Abstract
The plant endoplasmic reticulum (ER) contains functionally distinct subdomains at which cargo molecules are packed into transport carriers. To study these ER export sites (ERES), we used tobacco (Nicotiana tabacum) leaf epidermis as a model system and tested whether increased cargo dosage leads to their de novo formation. We have followed the subcellular distribution of the known ERES marker based on a yellow fluorescent protein (YFP) fusion of the Sec24 COPII coat component (YFP-Sec24), which, differently from the previously described ERES marker, tobacco Sar1-YFP, is visibly recruited at ERES in both the presence and absence of overexpressed membrane cargo. This allowed us to quantify variation in the ERES number and in the recruitment of Sec24 to ERES upon expression of cargo. We show that increased synthesis of membrane cargo leads to an increase in the number of ERES and induces the recruitment of Sec24 to these ER subdomains. Soluble proteins that are passively secreted were found to leave the ER with no apparent up-regulation of either the ERES number or the COPII marker, showing that bulk flow transport has spare capacity in vivo. However, de novo ERES formation, as well as increased recruitment of Sec24 to ERES, was found to be dependent on the presence of the diacidic ER export motif in the cytosolic domain of the membrane cargo. Our data suggest that the plant ER can adapt to a sudden increase in membrane cargo-stimulated secretory activity by signal-mediated recruitment of COPII machinery onto existing ERES, accompanied by de novo generation of new ERES.
The endoplasmic reticulum (ER) is an essential organelle responsible for the synthesis, assembly, and sorting of secretory proteins. Specialized ER subdomains, termed ER export sites (ERES), are dedicated to the export of proteins to the Golgi apparatus (Tang et al., 2005). In plants, the Golgi apparatus consists of several hundred individual stacks accompanied by closely linked ERES (Hanton et al., 2006). This feature permits the capture of high-resolution time series of individual ER-Golgi pairs.
In mammals and yeast (Saccharomyces cerevisiae), protein transport between the ER and Golgi apparatus is generally thought to be mediated by COPI and COPII coat complexes (Lee et al., 2004; Hanton et al., 2005a; Watson and Stephens, 2005). The Arf1-COPI coat associates with membranes of the Golgi apparatus and is believed to mediate retrograde transport back to the ER (Letourneur et al., 1994); the COPII coat performs the opposite function, assembling on ER membranes to induce the formation of anterograde carriers at ERES (Barlowe, 2003). The COPII coat consists of the GTPase Sar1 (Barlowe and Schekman, 1993; Barlowe et al., 1994; Weissman et al., 2001) plus two heterodimeric complexes: Sec23/24 and Sec13/31 (Matsuoka et al., 2001), which are thought to be recruited sequentially to the ER membrane at ERES (Aridor et al., 2001). Sec23 exhibits Sar1-specific GTPase-activating activity, whereas Sec24 is thought to select cargo and the Sec13/31 complex provides the structural properties to shape the membrane into a vesicle upon assembly (Stagg et al., 2006). Proteins such as p125 and Sec16 have been recognized as being fundamental for the establishment of the identity of ERES in mammalian and yeast cells (Espenshade et al., 1995; Shimoi et al., 2005; Watson et al., 2006). However, proteins with analogous functions have not yet been identified in plants. Plant homologs of components of the COPI and COPII vesicle coats have been identified and some characterization studies have been carried out (d'Enfert et al., 1992; Regad et al., 1993; Bar-Peled and Raikhel, 1997; Takeuchi et al., 1998; Movafeghi et al., 1999; Contreras et al., 2000; Pimpl et al., 2000; Vernoud et al., 2003; daSilva et al., 2004; Yang et al., 2005; Stefano et al., 2006). Although this area of research is currently one of great interest and activity, knowledge of transport in the early secretory pathway of plants still lags behind that from yeast and mammalian systems.
The function and behavior of plant ERES with regard to cargo recruitment is poorly understood. It is possible that cargo proteins are recruited to ERES for packaging and transport to the Golgi, as occurs in mammalian cells (Aridor et al., 2001), or, conversely, that cargo molecules recruit COPII components to ERES to facilitate their export to the Golgi. Studies on coexpression of Sar1-yellow fluorescent protein (YFP) and Golgi membrane markers (ER retention defective 2 [ERD2]-green fluorescent protein [GFP], ST-GFP, GONST1-GFP, and α-1,2-mannosidase-GFP) in tobacco (Nicotiana tabacum) leaf epidermal cells showed increased recruitment of the GTPase to ERES (daSilva et al., 2004). However, overexpression of soluble proteins, which are known to be exported from the ER via a passive bulk flow mechanism (Denecke et al., 1990; Phillipson et al., 2001), did not induce this effect. This suggests that, in plants, the quantity of membrane cargo controls the level of COPII recruitment to ERES (daSilva et al., 2004).
Emerging evidence suggests that maintenance of the identity of plant ERES is a regulated process that involves balanced traffic of materials between the ER and Golgi (daSilva et al., 2004; Yang et al., 2005; Stefano et al., 2006). Disruption of the integrity of retrograde protein transport by either the drug brefeldin A or a dominant negative mutant of Arf1 determines redistribution of fluorescent fusions of the ERES markers Sar1, Sec23, and Sec24 into the cytosol, as well as accumulation of Golgi markers in the ER in tobacco leaf epidermis (daSilva et al., 2004; Stefano et al., 2006). Upon brefeldin A washout, however, Sar1, Sec23, and Sec24 are recruited to the area of newly formed Golgi bodies (daSilva et al., 2004; Stefano et al., 2006). This effect suggests that the plant ER is capable of accommodating reversible changes in COPII assembly at ERES.
Together, these findings lead to the fundamental biological question of how cells respond to sudden synthesis of secretory proteins directed to the Golgi apparatus. Does cargo select COPII coats for membrane recruitment and is this process dependent on ER export signals? Can ERES be differentiated de novo and does the total number of ERES increase with increased cargo synthesis?
To address these important questions, we have used tobacco leaf epidermis and live cell-imaging analyses to investigate the consequences of sudden synthesis of protein cargo destined to the Golgi apparatus, using the Arabidopsis (Arabidopsis thaliana) COPII component Sec24, which associates with ERES in both the presence and absence of overexpressed membrane cargo in leaf tissue from Arabidopsis and tobacco (Matheson et al., 2006; Stefano et al., 2006). Our findings provide evidence for increased recruitment of Sec24 to ERES upon coexpression of the Golgi membrane markers ERD2-GFP or TMcCCASP (Hanton et al., 2005b). Mutagenesis experiments revealed that this effect is strictly dependent on the diacidic ER export signal DXE (Hanton et al., 2005b). In addition, we show that increased volume of secretory membrane cargo correlates to a higher number of ERES units, suggesting cargo-induced de novo differentiation of ERES. The results obtained shed new light on the causal relationship between COPII coats and their cargo.
RESULTS
Overexpression of Sec24 Does Not Affect Movement of Proteins to the Golgi Apparatus
To visualize ERES in tobacco leaf epidermal cells, we used a key component of the COPII coat, Sec24. A YFP fusion (YFP-Sec24) can be used to label ERES in both the presence and absence of overexpressed cargo in leaf epidermis of tobacco and Arabidopsis (Matheson et al., 2006; Stefano et al., 2006) in contrast to tobacco Sar1-YFP, which is only visible at ERES in the presence of overexpressed membrane cargo (daSilva et al., 2004). YFP-Sec24 would thus permit detection of ERES before and after induction of increased cargo biosynthesis.
To use YFP-Sec24 as a neutral marker for ERES, it was first necessary to demonstrate that its expression does not affect constitutive secretion. This was important because it is known that some components of the COPII machinery, such as the Sar1 guanine nucleotide exchange factor Sec12 (d'Enfert et al., 1991; Barlowe and Schekman, 1993; Barlowe et al., 1994), inhibit COPII-mediated ER export when present at increased levels, most likely by titration of Sar1 (d'Enfert et al., 1991; Phillipson et al., 2001; daSilva et al., 2004). Tobacco leaf protoplasts were cotransfected with the secretory marker α-amylase (Phillipson et al., 2001) and a dilution series of YFP-Sec24. As a positive control, we used a dilution series of a fluorescent fusion of Sec12, Sec12-GFP, which was previously shown to inhibit secretion in a similar manner to that of its untagged counterpart (daSilva et al., 2004). We used Sec12 overexpression to inhibit COPII transport rather than an alternative, such as the GTP-restricted mutant of Sar1 [Sar1(H74L); Takeuchi et al., 2000] because a Sec12 overexpression approach provides specific proof of COPII dependence of ER export of cargo. Inhibition of ER export in conditions of overexpression of Sec12 is believed to be the result of titration of Sar1, thus leading to inhibition of COPII vesicle budding that is dependent on Sar1 (d'Enfert et al., 1991; Barlowe and Schekman, 1993; Phillipson et al., 2001; daSilva et al., 2004). On the other hand, overexpression of Sar1(H74L) leads to rapid collapse of Golgi membranes into the ER (Takeuchi et al., 2000; daSilva et al., 2004). This means that the point of arrival of cargo exported from the ER via either the COPII-dependent pathway or other means (Törmäkangas et al., 2001) is disrupted. In these conditions, it is not possible to distinguish whether the ER export of cargo is dependent on or independent of COPII.
In each repetition of the experiment shown in Figure 1, all samples contained a homogeneous suspension of protoplasts from the same pool. This ensured that the data obtained were comparable between samples and that any stress caused to the secretory pathway through the preparation of protoplasts was consistent across all samples.
Figure 1.
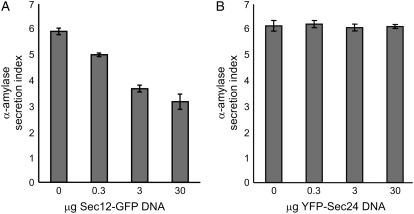
YFP-Sec24 overexpression does not disrupt constitutive ER export. Tobacco leaf protoplasts were transfected with a constant amount of α-amylase DNA, with an increasing concentration of DNA encoding either Sec12-GFP (A) or YFP-Sec24 (B). The α-amylase activities in the extracellular medium and cellular fraction were measured and the secretion index, which represents the ratio between the intracellular and extracellular activities, was calculated as previously described (Phillipson et al., 2001). Error bars represent se for three independent experiments.
We found that secretion was reduced noticeably upon coexpression of low levels of Sec12-GFP (Fig. 1A), corresponding to the findings of daSilva et al. (2004). However, even at high levels of expression of YFP-Sec24, α-amylase secretion was unaffected (Fig. 1B). This indicates that, unlike Sec12-GFP, YFP-Sec24 does not interfere with constitutive secretion and can therefore be used as a neutral marker for ERES. This offers a unique opportunity to monitor the response of the ER and ERES to increased levels of secretory cargo proteins.
The Golgi Marker ERD2-GFP Exits the ER in a COPII-Dependent Manner
Having established that YFP-Sec24 was not interfering with secretion, we next wanted to determine whether its distribution was influenced by the presence of increased availability of cargo destined for the Golgi apparatus. We selected ERD2-GFP, a fluorescent fusion of the Arabidopsis H/KDEL receptor, because it is an established ER-Golgi marker (Boevink et al., 1998; Brandizzi et al., 2002c; Saint-Jore et al., 2002) and has been shown to recruit the GTPase Sar1 to ERES (daSilva et al., 2004). We therefore wanted to test whether, in addition to recruiting the GTPase Sar1, ERD2 can also recruit COPII components such as Sec24.
ERD2 must recycle between the ER and Golgi apparatus to carry out its function and, although its recycling via COPI vesicles is well established (Lee et al., 1993), much less is known about its ER export properties. Because it has been shown that a COPII-independent pathway for ER export exists in plants (Törmäkangas et al., 2001), we first wanted to ensure that the ER export of ERD2 is dependent on COPII machinery. We therefore coexpressed ERD2-GFP with Sec12-YFP (Fig. 2, A–D), using a leaf infiltration protocol based on Agrobacterium-mediated integration into the plant genome (Batoko et al., 2000). An added advantage of this approach was that the fluorescent fusion allowed us to monitor expression levels of Sec12-YFP.
Figure 2.
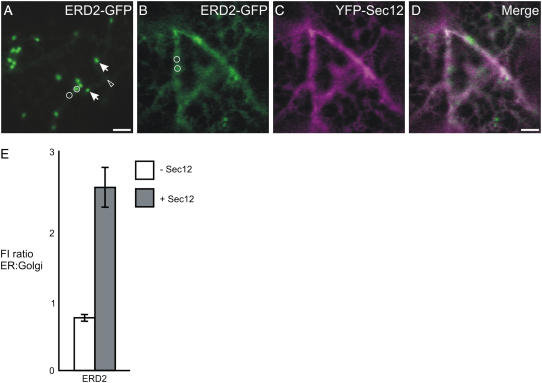
Overexpression of Sec12-YFP inhibits transport of ERD2-GFP to the Golgi apparatus. A, Confocal image of a cell expressing ERD2-GFP, which labels the Golgi apparatus (arrows) and ER (arrowhead). B to D, On coexpression of Sec12-YFP (C), ERD2-GFP is partially redistributed to the ER (B and D). Bars = 5 μm; circles represent the size of the areas used to measure fluorescence intensity in the ER and Golgi. E, Quantification of the ER-localized ERD2-GFP fluorescence relative to that in the Golgi apparatus in the absence (−Sec12) or presence (+Sec12) of Sec12-YFP. A significant increase in the ratio was verified when Sec12-YFP was expressed in comparison with the control. Sample size: 240 Golgi bodies and 240 ER measurements were analyzed for each sample. Error bars represent se of the mean.
Figure 2A shows that ERD2-GFP fluorescence is detected in punctate structures representing Golgi stacks, as well as in the ER network, as previously shown (Boevink et al., 1998). Upon coexpression with Sec12-YFP, most of the punctate fluorescence was lost, accompanied by an increase in ER fluorescence (Fig. 2B). We also quantified the intensity of fluorescence of ERD2-GFP in the ER and in the Golgi apparatus either alone or coexpressed with Sec12-YFP, using fixed parameters of imaging conditions, measurement areas, and levels of saturation of imaging pixels to allow accurate fluorescence intensity measurements in the GFP channel. This approach was feasible because the GFP variant used in this study (Haseloff et al., 1997) has sufficient spectral separation from YFP when excited with the 458-nm laser line (Brandizzi et al., 2002b).
We were able to select cells with similar levels of Sec12-YFP expression during the course of the experiment by comparing levels of saturation of the YFP-imaging pixels and selecting for those cells with similar levels of YFP fluorescence (see also “Materials and Methods”). We then calculated the ratio of the ER fluorescence intensity of ERD2-GFP to its value in the Golgi apparatus and noted a clear Sec12-YFP-induced increase in the ER-to-Golgi fluorescence signal ratio (Fig. 2E). The results show that Sec12 overexpression causes reduced ER export of ERD2-GFP, indicating that this protein is dependent on the COPII machinery for transport to the Golgi apparatus.
Overexpression of COPII-Dependent Membrane Cargo Induces YFP-Sec24 Recruitment to ERES
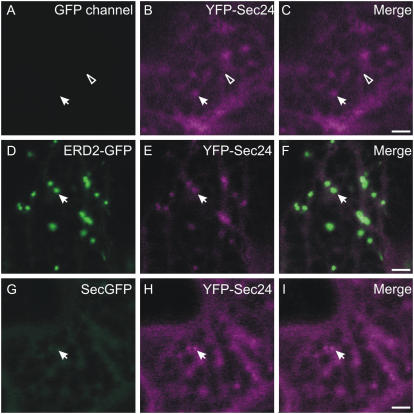
When expressed alone in tobacco leaf epidermal cells, YFP-Sec24 fluorescence was distributed in the cytosol and at punctate structures (Fig. 3, A–C). Upon coexpression with ERD2-GFP, the Sec24-labeled ERES were found in close association with Golgi bodies, although occasional bright dots that do not colocalize with Golgi were also observed (Supplemental Fig. S1) in accordance with previous observations (Matheson et al., 2006; Stefano et al., 2006). Interestingly, YFP-Sec24 cytosolic fluorescence appeared to be reduced when ERD2-GFP was coexpressed in comparison to cells expressing YFP-Sec24 alone (Fig. 3, D–F). We formulated two possible explanations for these observations. First, YFP-Sec24 might behave in a similar manner to Sar1-YFP in response to expression of membrane cargo (daSilva et al., 2004), in which case the fluorescence intensity of YFP-Sec24 at ERES would increase. Second, the total number of ERES per cell might increase, which would also explain the apparently reduced cytosolic fluorescence of YFP-Sec24.
Figure 3.
Coexpression of ERD2-GFP with YFP-Sec24 increases the fluorescence and number of ERES. A to C, YFP-Sec24 labels the cytosol (arrowhead) and punctate structures (ERES, arrow) when expressed alone. Note that there is no signal in the GFP channel, excluding the possibility that colocalization of the signals is due to imaging cross talk (Brandizzi et al., 2002b). Coexpression of ERD2-GFP (D) with YFP-Sec24 (E) results in an apparent increase in the fluorescence intensity of the YFP-Sec24 at ERES (E). F, Merged image of D and E. Coexpression of the soluble marker secGFP (G) with YFP-Sec24 (H) does not appear to affect the number or fluorescence of ERES (I, merge). Bars = 5 μm.
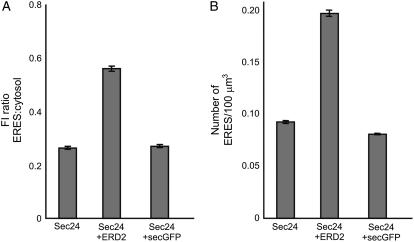
To test whether Sec24 can be recruited from the cytosol to ERES in a membrane cargo-induced fashion, as is the case for Sar1 (daSilva et al., 2004), we aimed to quantify the intensity of fluorescent punctate structures in cells expressing YFP-Sec24 with and without coexpressed ERD2-GFP. We measured the levels of YFP fluorescence intensity at ERES and in the cytosol of cells expressing YFP-Sec24 alone or with ERD2-GFP, and then calculated the ratio of the values of fluorescence intensity (Fig. 4A). Increased recruitment of YFP-Sec24 at ERES would lead to an increase in the ERES-to-cytosol fluorescence ratio. We found that, in cells expressing YFP-Sec24 alone, the average YFP fluorescence ratio was 0.26 (Fig. 4A). We observed that this ratio increased to 0.56 (P = 5.5 × 10−62) in cells coexpressing ERD2-GFP, confirming our initial observations that expression of ERD2-GFP increases the recruitment of YFP-Sec24 to ERES (Fig. 3, A–F).
Figure 4.
Quantification of the ERD2-mediated increase in fluorescence intensity and number of ERES. A, Quantification of the YFP-Sec24 fluorescence intensity at ERES relative to that in the cytosol, shown as a ratio, indicates that in the presence of ERD2-GFP, the fluorescence intensity at ERES increases in comparison to that in cells expressing YFP-Sec24 alone. However, no increase in fluorescence intensity is observed on coexpression of secGFP, a soluble cargo protein. Fluorescence was measured for at least 10 ERES and cytosol areas per cell in 150 cells, giving at least 1,500 ERES and 1,500 cytosol measurements for each combination of markers. B, Number of ERES per 100 μm3 in cells expressing YFP-Sec24 in the presence of ERD2-GFP increases in comparison to cells expressing YFP-Sec24 alone. However, coexpression of the soluble marker secGFP has no effect on the number of ERES per 100 μm3. Error bars represent se of the mean.
We next wanted to test whether soluble cargo would also be capable of recruiting YFP-Sec24 to ERES. Because soluble proteins are thought to leave the ER by passive transport (Phillipson et al., 2001) and fail to recruit Sar1 to ER membranes (daSilva et al., 2004), we postulated that recruitment of YFP-Sec24 to ERES would not increase either. To test this experimentally, we overexpressed a secreted form of GFP (secGFP) as a soluble cargo (Fig. 3, G–I; Batoko et al., 2000). secGFP is known to exit the ER in a COPII-mediated manner (daSilva et al., 2004). To maintain the same conditions of expression for soluble and membrane markers, secGFP was subcloned into the binary vector used for expression of ERD2-GFP. We found that, in conditions of coexpression using stable integration with Agrobacterium, secGFP had no significant effect on the YFP fluorescence ratio (Fig. 4A; ratio = 0.27; P = 0.46). The results confirm that secGFP is not able to recruit COPII components to ERES (Fig. 3, G–I).
The Number of ERES Increases upon Overexpression of ERD2-GFP
To test our second hypothesis, which would involve de novo differentiation of ERES from ER membranes, we aimed to quantify the number of ERES labeled by YFP-Sec24 in cells expressing the ERES marker alone or with ERD2-GFP.
We counted the YFP-Sec24-labeled ERES in 150 tobacco leaf epidermal cells for each combination of markers (Fig. 4B; see also “Materials and Methods”). We found that, in cells expressing YFP-Sec24 alone, there were on average 0.09 ERES per 100 μm3. In the presence of ERD2-GFP, however, we found that the number of ERES per 100 μm3 increased significantly to 0.2 (P = 1.7 × 10−78). In contrast, when we used secGFP as a secretory cargo protein, the number of ERES was almost unchanged with respect to the control (Fig. 4B; ERES number per 100 μm3 = 0.8; P = 0.07). This shows that bulk flow does not induce de novo synthesis of ERES.
These data indicate that the plant ER can respond to an increased necessity to secrete membrane cargo not only by increased recruitment of another COPII component, besides Sar1 (daSilva et al., 2004), to existing ERES, but also by differentiation of new ERES on the ER membranes. The lack of recruitment of YFP-Sec24 and unchanged numbers of ERES in the presence of secGFP not only confirm that soluble proteins can exit the ER by passive transport and do not influence COPII assembly (daSilva et al., 2004), but also that the cellular response observed for YFP-Sec24 in the presence of ERD2-GFP is specific to the concentration of membrane proteins in the ER rather than an unrelated response to the experimental conditions, such as Agrobacterium-mediated plant transformation and factors expressed by the binary vector.
ERES Formation and COPII Recruitment to ERES Are Signal Dependent
We next wanted to investigate the mechanism by which cargo-mediated recruitment of YFP-Sec24 to ERES occurs. To test whether recruitment of Sec24 and de novo ERES differentiation is restricted to coexpression of ERD2-GFP or is a widespread feature of cargo molecules that do not travel via bulk flow, we asked the following question: Is Sec24 recruitment a general feature of membrane cargo or is it dependent on specific ER export signals? An ER export signal for ERD2 has yet to be discovered, but it has been shown in yeast that Sec24 can interact with diacidic motifs in the cytosolic tails of membrane proteins (Votsmeier and Gallwitz, 2001). We have previously shown that diacidic export motifs are functional in plants because mutation of these signals strongly reduced the export of different membrane proteins from the ER (Hanton et al., 2005b).
Of particular use for our present investigation is TMcCCASP, a chimeric type I protein (Hanton et al., 2005b) consisting of a form of GFP carrying a signal peptide for entry into the ER and an N-glycosylation site (Batoko et al., 2000) fused to a 17-amino acid transmembrane domain, the short length of which restricts type I proteins to the ER (Brandizzi et al., 2002a). Adjacent to the transmembrane domain are 78 amino acids of the cytosolic domain of CASP (Hanton et al., 2005b; Renna et al., 2005), containing a functional diacidic ER export motif that is dominant over the properties of the transmembrane domain and permits the protein to travel to the Golgi apparatus (Hanton et al., 2005b).
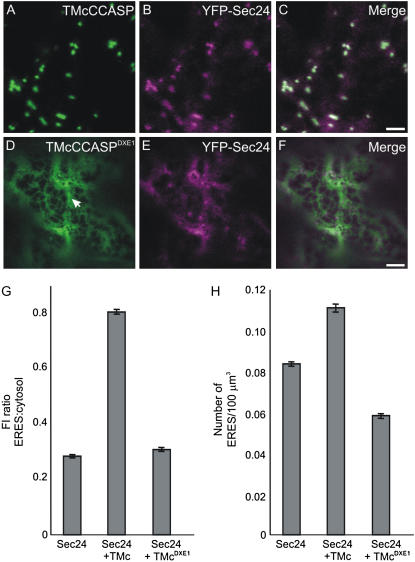
Our data demonstrate that TMcCCASP leaves the ER in a COPII-dependent manner, tested in the same manner as given for ERD2-GFP (Supplemental Fig. S2). We therefore postulated that TMcCCASP would be an ideal candidate to test whether ERES differentiation is signal dependent because the effect of TMcCCASP coexpression can be compared with that of TMcCCASPDXE1, a derivative carrying point mutations that render the DXE motif nonfunctional (Hanton et al., 2005b). Confocal imaging of TMcCCASP and TMcCCASPDXE1 confirmed that the mutation causes redistribution of the fluorescence pattern from Golgi bodies (Fig. 5A) to a pattern typical of the ER (Fig. 5D), including a high proportion of sheets that indicate increased levels of membrane proteins in the ER (Runions et al., 2006). We coexpressed each cargo molecule with YFP-Sec24 to test whether the mutated export motif in TMcCCASPDXE1 would affect the distribution of the ERES marker and quantified the YFP fluorescence intensity at ERES and in the cytosol in cells coexpressing YFP-Sec24 and either TMcCCASP or TMcCCASPDXE1, followed by calculation of the ratio of these values (Fig. 5, A–F). TMcCCASP appears to have a similar effect on the fluorescence distribution of YFP-Sec24 to that of ERD2-GFP (compare Figs. 5G and 4A), showing marked increase in the YFP fluorescence intensity ratio (ratio = 0.74; P = 4.5 × 10−127). Noticeably, this value was significantly lower in the presence of the DXE mutant compared to the wild-type protein (Fig. 5G; ratio = 0.37; P = 0.04), indicating an inability to recruit Sec24 to ERES. We also measured the number of ERES per 100 μm3 in cells from the same samples. Interestingly, whereas TMcCCASP caused an increase in the number of ERES per 100 μm3 (0.12; P = 2.2 × 10−9), expression of the DXE mutant caused a slight, but significant, reduction in ERES number compared with the control (Fig. 5H; number of ERES per 100 μm3 = 0.06; P = 1.0 × 10−32).
Figure 5.
Mutation of a cytosolic diacidic motif prevents recruitment of YFP-Sec24 to ERES. A to C, TMcCCASP (A) predominantly labels the Golgi apparatus. Coexpression with YFP-Sec24 (B) leads to an apparent increase in both YFP fluorescence intensity at ERES and ERES number. C, Merged image of A and B. D to F, Mutation of a diacidic motif in TMcCCASP results in redistribution of the marker to the ER membranes (D, arrow). Coexpression of TMcCCASPDXE1 with YFP-Sec24 (E) does not noticeably affect YFP fluorescence intensity or ERES number. F, Merged image of D and E. Bars = 5 μm. G, Quantification of the YFP fluorescence intensity at ERES relative to that in the cytosol, shown as a ratio, indicates that in the presence of TMcCCASP (TMc), the fluorescence intensity at ERES increases relative to that of YFP-Sec24 alone. In comparison, no obvious increase in fluorescence intensity is observed on coexpression of TMcCCASPDXE1 (TMcDXE1). Fluorescence was measured for at least 10 ERES and cytosol areas per cell, giving an average of 1,500 ERES for each combination of markers. H, Number of ERES per 100 μm3 (sample size = 150 cells) expressing YFP-Sec24 in the presence of TMcCCASP increases in comparison to cells expressing YFP-Sec24 alone. However, coexpression of the export-incompetent TMcCCASPDXE1 slightly reduces the number of ERES per 100 μm3. Error bars represent se of the mean.
These results indicate the ERES differentiation and COPII recruitment are not due to a general increase of membrane proteins in the ER. Despite considerable accumulation of TMcCCASPDXE1 in the ER, no induced ERES differentiation was observed (Fig. 5, D–F). Moreover, the data indicate that overexpression of mutant proteins carrying a nonfunctional export signal interferes with the differentiation of ERES. Because the presence of the diacidic signal is necessary for de novo differentiation of ERES and YFP-Sec24 recruitment to ERES, we postulate that it is the exposure of ER export signals on the ER surface that leads to COPII recruitment and ERES differentiation.
DISCUSSION
This study shows that in tobacco leaf epidermal cells, de novo differentiation of ERES and increased recruitment of COPII proteins onto existing ERES contribute to accommodate an influx of secretory membrane cargo in the ER. Although it was previously established that the COPII component Sec24 can interact with ER export signals (Votsmeier and Gallwitz, 2001), it is not clear which event occurs first: Sec24 recruitment to the membrane or cargo binding. Based on our present results, it appears that an increased concentration of cargo proteins with cytosolic export signals can recruit Sec24 to ERES in a similar manner to the GTPase Sar1 (daSilva et al., 2004). This leads to increased numbers of ERES and strongly suggests that ERES can form de novo. This model takes into account the data from mammals and yeast showing that Sec24 is capable of interaction with a variety of cargo molecules (Aridor et al., 1998; Roberg et al., 1999; Peng et al., 2000; Shimoni et al., 2000; Votsmeier and Gallwitz, 2001; Miller et al., 2003; Mossessova et al., 2003; Wang et al., 2004), but also provides a novel interpretation. Instead of postulating that COPII coats are constitutively recruited to ERES, regardless of the presence of cargo, followed by the process of cargo selection, our model argues that export structures are formed when the presence of cargo determines a requirement for transport.
ERES Biogenesis Is Cargo Induced
The fusion protein YFP-Sec24 was instrumental in allowing us to follow the behavior of COPII at ERES in this study because its association with ERES in the presence and absence of cargo proteins allowed us to quantify the effects of coexpression of soluble or membrane proteins on ERES, in contrast with the all-or-nothing behavior of tobacco Sar1 (daSilva et al., 2004). Alternative fluorescent ERES markers in plants are Sec23 (Stefano et al., 2006) and Sec13 (Yang et al., 2005); however, neither of these markers appeared suitable for our purpose. Sec23 overexpression might affect secretion due to its GTPase-activating protein activity (Yoshihisa et al., 1993; Antonny et al., 2001), whereas, although Sec13 labels cytosolic structures in Bright-Yellow 2 cells in the absence of Golgi cargo, these loci have not yet been equated unequivocally with ERES (Yang et al., 2005). We therefore chose to use Sec24, which is an established ERES marker in plants and other systems (Stephens et al., 2000; Forster et al., 2006; Matheson et al., 2006; Stefano et al., 2006). Moreover, we have demonstrated that Sec24 overexpression does not affect ER export (Fig. 1), confirming that it is a neutral marker.
We found that, in addition to quantitative redistribution of YFP-Sec24 from the cytosol to ERES, the number of ERES increased in the presence of cargo molecules such as ERD2-GFP and TMcCCASP (Figs. 4 and 5). We observed variation in the number of ERES on coexpression of different membrane cargo proteins in that ERD2-GFP appears to be more able to stimulate the formation of new ERES than TMcCCASP (compare Figs. 4B and 5H).
It may be the case that the transport properties of the markers influence the formation of ERES. ERD2-GFP is a well-known receptor that retrieves escaped soluble proteins to the ER and cycles continuously between the ER and Golgi apparatus (Lee et al., 1993; Brandizzi et al., 2002c) in a COPII-dependent manner (Fig. 2). Although mutagenesis has revealed a number of important residues responsible for the correct retrieval of this receptor (Townsley et al., 1993), an ER export signal remains to be established. In contrast, the synthetic reporter TMcCCASP (Hanton et al., 2005b) may not recycle as actively as ERD2 and carries the well-characterized ER export signal DXE (Nishimura and Balch, 1997; Sevier et al., 2000; Votsmeier and Gallwitz, 2001; Epping and Moye-Rowley, 2002; Hanton et al., 2005b). Active recycling of ERD2 to the ER means that every de novo synthesized molecule will complete several rounds of ER export steps and could explain an increased tendency to stimulate ERES formation compared to TMcCCASP (Figs. 4B and 5H).
Together, our data clearly show that increased expression of membrane proteins is paralleled by de novo generation of additional ERES units. This is a phenomenon that has yet to be reported in other cellular systems.
ERES Formation Is Signal Mediated
The principle that the display of Golgi and post-Golgi cargo molecules on the ER surface can result in an increase in the amount of COPII components at ERES appears to be conserved across kingdoms (Guo and Linstedt, 2006). We have demonstrated a further level of complexity by showing that the level of Sec24 recruitment to ERES and ERES biogenesis is dependent on the nature of the membrane protein cargo (Figs. 4 and 5). Furthermore, soluble cargo that is unable to be recognized directly by Sec24 failed to exhibit an effect (Figs. 3 and 4). This led us to postulate that Sec24 recruitment and ERES formation is a signal-mediated process. Experimental proof for this was obtained through mutation of the DXE export motif, which disrupted these two processes. TMcCCASPDXE1 is ER export incompetent (Hanton et al., 2005b) and fails to recruit YFP-Sec24 to existing ERES in the manner of the wild-type TMcCCASP (Fig. 5). This indicates a crucial role for diacidic export motifs in the assembly of COPII coats at ERES. The lack of recruitment of YFP-Sec24 to ERES in the presence of the bulk flow marker secGFP is consistent with these data.
We detected a small, but significant, reduction in the number of ERES when YFP-Sec24 was coexpressed with the ER export-incompetent molecule, TMcCCASPDXE1 (Fig. 5H). This effect was not general to proteins lacking an active ER export motif because it was not visible when secGFP was used as a cargo (Fig. 4B). We propose that overexpression of TMcCCASPDXE1 could lead to an accumulation of export-incompetent proteins at the ER membrane, preventing productive interactions of export-competent proteins with YFP-Sec24 that would normally generate ERES formation. The very slight increase in fluorescence intensity of YFP-Sec24 on coexpression of TMcCCASPDXE1 (Fig. 5G) can be explained by the hypothesis that other cargo proteins can only exit the ER through existing ERES, leading to increased recruitment of COPII components at these areas. This theory is supported by the observation that expression of TMcCCASPDXE1 did not noticeably disrupt the transport of the Golgi marker sialyltransferase-monomeric red fluorescence protein (Hanton et al., 2005b), although the mutant protein itself is unable to exit the ER.
ERES and the Golgi Apparatus in Plants: Which Came First?
Our results have implications for the mechanisms underlying the formation of Golgi bodies in plant cells. Given the close association of ERES and Golgi bodies in plant cells (Matheson et al., 2006), logical speculation is that the number of Golgi bodies may increase along with that of ERES. Therefore, formation of ERES could correlate with de novo formation of Golgi bodies. In this view, COPII elements would cooperate to form a domain of the ER that generates a pre-Golgi element, which would then mature into a cis-cisterna. The cis-Golgi elements would progress in further maturation and then dissipate into non-Golgi elements along the secretory pathway. This model views the Golgi apparatus not as a stable entity, but as a transient structure subject to continuous maturation.
Furthermore, the stronger effect of the ERD2 fusion in the de novo formation of ERES (Fig. 4) could indicate that Golgi and ERES may be interlinked. Recycling of components from the Golgi apparatus is a crucial process and not only involves ER chaperones, but also ER export machinery that continuously leaves the ER. Therefore, it cannot be ruled out that ERES formation is directly stimulated by retrograde, as well as anterograde, transport. Identification of putative ER export signals in ERD2 may provide further tools to differentiate between the possible ways in which ERES formation can be regulated in vivo.
MATERIALS AND METHODS
Fluorescent Proteins and Molecular Cloning
The fluorescent proteins used in this study are encoded by previously published constructs based on fusions with either mGFP5 (Haseloff et al., 1997) or enhanced YFP (CLONTECH). The spectral properties of mGFP5 allow efficient spectral separation from enhanced YFP (Haseloff et al., 1997; Brandizzi et al., 2002c). The binary vector pVKH18En6 (Batoko et al., 2000) is the binary vector used for Agrobacterium tumefaciens-mediated transformation in this study. The GFP-Golgi markers used were the Arabidopsis (Arabidopsis thaliana) H/KDEL receptor ERD2 (Lee et al., 1993; Boevink et al., 1998; Brandizzi et al., 2002c; Saint-Jore et al., 2002) and the synthetic secretory membrane cargo protein TMcCCASP (Hanton et al., 2005b). A mutant of TMcCCASP (TMcCCASPDXE1) that is impaired in ER to Golgi transport was also utilized (Hanton et al., 2005b). We used secGFP (Batoko et al., 2000) as a soluble marker protein. Arabidopsis Sec12 (Phillipson et al., 2001) was N-terminally fused to YFP (daSilva et al., 2004), whereas Arabidopsis Sec24 was C-terminally fused to YFP (Stefano et al., 2006).
Plant Material, α-Amylase Assay, and Western Blot
Four-week-old tobacco (Nicotiana tabacum cv Petit Havana) greenhouse plants grown at 25°C were used for A. tumefaciens (strain GV3101)-mediated stable DNA integration (Batoko et al., 2000). The bacterial optical density (OD600) used for plant transformation was 0.05 for YFP-Sec24 and Sec12-YFP, 0.2 for all cargo proteins.
For transient expression in protoplasts, tobacco (cv Petit Havana) was grown in Murashige and Skoog medium (Murashige and Skoog, 1962) supplemented with 2% Suc at 25°C with a 16-h light/8-h dark regime at light irradiance of 200 mE m−2 s−1. Tobacco leaf protoplast preparation and subsequent DNA transfection via electroporation were performed as described by Phillipson et al. (2001). Plasmid concentrations used are given in Figure 1. Extraction of protoplasts and α-amylase assays were performed as described by Crofts et al. (1999) and Phillipson et al. (2001). The secretion index was calculated as a ratio between extracellular and intracellular enzyme activities.
Sampling, Imaging, and Quantification
Imaging was performed using an upright laser-scanning confocal microscope (Zeiss LSM510 META; Zeiss) with a 63× water immersion objective. Transformed leaves were analyzed between 68 to 72 h after infection of the lower epidermis for experiments with YFP-Sec24, or between 44 to 48 h after infection for experiments with Sec12-YFP. For imaging expression and quantification of GFP constructs, YFP constructs or both nonsaturating imaging settings using low laser output as described by Brandizzi et al. (2002c) were used. This allows linear quantification of fluorescence intensity as well as exclusion of the possibility of cross talk between fluorochromes (Brandizzi et al., 2002c; daSilva et al., 2004).
Individual experiments for quantification of YFP-Sec24 fluorescence and ERES number were performed by single-imaging frame collection from cells expressing YFP-Sec24, either alone or with the Golgi markers, using identical laser output levels and imaging conditions. The standardized frame size used was 47.5 × 47.5 μm. These imaging settings were chosen so that the entire area shown was within the cell of interest and that no areas outside the cell were included in the image. Because plant ERES are on average 1 μm in diameter when viewed by fluorescence microscopy (daSilva et al., 2004) and exist within a thin layer of cytosol, images were collected using a 7.8-μm optical section as a compromise between ensuring maximal light collection and simultaneously reducing the out-of-focus background fluorescence in the Z direction. To obtain values for the confocal imaging volume of the images, the frame area was multiplied by the optical slice, giving a total volume of 17,598.75 μm3. For ERES number quantification, visible ERES within these images were counted and the average number of ERES per 100 μm3 was calculated (sample size for ERES quantification = 150 cells). For ERES fluorescence quantification, the fluorescence pixel intensity levels of visible ERES that were in focus within manually selected regions of interest of equal dimension in the planar axes were measured for the ERES of 150 cells. Measurements of the fluorescence of at least 10 ERES per cell, giving measurements of at least 1,500 ERES for each marker, for each combination of markers were performed. An identical procedure and sampling size were used to measure the fluorescence intensity from the cytosol adjacent to ERES, allowing normalization of the fluorescence intensity at the ERES.
Measurements of the YFP-Sec24 fluorescence levels at the ERES and cytosol were made within a 2-μm2 circle using ImageJ 1.34-s software in postacquisition analysis. Similar measurements on Golgi bodies and ER were made for quantification of the effects of Sec12-YFP on ERD2-GFP. Statistical analyses used the Student's two-tailed t test assuming equal variance and data with a P value < 0.05 were considered significant.
Postacquisition image processing was carried out using CorelDraw 12 software.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At3g07100, NM_111590 (Sec24); and At2g01470, NM_126208 (Sec12).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. YFP-Sec24 labels ERES and additional punctate structures.
Supplemental Figure S2. Overexpression of Sec12-YFP inhibits transport of TMcCCASP to the Golgi apparatus.
Supplementary Material
This work was supported by Canada Foundation for Innovation, Canada Research Chair, Natural Sciences and Engineering Research Council of Canada, and Department of Energy (Michigan State University) grants to F.B.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Federica Brandizzi (brandizz@msu.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R (2001) Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol 3 531–537 [DOI] [PubMed] [Google Scholar]
- Aridor M, Fish KN, Bannykh S, Weissman J, Roberts TH, Lippincott-Schwartz J, Balch WE (2001) The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J Cell Biol 152 213–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Weissman J, Bannykh S, Nuoffer C, Balch WE (1998) Cargo selection by the COPII budding machinery during export from the ER. J Cell Biol 141 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C (2003) Signals for COPII-dependent export from the ER: what's the ticket out? Trends Cell Biol 13 295–300 [DOI] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77 895–907 [DOI] [PubMed] [Google Scholar]
- Barlowe C, Schekman R (1993) Sec12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature 365 347–349 [DOI] [PubMed] [Google Scholar]
- Bar-Peled M, Raikhel NV (1997) Characterization of AtSec12 and AtSar1: proteins likely involved in endoplasmic reticulum and Golgi transport. Plant Physiol 114 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoko H, Zheng HQ, Hawes C, Moore I (2000) A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15 441–447 [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Frangne N, Marc-Martin S, Hawes C, Neuhaus JM, Paris N (2002. a) The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14 1077–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Fricker M, Hawes C (2002. b) A greener world: the revolution in plant bioimaging. Nat Rev Mol Cell Biol 3 520–530 [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Snapp EL, Roberts AG, Lippincott-Schwartz J, Hawes C (2002. c) Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching. Plant Cell 14 1293–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras I, Ortiz-Zapater E, Castilho LM, Aniento F (2000) Characterization of Cop I coat proteins in plant cells. Biochem Biophys Res Commun 273 176–182 [DOI] [PubMed] [Google Scholar]
- Crofts AJ, Leborgne-Castel N, Hillmer S, Robinson DG, Phillipson B, Carlsson LE, Ashford DA, Denecke J (1999) Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11 2233–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva LL, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F (2004) Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16 1753–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J, Botterman J, Deblaere R (1990) Protein secretion in plant cells can occur via a default pathway. Plant Cell 2 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C, Gensse M, Gaillardin C (1992) Fission yeast and a plant have functional homologues of the Sar1 and Sec12 proteins involved in ER to Golgi traffic in budding yeast. EMBO J 11 4205–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C, Wuestehube LJ, Lila T, Schekman R (1991) Sec12p-dependent membrane binding of the small GTP-binding protein Sar1p promotes formation of transport vesicles from the ER. J Cell Biol 114 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping EA, Moye-Rowley WS (2002) Identification of interdependent signals required for anterograde traffic of the ATP-binding cassette transporter protein Yor1p. J Biol Chem 277 34860–34869 [DOI] [PubMed] [Google Scholar]
- Espenshade P, Gimeno RE, Holzmacher E, Teung P, Kaiser CA (1995) Yeast Sec16 gene encodes a multidomain vesicle coat protein that interacts with Sec23p. J Cell Biol 131 311–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Weiss M, Zimmermann T, Reynaud EG, Verissimo F, Stephens DJ, Pepperkok R (2006) Secretory cargo regulates the turnover of COPII subunits at single ER exit sites. Curr Biol 16 173–179 [DOI] [PubMed] [Google Scholar]
- Guo Y, Linstedt AD (2006) COPII-Golgi protein interactions regulate COPII coat assembly and Golgi size. J Cell Biol 174 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton SL, Bortolotti LE, Renna L, Stefano G, Brandizzi F (2005. a) Crossing the divide—transport between the endoplasmic reticulum and Golgi apparatus in plants. Traffic 6 267–277 [DOI] [PubMed] [Google Scholar]
- Hanton SL, Matheson LA, Brandizzi F (2006) Seeking a way out: export of proteins from the plant endoplasmic reticulum. Trends Plant Sci 11 335–343 [DOI] [PubMed] [Google Scholar]
- Hanton SL, Renna L, Bortolotti LE, Chatre L, Stefano G, Brandizzi F (2005. b) Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells. Plant Cell 17 3081–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HI, Gal S, Newman TC, Raikhel NV (1993) The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proc Natl Acad Sci USA 90 11433–11437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R (2004) Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20 87–123 [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P (1994) Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 79 1199–1207 [DOI] [PubMed] [Google Scholar]
- Matheson LA, Hanton SL, Brandizzi F (2006) Traffic between the plant endoplasmic reticulum and Golgi apparatus: to the Golgi and beyond. Curr Opin Plant Biol 9 601–609 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Schekman R, Orci L, Heuser JE (2001) Surface structure of the COPII-coated vesicle. Proc Natl Acad Sci USA 98 13705–13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Beilharz TH, Malkus PN, Lee MC, Hamamoto S, Orci L, Schekman R (2003) Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114 497–509 [DOI] [PubMed] [Google Scholar]
- Mossessova E, Bickford LC, Goldberg J (2003) Snare selectivity of the COPII coat. Cell 114 483–495 [DOI] [PubMed] [Google Scholar]
- Movafeghi A, Happel N, Pimpl P, Tai GH, Robinson DG (1999) Arabidopsis Sec21p and Sec23p homologs: probable coat proteins of plant cop-coated vesicles. Plant Physiol 119 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Nishimura N, Balch WE (1997) A di-acidic signal required for selective export from the endoplasmic reticulum. Science 277 556–558 [DOI] [PubMed] [Google Scholar]
- Peng R, De Antoni A, Gallwitz D (2000) Evidence for overlapping and distinct functions in protein transport of coat protein Sec24p family members. J Biol Chem 275 11521–11528 [DOI] [PubMed] [Google Scholar]
- Phillipson BA, Pimpl P, daSilva LL, Crofts AJ, Taylor JP, Movafeghi A, Robinson DG, Denecke J (2001) Secretory bulk flow of soluble proteins is efficient and COPII dependent. Plant Cell 13 2005–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl P, Movafeghi A, Coughlan S, Denecke J, Hillmer S, Robinson DG (2000) In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12 2219–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regad F, Bardet C, Tremousaygue D, Moisan A, Lescure B, Axelos M (1993) cDNA cloning and expression of an Arabidopsis GTP-binding protein of the Arf family. FEBS Lett 316 133–136 [DOI] [PubMed] [Google Scholar]
- Renna L, Hanton SL, Stefano G, Bortolotti L, Misra V, Brandizzi F (2005) Identification and characterization of Atcasp, a plant transmembrane Golgi matrix protein. Plant Mol Biol 58 109–122 [DOI] [PubMed] [Google Scholar]
- Roberg KJ, Crotwell M, Espenshade P, Gimeno R, Kaiser CA (1999) Lst1 is a Sec24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. J Cell Biol 145 659–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runions J, Brach T, Kuhner S, Hawes C (2006) Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane. J Exp Bot 57 43–50 [DOI] [PubMed] [Google Scholar]
- Saint-Jore CM, Evins J, Batoko H, Brandizzi F, Moore I, Hawes C (2002) Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks. Plant J 29 661–678 [DOI] [PubMed] [Google Scholar]
- Sevier CS, Weisz OA, Davis M, Machamer CE (2000) Efficient export of the vesicular stomatitis virus G protein from the endoplasmic reticulum requires a signal in the cytoplasmic tail that includes both tyrosine-based and di-acidic motifs. Mol Biol Cell 11 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoi W, Ezawa I, Nakamoto K, Uesaki S, Gabreski G, Aridor M, Yamamoto A, Nagahama M, Tagaya M, Tani K (2005) P125 is localized in endoplasmic reticulum exit sites and involved in their organization. J Biol Chem 280 10141–10148 [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Kurihara T, Ravazzola M, Amherdt M, Orci L, Schekman R (2000) Lst1p and Sec24p cooperate in sorting of the plasma membrane ATPase into COPII vesicles in Saccharomyces cerevisiae. J Cell Biol 151 973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg SM, Gurkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, Carragher B, Balch WE (2006) Structure of the Sec13/31 COPII coat cage. Nature 439 234–238 [DOI] [PubMed] [Google Scholar]
- Stefano G, Renna L, Chatre L, Hanton SL, Moreau P, Hawes C, Brandizzi F (2006) In tobacco leaf epidermal cells, the integrity of protein export from the endoplasmic reticulum and of ER export sites depends on active COPI machinery. Plant J 46 95–110 [DOI] [PubMed] [Google Scholar]
- Stephens DJ, Lin-Marq N, Pagano A, Pepperkok R, Paccaud JP (2000) COPI-coated ER-to-Golgi transport complexes segregate from COPII in close proximity to ER exit sites. J Cell Sci 113 2177–2185 [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Tada M, Saito C, Yashiroda H, Nakano A (1998) Isolation of a tobacco cDNA encoding Sar1 GTPase and analysis of its dominant mutations in vesicular traffic using a yeast complementation system. Plant Cell Physiol 39 590–599 [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Ueda T, Sato K, Abe H, Nagata T, Nakano A (2000) A dominant negative mutant of Sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J 23 517–525 [DOI] [PubMed] [Google Scholar]
- Tang BL, Wang Y, Ong YS, Hong W (2005) COPII and exit from the endoplasmic reticulum. Biochim Biophys Acta 1744 293–303 [DOI] [PubMed] [Google Scholar]
- Törmäkangas K, Hadlington JL, Pimpl P, Hillmer S, Brandizzi F, Teeri TH, Denecke J (2001) A vacuolar sorting domain may also influence the way in which proteins leave the endoplasmic reticulum. Plant Cell 13 2021–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Wilson DW, Pelham HR (1993) Mutational analysis of the human KDEL receptor: distinct structural requirements for Golgi retention, ligand binding and retrograde transport. EMBO J 12 2821–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud V, Horton AC, Yang Z, Nielsen E (2003) Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol 131 1191–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votsmeier C, Gallwitz D (2001) An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export. EMBO J 20 6742–6750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Matteson J, An Y, Moyer B, Yoo JS, Bannykh S, Wilson IA, Riordan JR, Balch WE (2004) COPII-dependent export of cystic fibrosis transmembrane conductance regulator from the ER uses a di-acidic exit code. J Cell Biol 167 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Stephens DJ (2005) ER-to-Golgi transport: form and formation of vesicular and tubular carriers. Biochim Biophys Acta 1744 304–315 [DOI] [PubMed] [Google Scholar]
- Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ (2006) Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic 7 1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman JT, Aridor M, Balch WE (2001) Purification and properties of rat liver Sec23-Sec24 complex. Methods Enzymol 329 431–438 [DOI] [PubMed] [Google Scholar]
- Yang YD, Elamawi R, Bubeck J, Pepperkok R, Ritzenthaler C, Robinson DG (2005) Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites. Plant Cell 17 1513–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R (1993) Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science 259 1466–1468 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.