Abstract
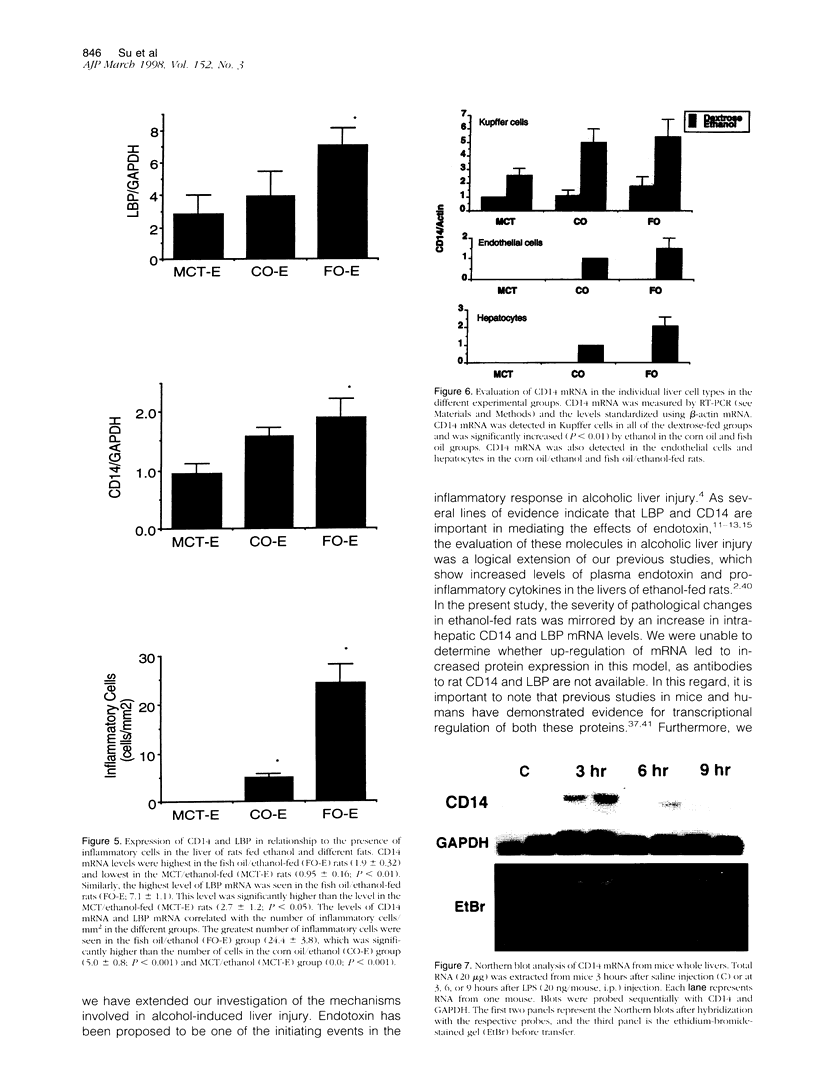
Lipopolysaccharide-binding protein (LBP) and CD14 play key intermediary roles in the activation of cells by endotoxin. As endotoxin has been postulated to participate in promoting pathological liver injury in alcoholic liver disease, we investigated the role of LBP and CD14 in alcoholic liver injury. Rats were fed intragastrically ethanol or dextrose and either medium-chain triglycerides, corn oil, or fish oil for 4 weeks. Kupffer cells, endothelial cells, and hepatocytes were isolated. LBP and CD14 mRNA levels were measured in liver and individual cell types. The highest levels of LBP and CD14 mRNA levels in the liver were found in the fish oil/ethanol group, which was also the group with the greatest degree of pathological injury and inflammation. CD14 mRNA levels were also significantly elevated in groups fed unsaturated fatty acids with dextrose. CD14 expression was localized to the Kupffer cells and LBP expression to the hepatocytes. Expression of CD14 mRNA was also found in nonmyeloid cells in the two experimental groups (fish oil/ethanol and corn oil/ethanol) that had liver necrosis and inflammation. Our results suggest that enhanced LBP and CD14 expression correlates with the presence of pathological liver injury in alcoholic liver injury. Furthermore, unsaturated fatty acids may prime cells to respond to endotoxin by enhancing CD14 expression.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Bradford B. U., Gao W., Bojes H. K., Thurman R. G. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994 Aug;20(2):453–460. [PubMed] [Google Scholar]
- Adachi Y., Moore L. E., Bradford B. U., Gao W., Thurman R. G. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995 Jan;108(1):218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Bautista A. P., Spitzer J. J. Superoxide anion generation by in situ perfused rat liver: effect of in vivo endotoxin. Am J Physiol. 1990 Dec;259(6 Pt 1):G907–G912. doi: 10.1152/ajpgi.1990.259.6.G907. [DOI] [PubMed] [Google Scholar]
- Bellezzo J. M., Britton R. S., Bacon B. R., Fox E. S. LPS-mediated NF-kappa beta activation in rat Kupffer cells can be induced independently of CD14. Am J Physiol. 1996 Jun;270(6 Pt 1):G956–G961. doi: 10.1152/ajpgi.1996.270.6.G956. [DOI] [PubMed] [Google Scholar]
- Bode C., Kugler V., Bode J. C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987 Feb;4(1):8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dentener M. A., Bazil V., Von Asmuth E. J., Ceska M., Buurman W. A. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993 Apr 1;150(7):2885–2891. [PubMed] [Google Scholar]
- Fearns C., Kravchenko V. V., Ulevitch R. J., Loskutoff D. J. Murine CD14 gene expression in vivo: extramyeloid synthesis and regulation by lipopolysaccharide. J Exp Med. 1995 Mar 1;181(3):857–866. doi: 10.1084/jem.181.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero E., Jiao D., Tsuberi B. Z., Tesio L., Rong G. W., Haziot A., Goyert S. M. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2380–2384. doi: 10.1073/pnas.90.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French S. W., Miyamoto K., Tsukamoto H. Ethanol-induced hepatic fibrosis in the rat: role of the amount of dietary fat. Alcohol Clin Exp Res. 1986;10(6 Suppl):13S–19S. doi: 10.1111/j.1530-0277.1986.tb05175.x. [DOI] [PubMed] [Google Scholar]
- Gegner J. A., Ulevitch R. J., Tobias P. S. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J Biol Chem. 1995 Mar 10;270(10):5320–5325. doi: 10.1074/jbc.270.10.5320. [DOI] [PubMed] [Google Scholar]
- Geller D. A., Kispert P. H., Su G. L., Wang S. C., Di Silvio M., Tweardy D. J., Billiar T. R., Simmons R. L. Induction of hepatocyte lipopolysaccharide binding protein in models of sepsis and the acute-phase response. Arch Surg. 1993 Jan;128(1):22–28. doi: 10.1001/archsurg.1993.01420130026005. [DOI] [PubMed] [Google Scholar]
- Gupta D., Kirkland T. N., Viriyakosol S., Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996 Sep 20;271(38):23310–23316. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- Hailman E., Lichenstein H. S., Wurfel M. M., Miller D. S., Johnson D. A., Kelley M., Busse L. A., Zukowski M. M., Wright S. D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994 Jan 1;179(1):269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Cherwitz D. L., Allen J. I. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994 Aug;20(2):461–474. [PubMed] [Google Scholar]
- Haziot A., Ferrero E., Lin X. Y., Stewart C. L., Goyert S. M. CD14-deficient mice are exquisitely insensitive to the effects of LPS. Prog Clin Biol Res. 1995;392:349–351. [PubMed] [Google Scholar]
- Haziot A., Rong G. W., Bazil V., Silver J., Goyert S. M. Recombinant soluble CD14 inhibits LPS-induced tumor necrosis factor-alpha production by cells in whole blood. J Immunol. 1994 Jun 15;152(12):5868–5876. [PubMed] [Google Scholar]
- Kamimura S., Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995 Oct;22(4 Pt 1):1304–1309. [PubMed] [Google Scholar]
- Kielian T. L., Blecha F. CD14 and other recognition molecules for lipopolysaccharide: a review. Immunopharmacology. 1995 Apr;29(3):187–205. doi: 10.1016/0162-3109(95)00003-c. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. D., Kato K., Tobias P. S., Kirkland T. N., Ulevitch R. J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992 Jun 1;175(6):1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliszewski C. R. CD14 and immune response to lipopolysaccharide. Science. 1991 May 31;252(5010):1321–1322. doi: 10.1126/science.1718034. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Mongovin S. M., Tobias P. S., Mathison J. C., Moriarty A. M., Leturcq D. J., Ulevitch R. J. The CD14 differentiation antigen mediates the development of endotoxin responsiveness during differentiation of mononuclear phagocytes. J Leukoc Biol. 1994 Jul;56(1):1–9. doi: 10.1002/jlb.56.1.1. [DOI] [PubMed] [Google Scholar]
- Matsuura K., Ishida T., Setoguchi M., Higuchi Y., Akizuki S., Yamamoto S. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J Exp Med. 1994 May 1;179(5):1671–1676. doi: 10.1084/jem.179.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain C., Hill D., Schmidt J., Diehl A. M. Cytokines and alcoholic liver disease. Semin Liver Dis. 1993 May;13(2):170–182. doi: 10.1055/s-2007-1007347. [DOI] [PubMed] [Google Scholar]
- Morimoto M., Zern M. A., Hagbjörk A. L., Ingelman-Sundberg M., French S. W. Fish oil, alcohol, and liver pathology: role of cytochrome P450 2E1. Proc Soc Exp Biol Med. 1994 Nov;207(2):197–205. doi: 10.3181/00379727-207-43807. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Nanji A. A., French S. W. Dietary factors and alcoholic cirrhosis. Alcohol Clin Exp Res. 1986 Jun;10(3):271–273. doi: 10.1111/j.1530-0277.1986.tb05088.x. [DOI] [PubMed] [Google Scholar]
- Nanji A. A., French S. W. Dietary linoleic acid is required for development of experimentally induced alcoholic liver injury. Life Sci. 1989;44(3):223–227. doi: 10.1016/0024-3205(89)90599-7. [DOI] [PubMed] [Google Scholar]
- Nanji A. A., Greenberg S. S., Tahan S. R., Fogt F., Loscalzo J., Sadrzadeh S. M., Xie J., Stamler J. S. Nitric oxide production in experimental alcoholic liver disease in the rat: role in protection from injury. Gastroenterology. 1995 Sep;109(3):899–907. doi: 10.1016/0016-5085(95)90400-x. [DOI] [PubMed] [Google Scholar]
- Nanji A. A., Griniuviene B., Sadrzadeh S. M., Levitsky S., McCully J. D. Effect of type of dietary fat and ethanol on antioxidant enzyme mRNA induction in rat liver. J Lipid Res. 1995 Apr;36(4):736–744. [PubMed] [Google Scholar]
- Nanji A. A., Griniuviene B., Yacoub L. K., Fogt F., Tahan S. R. Intercellular adhesion molecule-1 expression in experimental alcoholic liver disease: relationship to endotoxemia and TNF alpha messenger RNA. Exp Mol Pathol. 1995 Feb;62(1):42–51. doi: 10.1006/exmp.1995.1005. [DOI] [PubMed] [Google Scholar]
- Nanji A. A., Khettry U., Sadrzadeh S. M. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994 Mar;205(3):243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- Nanji A. A., Khettry U., Sadrzadeh S. M., Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993 Feb;142(2):367–373. [PMC free article] [PubMed] [Google Scholar]
- Nanji A. A., Khwaja S., Tahan S. R., Sadrzadeh S. M. Plasma levels of a novel noncyclooxygenase-derived prostanoid (8-isoprostane) correlate with severity of liver injury in experimental alcoholic liver disease. J Pharmacol Exp Ther. 1994 Jun;269(3):1280–1285. [PubMed] [Google Scholar]
- Nanji A. A., Mendenhall C. L., French S. W. Beef fat prevents alcoholic liver disease in the rat. Alcohol Clin Exp Res. 1989 Feb;13(1):15–19. doi: 10.1111/j.1530-0277.1989.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Nanji A. A., Miao L., Thomas P., Rahemtulla A., Khwaja S., Zhao S., Peters D., Tahan S. R., Dannenberg A. J. Enhanced cyclooxygenase-2 gene expression in alcoholic liver disease in the rat. Gastroenterology. 1997 Mar;112(3):943–951. doi: 10.1053/gast.1997.v112.pm9041257. [DOI] [PubMed] [Google Scholar]
- Nanji A. A., Sadrzadeh S. M., Thomas P., Yamanaka T. Eicosanoid profile and evidence for endotoxin tolerance in chronic ethanol-fed rats. Life Sci. 1994;55(8):611–620. doi: 10.1016/0024-3205(94)00487-0. [DOI] [PubMed] [Google Scholar]
- Nanji A. A., Zhao S., Sadrzadeh S. M., Waxman D. J. Use of reverse transcription-polymerase chain reaction to evaluate in vivo cytokine gene expression in rats fed ethanol for long periods. Hepatology. 1994 Jun;19(6):1483–1487. [PubMed] [Google Scholar]
- Petrick A. T., Meterissian S., Steele G., Jr, Thomas P. Desialylation of metastatic human colorectal carcinoma cells facilitates binding to Kupffer cells. Clin Exp Metastasis. 1994 Mar;12(2):108–116. doi: 10.1007/BF01753977. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Meyer zum Buschenfelde K. H., Tobias P. S., Mathison J. C., Ulevitch R. J. Biosynthesis of lipopolysaccharide-binding protein in rabbit hepatocytes. Pathobiology. 1990;58(2):89–94. doi: 10.1159/000163569. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Schumann R. R. Mechanisms of transcriptional activation of lipopolysaccharide binding protein (LBP). Prog Clin Biol Res. 1995;392:297–304. [PubMed] [Google Scholar]
- Su G. L., Freeswick P. D., Geller D. A., Wang Q., Shapiro R. A., Wan Y. H., Billiar T. R., Tweardy D. J., Simmons R. L., Wang S. C. Molecular cloning, characterization, and tissue distribution of rat lipopolysaccharide binding protein. Evidence for extrahepatic expression. J Immunol. 1994 Jul 15;153(2):743–752. [PubMed] [Google Scholar]
- Su G. L., Simmons R. L., Wang S. C. Lipopolysaccharide binding protein participation in cellular activation by LPS. Crit Rev Immunol. 1995;15(3-4):201–214. doi: 10.1615/critrevimmunol.v15.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H., Towner S. J., Ciofalo L. M., French S. W. Ethanol-induced liver fibrosis in rats fed high fat diet. Hepatology. 1986 Sep-Oct;6(5):814–822. doi: 10.1002/hep.1840060503. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Tobias P. S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- Wan Y., Freeswick P. D., Khemlani L. S., Kispert P. H., Wang S. C., Su G. L., Billiar T. R. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect Immun. 1995 Jul;63(7):2435–2442. doi: 10.1128/iai.63.7.2435-2442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Wurfel M. M., Hailman E., Wright S. D. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995 May 1;181(5):1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurfel M. M., Kunitake S. T., Lichenstein H., Kane J. P., Wright S. D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994 Sep 1;180(3):1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]