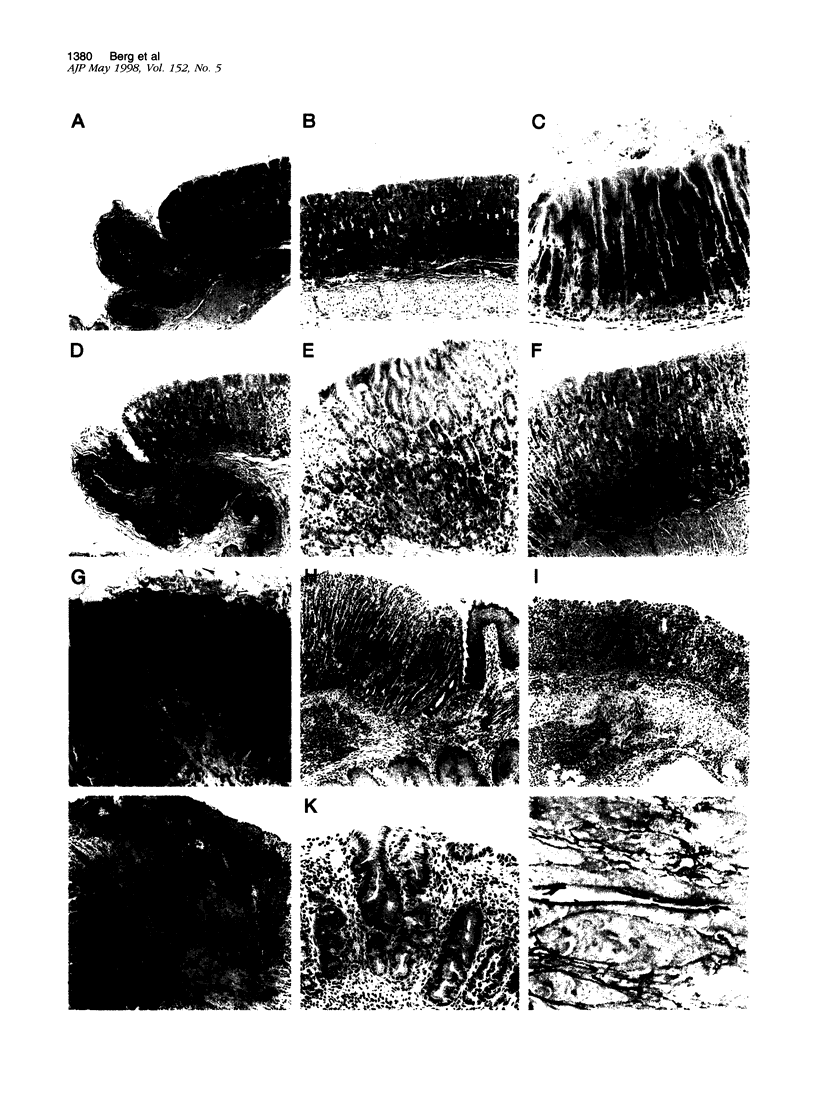
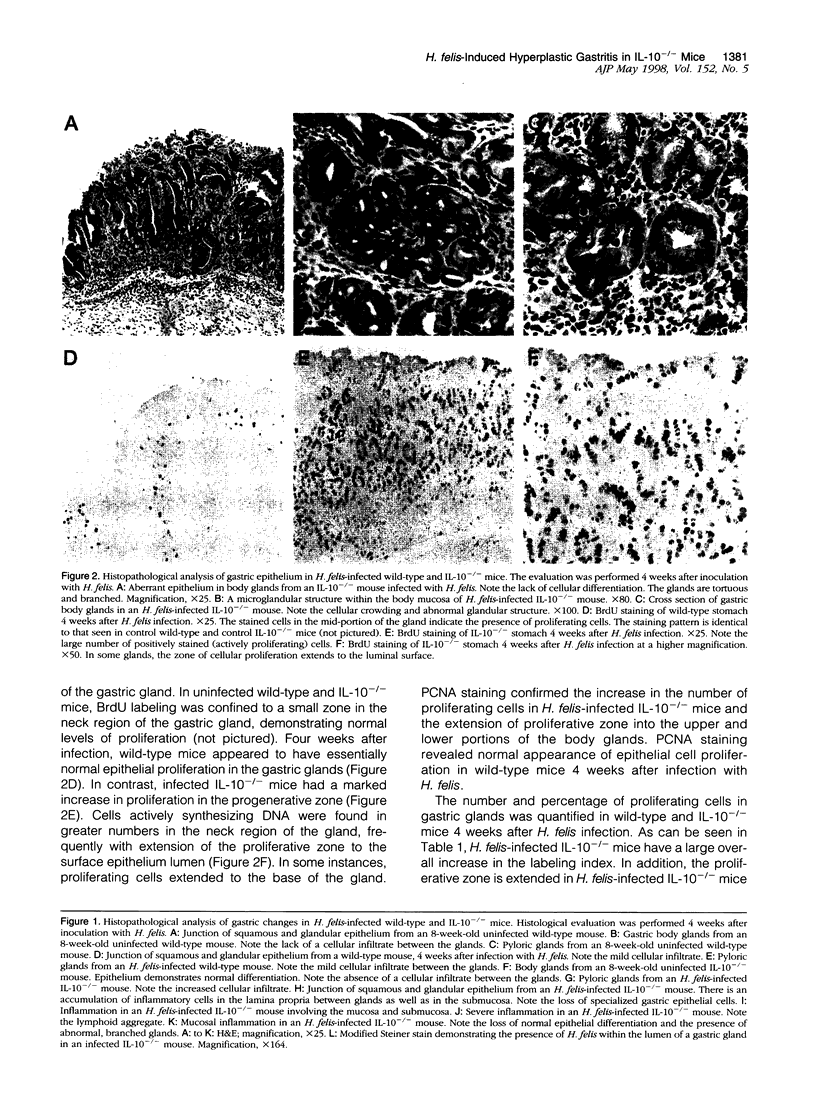
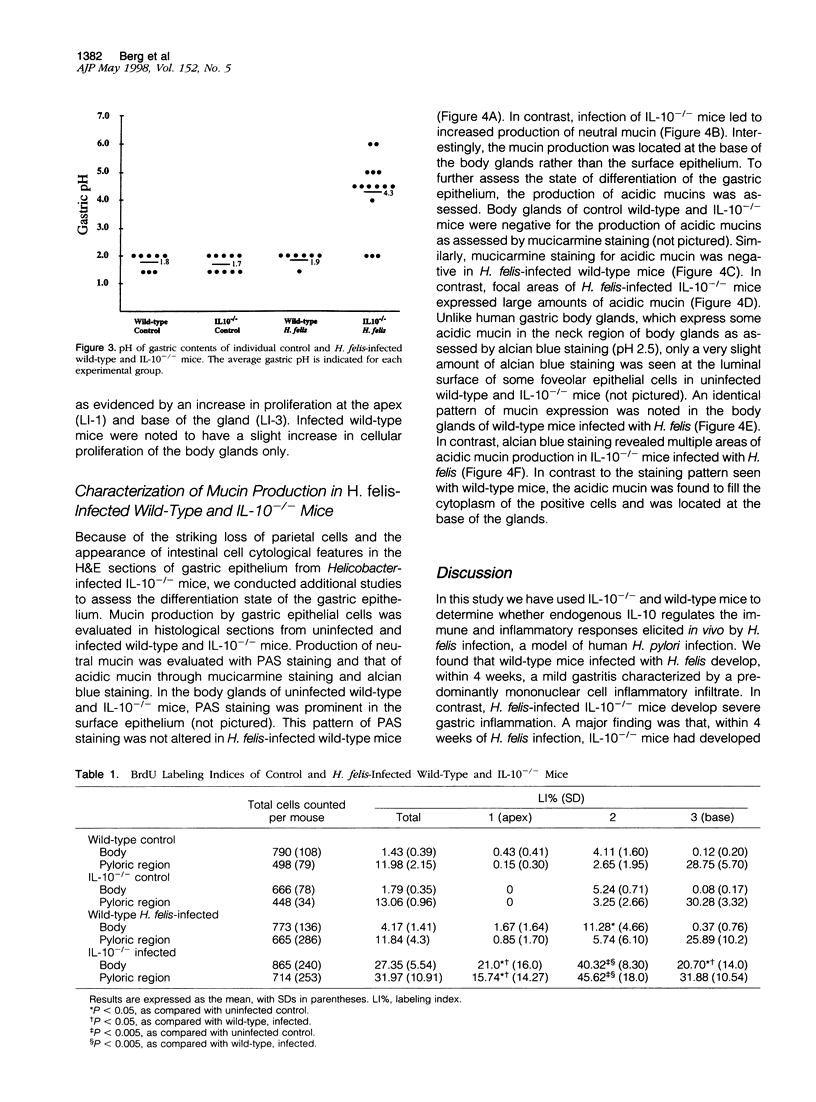
Abstract
Interleukin (IL)-10 is a potent anti-inflammatory and immune-regulatory cytokine. Mice deficient in IL-10 production (IL-10(-/-)) develop a spontaneous inflammatory bowel disease, indicating that IL-10 is an important regulator of the mucosal immune response in vivo. To study the role of IL-10 in the host response to gastric Helicobacter infection, stomachs of IL-10(-/-) and wild-type mice were colonized with Helicobacter felis, as a model of human H. pylori infection. Within 4 weeks of H. felis infection, wild-type mice develop a mild, focal chronic gastritis. In contrast, H. felis-infected IL-10(-/-) mice develop a severe hyperplastic gastritis, characterized by a dense, predominantly mononuclear cell inflammation of the mucosa and submucosa and epithelial cell proliferation and dedifferentiation. Within 4 weeks of H. felis infection, there are striking alterations in the character of the gastric epithelium from IL-10(-/-) mice, including a profound loss of parietal and chief cells, focal de novo production of acidic mucins, and marked epithelial proliferation with disordered epithelial architecture. These findings indicate that, in the absence of IL-10, the inflammatory and immunological responses of the murine host to gastric colonization with Helicobacter is a rapidly evolving pathological process with features that mimic those associated with H. pylori infection in humans. H. felis-infected IL-10(-/-) mice may provide a model with which to investigate the cellular and molecular changes that stem from gastric infection with H. pylori.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. J., Davidson N., Kühn R., Müller W., Menon S., Holland G., Thompson-Snipes L., Leach M. W., Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996 Aug 15;98(4):1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. J., Kühn R., Rajewsky K., Müller W., Menon S., Davidson N., Grünig G., Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995 Nov;96(5):2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkholz S., Knipp U., Nietzki C., Adamek R. J., Opferkuch W. Immunological activity of lipopolysaccharide of Helicobacter pylori on human peripheral mononuclear blood cells in comparison to lipopolysaccharides of other intestinal bacteria. FEMS Immunol Med Microbiol. 1993 Apr;6(4):317–324. doi: 10.1111/j.1574-695X.1993.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Birkholz S., Knipp U., Opferkuch W. Stimulatory effects of Helicobacter pylori on human peripheral blood mononuclear cells of H. pylori infected patients and healthy blood donors. Zentralbl Bakteriol. 1993 Sep;280(1-2):166–176. doi: 10.1016/s0934-8840(11)80953-9. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Parsonnet J. Parasitism by the "slow" bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994 Jul;94(1):4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauchle M., Angermeyer K., Hübner G., Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994 Nov;9(11):3199–3204. [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Inability of human clinical strains of Helicobacter pylori to colonize the alimentary tract of germfree rodents. Can J Microbiol. 1990 Apr;36(4):237–241. doi: 10.1139/m90-041. [DOI] [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 (Suppl 1):S37–S43. [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992 May 25;267(15):10570–10575. [PubMed] [Google Scholar]
- D'Elios M. M., Manghetti M., De Carli M., Costa F., Baldari C. T., Burroni D., Telford J. L., Romagnani S., Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997 Jan 15;158(2):962–967. [PubMed] [Google Scholar]
- Danon S. J., O'Rourke J. L., Moss N. D., Lee A. The importance of local acid production in the distribution of Helicobacter felis in the mouse stomach. Gastroenterology. 1995 May;108(5):1386–1395. doi: 10.1016/0016-5085(95)90686-x. [DOI] [PubMed] [Google Scholar]
- Davidson N. J., Leach M. W., Fort M. M., Thompson-Snipes L., Kühn R., Müller W., Berg D. J., Rennick D. M. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996 Jul 1;184(1):241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso A., Xiang Z., Bugnoli M., Pileri P., Figura N., Bayeli P. F., Rappuoli R., Abrignani S., De Magistris M. T. Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infect Immun. 1995 Mar;63(3):1102–1106. doi: 10.1128/iai.63.3.1102-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick-Hegedus E., Lee A. Use of a mouse model to examine anti-Helicobacter pylori agents. Scand J Gastroenterol. 1991 Sep;26(9):909–915. doi: 10.3109/00365529108996241. [DOI] [PubMed] [Google Scholar]
- Dixon M. F. Pathophysiology of Helicobacter pylori infection. Scand J Gastroenterol Suppl. 1994;201:7–10. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fox J. G., Blanco M., Murphy J. C., Taylor N. S., Lee A., Kabok Z., Pappo J. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infect Immun. 1993 Jun;61(6):2309–2315. doi: 10.1128/iai.61.6.2309-2315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Correa P., Taylor N. S., Zavala D., Fontham E., Janney F., Rodriguez E., Hunter F., Diavolitsis S. Campylobacter pylori-associated gastritis and immune response in a population at increased risk of gastric carcinoma. Am J Gastroenterol. 1989 Jul;84(7):775–781. [PubMed] [Google Scholar]
- Fox J. G., Lee A. The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals. Lab Anim Sci. 1997 Jun;47(3):222–255. [PubMed] [Google Scholar]
- Fox J. G., Li X., Cahill R. J., Andrutis K., Rustgi A. K., Odze R., Wang T. C. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996 Jan;110(1):155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- Karttunen R., Karttunen T., Ekre H. P., MacDonald T. T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995 Mar;36(3):341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipp U., Birkholz S., Kaup W., Mahnke K., Opferkuch W. Suppression of human mononuclear cell response by Helicobacter pylori: effects on isolated monocytes and lymphocytes. FEMS Immunol Med Microbiol. 1994 Feb;8(2):157–166. doi: 10.1111/j.1574-695X.1994.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L. Th1- and Th2-type cytokines regulate chemokine expression. Biol Signals. 1996 Jul-Aug;5(4):197–202. doi: 10.1159/000109190. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lee A., Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect Immun. 1994 Aug;62(8):3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. Helicobacter infections in laboratory animals: a model for gastric neoplasias? Ann Med. 1995 Oct;27(5):575–582. doi: 10.3109/07853899509002472. [DOI] [PubMed] [Google Scholar]
- Lee A. Helicobacter infections in laboratory animals: a model for gastric neoplasias? Ann Med. 1995 Oct;27(5):575–582. doi: 10.3109/07853899509002472. [DOI] [PubMed] [Google Scholar]
- Lee A., O'Rourke J., De Ungria M. C., Robertson B., Daskalopoulos G., Dixon M. F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997 Apr;112(4):1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- Lee A. The use of a mouse model in the study of Helicobacter sp.-associated gastric cancer. Eur J Gastroenterol Hepatol. 1994 Dec;6 (Suppl 1):S67–S71. [PubMed] [Google Scholar]
- Macatonia S. E., Doherty T. M., Knight S. C., O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993 May 1;150(9):3755–3765. [PubMed] [Google Scholar]
- Mannick E. E., Bravo L. E., Zarama G., Realpe J. L., Zhang X. J., Ruiz B., Fontham E. T., Mera R., Miller M. J., Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996 Jul 15;56(14):3238–3243. [PubMed] [Google Scholar]
- Mohammadi M., Czinn S., Redline R., Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996 Jun 15;156(12):4729–4738. [PubMed] [Google Scholar]
- Mohammadi M., Czinn S., Redline R., Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996 Jun 15;156(12):4729–4738. [PubMed] [Google Scholar]
- Moran A. P., Helander I. M., Kosunen T. U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J Bacteriol. 1992 Feb;174(4):1370–1377. doi: 10.1128/jb.174.4.1370-1377.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P. The role of lipopolysaccharide in Helicobacter pylori pathogenesis. Aliment Pharmacol Ther. 1996 Apr;10 (Suppl 1):39–50. doi: 10.1046/j.1365-2036.1996.22164004.x. [DOI] [PubMed] [Google Scholar]
- Muotiala A., Helander I. M., Pyhälä L., Kosunen T. U., Moran A. P. Low biological activity of Helicobacter pylori lipopolysaccharide. Infect Immun. 1992 Apr;60(4):1714–1716. doi: 10.1128/iai.60.4.1714-1716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini R., Savio A., Poiesi C., Appelmelk B. J., Buffoli F., Paterlini A., Cesari P., Graffeo M., Vaira D., Franzin G. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996 Sep;111(3):655–665. doi: 10.1053/gast.1996.v111.pm8780570. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Birkholz S., Andersen L. P., Moran A. P. Neutrophil activation by Helicobacter pylori lipopolysaccharides. J Infect Dis. 1994 Jul;170(1):135–139. doi: 10.1093/infdis/170.1.135. [DOI] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Paster B. J., Lee A., Fox J. G., Dewhirst F. E., Tordoff L. A., Fraser G. J., O'Rourke J. L., Taylor N. S., Ferrero R. Phylogeny of Helicobacter felis sp. nov., Helicobacter mustelae, and related bacteria. Int J Syst Bacteriol. 1991 Jan;41(1):31–38. doi: 10.1099/00207713-41-1-31. [DOI] [PubMed] [Google Scholar]
- Peek R. M., Jr, Miller G. G., Tham K. T., Perez-Perez G. I., Zhao X., Atherton J. C., Blaser M. J. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995 Dec;73(6):760–770. [PubMed] [Google Scholar]
- Phull P. S., Green C. J., Jacyna M. R. A radical view of the stomach: the role of oxygen-derived free radicals and anti-oxidants in gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995 Mar;7(3):265–274. [PubMed] [Google Scholar]
- Rennick D., Davidson N., Berg D. Interleukin-10 gene knock-out mice: a model of chronic inflammation. Clin Immunol Immunopathol. 1995 Sep;76(3 Pt 2):S174–S178. doi: 10.1016/s0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Riley L. K., Franklin C. L., Hook R. R., Jr, Besch-Williford C. Identification of murine helicobacters by PCR and restriction enzyme analyses. J Clin Microbiol. 1996 Apr;34(4):942–946. doi: 10.1128/jcm.34.4.942-946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami T., Dixon M., O'Rourke J., Howlett R., Alderuccio F., Vella J., Shimoyama T., Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996 Nov;39(5):639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami T., Vella J., Dixon M. F., O'Rourke J., Radcliff F., Sutton P., Shimoyama T., Beagley K., Lee A. The endotoxin of Helicobacter pylori is a modulator of host-dependent gastritis. Infect Immun. 1997 Aug;65(8):3310–3316. doi: 10.1128/iai.65.8.3310-3316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semeraro N., Montemurro P., Piccoli C., Muolo V., Colucci M., Giuliani G., Fumarola D., Pece S., Moran A. P. Effect of Helicobacter pylori lipopolysaccharide (LPS) and LPS derivatives on the production of tissue factor and plasminogen activator inhibitor type 2 by human blood mononuclear cells. J Infect Dis. 1996 Dec;174(6):1255–1260. doi: 10.1093/infdis/174.6.1255. [DOI] [PubMed] [Google Scholar]
- Sharma S. A., Miller G. G., Perez-Perez G. I., Gupta R. S., Blaser M. J. Humoral and cellular immune recognition of Helicobacter pylori proteins are not concordant. Clin Exp Immunol. 1994 Jul;97(1):126–132. doi: 10.1111/j.1365-2249.1994.tb06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., Blaser M. J. The epidemiology of Helicobacter pylori infection. Epidemiol Rev. 1991;13:42–59. doi: 10.1093/oxfordjournals.epirev.a036078. [DOI] [PubMed] [Google Scholar]
- Telford J. L., Ghiara P., Dell'Orco M., Comanducci M., Burroni D., Bugnoli M., Tecce M. F., Censini S., Covacci A., Xiang Z. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994 May 1;179(5):1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S., Kawano S., Tsujii M., Fusamoto H., Kamada T. Roles of hepatocyte growth factor and its receptor in gastric mucosa. A cell biological and molecular biological study. Dig Dis Sci. 1995 May;40(5):1132–1139. doi: 10.1007/BF02064211. [DOI] [PubMed] [Google Scholar]
- Tsujii M., Kawano S., Tsuji S., Ito T., Hayashi N., Horimoto M., Mita E., Nagano K., Masuda E., Hayashi N. Increased expression of c-met messenger RNA following acute gastric injury in rats. Biochem Biophys Res Commun. 1994 Apr 15;200(1):536–541. doi: 10.1006/bbrc.1994.1481. [DOI] [PubMed] [Google Scholar]
- Wadström T., Rydberg J., Rozalska B., Lelwala-Guruge J. Intravenous Helicobacter pylori induces low levels of TNF-alpha and IL-1 alpha in a murine model. APMIS. 1994 Jan;102(1):49–52. [PubMed] [Google Scholar]
- Wolf H. K., Dittrich K. L. Detection of proliferating cell nuclear antigen in diagnostic histopathology. J Histochem Cytochem. 1992 Sep;40(9):1269–1273. doi: 10.1177/40.9.1354677. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Kita M., Kodama T., Sawai N., Kashima K., Imanishi J. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand J Gastroenterol. 1995 Dec;30(12):1153–1159. doi: 10.3109/00365529509101624. [DOI] [PubMed] [Google Scholar]