Abstract
Blood cell transplantation is largely replacing bone marrow transplantation because engraftment is more rapid. This accelerated engraftment is thought to be mediated by relatively mature committed hematopoietic progenitor cells. Herein, we have used a modified rhodamine (Rho) staining procedure to identify and purify Rho+/++ (dull/bright) and Rho− (negative) subpopulations of hematopoietic progenitor cells in murine cytokine-mobilized blood. The Rho+/++ cell population contained >99% of committed progenitor cells with in vitro colony-forming ability. The Rho− cell population contained the majority of hematopoietic stem cells with in vivo marrow repopulating ability. The rate of hematopoietic reconstitution was identical in recipients of grafts containing only purified Rho− stem cells or purified Rho− stem cells in combination with large numbers of Rho+/++ committed progenitor cells. In contrast, transplantation of 3-fold more hematopoietic stem cells resulted in accelerated reconstitution, indicating that the reconstitution rate was determined by the absolute numbers of Rho− stem cells in the graft. In addition, we observed a 5- to 8-fold reduced frequency of the subset of hematopoietic stem cells with long-term repopulating ability in cytokine-mobilized blood in comparison to steady-state bone marrow. Our results indicate that hematopoietic stem cells and not committed progenitor cells mediate early hematopoietic reconstitution after blood cell transplantation and that relative to bone marrow, the frequency of stem cells with long-term repopulating ability is reduced in mobilized blood.
Mature blood cells are produced by hematopoietic progenitor cells in the bone marrow. Within this progenitor cell population, relatively immature stem cells and more mature committed progenitor cells can be distinguished (for reviews, see refs. 1 and 2). After myelo-ablative treatment and bone marrow transplantation, mature blood cell production (i.e., engraftment) is resumed after an interval of 2–3 weeks (3). Engraftment is thought to be mediated initially by committed progenitor cells and finally by stem cells. Several arguments support this concept. (i) Multiple cell divisions and maturation steps separate the most immature stem cells from mature blood cells (1). Less-immature committed progenitor cells may require fewer cell divisions to produce mature blood cells. (ii) Several murine in vivo studies using different cell separation methods showed that early engraftment is mediated by cell populations that were relatively enriched for committed progenitors (4–9). (iii) Cytokine-mobilized blood cell grafts contain increased numbers of committed progenitor cells compared with bone marrow grafts, and transplantation of these grafts results in an earlier engraftment (10–12). Although these arguments do not provide formal proof for the concept, large-scale ex vivo expansion of committed progenitor cells is being developed with the aim to further enhance engraftment (8, 13–16).
To identify the cell population mediating the early phase of hematopoietic reconstitution after blood cell transplantation, we have used a recently developed method to purify subpopulations of murine hematopoietic progenitor cells (17). Herein, we identified purified populations of immature stem cells or committed progenitor cells in murine cytokine-mobilized blood. Engraftment was accelerated by transplanting higher numbers of stem cells and not by adding large numbers of committed progenitor cells. In cotransplantation experiments, a severely reduced long-term repopulating ability (LTRA) of mobilized blood cells in comparison to bone marrow cells was observed. We conclude that stem cells and not committed progenitor cells mediate the early phase of engraftment after blood cell transplantation and that the rate of engraftment is determined by the absolute number of stem cells in the graft.
MATERIALS AND METHODS
Stem Cell Mobilization.
Male BALB/c donor mice (Broekman B.V., Someren, The Netherlands), ranging from 8 to 12 weeks of age, were treated with cyclophosphamide at 400 mg/kg i.p. on day 0 and 5 μg of human granulocyte colony-stimulating factor (Amgen Biologicals) per mouse daily s.c. on days 1–5. On day 5, mice were killed by CO2 asphyxia. Blood was harvested by cardiac puncture and collected in heparin-containing tubes. Steady-state bone marrow cells were harvested from the femurs of untreated animals. The protocol was approved by the institutional committee on animal experiments.
Purification of Subpopulations of Progenitor Cells.
All washing, incubation, and purification procedures were done in RPMI 1640 medium supplemented with 2% fetal bovine serum, penicillin (500 μg/ml), and streptomycin (250 μg/ml) (GIBCO/BRL). Low-density cells (Ficoll/Isopaque; 1.077 g/cm3), derived from mobilized blood or steady-state bone marrow, were labeled with mAb 15-1.1 (Rat IgG2b), binding to cells from myelo-monocytic lineages (Lin) (17). Cells were washed and incubated with fluorescein isothiocyanate-conjugated wheat germ agglutinin (WGA, 0.2 μg/ml, Vector Laboratories) and phycoerythrin-conjugated goat anti-Rat IgG (Caltag, South San Francisco, CA). WGA+/Lin− cells (representing 18 ± 6% and 5 ± 2% in blood and bone marrow, respectively) were sorted by using a FACStar flow cytometer (Becton Dickinson) equipped with a 5-W argon laser tuned at 488 nm (0.2 W). Sorted WGA+/Lin− cells were stained with rhodamine-123 (Rho, Molecular Probes) at a concentration of 0.1 μg/ml (20 min, 37°C), washed twice (20°C), and then incubated in Rho-free medium (20 min, 37°C). Rho fluorescence was measured by using excitation and emission wave lengths of 514 and 580 nm, respectively (17). In some experiments, sorted WGA+/Lin−/Rho− cells were subjected to a second Rho staining procedure with the use of verapamil (VP) (17). After restaining with Rho (0.1 μg/ml, 20 min, 37°C) and washing twice (4°C), the cells were incubated in medium without Rho (20 min, 37°C) in the presence of VP (10 μM). Distinct populations of Rho(VP)+ and Rho(VP)− cells could be identified.
Characterization of Committed Progenitor Cells.
A total of 200–750 WGA+/Lin−/Rho− or WGA+/Lin−/Rho+/++ sorted cells from blood or bone marrow were cultured in 35-mm tissue culture dishes in Iscove’s modified Dulbecco’s medium, containing 30% fetal calf serum, 1% deionized BSA, 2 × 10−5 M 2-mercaptoethanol, human transferrin (0.47 g/liter) saturated with FeCl3⋅H2O and 1.1% methylcellulose in the presence of hematopoietic growth factors, i.e., murine granulocyte–macrophage colony-stimulating factor (10 ng/ml) and murine interleukin 3 (25 ng/ml), both provided by I. Lindley, Sandoz, Basel, Switzerland; human granulocyte colony-stimulating factor (10 ng/ml) and human erythropoietin (2 units/ml; Organon Technica, Turnhout, Belgium). Colonies were counted on day 12 of culture.
Characterization of Hematopoietic Stem Cells.
BALB/c recipient mice (8–12 weeks of age) were given total body irradiation of 8.5 Gy divided in two equal parts in posterior–anterior and anterior–posterior position, at a dose of 4 Gy/min with a Philips SL 75-5/6 mV linear accelerator (Philips Medical Systems, Best, The Netherlands). All control mice (n = 21), not transplanted with donor cells, died between 12 and 18 days after irradiation. The presence of donor-derived hematopoietic stem cells in a graft was assessed by recipient mice survival for at least 4 weeks after irradiation and transplantation. Recipient mice were housed in laminar flow cabins for the duration of the experiment and were fed commercially available rodent chow. From 1 week before to 4 weeks after total body irradiation, the drinking fluid was supplemented with ciprofloxacin (85 mg/ml) and polymyxin B (70 mg/ml).
Early Phase of Engraftment.
Recipient mice were lethally irradiated (8.5 Gy) and transplanted with purified or unseparated donor-derived blood or bone marrow grafts. After transplantation, blood was obtained by tail bleeding every 3–4 days until day 30. White blood cells, red blood cells, and platelets were counted on a Sysmex F800 counter (TOA Medical Electronics Co., Kobe, Japan).
Late Phase of Engraftment.
Because the recovery of autologous stem cells in our transplantation model for at least 6 months after the lethal irradiation is very low, LTRA was assessed by long-term survival and long-term bone marrow function, i.e., the level of circulating mature blood cells (17). Also, a direct comparison of the short-term repopulating ability (STRA) and LTRA of bone marrow and mobilized blood cells was investigated in a cotransplantation assay. Female recipient mice were cotransplanted with bone-marrow-derived cells from a male donor and blood-derived cells from a female donor or with bone-marrow-derived cells from a female donor and blood-derived cells from a male donor. The bone marrow grafts contained 4 × 104 unseparated cells and the blood grafts contained 15 × 104 unseparated cells. From 3 to 16 weeks after transplantation, the percentage male cells was determined in blood obtained by tail bleeding by using fluorescence in situ hybridization with the Y chromosome-specific probe M34 as described (17). Blinded samples were scored by using a Leitz Diaplan microscope by two observers, each examining 200 nuclei.
RESULTS
Identification of Purified Populations of Stem Cells and Committed Progenitor Cells in Cytokine-Mobilized Blood.
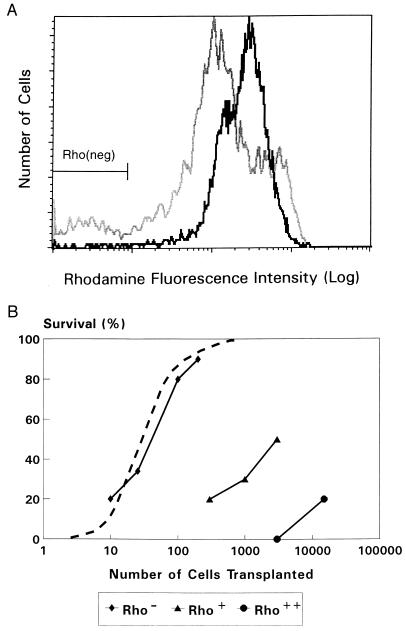
Stem cell mobilization was induced by treating donor mice with cyclophosphamide and granulocyte colony-stimulating factor. To purify cell fractions containing either stem cells or committed progenitor cells, WGA+/Lin− cells were sorted and subjected to a modified Rho staining procedure resulting in distinct populations of Rho++(bright), Rho+(dull), and Rho−(negative) cells (Fig. 1A). In comparison with steady-state bone marrow, WGA+/Lin− cells derived from mobilized blood contained more Rho++ cells and fewer Rho+ and Rho− cells. Committed progenitor cells, defined by their in vitro responsiveness to a combination of hematopoietic growth factors, resided in the Rho+/++ fractions of WGA+/Lin− cells for >99% (Table 1). After transplantation into lethally irradiated recipients, these committed progenitor cell populations had a poor radioprotective capacity because 50% survival required transplantation of 3,000 Rho+ cells or >20,000 Rho++ cells (Fig. 1B). In contrast, 50% survival was observed after transplantation of only 50 Rho− cells. These data indicate that in vitro colony-forming cells (committed progenitor cells) and in vivo radioprotective stem cells were highly separated in the Rho+/++ and Rho− fractions. In addition, radioprotection after blood cell transplantation appears to be mediated by the same WGA+/Lin−/Rho− cell population that has been found to mediate radioprotection and LTRA after bone marrow transplantation (17).
Figure 1.
(A) Rho fluorescence histograms of sorted WGA+/Lin− cells derived from mobilized blood (solid line) or steady-state bone marrow (shaded line). The Rho− cell fraction contained 0.9 ± 0.3% and 6.5 ± 1.0% of all WGA+/Lin− cells from blood and bone marrow, respectively. (B) Recipient mice survival for at least 4 weeks after lethal irradiation (8.5 Gy) and transplantation of cells from the Rho−, Rho+, and Rho++ fractions of WGA+/Lin− cells derived from mobilized blood (solid lines). In three different experiments a total of 18–28 mice was transplanted for each cell dose. The survival of mice transplanted with steady-state bone-marrow-derived WGA+/Lin−/Rho− cells (dashed line) is derived from previous studies (17).
Table 1.
CFCs in the Rho− and Rho+/++ fractions of WGA+/Lin− cells derived from mobilized blood or steady-state bone marrow
| Mobilized blood
|
Bone marrow
|
|||
|---|---|---|---|---|
| Rho− | Rho+/++ | Rho− | Rho+/++ | |
| CFC/750 sorted cells, no. | 3 ± 4 | 25 ± 18 | 1 ± 1 | 36 ± 20 |
| Graft composition | ||||
| Cells, no. | 74 ± 18 | 6,600 ± 2,200 | 67 ± 7 | 910 ± 110 |
| CFCs, no. | 0.3 ± 0.3 | 260 ± 170 | 0.1 ± 0.1 | 47 ± 20 |
Absolute numbers in the grafts represent the analysis of a blood cell graft of 5 × 104 cells and a bone marrow graft of 2 × 104 cells, i.e., the minimal graft size that was required for 90% survival of transplanted lethally irradiated recipients. The absolute numbers of CFCs in unseparated blood and bone marrow grafts of similar size were 211 ± 45 and 40 ± 14 cells, respectively. Results are expressed as the mean ± SD of at least three experiments involving 30 mice.
Early Phase of Engraftment.
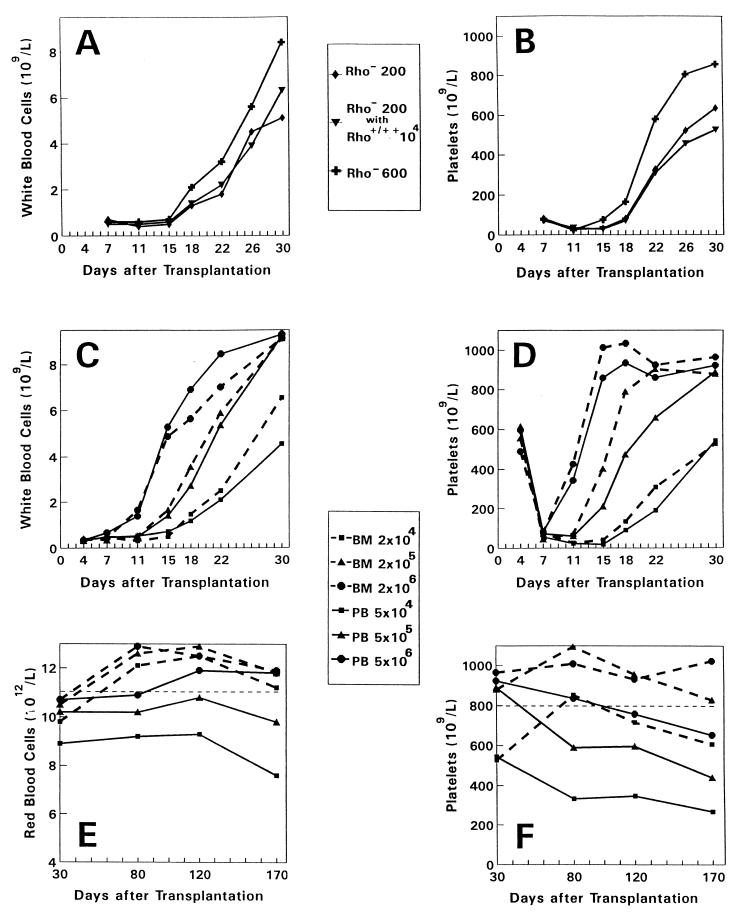
To investigate the role of committed progenitor cells in the early phase of engraftment, hematologic reconstitution rates were determined in recipients of purified Rho− stem cells only or in recipients transplanted with a combination of purified Rho− stem cells and an excess number of Rho+/++ cells (Fig. 2 A and B). Reconstitution of white blood cells, red blood cells, and platelets was not influenced by adding a 50-fold excess of Rho+/++ cells (104 Rho+/++ cells containing ±390 colony-forming cells (CFCs) per mouse (see Table 1), i.e., ±1.5 × 104 CFCs per kg (body weight). In contrast, hematologic reconstitution was significantly accelerated by transplanting 3-fold more Rho− cells (±2 CFCs per mouse, see Table 1). Groups of mice were then transplanted with increasing cell numbers of unseparated blood (5 × 104, 5 × 105, or 5 × 106 cells) or bone marrow grafts (2 × 104, 2 × 105, or 2 × 106 cells) (Fig. 2 C and D). The respective blood and bone marrow grafts contained equal numbers of Rho− stem cells but the blood cell grafts contained 7-fold more Rho+/++ cells and 5-fold more CFCs (Table 1). The rate of reconstitution was identical for recipients transplanted with blood or bone marrow cells at all three cell doses studied. Thus, the rate of engraftment appeared to be determined by the absolute numbers of Rho− stem cells transplanted irrespective of their origin from blood or bone marrow. Furthermore, committed progenitor cells did not influence the kinetics of the early phase of engraftment.
Figure 2.
Hematologic reconstitution and long-term graft function after transplantation of purified or unseparated mobilized peripheral blood (PB) or steady-state bone marrow (BM) grafts. (A and B) Groups of 10 mice were transplanted with purified PB-derived WGA+/Lin−/Rho− cells only (200 or 600 cells per mouse) or in combination with an excess number of Rho+/++ cells (10,000 cells per mouse). (C–F) Groups of 20 mice were transplanted with unseparated BM-derived (2 × 104, 2 × 105, or 2 × 106 cells; dashed lines) or PB-derived (5 × 104, 5 × 105, or 5 × 106 cells; solid lines) grafts. The respective BM and PB grafts contained equal numbers of Rho− cells (±70, ±700, and ±7,000, respectively), and the PB grafts contained 7-fold more Rho+/++ cells and 5-fold more committed progenitor cells (see Table 1). By using a two-tailed t test for paired samples, a significant (P < 0.05) difference was observed for the means of platelet and white blood cell counts on days 15, 18, 22, 26, and 30 between the groups transplanted with 200 or 600 Rho− cells. No difference was observed for platelet (P = 0.25) and white blood cell counts (P = 0.45) between the groups transplanted with 200 Rho− cells only or 200 Rho− cells in combination with 10,000 Rho+/++ cells.
Late Phase of Engraftment.
Previously, we demonstrated that the bone-marrow-derived WGA+/Lin−/Rho− stem cell population can be further separated by using a second Rho staining procedure using the P-glycoprotein-blocker VP (17, 18). In those experiments, most of the bone marrow-derived WGA+/Lin−/Rho− stem cells became Rho-positive [Rho(VP)+] and exhibited predominantly STRA. A minority of the cells (15 ± 6%) remained Rho-negative [Rho(VP)−] and exhibited predominantly LTRA (17). In the present study, we applied an identical second Rho staining with VP to investigate the blood-derived WGA+/Lin−/Rho− cell population. The percentage of Rho(VP)− cells was only 1.8 ± 0.5%, suggesting that the frequency of cells with LTRA is approximately 8-fold reduced in mobilized blood. Because the recovery of autologous stem cells in our transplantation model for at least 6 months after the lethal irradiation is very low (17), LTRA was also assessed by long-term bone marrow function and long-term survival. Between 1 and 6 months after transplantation, we observed reduced red blood cell and platelet counts (Fig. 2 E and F) in mice transplanted with blood-derived cells, supporting the relatively reduced LTRA of blood-derived stem cells. The survival percentage of these mice at 6 months after transplantation was 67%, 88%, and 85% for mice transplanted with increasing bone marrow-derived grafts and 30%, 68%, and 75% for recipients of increasing blood-derived grafts. To directly compare the STRA and the LTRA of bone marrow-derived and blood-derived hematopoietic stem cells, we cotransplanted sex-mismatched bone marrow and mobilized blood cells in individual recipient mice (Fig. 3). From 3 to 16 weeks after transplantation, a gradual decrease in white blood cells derived from the blood graft was observed concommitant with an increase in white blood cells derived from the bone marrow graft. To exclude lineage-specific chimerism, four mice from each group were sacrificed on day 120 and blood was obtained by cardiac puncture. Granulocytes (Gr-1+), T lymphocytes (Thy-1+), and B lymphocytes (B220+) were separately sorted from each individual mouse and hybridized with the Y chromosome-specific probe. The percentage male cells (mean of four mice) for the three lineages was 73%, 65%, and 66% in group A and 26%, 20%, and 23% in group B, showing that chimerism was multilineage.
Figure 3.
Peripheral blood chimerism of individual mice at various times after cotransplantation of bone-marrow- and blood-derived cells. Female recipient mice were transplanted with bone-marrow-derived cells from a male donor and blood-derived cells from a female donor (group A, dashed lines) or with bone-marrow-derived cells from a female donor and blood-derived cells from a male donor (group B, solid lines). Each line represents the analysis of an individual animal. The mean percentage of male cells increased from 41 to 75% in group A and decreased from 65 to 24% in group B. Because the blood graft (15 × 104 unseparated cells) and bone marrow graft (4 × 104 unseparated cells) contained WGA+/Lin−/Rho− stem cells at a ratio of 3:2 (see Table 1), the results indicate an equal STRA of blood- and bone-marrow-derived stem cells at day 20, but a ±5-fold reduced LTRA of blood-derived stem cells at day 110.
Thus, LTRA of cytokine-mobilized blood cells appeared to be reduced in comparison with bone marrow according to the following several lines of evidence: (i) the frequency of WGA+/Lin−/Rho−/Rho(VP)− cells was 8-fold lower, (ii) peripheral blood cell counts were reduced at 16 weeks after transplantation, (iii) late mortality was observed after blood cell transplantation, and (iv) cytokine-mobilized blood cells could not compete for LTRA with bone marrow cells in a cotransplantation assay.
DISCUSSION
In the present study, we have used a cell purification method based on Rho uptake and efflux properties to isolate subpopulations of hematopoietic progenitor cells in murine cytokine-mobilized blood. We identified a Rho+/++ cell population containing 99% of the relatively mature committed progenitor cells and a Rho− cell population containing immature stem cells. Engraftment after transplantation was mediated by the Rho− cells only and the time to engraftment was determined by the absolute number of these Rho− cells in the graft.
Several studies have focused on the heterogeneity of hematopoietic progenitor cells in murine bone marrow (4–7, 9, 17, 19–21). These studies showed that two populations can be discriminated, i.e., relatively mature committed progenitors with in vitro colony-forming ability and primitive hematopoietic stem cells with long-term (>4 months) in vivo bone marrow repopulating ability (LTRA). It was concluded that short-term repopulation (STRA) is mediated by committed progenitor cells because the cell populations mediating STRA are relatively enriched for committed progenitor cells (4–9). Herein we show that in mobilized blood, the committed progenitor cell population can be completely separated from the cells with STRA. Also in bone marrow, correlative engraftment data indicate a similar distinction between committed progenitors and cells with STRA. Because many studies have described the distinction between cells mediating STRA and LTRA (4–7, 9, 17, 19–21), we can now distinguish three populations of hematopoietic cells, (i) committed progenitor cells, (ii) stem cells with STRA, and (iii) stem cells with LTRA. The cell population with STRA can best be classified as a stem cell population. We show herein that these cells, derived from steady-state bone marrow or mobilized blood, are in vitro not responsive to a combination of interleukin 3/murine granulocyte–macrophage colony-stimulating factor/granulocyte colony-stimulating factor. It has been shown that they are characterized by the Rho−/Rho(VP)+ phenotype (17) or the Thy-1.1loLin−Sca-1+ phenotype (22) and by in vivo lymphomyeloid differentiation and extensive proliferation potential (17, 22, 23). The main difference between the two subsets of stem cells is the preference for, respectively, early or late bone marrow repopulation after transplantation, which may relate to differences in cell cycle status.
We observed that the engraftment kinetics in the first weeks after transplantation are determined by the absolute number of Rho− stem cells in the graft. In transplantations with progenitor cell numbers comparable to human transplantations, we show that a 3-fold higher number of purified Rho− stem cells in the graft results in enhancement of engraftment with 2–3 days to day 15 after transplantation. In transplantations with unseparated blood and marrow grafts containing a 10- or 100-fold higher cell number, engraftment was further enhanced to day 7. Still, the engraftment kinetics are related to the number of Rho− stem cells in the graft rather than to the number of Rho+/++ committed progenitors. These results indicate that stem cells are producing mature blood cells as early as 7 days after transplantation. In transplantations with a low number of stem cells, the production rate of mature cells may initially be insufficient to result in detectable levels in the peripheral blood. Then, engraftment is delayed until the stem cell population has made sufficient self-renewal cell divisions. Our results also suggest that the earlier engraftment observed in human blood cell transplantation in comparison to bone marrow transplantation (10–12, 16) is mediated by increased numbers of STRA stem cells in blood cell grafts rather than by increased numbers of committed progenitor cells.
Our results argue against a role for committed progenitor cells in engraftment after bone marrow or blood cell transplantation. A limited role for committed progenitor cells in the early phase of hematopoietic reconstitution after bone marrow transplantation has been suggested in both mice (22) and humans (24). Committed progenitors may be unable to home to the bone marrow after intravenous injection in the transplantation procedure. Alternatively, these cells may have a proliferative capacity that is too low to contribute significantly to mature blood cell production after transplantation. Then, the transplantation of very high (>100-fold expanded) numbers of committed progenitors might result in significant mature blood cell production in the first days after transplantation. Currently, these high numbers are not obtained with ex vivo expansion procedures (8, 13, 14, 16).
In cotransplantation experiments, a reduced LTRA of mobilized blood cells is observed in comparison to bone marrow cells. We have shown that within the bone-marrow-derived Rho− stem cell population, cells with STRA [Rho−/Rho(VP)+] and cells with LTRA [Rho−/Rho(VP)−] can be discriminated (17). Herein, identical subpopulations are identified in the Rho− stem cell population derived from mobilized blood. The reduction in LTRA of mobilized blood is accompanied by a similar reduction in the frequency of Rho−/Rho(VP)− cells. This suggests that LTRA and STRA mediating stem cell subsets can be similarly identified in steady-state bone marrow and in mobilized blood by using our purification method that is based on functional Rho uptake and efflux properties. This is in contrast to other stem cell characteristics such as the membrane markers c-kit and Mac-1 that are differentially expressed in the LTRA-mediating stem cells of steady-state and post-5-fluorouracil bone marrow (25). Our results also suggest that quantitative differences in stem cell subsets rather than qualitative differences in the stem cells account for the reduced LTRA of mobilized blood cells.
In conclusion, we showed that three separate populations of hematopoietic progenitor cells can be distinguished, i.e., committed progenitor cells, stem cells with STRA, and stem cells with LTRA. The committed progenitor cell population has no role in the early phase of engraftment after murine blood cell transplantation. Engraftment could only be accelerated by transplanting higher numbers of stem cells. Therefore, our results suggest that the stem cell population rather than the committed progenitor population is to be expanded ex vivo to accelerate hematologic reconstitution after transplantation.
ABBREVIATIONS
- LTRA
long-term repopulating ability
- STRA
short-term repopulating ability
- Rho
rhodamine
- VP
verapamil
- CFC
colony-forming cell
- WGA
wheat germ agglutinin
References
- 1.Visser J W M, Van Bekkum D W. Exp Hematol. 1990;18:248–256. [PubMed] [Google Scholar]
- 2.Uchida N, Fleming W H, Alpern E P, Weissman I L. Curr Opin Immunol. 1993;5:177–184. doi: 10.1016/0952-7915(93)90002-a. [DOI] [PubMed] [Google Scholar]
- 3.Armitage J O. N Engl J Med. 1994;330:827–838. doi: 10.1056/NEJM199403243301206. [DOI] [PubMed] [Google Scholar]
- 4.Jones R J, Celano P, Sharkis S J, Sensenbrenner L L. Blood. 1989;73:397–401. [PubMed] [Google Scholar]
- 5.Jones R J, Wagner J E, Celano P, Zicha M S, Sharkis S J. Nature (London) 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 6.Li C L, Johnson G R. J Exp Med. 1992;175:1443–1447. doi: 10.1084/jem.175.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ploemacher R E, Van der Loo J C M, Van Beurden C A J, Baert M R M. Leukemia. 1993;7:120–130. [PubMed] [Google Scholar]
- 8.Muench M O, Firpo M T, Moore M A S. Blood. 1993;81:3463–3473. [PubMed] [Google Scholar]
- 9.Trevisan M, Iscove N N. J Exp Med. 1995;181:93–103. doi: 10.1084/jem.181.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianni A M, Siena S, Bregni M, Tarella C, Stern A C, Pileri A, Bonadonna G. Lancet. 1989;ii:580–585. doi: 10.1016/s0140-6736(89)90711-3. [DOI] [PubMed] [Google Scholar]
- 11.Siena S, Bregni M, Brando B, Ravagnani F, Bonadona G, Gianni A M. Blood. 1989;74:1905–1914. [PubMed] [Google Scholar]
- 12.Sheridan W P, Begley C G, Juttner C A, Szer J, To L B, Maher D, McGrath K M, Morstyn G, Fox R M. Lancet. 1992;339:640–644. doi: 10.1016/0140-6736(92)90795-5. [DOI] [PubMed] [Google Scholar]
- 13.Iscove N N, Shaw A R, Keller G. J Immunol. 1989;142:2332–2337. [PubMed] [Google Scholar]
- 14.Haylock D N, To L B, Dowse T L, Juttner C A, Simmons P J. Blood. 1992;80:1405–1412. [PubMed] [Google Scholar]
- 15.Juttner C A, Fibbe W E, Nemunaitis J, Kanz L, Gianni A M. Bone Marrow Transplant. 1994;14:689–693. [PubMed] [Google Scholar]
- 16.Brugger W, Heimfeld S, Berenson R J, Mertelsmann R, Kanz L. N Engl J Med. 1995;333:283–287. doi: 10.1056/NEJM199508033330503. [DOI] [PubMed] [Google Scholar]
- 17.Zijlmans J M J M, Visser J W M, Kleiverda K, Kluin Ph M, Willemze R, Fibbe W E. Proc Natl Acad Sci USA. 1995;92:8901–8905. doi: 10.1073/pnas.92.19.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhary P M, Roninson I B. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 19.Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 20.Morrison S J, Weissman I L. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 21.Jordan C T, Lemischka I R. Genes Dev. 1990;4:220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 22.Uchida N, Aguila H L, Fleming W H, Jerabek L, Weissman I L. Blood. 1994;83:3758–3779. [PubMed] [Google Scholar]
- 23.Harrison D E, Zhong R-K. Proc Natl Acad Sci USA. 1992;89:10134–10138. doi: 10.1073/pnas.89.21.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanania E G, Giles R E, Kavanagh J, Ellerson D, Zu Z, et al. Proc Natl Acad Sci USA. 1996;93:15346–15351. doi: 10.1073/pnas.93.26.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randall T D, Weissman I L. Blood. 1997;89:3596–3606. [PubMed] [Google Scholar]