Abstract
Neurophysiological and functional imaging experiments remain in apparent disagreement on the role played by the earliest stages of the visual cortex in supporting a visual percept. Here, we report electrophysiological findings that shed light on this issue. We monitored neural activity in the visual cortex of monkeys as they reported their perception of a high-contrast visual stimulus that was induced to vanish completely from perception on a subset of trials. We found that the spiking of neurons in cortical areas V1 and V2 was uncorrelated with the perceptual visibility of the target, whereas that in area V4 showed significant perception-related changes. In contrast, power changes in the lower frequency bands (particularly 9–30 Hz) of the local field potential (LFP), collected on the same trials, showed consistent and sustained perceptual modulation in all three areas. In addition, for the gamma frequency range (30–50 Hz), the responses during perceptual suppression of the target were correlated significantly with the responses to its physical removal in all areas, although the modulation magnitude was considerably higher in area V4 than in V1 and V2. These results, taken together, suggest that low-frequency LFP power in early cortical processing is more closely related to the representation of stimulus visibility than is spiking or higher frequency LFP activity.
Keywords: attention, perception, rivalry, V1, consciousness
What kind of neural processes underlie our basic subjective impression of a sensory stimulus? This question might be reserved for philosophical speculation were it not for a number of visual illusions where salient images are physically present, yet escape perception entirely (1–5). The existence of such phenomena illustrates that the contents of our conscious perception are not simply a reconstitution of the external world, but instead reflect internal processes in the brain that organize and interpret sensory patterns. In the last years, visual suppression paradigms have emerged as a powerful means to study the neuronal underpinnings of perception in both humans and nonhuman primates. The neural basis of binocular rivalry, for example, where dissimilar stimuli presented to the two eyes are alternately perceived as being perceptually dominant (6, 7), has been studied by using microelectrode recordings in animals (8–12) and humans (13), electroencephalography (14), magnetoencephalography (15), and functional magnetic resonance imaging (fMRI) (16–20).
The abundant research on this topic nonetheless has failed to provide a clear picture regarding the origin and expression of perceptual suppression at the neuronal level. Fundamental questions such as whether perceptual suppression is a consequence of activity changes in primary visual cortex (V1) remain a topic of intense debate. In general, single-cell recordings in this area and in adjacent extrastriate area V2 have found minimal modulation in neural firing rate during perceptual suppression (9, 11, 21), suggesting that the earliest cortical processing stages have little role in determining the perceptual visibility of a stimulus. In contrast, functional imaging (fMRI) studies have revealed a strong correlation of functional imaging signals with visibility in the corresponding cortical area of humans (17, 18, 22, 23). Although the basis of this discrepancy is unknown, it is possible that the local field potential might provide a link to perception, because it has been demonstrated to be more closely related to the fMRI signal than is spiking activity (24, 25). This possibility is attractive, because it could potentially reconcile single-unit recordings performed in monkeys with human neuroimaging results (11, 26) and might also provide an additional dimension for understanding how percepts are expressed in the brain.
In the present study, we address this issue in behaving monkeys, examining how spiking activity and the LFP power in different frequency bands are differentially affected by perceptual suppression. Using the paradigm of generalized flash suppression (GFS; ref. 5), we created stimuli in which salient visual targets subjectively disappeared on approximately half of the trials. We asked how the visibility of this pattern affected neural responses in the early visual cortical areas V1, V2, and V4. We report here that the local field potential (LFP) power at low frequencies (especially in the α-range, 9–14 Hz, and β-range, 15–30 Hz) is significantly and consistently decreased during periods of perceptual suppression throughout all three cortical areas. In contrast, perception-related changes in both the spiking and γ-range (30–50 Hz) LFP power were pronounced in area V4, but modest in V1 and V2. These findings, taken together, suggest that mechanisms shaping the contents of our perception may involve large-scale, coordinated processes that are most prominently reflected in low-frequency changes of the local field.
Results
We recorded multiunit activity (MUA) and LFPs from a total of 248 visually responsive sites in areas V1 (78), V2 (58), and V4 (112) in three hemispheres of three monkeys (see Materials and Methods and Table 1, which is published as supporting information on the PNAS web site). The behavioral paradigm is diagrammed in Fig. 1, which shows the structure of an individual trial. Shortly after the presentation of a salient target stimulus, a dense pattern of moving random dots was added abruptly to the regions of the screen surrounding the target. Monkeys were trained, initially with unambiguous stimuli, to respond whether the target disappeared on each trial (see Fig. 1B; see Supporting Text, which is published as supporting information on the PNAS web site, for details on the training). On trials where the target vanished, the animals released a lever. On trials where the target remained visible, they held the lever throughout. Only after the monkeys reached a criterion of ≈95% correct during these control trials were they tested with the ambiguous variants of GFS. In the present study, the stimulus parameters were adjusted to give an ≈50% disappearance probability, as determined with psychophysical testing (Fig. 2). During this testing, the monkeys, like human subjects (5), reported an increased probability of target disappearance with both increases in the density of the surrounding dots, and decreases in the size of the margin between the target and the dots. During neurophysiological testing, the ambiguous condition of interest was interleaved with a much larger fraction of unambiguous catch trials, for which the three animals responded with 94.5% accuracy on average.
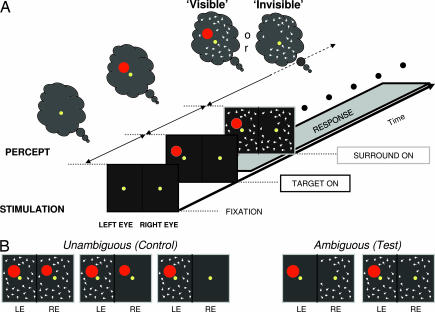
Fig. 1.
Illustration of GFS stimulation sequence and monkey task. (A) Structure of an (ambiguous) test trial. Monkeys fixated a central spot for 300 ms before the target stimulus (red disk) was presented parafoveally. After 1,400 ms of target presentation, a surrounding pattern consisting of randomly moving white dots was added to the screen. Monkeys were required to maintain fixation throughout the whole trial and to hold a lever as long as the target was visible. If the target became invisible, either through perceptual suppression or physical removal, the monkey released the lever and had to maintain fixation for an additional 800 ms to receive a juice reward. (B) Ocular configurations used to create ambiguous (test) and unambiguous (control) trials. In addition to these controls shown, other control trials involved physically removing the target. Compared with the numerous control trials, the test trials represented a relatively small fraction. This was done in order to ensure the monkey's proper behavior.
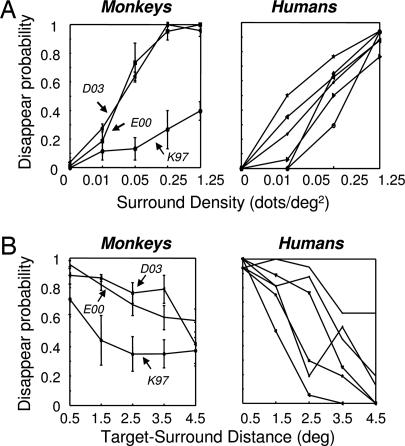
Fig. 2.
Comparison of psychophysical results for monkey and human observers. For the monkeys, the target consisted of a red monocular disk or gabor patch presented alone for 1,400 ms, followed by the additional presentation of a binocular surround (see Fig. 1B, ambiguous). For the humans, the target consisted of a red monocular disk presented for 2,000 ms before a binocular surround was added. In both cases, the targets were shown at an eccentricity of 1.4° in the left lower or upper quadrant or in the right lower or upper quadrant, respectively. Each point corresponds to the probability of disappearance within the first 1,200 ms after surround onset. Human subject data from ref. 5. (A) Effects of surround dot density on disappearance probability. (B) Effect of variable target-surround distance on disappearance probability (dot density 1.25 dots/deg2).
Spiking Responses Showed Minimal Modulation with Subjective Visibility.
We began by considering the spiking responses of neurons in cortical areas V1, V2, and V4 to the perceptual disappearance of the target, as reported by the monkey. Previous work with binocular rivalry has shown that neurons in these areas are only modestly affected when a preferred stimulus is perceptually suppressed, particularly in areas V1 and V2 (9, 12). In contrast to binocular rivalry, GFS does not rely on perceptual conflict between two stimuli occupying the same position in space but rather requires a more general conflict across the visual field such as the temporally asynchronous onset of nonoverlapping stimuli. We wondered whether a paradigm such as GFS, in which the disappearance of the target is not accompanied by the appearance of a competing pattern at the same position in space, would lead to a larger modulation of neural activity.
To address this question, we first identified sites for which the spiking activity (i.e., MUA) was modulated by the physical addition and removal of a target image. We then compared the MUA under GFS conditions when the target was perceptually suppressed, comparing it with when it remained visible. The population results are shown in Fig. 3. In contrast to our expectations, the magnitude of perceptual modulation in the MUA during GFS was even less than previously reported in binocular rivalry. In fact, Fig. 3 A and B shows that, in areas V1 and V2, the spiking activity during periods of perceptual suppression (orange) was statistically indistinguishable from that when the target remained subjectively visible (black). Only in area V4 was there clear and significant perceptual modulation as a function of the visibility of the target (two-sample t test, P < 0.01). Note that the data in Fig. 3A represent the population means from the subset of sites in each area that showed a decrease in spiking after the physical removal of the target. We used the response to the physical removal as a means to sort the data for two reasons. First, we found that there were approximately equal numbers of sites for which removal of the target produced excitatory and inhibitory responses, and when we pooled them for the population analysis, it was clear that we observed cancellation effects. Second, restricting analysis to sites showing a particular type of offset response (either positive or negative) provided a clear prediction regarding what might be observed during perceptual suppression. In contrast to Fig. 3A, the data in Fig. 3B represent only those sites that showed activity increases when the target pattern was physically removed, which represented more than half of the sites from which we recorded. These sites showed the same basic pattern of responses according to the reported percept, but with a difference in polarity reflecting the neurons' positive off-responses.
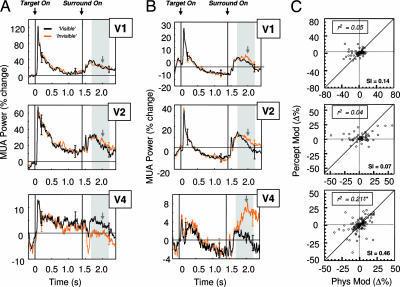
Fig. 3.
Perceptual modulation measured in the multiunit activity. (A) Grand mean of multiunit response (in percent change) during test trials, shown for areas V1 (n = 26), V2 (n = 20), and V4 (n = 46). Only sites showing negative responses to the physical removal of a stimulus are shown. Lines correspond to the mean activity for all sites (error bars: ±1 SEM) on trials in which the target was reported to disappear (orange) and on those in which it remained visible (black). Note that the mean activity differs between these two conditions. Gray arrows correspond to the mean latency of reported target disappearance by the monkey (mean latency: 611 ms; range: 492–814 ms). Gray shaded areas depict the time between 300 and 800 ms after surround onset, corresponding to the time interval considered for the correlation plots. (B) Same as A, but for sites showing positive responses to the physical removal of the stimulus, for areas V1 (n = 52), V2 (n = 38), and V4 (n = 66). (C) Scatter plots of all sites collected V1 (n = 78), V2 (n = 58), and V4 (n = 112), comparing the modulation during the physical removal of a stimulus with that observed during perceptual suppression. Again, these variables are only significantly correlated in area V4. Slopes of the regression are plotted at the bottom the figures (Sl). ∗∗, P < 0.01.
The perceptual and physical modulation of all sites in each area is shown as a scatterplot in Fig. 3C. Each point represents the modulation of a single site, with positive values corresponding to relative increases in activity, whereas negative values correspond to relative decreases. Note that there is a significant correlation between the degree (and sign) of physical and perceptual modulation in area V4, but that this is not the case in areas V1 and V2. This result demonstrates that for the area showing perceptual modulation, the degree of subjective modulation depends on the strength of activity changes in response to a physical target removal. This observation is consistent with previous single-unit studies demonstrating a cell-to-cell correlation between the strength of neural tuning and the degree of perceptual modulation (27).
Finally, additional analyses, presented in Fig. 6, which is published as supporting information on the PNAS web site, show that differences in the receptive field sizes per se are unlikely to account for the increased perceptual modulation in V4. Specifically, we found that within each area, the degree of modulation was uncorrelated with the receptive field size (except for area V2, where we found a weak but significant correlation). These results, taken together with the previous studies, strongly suggest that cortical mechanisms leading to the visibility of a salient pattern are reflected only minimally in the responses of neurons in the primary visual cortex.
Low-Frequency LFP Activity Reliably Correlates with Target Visibility.
We next analyzed the LFP signals in an analogous manner to the MUA to determine whether they better reflected changes in target visibility. We found that the power in the low frequencies (α and β, 9–30 Hz), in sharp contrast to the MUA activity, was strongly and reliably modulated by the perceptual visibility of the target. This can be seen in Fig. 4A, which shows the perceptual modulation in the α band. Fig. 4A Left shows that, in addition to an overall decrease in alpha power after the presentation of the surround stimulus, there is a significant and sizeable difference in the alpha power level depending on whether the target stimulus was perceived on a given trial. The three different areas showed different latencies with respect to their perceptual modulation. After the surround presentation, such modulation (corresponding to the separation between the black and orange traces) was observable first in V1, and then reached V2, and then V4, with increasing latencies.
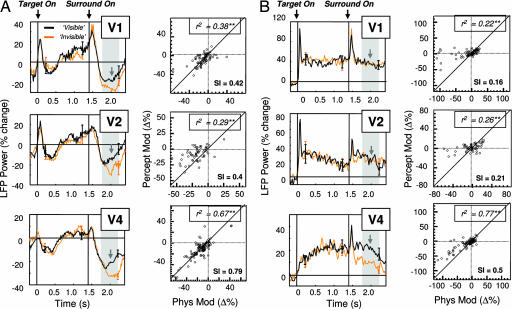
Fig. 4.
Perceptual modulation measured in the LFP power, same conventions as Fig. 3. (A Left) The mean power in the α-band range (9–14 Hz) is strongly modulated by the perceived visibility of the target on test trials. (A Right) There is a strong site-to-site correlation between the strength of the response to physical removal and that to perceptual suppression. The response magnitudes of physical and subjective modulation are approximately equal. (B) Gamma range (30–50 Hz) power modulation closely resembles the pattern observed in the MUA in Fig. 3, correlated in area V4. Slopes of the regression are plotted at the bottom the figures (Sl). ∗∗, P < 0.01.
The Fig. 4A Right compares, for each area, the modulation of individual sites during the physical and perceptual conditions. In the case of the alpha power, there was a very close correspondence between the degree of physical and perceptual modulation. Interestingly, in contrast to the MUA modulation above, physical removal and perceptual suppression nearly always involved a decrease in alpha power. This can be seen by the majority of points residing in the lower left quadrant. Thus, these data demonstrate that in areas V1, V2, and V4, modulation of activity in the alpha range strongly and reliably reflected the perceptual state. Might such an LFP signal provide clues regarding the nature of the aforementioned discrepancy between the lack of perceptual modulation observed in single-unit V1 and the clear modulation seen in the same area by using fMRI? We will return to this point later.
Gamma Frequency LFP Activity Correlates with Visibility in Area V4.
Fig. 4B shows modulation in the gamma range (30–50 Hz). Interestingly, we found that the pattern of gamma-range modulation did not resemble that of the alpha range but was instead very similar to that observed in the MUA traces shown in Fig. 3. Specifically, the gamma range power in V1 and V2 responded with a short latency burst after the onset of the surround but did not show any differences between trials in which the target was seen and those in which it was not. However, in V4, there was a significant and large, sustained difference in gamma power according to the perceptual state, resembling the pattern observed in the MUA, as shown in Fig. 3. In addition, there was a very strong correlation in area V4 between the magnitude of site-by-site modulation between the physical and perceptual conditions (Fig. 4B Bottom Right). Thus, gamma modulation in area V4 strongly reflected perceptual visibility for a subset of sites, a result that agrees with the pattern observed for both single-unit and multiunit recordings during binocular rivalry (9, 12). This correlation was weaker in areas V1 and V2 but still significant. However, the slope of the regressions was quite low, with sites changing their gamma power on average only 16% (V1) or 21% (V2) of that compared with the physical removal of the stimulus. In area V4, the magnitude of this modulation was ≈50%. Although the pattern of modulation and correlation was higher than that for the multiunit responses, the general pattern was similar.
Interestingly, in comparing the visibility modulation in V4 in the different frequency bands, it is clear that the modulation in the gamma and multiunit emerged several hundred milliseconds earlier than modulation in the alpha range. Accordingly, when we repeated our correlation analysis by calculating perceptual modulation during an earlier time window (200–500 ms after surround onset), we found that the correlation coefficients for the physical removal and subjective target disappearance remained approximately the same in the multiunit and gamma range, whereas coefficients were slightly smaller in the alpha range. This difference may be important for understanding how each of these signals relates to the visual percept per se or also may contribute to other cognitive aspects of the task such as attention.
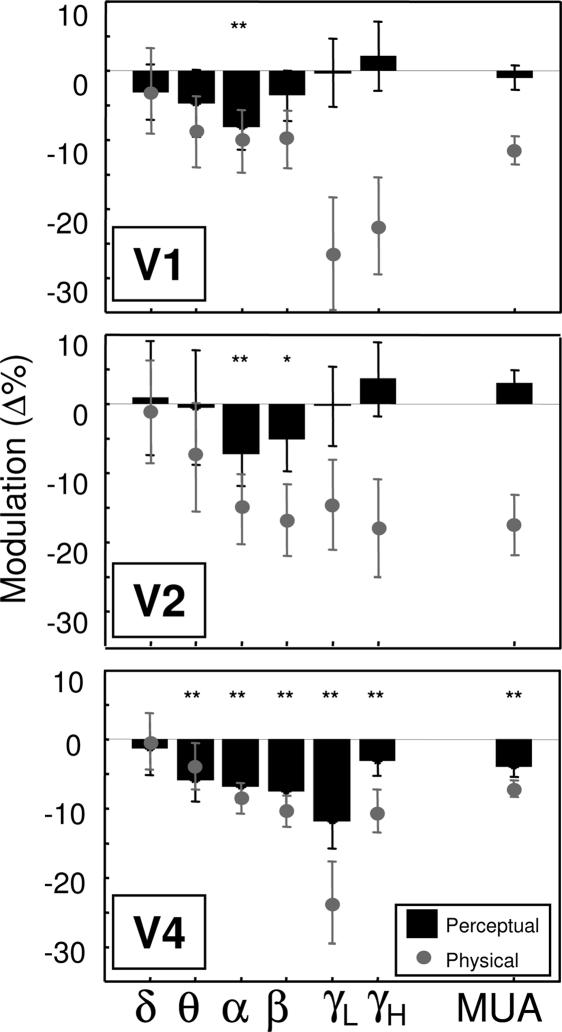
Perceptual modulation over several different frequency bands is shown in Fig. 5 for each of the areas. In this figure, it is possible to observe that not only is the perceptual modulation (black bars) strongest in the lower frequency bands in V1 and V2, but that it is nearly as strong as the physical removal (gray circles) of the stimulus in V1. Area V4 showed the strongest modulation overall, showing significant perceptual modulation for nearly every frequency band. As in Fig. 3A, the population data here were restricted to those sites showing decreased activity when the target was removed. Removal of this constraint led to cancellation effects (Fig. 7, which is published as supporting information on the PNAS web site), leading to difficult interpretation, particularly for the higher frequencies. Interestingly, the effects of the low frequencies were largely unaffected by pooling over all sites, as can be seen in the maintained α- and β-band modulation.
Fig. 5.
Perceptual modulation in all measured frequency bands in areas V1, V2, and V4. Positive values correspond to increased power during perceptual suppression, whereas negative values correspond to decreased power. Only sites are included for which the physical removal of a stimulus caused a decrease in the multiunit response. Error bars refer to the mean modulation computed over the period between 300 and 800 ms after surround onset. Asterisks depict the significance value for the visibility modulation (one-sample t test, ∗, P < 0.05; ∗∗, P < 0.01).
Discussion
We report a strong and widespread decrease of low frequency local field power in areas V1, V2, and V4 during the perceptual disappearance of a salient stimulus. This pattern was markedly different from spiking activity and gamma-range power, which both showed marked differences only in area V4, but not in areas V1 or V2. Because all signals were collected simultaneously from the same electrode, the observed differences cannot be attributed to sampling biases, fluctuations in animal performance, or instability in the recordings.
The modulation of spiking activity associated with subjective visibility was remarkably modest, given the complete subjective disappearance of the target. It was, in fact, even weaker than activity changes found in previous studies of binocular rivalry, where interocular competition dictates that the disappearance of a stimulus is always accompanied by the appearance of another stimulus at the same point in space (9, 11).
This absence of perceptual modulation in the spiking of neurons in V1 is not true for all forms of induced suppression, such as visual masking (4, 28–30). However, these studies have generally compared responses to a target in the presence and absence of a visual mask. It is interesting to note that when the stimulus is always identical, and responses to a weakly masked target are sorted based on the visibility reported by a monkey, perceptual modulation disappears (30). The present results, in combination with the other studies mentioned, support the notion that the spiking of neurons in the primary visual cortex is first and foremost determined by the structure of the sensory input.
Because analysis was restricted to multiunit signals, it cannot be excluded that we would have found more pronounced visibility-related modulation by optimally stimulating well isolated single neurons. And, although it is possible that the perceptual modulation we observed might be affected by the extent to which our stimuli matched the specific receptive field properties of the recorded neurons, the consistent differences between areas for a range of stimulus sizes and positions and the agreement with previous studies single unit studies suggest that the pattern of modulation cannot be attributed to our specific stimulus set.
Interestingly, gamma-range power showed nearly the same pattern of perceptual modulation across the different areas as the MUA responses. Similar to the spiking, significant gamma modulation was only measurable in area V4 and all but absent in the earlier areas. Whether this observation is the consequence of a generally tight correspondence between the gamma and MUA power, or whether each contributes uniquely in the representation of stimulus visibility in V4, remains unanswered. It is interesting, however, that the gamma-range power did not show more perceptual modulation in V1. Based on previous studies, we expected there to be more gamma-band modulation given the profound blood oxygenation level-dependent (BOLD) responses in V1 associated with perceptual changes (18, 20, 22), and the recently reported tight coupling between gamma range activity and the BOLD signal (31).
Much to our surprise, the power changes in the lower frequencies of the LFP signal were strongly and reliably correlated with the perceptual visibility of the target. Not only did these changes significantly reflect the perceptual suppression of the stimulus, but their amplitude was approximately the same as the control condition where the stimulus was physically removed. Thus the low-frequency LFP power, rather than the gamma or the MUA, appears to reflect the visibility of a stimulus in V1. It is interesting to consider whether these low-frequency LFP changes might reflect the fact that the attentional focus of the monkey shifts as soon as he detected the target disappearance? Although a contribution of attentional factors on the low-frequency LFP modulation during perceptual suppression cannot be excluded, especially because GFS is an asymmetrical paradigm, perceptual modulation was observed well before the lever response. Thereby, it seems at least unlikely that the neural modulation was directly related to the execution of the monkey response and, thereby, related to a general release of attention.
A previous study by Gail et al. (11) examined modulation in area V1 during binocular rivalry and reported perception-related changes in the low-frequency LFP but not in the MUA. Those findings previously have been difficult to interpret, because the timing of the perceptual modulations they observed was nearly synchronous with the monkeys' manual indications of a perceptual transition. Previous single-unit work has suggested that perceptual modulation during rivalry, when present, should appear several hundred milliseconds earlier, because manual reaction time to the perceptual change requires a certain delay (7). Interestingly, the present GFS results shed light on this puzzle, because the timing of the low-frequency modulation was also considerably later than the gamma and MUA modulation in area V4. Changes in this frequency range, it appears, do not impact the visual cortex until well after a new perceptual state has been initiated, perhaps via intervention from other cortical and possibly subcortical areas. Although this raises questions regarding the role of such activity in the formation of a visible percept, it may suggest that the maintenance of such a state is explicitly represented in the visual cortex. Interestingly, the present findings seem to indicate that area V1 is not involved in the formation of a visual percept, but is somehow invested in its maintenance.
Finally, given the large discrepancy between single-unit and BOLD fMRI studies in regard to the role of the primary visual cortex in attention and perception (6), it is interesting to speculate whether these alpha-range LFP fluctuations ultimately might provide a link between electrical activity and functional imaging responses. Although this is an attractive prospect, it is probably too early to draw this conclusion. Previous work examining alpha range electrical activity has combined EEG and fMRI techniques simultaneously. These studies have revealed that alpha-range electrical activity is indeed closely related to the BOLD signal in the cortex (32, 33). However, these studies have typically tracked power changes in the alpha rhythm (34), which may or may not bear relation to the changes in alpha power reported here, and have generally found a negative, rather than a positive, correlation in the cortex. Clearly additional studies are required to unravel the complex relationship between the various types of neural signals measured with a microelectrode, the BOLD signal measured by using fMRI, and brain mechanisms that create and support a visual percept.
Materials and Methods
Three adult male Macaca mulatta monkeys (E00, K97, and D03), weighing 6–14.5 kg, participated in the experiments. During each session, data were recorded while the animal reported target visibility while maintaining fixation. All experimental procedures were performed in accordance with the guidelines of the local authorities (Regierungspraesidium) as well as the European Community (EUVD 86/609/EEC) for the care and use of laboratory animals. Results from all animals were similar and are, unless otherwise mentioned, considered together. Details about the surgery, mapping procedure, animal training, and data acquisition are available in the Supporting Text.
Stimuli.
The GFS paradigm has been described in great detail in ref. 5. Briefly, when a monocular target pattern is followed after 1–2 seconds by a large, surrounding dynamic random dot pattern, either in the opposite eye or in perfect correspondence in the two eyes, the monocular target can have a high probability of completely disappearing, and remaining perceptually suppressed for several seconds. This phenomenon is highly robust to different stimulus/surround types, but also sensitive to patterns such as the dot density of the surround, distance between the surround and the target, and the ocular configuration of the surround and the target.
For the present study, both the target and surround were of high luminance contrast, and were always presented against a dark background. The surround always consisted of randomly moving dots. Dot count and target-surround distance varied between sessions. Stimulus sizes ranged between 0.6° and 3.2°. Generally, larger stimuli were chosen for more peripheral screen locations. Target surround distance ranged between 0.5° and 5.0°. In most sessions, the target consisted of a sinusoidal grating or a uniform disk (see Fig. 1A) with a size of 1.0° and a surround density of 1.25 dots per deg2 (dot speed = 10.8°/sec). “Optimal” and “suboptimal” target-surround distances were defined based on human/monkey psychophysical results leading to reliable or unreliable target suppression upon surround onset, respectively. The target position was selected based on receptive field properties mapped in each session for at least one of the recording sites. For additional details, see Supporting Text.
Behavioral Task.
We were most interested in measuring those changes in neural activity associated with purely perceptual changes and unpolluted by physical stimulus manipulations such as ocular configuration. To this end, all analysis related to the perceptual modulation was performed on responses to stimuli that remained physically identical within a session, but that were sorted according to the perceived visibility reported by the monkey. Note that suppression in GFS is, to a first approximation, all-or-none (5), with only the probability of disappearance changing as a function of the physical target and surround stimulus parameters. In optimizing the experimental design, stimulus parameters were chosen based on the monkeys' previous psychophysical performance, and we tailored the stimuli in a way that complete suppression of the target would be achieved on approximately half the trials. This was generally titrated within a session by starting with stimuli that favored target disappearance (e.g., a monocular target followed by a binocular surround, “ambiguous” in Fig. 1B), and then enlarging the target-surround distance to make the fraction disappearing and persisting targets approximately equal. Because enforcing the truthful responses of monkeys is challenging (35), a number of control measures were taken. First, during all experimental sessions, we used between 3 and 6 times more unambiguous catch trials, of the type described above, than ambiguous test trials. These catch trials are created by changing the ocular configuration of the target and surround. They are highly effective, as even experienced subjects cannot reliably discriminate the physical removal condition from perceptual suppression (5).
A typical test trial started with a warning tone followed by the onset of a fixation spot (0.15°). After the monkey maintained fixation for 300 ms (in some sessions 500 ms), the target was turned on. Monkeys were required to hold the lever at least during the time period before surround onset during which only the target was presented. Then, 1,400 ms after target onset, the surround stimulus was added to the target presentation. When the monkey reported target suppression by releasing the lever, the target stayed physically on the screen for additional 800 ms, and was then removed. In trials where the monkey did not release the lever within 4,000 ms after surround presentation (in some sessions the upper time limit was set to 2,000 ms), thereby indicating sustained target visibility, the target was physically removed from the screen and the monkey had to release the lever within an 800 ms time window to receive juice reward. Trials were automatically aborted when the monkey moved his eyes outside of the fixation window. In case of monocular stimulus presentation, the eye of target or/and surround presentation was randomly interleaved. Monkeys maintained fixation within a 0.5°–0.6° (radius) window around the fixation spot (see Supporting Text).
Data Analysis.
Neuronal recordings were conducted through a surgically implanted chamber situated over the lunate sulcus, allowing access to areas V1, V2, and V4. Recordings were made from three hemispheres of three monkeys (E00: 35 sessions; K97: 11 sessions; D03: 9 sessions). Details about the number of visually responsive recording sites in the different areas of all monkeys are listed in Table 1. Additional details about the microelectrode recordings are available in Supporting Text.
In the test condition, each trial was classified post hoc as either “visible” or “invisible,” based on the responses of the animal. For a trial to be considered invisible, the monkey had to release the lever within 1,000 ms after the onset of the surround. Trials in which the monkey continued to depress the lever >2,000 ms after surround onset were thus classified as visible. Trials in which the lever was released between 1,000 and 2,000 ms after the onset of the surround were not considered.
To facilitate comparison of the different neural signals, all multiunit and BLP data for a given channel were expressed as the percent change with respect to the baseline activity measured during the initial fixation period of each trial, in the interval between 50 ms to target onset
The modulation of the perceptual (and physical) modulation, as shown in Figs. 3B, 4, and 5, represent differences in the percent change activation for different conditions. The mean difference percentage was computed over the time interval 300–800 ms after the surround onset, where perceptual suppression typically occurred. The perceptual modulation is the difference in the percent modulation for the visible and invisible conditions, as reported by the monkey, whereas the physical control condition is the difference in the percent modulation between the physical removal of the stimulus and the visible condition.
Importantly, the computing the mean responses in the population followed a selection criterion based on the spiking responses to the physical removal of the target. Because approximately half of all multiunit sites responded with an activity increase to target removal during surround presentation, pooling all data would have resulted in cancellation of heterogeneous signals (see Results). This preselection of sites is in some ways analogous to the classification of “preferred” vs. “null” conditions in previous studies on binocular rivalry and other bistable stimuli (9, 10).
Supporting Information.
Additional details can be found in Figs. 8–11, which are published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank J. Werner, O. Nakrou, and A. Oeltermann for excellent technical assistance; Dr. D. Sheinberg for help with the visual stimulation; Dr. Y. Murayama for help with the data acquisition software; M. Augath for conducting the anatomical MRI scans; and Kai-Markus Mueller and Drs. A. R. Mitz and A. Maier for their very helpful comments on an earlier version of the manuscript. This work was supported by the Max-Planck-Society.
Abbreviations
- BOLD
blood oxygenation level-dependent
- fMRI
functional MRI
- GFS
generalized flash suppression
- LFP
local field potential
- MUA
multiunit activity.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Bonneh YS, Cooperman A, Sagi D. Nature. 2001;411:798–801. doi: 10.1038/35081073. [DOI] [PubMed] [Google Scholar]
- 2.Breitmeyer BG. Visual Masking: An Integrative Approach. New York: Oxford Univ Press; 1984. [Google Scholar]
- 3.Kanai R, Kamitani Y. J Cogn Neurosci. 2003;15:664–672. doi: 10.1162/089892903322307384. [DOI] [PubMed] [Google Scholar]
- 4.Macknik SL, Livingstone MS. Nat Neurosci. 1998;1:144–149. doi: 10.1038/393. [DOI] [PubMed] [Google Scholar]
- 5.Wilke M, Logothetis NK, Leopold DA. Neuron. 2003;39:1043–1052. doi: 10.1016/j.neuron.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Blake R, Logothetis NK. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 7.Leopold DA, Logothetis NK. Trends Cogn Sci. 1999;3:254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis NK, Schall JD. Science. 1989;245:761–763. doi: 10.1126/science.2772635. [DOI] [PubMed] [Google Scholar]
- 9.Leopold DA, Logothetis NK. Nature. 1996;379:549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- 10.Sheinberg DL, Logothetis NK. Proc Natl Acad Sci USA. 1997;94:3408–3413. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gail A, Brinksmeyer HJ, Eckhorn R. Cereb Cortex. 2004;14:300–313. doi: 10.1093/cercor/bhg129. [DOI] [PubMed] [Google Scholar]
- 12.Leopold DA, Maier A, Wilke M, Logothetis NK. In: Binocular Rivalry. Alais D, Blake R, editors. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 13.Kreiman G, Fried I, Koch C. Proc Natl Acad Sci USA. 2002;99:8378–8383. doi: 10.1073/pnas.072194099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown RJ, Norcia AM. Vision Res. 1997;37:2401–2408. doi: 10.1016/s0042-6989(97)00045-x. [DOI] [PubMed] [Google Scholar]
- 15.Tononi G, Srinivasan R, Russell DP, Edelman GM. Proc Natl Acad Sci USA. 1998;95:3198–3203. doi: 10.1073/pnas.95.6.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumer ED, Friston KJ, Rees G. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- 17.Polonsky A, Blake R, Braun J, Heeger DJ. Nat Neurosci. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- 18.Tong F, Engel SA. Nature. 2001;411:195–199. doi: 10.1038/35075583. [DOI] [PubMed] [Google Scholar]
- 19.Tong F, Nakayama K, Vaughan JT, Kanwisher N. Neuron. 1998;21:753–759. doi: 10.1016/s0896-6273(00)80592-9. [DOI] [PubMed] [Google Scholar]
- 20.Haynes JD, Rees G. Curr Biol. 2005;15:1301–1307. doi: 10.1016/j.cub.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Lehky SR, Maunsell JH. Vision Res. 1996;36:1225–1234. doi: 10.1016/0042-6989(95)00232-4. [DOI] [PubMed] [Google Scholar]
- 22.Haynes JD, Deichmann R, Rees G. Nature. 2005;438:496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wunderlich K, Schneider KA, Kastner S. Nat Neurosci. 2005;8:1595–1602. doi: 10.1038/nn1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logothetis NK. Philos Trans R Soc London B. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 26.Fries P, Roelfsema PR, Engel AK, Konig P, Singer W. Proc Natl Acad Sci USA. 1997;94:12699–12704. doi: 10.1073/pnas.94.23.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purushothaman G, Bradley DC. Nat Neurosci. 2005;8:99–106. doi: 10.1038/nn1373. [DOI] [PubMed] [Google Scholar]
- 28.Bridgeman B. Brain Res. 1980;196:347–364. doi: 10.1016/0006-8993(80)90400-x. [DOI] [PubMed] [Google Scholar]
- 29.Schiller PH. Vision Res. 1968;8:855–866. doi: 10.1016/0042-6989(68)90135-1. [DOI] [PubMed] [Google Scholar]
- 30.Lamme VA, Zipser K, Spekreijse H. J Cogn Neurosci. 2002;14:1044–1053. doi: 10.1162/089892902320474490. [DOI] [PubMed] [Google Scholar]
- 31.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 32.Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. NeuroImage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- 33.Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. NeuroImage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- 34.Berger H. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- 35.Leopold DA, Maier A, Logothetis NK. J Conscious Stud. 2003;10:115–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.