Abstract
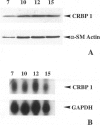
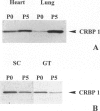
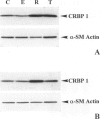
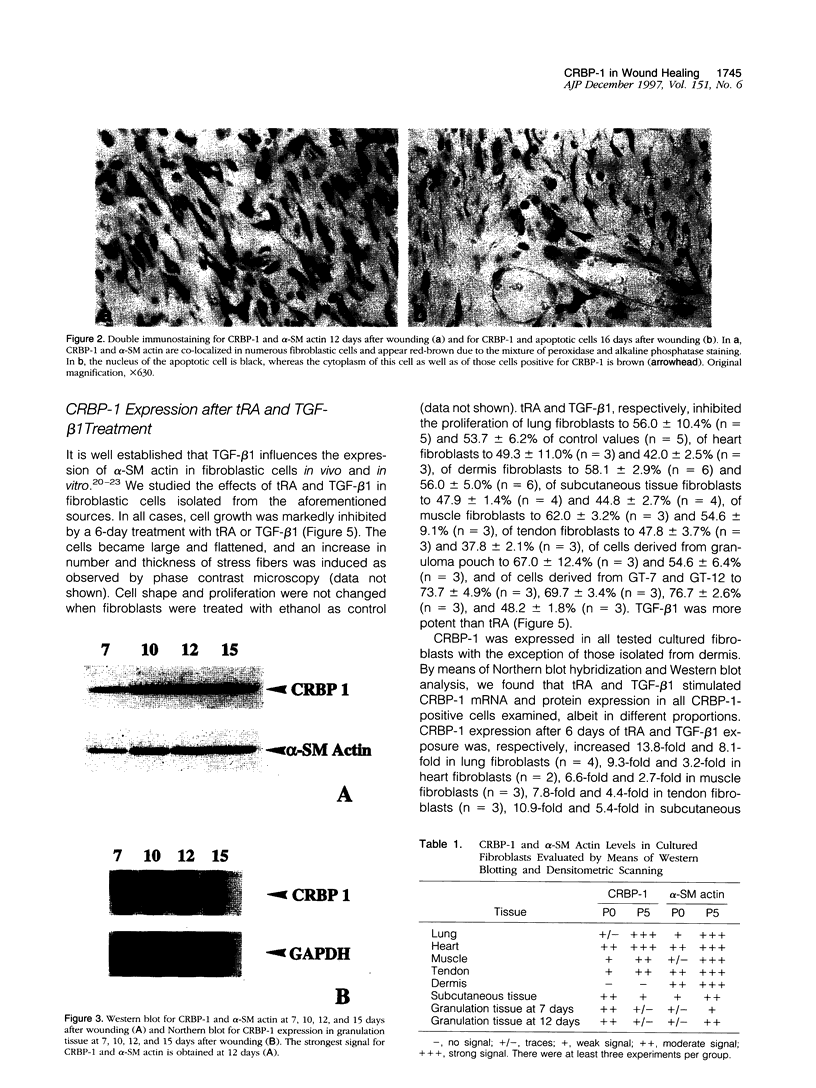
We have reported that cellular retinol-binding protein-1 (CRBP-1) is transiently expressed by arterial smooth muscle cells during experimental intimal repair (P. Neuville, A. Geinoz, G. Benzonana, M. Redard, F. Gabbiani, P. Ropraz, G. Gabbiani: Am J Pathol 1997, 150:509-521). We have examined here the expression of CRBP-1 during wound healing after a full-thickness rat skin wound. CRBP-1 was transiently expressed by a significant proportion of fibroblastic cells including myofibroblasts. Expression started 4 days after wounding, reached a maximum at 12 days, and persisted up to 30 days when a scar was formed. After wound closure, most CRBP-1-containing fibroblastic cells underwent apoptosis. We have further investigated CRBP-1 expression in rat fibroblasts cultured from different organs. CRBP-1 was abundant in lung and heart fibroblasts and was detected in decreasing amounts in muscle, tendon, subcutaneous tissue, and granulation tissue fibroblasts. Dermis fibroblasts contained no detectable levels of CRBP-1. All-trans retinoic acid and transforming growth factor-beta1 inhibited cell proliferation and increased CRBP-1 expression in fibroblastic populations except dermis fibroblasts. We demonstrate that during granulation tissue formation a subpopulation of fibroblastic cells express CRBP-1 de novo. We also demonstrate that CRBP-1 expression by fibroblasts is regulated in vitro by retinoic acid and transforming growth factor-beta1. Our results suggest that CRBP-1 and possibly retinoic acid play a role in the evolution of granulation tissue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achkar C. C., Derguini F., Blumberg B., Langston A., Levin A. A., Speck J., Evans R. M., Bolado J., Jr, Nakanishi K., Buck J. 4-Oxoretinol, a new natural ligand and transactivator of the retinoic acid receptors. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4879–4884. doi: 10.1073/pnas.93.10.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Takeshita A., Ozaki K., Kitano S., Hanazawa S. Transcriptional regulation by transforming growth factor beta of the expression of retinoic acid and retinoid X receptor genes in osteoblastic cells is mediated through AP-1. J Biol Chem. 1996 Dec 6;271(49):31602–31606. doi: 10.1074/jbc.271.49.31602. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Letterio J. J., Gaetano C., Chan J., Matsumoto K., Sporn M. B., Thiele C. J. Induction of transforming growth factor beta 1 and its receptors during all-trans-retinoic acid (RA) treatment of RA-responsive human neuroblastoma cell lines. Cancer Res. 1995 Jun 1;55(11):2380–2386. [PubMed] [Google Scholar]
- Cosgaya J. M., Perona R., Aranda A. Retinoic acid induces secretion of transforming growth factors by PC12 pheochromocytoma cells. Oncogene. 1997 Feb 6;14(5):579–587. doi: 10.1038/sj.onc.1200865. [DOI] [PubMed] [Google Scholar]
- Danielpour D. Induction of transforming growth factor-beta autocrine activity by all-trans-retinoic acid and 1 alpha,25-dihydroxyvitamin D3 in NRP-152 rat prostatic epithelial cells. J Cell Physiol. 1996 Jan;166(1):231–239. doi: 10.1002/(SICI)1097-4652(199601)166:1<231::AID-JCP24>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Kim K. Y., Winokur T. S., Sporn M. B. Differential regulation of the expression of transforming growth factor-beta s 1 and 2 by retinoic acid, epidermal growth factor, and dexamethasone in NRK-49F and A549 cells. J Cell Physiol. 1991 Aug;148(2):235–244. doi: 10.1002/jcp.1041480208. [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995 Jan;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Rubbia-Brandt L., Abdiu A., Walz T., Macieira-Coelho A., Gabbiani G. Alpha-smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma-interferon. Exp Cell Res. 1992 Jul;201(1):64–73. doi: 10.1016/0014-4827(92)90348-c. [DOI] [PubMed] [Google Scholar]
- Falk L. A., De Benedetti F., Lohrey N., Birchenall-Roberts M. C., Ellingsworth L. W., Faltynek C. R., Ruscetti F. W. Induction of transforming growth factor-beta 1 (TGF-beta 1), receptor expression and TGF-beta 1 protein production in retinoic acid-treated HL-60 cells: possible TGF-beta 1-mediated autocrine inhibition. Blood. 1991 Mar 15;77(6):1248–1255. [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit D., Ebner R., Kahn A. J., Derynck R. Modulation of expression and cell surface binding of members of the transforming growth factor-beta superfamily during retinoic acid-induced osteoblastic differentiation of multipotential mesenchymal cells. Mol Endocrinol. 1993 Feb;7(2):189–198. doi: 10.1210/mend.7.2.8385738. [DOI] [PubMed] [Google Scholar]
- Glick A. B., Flanders K. C., Danielpour D., Yuspa S. H., Sporn M. B. Retinoic acid induces transforming growth factor-beta 2 in cultured keratinocytes and mouse epidermis. Cell Regul. 1989 Nov;1(1):87–97. doi: 10.1091/mbc.1.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. I., Sung J. J., Siebert J. W., Longaker M. T. Type I (RI) and type II (RII) receptors for transforming growth factor-beta isoforms are expressed subsequent to transforming growth factor-beta ligands during excisional wound repair. Am J Pathol. 1997 Jan;150(1):209–222. [PMC free article] [PubMed] [Google Scholar]
- Guérin E., Ludwig M. G., Basset P., Anglard P. Stromelysin-3 induction and interstitial collagenase repression by retinoic acid. Therapeutical implication of receptor-selective retinoids dissociating transactivation and AP-1-mediated transrepression. J Biol Chem. 1997 Apr 25;272(17):11088–11095. doi: 10.1074/jbc.272.17.11088. [DOI] [PubMed] [Google Scholar]
- Herrera R. E., Mäkelä T. P., Weinberg R. A. TGF beta-induced growth inhibition in primary fibroblasts requires the retinoblastoma protein. Mol Biol Cell. 1996 Sep;7(9):1335–1342. doi: 10.1091/mbc.7.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn V., Minucci S., Ogryzko V. V., Adamson E. D., Howard B. H., Levin A. A., Ozato K. RAR and RXR selective ligands cooperatively induce apoptosis and neuronal differentiation in P19 embryonal carcinoma cells. FASEB J. 1996 Jul;10(9):1071–1077. doi: 10.1096/fasebj.10.9.8801169. [DOI] [PubMed] [Google Scholar]
- Husmann M., Hoffmann B., Stump D. G., Chytil F., Pfahl M. A retinoic acid response element from the rat CRBPI promoter is activated by an RAR/RXR heterodimer. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1558–1564. doi: 10.1016/0006-291x(92)90480-9. [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Cubert J., Danielpour D., Sporn M. B., Roberts A. B. Differential regulation of the expression of transforming growth factor-beta mRNAs by growth factors and retinoic acid in chicken embryo chondrocytes, myocytes, and fibroblasts. J Cell Physiol. 1992 Feb;150(2):377–385. doi: 10.1002/jcp.1041500222. [DOI] [PubMed] [Google Scholar]
- Jarnagin W. R., Rockey D. C., Koteliansky V. E., Wang S. S., Bissell D. M. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994 Dec;127(6 Pt 2):2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T. Re-evaluation of fibroblasts and fibroblast-like cells. Anat Embryol (Berl) 1990;182(2):103–112. doi: 10.1007/BF00174011. [DOI] [PubMed] [Google Scholar]
- Lafyatis R., Kim S. J., Angel P., Roberts A. B., Sporn M. B., Karin M., Wilder R. L. Interleukin-1 stimulates and all-trans-retinoic acid inhibits collagenase gene expression through its 5' activator protein-1-binding site. Mol Endocrinol. 1990 Jul;4(7):973–980. doi: 10.1210/mend-4-7-973. [DOI] [PubMed] [Google Scholar]
- Malliri A., Yeudall W. A., Nikolic M., Crouch D. H., Parkinson E. K., Ozanne B. Sensitivity to transforming growth factor beta 1-induced growth arrest is common in human squamous cell carcinoma cell lines: c-MYC down-regulation and p21waf1 induction are important early events. Cell Growth Differ. 1996 Oct;7(10):1291–1304. [PubMed] [Google Scholar]
- Martin P., Nobes C., McCluskey J., Lewis J. Repair of excisional wounds in the embryo. Eye (Lond) 1994;8(Pt 2):155–160. doi: 10.1038/eye.1994.39. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997 Apr 4;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- McClain S. A., Simon M., Jones E., Nandi A., Gailit J. O., Tonnesen M. G., Newman D., Clark R. A. Mesenchymal cell activation is the rate-limiting step of granulation tissue induction. Am J Pathol. 1996 Oct;149(4):1257–1270. [PMC free article] [PubMed] [Google Scholar]
- McGowan S. E., Torday J. S. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- Moulin V. Growth factors in skin wound healing. Eur J Cell Biol. 1995 Sep;68(1):1–7. [PubMed] [Google Scholar]
- Nagy L., Thomázy V. A., Shipley G. L., Fésüs L., Lamph W., Heyman R. A., Chandraratna R. A., Davies P. J. Activation of retinoid X receptors induces apoptosis in HL-60 cell lines. Mol Cell Biol. 1995 Jul;15(7):3540–3551. doi: 10.1128/mcb.15.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli J. L. Retinoic acid biosynthesis and metabolism. FASEB J. 1996 Jul;10(9):993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- Neuville P., Geinoz A., Benzonana G., Redard M., Gabbiani F., Ropraz P., Gabbiani G. Cellular retinol-binding protein-1 is expressed by distinct subsets of rat arterial smooth muscle cells in vitro and in vivo. Am J Pathol. 1997 Feb;150(2):509–521. [PMC free article] [PubMed] [Google Scholar]
- Nicholson R. C., Mader S., Nagpal S., Leid M., Rochette-Egly C., Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990 Dec;9(13):4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottonello S., Scita G., Mantovani G., Cavazzini D., Rossi G. L. Retinol bound to cellular retinol-binding protein is a substrate for cytosolic retinoic acid synthesis. J Biol Chem. 1993 Dec 25;268(36):27133–27142. [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Mechanistic interrelationships between two superfamilies: the steroid/retinoid receptors and transforming growth factor-beta. Cancer Surv. 1992;14:205–220. [PubMed] [Google Scholar]
- Roberts A. B. Transforming growth factor-beta: activity and efficacy in animal models of wound healing. Wound Repair Regen. 1995 Oct–Dec;3(4):408–418. doi: 10.1046/j.1524-475X.1995.30405.x. [DOI] [PubMed] [Google Scholar]
- Ross A. C. Overview of retinoid metabolism. J Nutr. 1993 Feb;123(2 Suppl):346–350. doi: 10.1093/jn/123.suppl_2.346. [DOI] [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Rønnov-Jessen L., Petersen O. W. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993 Jun;68(6):696–707. [PubMed] [Google Scholar]
- Sappino A. P., Schürch W., Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990 Aug;63(2):144–161. [PubMed] [Google Scholar]
- Scarpa S., Coppa A., Ragano-Caracciolo M., Mincione G., Giuffrida A., Modesti A., Colletta G. Transforming growth factor beta regulates differentiation and proliferation of human neuroblastoma. Exp Cell Res. 1996 Nov 25;229(1):147–154. doi: 10.1006/excr.1996.0352. [DOI] [PubMed] [Google Scholar]
- Schmitt-Gräff A., Desmoulière A., Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch. 1994;425(1):3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- Scita G., Darwiche N., Greenwald E., Rosenberg M., Politi K., De Luca L. M. Retinoic acid down-regulation of fibronectin and retinoic acid receptor alpha proteins in NIH-3T3 cells. Blocks of this response by ras transformation. J Biol Chem. 1996 Mar 15;271(11):6502–6508. doi: 10.1074/jbc.271.11.6502. [DOI] [PubMed] [Google Scholar]
- Sempowski G. D., Borrello M. A., Blieden T. M., Barth R. K., Phipps R. P. Fibroblast heterogeneity in the healing wound. Wound Repair Regen. 1995 Apr–Jun;3(2):120–131. doi: 10.1046/j.1524-475X.1995.30204.x. [DOI] [PubMed] [Google Scholar]
- Serini G., Gabbiana G. Modulation of alpha-smooth muscle actin expression in fibroblasts by transforming growth factor-beta isoforms: an in vivo and in vitro study. Wound Repair Regen. 1996 Apr–Jun;4(2):278–287. doi: 10.1046/j.1524-475X.1996.40217.x. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Schürch W., Seemayer T., Lagacé R., Montandon D., Pittet B., Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989 Feb;60(2):275–285. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley J. M., Falk L. A., Ruscetti F. W., Kasper J. J., Francomano T., Fu T., Bang O. S., Birchenall-Roberts M. C. Transforming growth factor beta 1 functions in monocytic differentiation of hematopoietic cells through autocrine and paracrine mechanisms. Cell Growth Differ. 1996 Nov;7(11):1535–1544. [PubMed] [Google Scholar]
- Vande Berg J. S., Rudolph R., Woodward M. Comparative growth dynamics and morphology between cultured myofibroblasts from granulating wounds and dermal fibroblasts. Am J Pathol. 1984 Feb;114(2):187–200. [PMC free article] [PubMed] [Google Scholar]
- Wei L. N., Blaner W. S., Goodman D. S., Nguyen-Huu M. C. Regulation of the cellular retinoid-binding proteins and their messenger ribonucleic acids during P19 embryonal carcinoma cell differentiation induced by retinoic acid. Mol Endocrinol. 1989 Mar;3(3):454–463. doi: 10.1210/mend-3-3-454. [DOI] [PubMed] [Google Scholar]
- Yokozeki M., Moriyama K., Shimokawa H., Kuroda T. Transforming growth factor-beta 1 modulates myofibroblastic phenotype of rat palatal fibroblasts in vitro. Exp Cell Res. 1997 Mar 15;231(2):328–336. doi: 10.1006/excr.1997.3473. [DOI] [PubMed] [Google Scholar]