Abstract
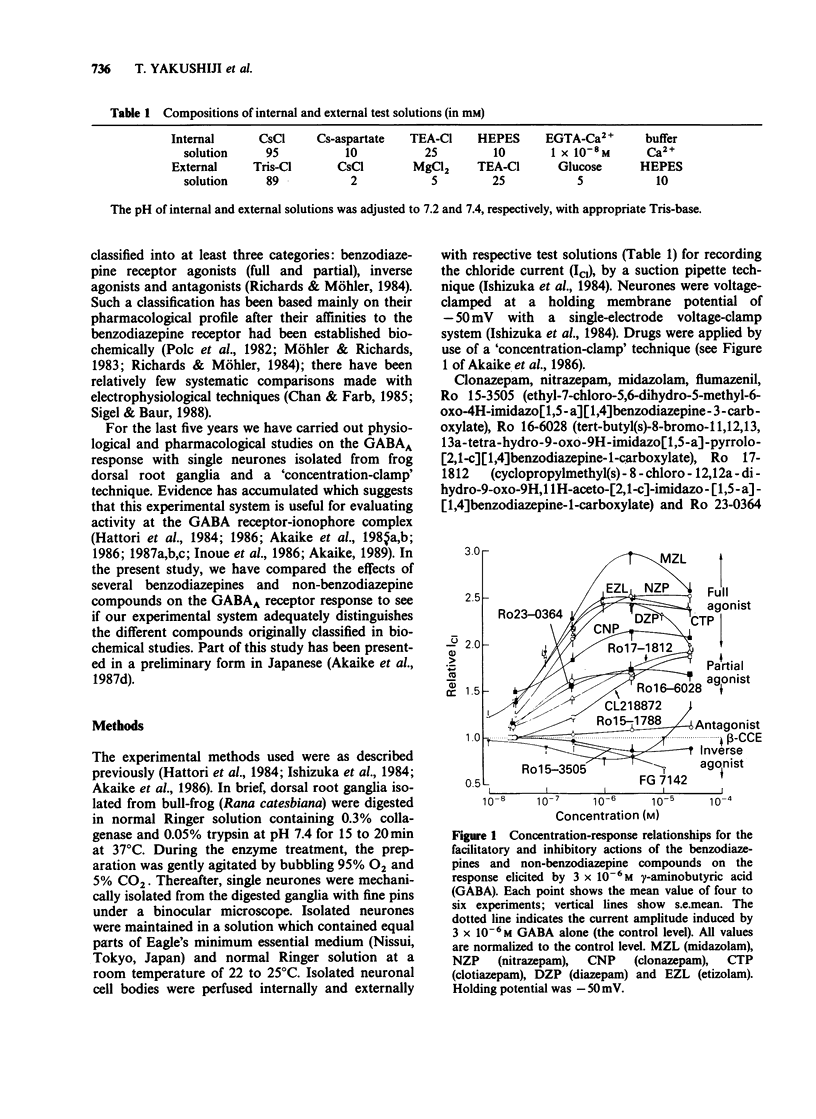
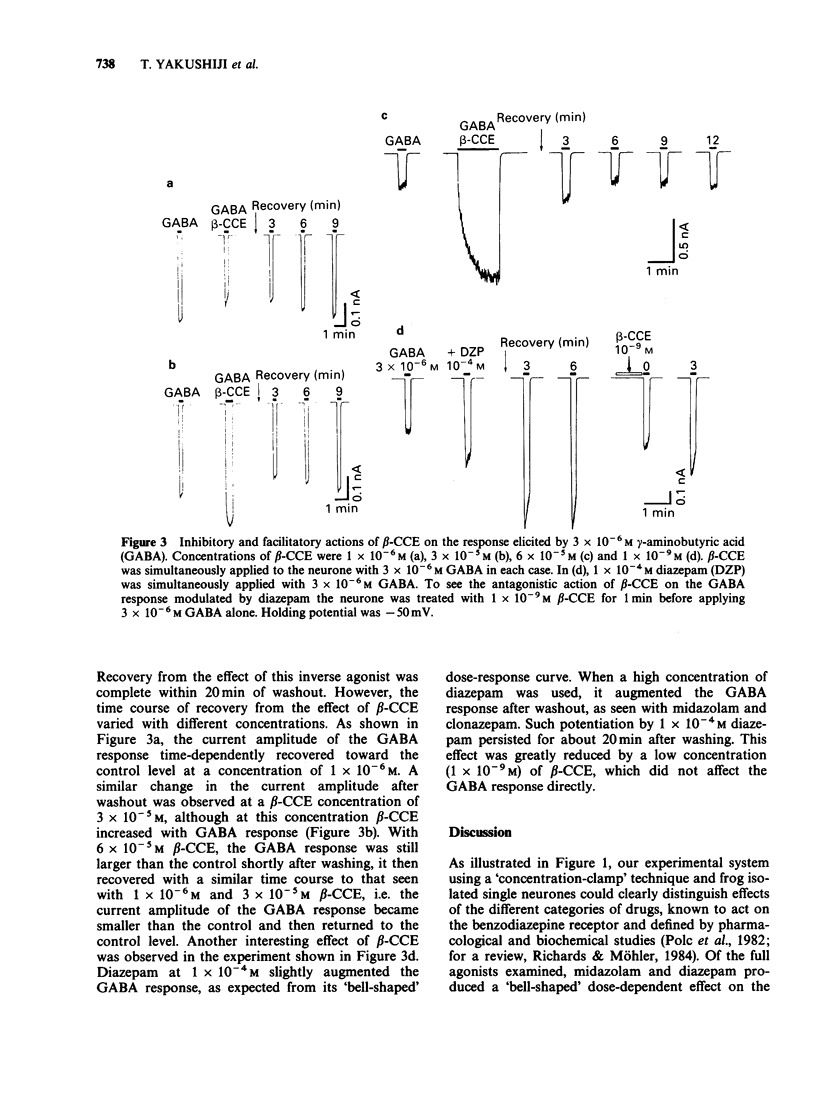
1. The effects of benzodiazepines and non-benzodiazepine compounds on the gamma-aminobutyric acid (GABA)-induced chloride current (ICl) were studied in frog isolated sensory neurones by use of a concentration-jump (termed 'concentration-clamp') technique, under single-electrode voltage-clamp conditions. The drugs used were classified into four categories as follows: full benzodiazepine receptor agonists (diazepam, clonazepam, nitrazepam, midazolam, clotiazepam and etizolam), partial agonists (CL 218,872, Ro 16-6028, Ro 17-1812 and Ro 23-0364), inverse agonists (Ro 15-3505, FG 7142 and beta-CCE) and a benzodiazepine receptor antagonist, Ro 15-1788 (flumazenil). 2. All full agonists at concentrations of 3 x 10(-6) M or less increased dose-dependently the peak amplitude of ICl elicited by 3 x 10(-6) M GABA to twice to three times larger than the control. However, no further augmentation of the GABA response was observed at concentrations of 1 x 10(-5) M or higher. Partial agonists also showed a dose-dependent augmentation of the GABA response at concentrations ranging from 3 x 10(-8) M to 3 x 10(-5) M, but their efficacies of augmentation of the GABA response were only about half or less of those of full agonists. Of the inverse agonists, beta-CCE had a unique dose-dependent effect on the GABA response. Beta-CCE reduced dose-dependently the GABA response at concentrations of less than 3 x 10(-6) M, but augmented it at concentrations of 3 x 10(-5) M and 6 x 10(-5) M. The inverse agonists reduced dose-dependently the GABA response.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Hattori K., Inomata N., Oomura Y. gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1985 Mar;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Hattori K., Oomura Y., Carpenter D. O. Bicuculline and picrotoxin block gamma-aminobutyric acid-gated Cl- conductance by different mechanisms. Experientia. 1985 Jan 15;41(1):70–71. doi: 10.1007/BF02005880. [DOI] [PubMed] [Google Scholar]
- Akaike N., Inomata N., Tokutomi N. Contribution of chloride shifts to the fade of gamma-aminobutyric acid-gated currents in frog dorsal root ganglion cells. J Physiol. 1987 Oct;391:219–234. doi: 10.1113/jphysiol.1987.sp016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Maruyama T., Sikdar S. K., Yasui S. Sodium-dependent suppression of gamma-aminobutyric-acid-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1987 Nov;392:543–562. doi: 10.1113/jphysiol.1987.sp016796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Maruyama T., Tokutomi N. Kinetic properties of the pentobarbitone-gated chloride current in frog sensory neurones. J Physiol. 1987 Dec;394:85–98. doi: 10.1113/jphysiol.1987.sp016861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard J. C., Boireau A., Garret C., Julou L. In vitro and in vivo inhibition by zopiclone of benzodiazepine binding to rodent brain receptors. Life Sci. 1979 Jun 25;24(26):2417–2420. doi: 10.1016/0024-3205(79)90449-1. [DOI] [PubMed] [Google Scholar]
- Blanchard J. C., Julou L. Suriclone: a new cyclopyrrolone derivative recognizing receptors labeled by benzodiazepines in rat hippocampus and cerebellum. J Neurochem. 1983 Mar;40(3):601–607. doi: 10.1111/j.1471-4159.1983.tb08023.x. [DOI] [PubMed] [Google Scholar]
- Chan C. Y., Farb D. H. Modulation of neurotransmitter action: control of the gamma-aminobutyric acid response through the benzodiazepine receptor. J Neurosci. 1985 Sep;5(9):2365–2373. doi: 10.1523/JNEUROSCI.05-09-02365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E., Guidotti A., Mao C. C. Evidence for involvement of GABA in the action of benzodiazepines: studies on rat cerebellum. Adv Biochem Psychopharmacol. 1975;(14):113–130. [PubMed] [Google Scholar]
- Haefely W., Kulcsár A., Möhler H., Pieri L., Polc P., Schaffner R. Possible involvement of GABA in the central actions of benzodiazepines. Adv Biochem Psychopharmacol. 1975;(14):131–151. [PubMed] [Google Scholar]
- Hattori K., Akaike N., Oomura Y., Kuraoka S. Internal perfusion studies demonstrating GABA-induced chloride responses in frog primary afferent neurons. Am J Physiol. 1984 Mar;246(3 Pt 1):C259–C265. doi: 10.1152/ajpcell.1984.246.3.C259. [DOI] [PubMed] [Google Scholar]
- Hattori K., Oomura Y., Akaike N. Diazepam action on gamma-aminobutyric acid-activated chloride currents in internally perfused frog sensory neurons. Cell Mol Neurobiol. 1986 Sep;6(3):307–323. doi: 10.1007/BF00711116. [DOI] [PubMed] [Google Scholar]
- Hunkeler W., Möhler H., Pieri L., Polc P., Bonetti E. P., Cumin R., Schaffner R., Haefely W. Selective antagonists of benzodiazepines. Nature. 1981 Apr 9;290(5806):514–516. doi: 10.1038/290514a0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Ishizuka S., Hattori K., Akaike N. Separation of ionic currents in the somatic membrane of frog sensory neurons. J Membr Biol. 1984;78(1):19–28. doi: 10.1007/BF01872528. [DOI] [PubMed] [Google Scholar]
- Lippa A. S., Critchett D., Sano M. C., Klepner C. A., Greenblatt E. N., Coupet J., Beer B. Benzodiazepine receptors: cellular and behavioral characteristics. Pharmacol Biochem Behav. 1979 May;10(5):831–843. doi: 10.1016/0091-3057(79)90342-3. [DOI] [PubMed] [Google Scholar]
- Möhler H., Okada T. Benzodiazepine receptor: demonstration in the central nervous system. Science. 1977 Nov 25;198(4319):849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- Polc P., Bonetti E. P., Schaffner R., Haefely W. A three-state model of the benzodiazepine receptor explains the interactions between the benzodiazepine antagonist Ro 15-1788, benzodiazepine tranquilizers, beta-carbolines, and phenobarbitone. Naunyn Schmiedebergs Arch Pharmacol. 1982 Dec;321(4):260–264. doi: 10.1007/BF00498510. [DOI] [PubMed] [Google Scholar]
- Richards J. G., Möhler H. Benzodiazepine receptors. Neuropharmacology. 1984 Feb;23(2B):233–242. doi: 10.1016/0028-3908(84)90064-9. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R. Allosteric modulation by benzodiazepine receptor ligands of the GABAA receptor channel expressed in Xenopus oocytes. J Neurosci. 1988 Jan;8(1):289–295. doi: 10.1523/JNEUROSCI.08-01-00289.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. Anxioselective anxiolytics. J Med Chem. 1983 May;26(5):619–628. doi: 10.1021/jm00359a001. [DOI] [PubMed] [Google Scholar]


