to be the principal component of the increased dry weight in zinc-supplemented cultures. Zinc-deficient cultures synthesized more lipid and lipidlike compounds, such as fusarin C, than did zinc-supplemented cultures. Microscopic examination showed that zinc-deficient hyphae contained numerous lipid globules which were not present in zinc-supplemented cultures. Addition of zinc (3,200 ppb) to 2- and 4-day-old cultures inhibited further fusarin C biosynthesis but did not stimulate additional dry weight accumulation. Iron (10.0 ppm) and cobalt (9.0 ppm) did not affect fusarin C biosynthesis or dry weight accumulation. Manganese (5.1 ppm) did not affect dry weight accumulation but did increase fusarin C biosynthesis in the absence of zinc. Maximum fusarin C levels, 32.3 micrograms/mg (dry weight), were produced when cultures were supplied manganese, whereas minimum fusarin C levels, 0.07 micrograms/mg (dry weight), were produced when zinc, iron, cobalt, and manganese were supplied. These results suggest a multifunctional role for zinc in affecting F. moniliforme metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjeldanes L. F., Thomson S. V. Mutagenic activity of Fusarium moniliforme isolates in the Salmonella typhimurium assay. Appl Environ Microbiol. 1979 Jun;37(6):1118–1121. doi: 10.1128/aem.37.6.1118-1121.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Sanders G. W. Fusarin C production by North American isolates of Fusarium moniliforme. Appl Environ Microbiol. 1986 Feb;51(2):381–384. doi: 10.1128/aem.51.2.381-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom W. C., Jaskiewicz K., Marasas W. F., Thiel P. G., Horak R. M., Vleggaar R., Kriek N. P. Fumonisins--novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol. 1988 Jul;54(7):1806–1811. doi: 10.1128/aem.54.7.1806-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom W. C., Thiel P. G., van der Merwe K. J., Marasas W. F., Spies H. S. A mutagen produced by Fusarium moniliforme. Toxicon. 1983;21(4):467–473. doi: 10.1016/0041-0101(83)90124-1. [DOI] [PubMed] [Google Scholar]
- KALYANASUNDARAM R., SARASWATHIDEVI L. Zinc in the metabolism of Fusarium vasinfectum Atk. Nature. 1955 May 28;175(4465):945–945. doi: 10.1038/175945a0. [DOI] [PubMed] [Google Scholar]
- Kriek N. P., Kellerman T. S., Marasas W. F. A comparative study of the toxicity of Fusarium verticillioides (= F. moniliforme) to horses, primates, pigs, sheep and rats. Onderstepoort J Vet Res. 1981 Jun;48(2):129–131. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marasas W. F., Kriek N. P., Fincham J. E., van Rensburg S. J. Primary liver cancer and oesophageal basal cell hyperplasia in rats caused by Fusarium moniliforme. Int J Cancer. 1984 Sep 15;34(3):383–387. doi: 10.1002/ijc.2910340315. [DOI] [PubMed] [Google Scholar]
- Marasas W. F., van Rensburg S. J., Mirocha C. J. Incidence of Fusarium species and the mycotoxins, deoxynivalenol and zearalenone, in corn produced in esophageal cancer areas in Transkei. J Agric Food Chem. 1979 Sep-Oct;27(5):1108–1112. doi: 10.1021/jf60225a013. [DOI] [PubMed] [Google Scholar]
- Marsh P. B., Simpson M. E., Trucksess M. W. Effects of trace metals on the production of aflatoxins by Aspergillus parasiticus. Appl Microbiol. 1975 Jul;30(1):52–57. doi: 10.1128/am.30.1.52-57.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasavada A. B., Hsieh D. P. Effects of Metals on 3-Acetyldeoxynivalenol Production by Fusarium graminearum R2118 in Submerged Cultures. Appl Environ Microbiol. 1988 Apr;54(4):1063–1065. doi: 10.1128/aem.54.4.1063-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. P., Johnson G. T. Zinc effects on growth and cynodontin production of helminthosporium cynodontis. Mycologia. 1971 May-Jun;63(3):548–561. [PubMed] [Google Scholar]