Abstract
A hypothetical gene with similarity to the ispD gene of Escherichia coli was cloned from Arabidopsis thaliana cDNA. The ORF of 909 bp specifies a protein of 302 amino acid residues. The cognate chromosomal gene consists of 2,071 bp and comprises 11 introns with a size range of 78–202 bp. A fragment comprising amino acid residues 76–302 was expressed in a recombinant E. coli strain. The protein was purified to homogeneity and was shown to catalyze the formation of 4-diphosphocytidyl-2C-methyl-d-erythritol from 2C-methyl-d-erythritol 4-phosphate with a specific activity of 67 μmol⋅min−1 mg−1. The Michaelis constants for 4-diphosphocytidyl-2C-methyl-d-erythritol and CTP were 500 μM and 114 μM, respectively.
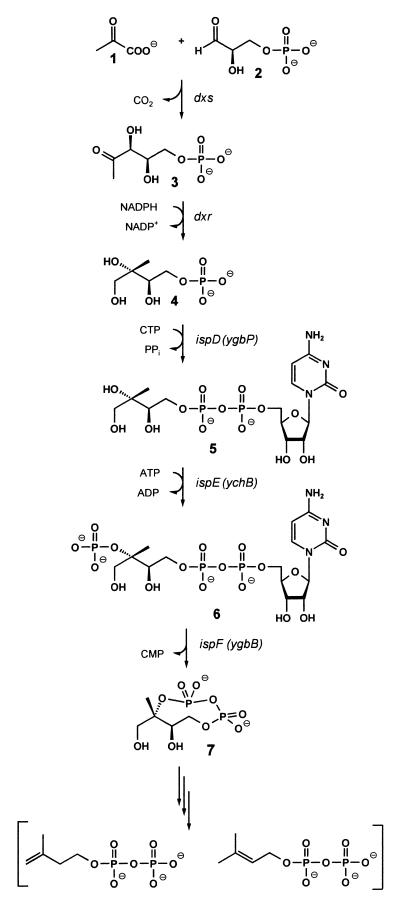
Independent studies by the research groups of Rohmer and Arigoni revealed the existence of a nonmevalonate pathway for the biosynthesis of terpenoids in certain bacteria (refs. 1, 2; for review, see refs. 3 and 4). Arigoni and coworkers observed the occurrence of this pathway in plants and demonstrated the incorporation of 1-deoxy-d-xylulose into terpenoids in plants and certain bacteria (2, 5).
More recently, it was found that 1-deoxy-d-xylulose 5-phosphate (3) biosynthesized from pyruvate (1) and glyceraldehyde 3-phosphate (2) serves as the first intermediate of the nonmevalonate pathway (6–9) and can be converted into 2C-methyl-d-erythritol 2,4-cyclodiphosphate (7) by the consecutive action of 1-deoxy-d-xylulose 5-phosphate reductoisomerase (10–13), 4-diphosphocytidyl-2C-methyl-d-erythritol synthase (14, 15), 4-diphosphocytidyl-2C-methyl-d-erythritol kinase (16), and 2C-methyl-d-erythritol 2,4-cyclodiphosphate synthase (17) (Fig. 1).
Figure 1.
The nonmevalonate pathway of isoprenoid biosynthesis.
The deoxyxylulose phosphate pathway supplies the isoprenoid moieties for the vast majority of plant terpenes. This paper describes the first plant ortholog of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase.
Experimental Procedures
Materials.
Oligonucleotides were custom synthesized by MWG Biotech, Ebersberg, Germany. 2C-Methyl-d-erythritol 4-phosphate was prepared as described earlier (18). The preparation of 13C-labeled 2C-methyl-d-erythritol 4-phosphate will be described elsewhere.
Microorganisms and Plasmids.
The bacterial strains and plasmids used in this study are summarized in Table 1.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristic | Reference or source |
|---|---|---|
| E. coli | ||
| XL1-Blue | RecA1, endA1, gyrA96, thi-1, hsdR17, supE44, relA1, lac, [F′, proAB, lacIqZΔM15, Tn10 (tetr)] | 20, Stratagene |
| M15(pREP4) | Lac, ara, gal, mtl, recA+, uvr+, [pREP4, lacI, kanr] | 21 |
| Plasmids | ||
| pQE30 | High copy His-tag expression vector | Qiagen |
| pNCO113 | High copy expression vector | ATTC, PTA-852 |
| pQEygbParakom | This study | |
| pNCOygbPara | This study |
Cloning and Expression of the ispD Gene of Arabidopsis thaliana.
RNA was isolated from 1 g (fresh weight) of A. thaliana var. Columbia plants by published procedures (19). A mixture containing 2.8 μg of RNA, 50 nmol of dNTPs, 1 μg of random hexameric primer, and 1 μg of T15 primer in 20% first strand ×5 buffer (Promega) in a total volume of 50 μl was incubated for 5 min at 95°C and then cooled on ice. Reverse transcriptase (Promega, 500 units) was added, and the mixture was incubated for 1 h at 42°C and subsequently at 92°C for 5 min. RNase A (20 units) and RNase H (2 units) were added, and the mixture was incubated for 30 min at 37°C.
PCR amplification by using the oligonucleotides ygbPara3 and ygbParahi1 (Table 2) as primers and A. thaliana cDNA as template afforded a 0.9-kb DNA fragment that was purified and used as PCR template with the oligonucleotides ygbPara4 and ygbParahi2 as primers. The resulting 0.9-kb fragment was purified by agarose gel electrophoresis, digested with BamHI and PstI, and ligated into the plasmid pQE30 (Qiagen, Hilden, Germany) that had been treated with the same enzymes. The resulting plasmid pQEygbParakom was electrotransformed into Escherichia coli strain XL1-Blue (20, Stratagene) yielding the strain XL1-pQEygbParakom.
Table 2.
Oligonucleotides used in this study
| Designation | Sequence |
|---|---|
| ygbParavo1 | 5′-TTGTTGTGAAGGAGAAGAGTG-3′ |
| ygbParahi1 | 5′-CATGCATACCCTTGACACGTC-3′ |
| ygbParavo2 | 5′-CAATGTTGTTGCCATGGAGAAG-3′ |
| ygbParahi2 | 5′-ACACGTCTTCTGCAGAAGTAAATG-3′ |
| ygbPara3 | 5′-CTTCTCTCAGGCGAGATAAAACATGG-3′ |
| ygbPara4 | 5′-GGCGAGAGGATCCATGGCGATGTCTCAGACG-3′ |
By using the same experimental approach, a 0.65-kb fragment was obtained by PCR amplification with the oligonucleotides ygbParavo1 and ygbParahi1 as primers and A. thaliana cDNA as template. Reamplification of the purified fragment with the oligonucleotides ygbParavo2 and ygbParahi2 afforded a DNA segment that was ligated into plasmid pNCO113 affording the plasmid pNCOygbPara. Electrotransformation of this plasmid into E. coli cells M15(pREP4) (21) yielded the recombinant E. coli strain M15-pNCOygbPara.
Assay of 4-Diphosphocytidyl-2C-methyl-d-erythritol Synthase Activity.
An indirect assay method for 4-diphosphocytidyl-2C-methyl-d-erythritol synthase was performed as described earlier (14).
Assay mixtures for direct detection of the enzyme product via HPLC contained 100 mM Tris⋅hydrochloride, pH 8.0, 5 mM MgCl2, 1 mM CTP, 1 mM 2C-methyl-d-erythritol 4-phosphate, 1 mM DTT, 0.1 unit inorganic pyrophosphatase, and protein in a total volume of 100 μl. The mixtures were incubated at 37°C for 20 min, heated to 80°C for 5 min, and centrifuged. Aliquots (10 μl) of the supernatant were applied to a Nucleosil RP 18 column (4.6 × 250 mm, Macherey & Nagel) that was developed with 4 mM tetra-N-butylammonium hydrogen sulfate in 20% (vol/vol) methanol. The flow rate was 2 ml/min. The effluent was monitored photometrically at 270 nm. The retention volume of 4-diphosphocytidyl-2C-methyl-d-erythritol was 4.36 ml.
Purification of 4-Diphosphocytidyl-2C-methyl-d-erythritol Synthase.
Cells of the recombinant E. coli strain M15-pNCOygbPara (1 g) were suspended in 10 ml of 50 mM Tris⋅hydrochloride, pH 8.0, containing 1 mM dithioerythritol and 0.02% sodium azide (buffer A). The suspension was submitted to ultrasonic treatment and centrifuged (Sorvall RC 5B Plus, 15,000 rpm, 40 min). The supernatant was loaded on top of a Source 15 Q column (1.6 × 10 cm, Amersham Pharmacia Biotech) that had been equilibrated with buffer A. The column was washed with 40 ml of buffer A and developed with a linear gradient of 0 − 1.0 M KCl in 200 ml of buffer A. Fractions were analyzed by SDS/PAGE, combined, and concentrated to 3 ml by ultrafiltration by using a Macrosep unit (10 k, Pall). The protein solution was loaded on top of a Superdex 200 column (2.6 × 60, Amersham Pharmacia Biotech) that had been equilibrated with 100 mM NaCl in buffer A at a flow rate of 3 ml/min. The retention volume was 203 ml. Fractions were combined and concentrated by ultrafiltration (10 k, Pall).
Sequence Determination.
DNA was sequenced by the automated dideoxynucleotide method by using a 377 Prism sequencer from Perkin–Elmer (21). N-terminal peptide sequences were obtained by Pulsed-Liquid Mode by using a PE Biosystems Model 492 (Perkin–Elmer).
NMR Spectroscopy.
NMR spectra were recorded by using an AVANCE DRX 500 spectrometer from Bruker, Karlsruhe, Germany. The transmitter frequencies were 500.1 MHz and 125.6 MHz for 1H and 13C, respectively. Chemical shifts were referenced to external trimethylsilylpropane sulfonate. Two-dimensional correlation experiments (gradient enhanced double quantum filtered correlated spectroscopy, heteronuclear multiple quantum correlation) were performed by using xwinnmr software from Bruker. 31P NMR spectra were recorded by using an AC 250 spectrometer from Bruker at a transmitter frequency of 101.3 MHz. Chemical shifts were referenced to external 85% H3PO4.
Analytical Ultracentrifugation.
Hydrodynamic studies were performed with an analytical ultracentrifuge Optima XL-I (Beckman Coulter) equipped with UV and interference optics. Experiments were performed with double sector cells equipped with aluminum centerpieces and sapphire windows. Partial specific volumes and buffer densities were estimated according to published procedures (23).
Results
Cloning and Expression of the ispD Gene of A. thaliana.
A similarity search by using the sequence of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase of E. coli (GenBank accession no. AF230736) and the blastp algorithm retrieved a hypothetical amino acid sequence predicted by a genomic DNA sequence of A. thaliana (GenBank accession no. AC004136). The putative ORF was cloned from A. thaliana cDNA.
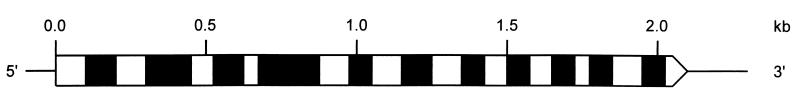
A comparison of the chromosomal gene (GenBank AC004136) with the cDNA reveals 11 introns ranging from 78 to 202 bp and a combined length of 1,164 bp (Fig. 2, Table 3).
Figure 2.
Intron/exon topology of the ispD gene of A. thaliana.
Table 3.
Intron–exon topology of the ispD gene of A. thaliana
| Intron no. | 5′ Sequence | Basepair position | 3′ Sequence |
|---|---|---|---|
| 1 | AG|GCAATTTCTCATACT | 110–201 | ATGTTGATTTTTCAG|TT |
| 2 | AT|GTAAGTGTTAGTTCA | 300–455 | TCTAATGTTTTTCAG|AG |
| 3 | AA|GTAAGTGTTTTTGGG | 528–621 | ATGTAATGTGAATAG|AT |
| 4 | AG|GTAACATTTCTTACT | 678–880 | GTTTCATTGTTGCAG|CT |
| 5 | AG|GTTTTTTAGTCTCTC | 960–1055 | TTTGTGGTGTTCTAG|AA |
| 6 | AG|GTTTATACCTCCGTT | 1139–1254 | GTTTATTTCTCATAG|GA |
| 7 | AG|GTATACTTATGAGAA | 1333–1425 | TTGTATTTTGTTAAG|GT |
| 8 | AG|GTACAAAATCTTAAG | 1495–1579 | TTTTGCGATATGCAG|GT |
| 9 | AG|GTTTTAAAGTATACT | 1647–1722 | ATGCTTTTTCTGTAG|GT |
| 10 | AG|GTTTGTGAACCTTCA | 1768–1854 | CTATATCTAAAACAG|TG |
| 11 | AG|GTAACAAAACACTAA | 1946–2024 | TCTCATCCATTGCAG|GT |
Consensus residues (24) are shown in bold type. Splice sites that had not been predicted correctly by computer analysis of the chromosomal gene sequence are underlined.
The cDNA sequence predicts an ORF of 909 bp specifying a peptide of 302 amino acids with a calculated mass of 35.5 Da (Fig. 3). An initial attempt to express the entire ORF preceded by the sequence MRGSH6 afforded recombinant E. coli cell extracts with a 4-diphosphocytidyl-2C-methyl-d-erythritol activity of 0.08 μmol⋅min−1 mg−1, well above the endogenous enzyme level of the host strain. SDS/PAGE indicated the presence of a recombinant protein accounting for about 1% of total protein and an apparent molecular mass of 41 kDa (Fig. 4, lane C).
Figure 3.
ORF and predicted amino acid sequence of the 4-diphosphocytidyl-2C-methyl-d-erythritol synthase gene of A. thaliana. The amino acid sequence of the pseudomaturated catalytic domain is shaded.
Figure 4.
SDS/PAGE. Lane A, molecular weight markers; B, cell extract of E. coli XL1-Blue; C, cell extract of recombinant E. coli expressing the full-length 4-diphosphocytidyl-2C-methyl-d-erythritol synthase of A. thaliana; D, cell extract of recombinant E. coli expressing the catalytic domain of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase of A. thaliana; E, catalytic domain of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase after Source 15 Q chromatography; catalytic domain of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase after Superdex G 200 gel filtration.
Sequence comparison suggested that the N terminus of the plant protein may serve as a plastid targeting sequence and may not be part of the catalytically active enzyme inside the plastids. A hypothetical cleavage site at amino acid residue 61 was predicted by the program chlorop V1.1 (27).§
A recombinant E. coli strain M15-pNCOygbPara directing the expression of amino acid residues 76–302 afforded cell extract with a 4-diphosphocytidyl-2C-methyl-d-erythritol synthase activity of 33 μmol⋅min−1 mg−1. SDS gel electrophoresis showed the presence of an abundant protein with an apparent mass of 29 kDa that was purified to homogeneity as described under Experimental Procedures (Fig. 4). A typical purification procedure is summarized in Table 4. The pure protein had a specific activity of 67 μmol⋅min−1·mg−1. All experiments described below were performed with that pseudomaturated protein.
Table 4.
Purification of recombinant 4-diphosphocytidyl-2C-methyl-d-erythritol synthase from A. thaliana (pseudomaturated protein comprising residues 76–302)
| Procedure | Total protein, mg | Specific activity, μmol⋅min−1· mg−1 | Total activity, μmol⋅min−1 | Yield, % | Purification factor |
|---|---|---|---|---|---|
| Cell extract | 128 | 33 | 4,224 | 100 | 1 |
| Source 15 Q | 17.5 | 67 | 1,172 | 28 | 2 |
| Superdex G 200 | 9.5 | 67 | 637 | 15 | 2 |
Structure Determination of the Enzyme Product.
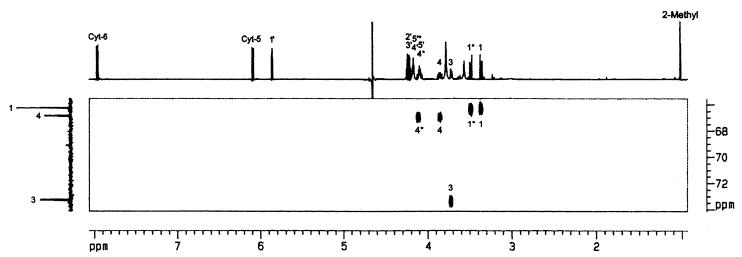
To assess the structure of the enzyme product, experiments were performed with [1,3,4-13C1]2C-methyl-d-erythritol 4-phosphate as substrate. The product of the reaction was isolated by HPLC and analyzed by NMR spectroscopy. The 13C NMR spectrum was dominated by three intense signals at 66.3 ppm, 66.9 ppm, and 73.3 ppm, reflecting carbon atoms that had acquired 13C label from the 13C enriched substrate (Fig. 5). The signals at 66.9 ppm and 73.3 ppm displayed 13C31P coupling of 7 Hz and 6 Hz, respectively. A two-dimensional 1H13C correlation experiment (heteronuclear multiple quantum correlation) revealed 1H NMR chemical shifts of 1H atoms coupled to 13C via one bond (1JCH) (Fig. 5). The observed coupling patterns as well as 1H, 13C, and 31P NMR chemical shifts were identical with the values reported earlier for 4-diphosphocytidyl-2C-methyl-d-erythritol (14). It follows that the recombinant A. thaliana protein catalyzes the transfer of a cytidyl phosphate group to 2C-methyl-d-erythritol 4-phosphate.
Figure 5.
Two-dimensional heteronuclear multiple quantum correlation spectrum of [1,3,4-13C]4-diphosphocytidyl-2C-methyl-d-erythritol (1H and 13C NMR signal assignments from ref. 14).
Properties of Recombinant 4-Diphosphoctidyl-2C-methyl-d-erythritol Synthase.
The enzyme displayed linear Lineweaver plots. The KM values of the recombinant enzyme for CTP and 2C-methyl-d-erythritol 4-phosphate were 114 μM and 500 μM, respectively, and vmax was 67 μmol⋅min−1 mg−1 (Table 5, Fig. 6). The enzyme could use several nucleotide triphosphates as substrates besides CTP; the maximum catalytic rate was found with CTP as substrate (Table 6).
Table 5.
Properties of the catalytic domain of recombinant 4-diphosphocytidyl-2C-methyl-d-erythritol synthase from A. thaliana and E. coli
| Property | Species
|
|
|---|---|---|
| A. thaliana | E. coli* | |
| vmax, μmol⋅min−1·mg−1 | 67 | 23 |
| Turnover number (s−1 per subunit) | 26 | 9 |
| KM for CTP, μM | 114 | 3 |
| KM for 2C-methyl-d-erythritol 4-phosphate, μM | 500 | 131 |
| Predicted subunit mass, Da | 25.472 | 25.737 |
Data from ref. 14.
Figure 6.
Double reciprocal Lineweaver–Burk plot of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase from A. thaliana. (A) Various amounts of CTP; (B) various amounts of 2C-methyl-d-erythritol 4-phosphate.
Table 6.
Substrate specificity of recombinant catalytic domain of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase from A. thaliana
| Substrate | Relative activity, % | Retention volume of product, ml |
|---|---|---|
| CTP | 100 | 2.5 |
| ATP | 3.9 | 3.6 |
| UTP | <0.1 | ND* |
| GTP | <0.1 | ND |
| ITP | <0.1 | ND |
| dTTP | <0.1 | ND |
| dGTP | <0.1 | ND |
| dCTP | 8.9 | 2.6 |
| dATP | 5.3 | 4.2 |
ND, not determined.
The recombinant plant enzyme has an absolute requirement for a divalent metal ion for catalytic activity. The maximum activity was observed with Mg2+ (Table 7).
Table 7.
Activation of the recombinant catalytic domain of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase from A. thaliana by divalent cations
| Metal ion | Relative activity, % |
|---|---|
| Mg2+ | 100 |
| Ni2+ | 91.4 |
| Mn2+ | 91.0 |
| Co2+ | 60.5 |
| Ca2+ | 47.2 |
| Cu2+ | 2.3 |
| Zn2+ | <0.1 |
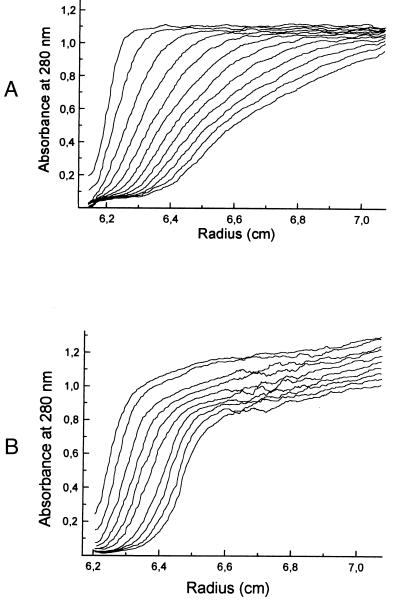
Analytical ultracentrifugation studies gave a complex picture of a protein undergoing reversible association on the time scale of hydrodynamic experiments. Sedimentation experiments in 50 mM Tris⋅hydrochloride, pH 8.0 (Fig. 7A) show a rapidly broadening boundary with components sedimenting at velocities ranging from about 4 to 17 S. Sedimentation experiments in buffer containing 20 mM potassium citrate and 200 mM potassium phosphate, pH 6.6, show a relatively narrow boundary at an approximate sedimentation velocity of 4 S, but the presence of more rapidly sedimenting components is also apparent (Fig. 7B). Apparent molecular weights observed in sedimentation equilibrium experiments were highly variable with different protein concentrations and rotor speeds.
Figure 7.
Boundary sedimentation of the recombinant catalytic domain of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase from A. thaliana at 60,000 rpm and 20°C. (A) Low-salt buffer (50 mM Tris⋅hydrochloride, pH 8.0; (B) high-salt buffer (20 mM potassium citrate/200 mM potassium phosphate, pH 6.6). Scans were obtained at intervals of 5 min. Absorbance was monitored at 280 nm.
Discussion
The sequence of the chromosomal ispD gene of A. thaliana (GenBank accession no. AC004136) had been determined earlier in the context of whole genome sequencing. However, only 7 of 12 exons had been predicted correctly by computer analysis of the genomic sequence. Three exons had not been predicted at all, and two exons had been predicted with incorrect boundaries. Moreover, the computer had predicted a nonexisting exon. In retrospect, the relatively low success of the computer prediction is easily explained by the DNA sequences of several intron/exons boundaries that were found to deviate substantially from the A. thaliana consensus sequence (24).
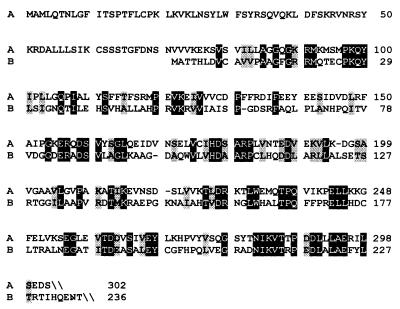
The amino acid sequence of 4-diphosphocytidyl-2C-methyl-d-erythritol synthase of A. thaliana comprises a putative plastid transit leader sequence, well in line with the assumed plastid location of the deoxyxylulose phosphate pathway (5, 25, 26). The catalytic domain of the plant enzyme is about 30% identical with the E. coli enzyme (Fig. 8).
Figure 8.
Alignment of deduced amino acid residues of 4-diphosphocytidyl-2C-methyl-d-erythritol synthases. Lane A, A. thaliana [AF230737]; Lane B, E. coli [AF230736]. Identical residues are shown in inverse contrast; similar residues are shaded.
The catalytic rates of 4-diphosphocytidyl-2C-methylerythritol synthases from A. thaliana and E. coli are similar (Table 5). The A. thaliana enzyme can use several nucleotide triphosphates besides CTP as substrates with significant rates. It is as yet unknown whether each of the respective products can serve as substrates for the consecutive enzyme reaction catalyzed by 4-diphosphocytidyl-2C-methyl-d-erythritol kinase.
Analytical ultracentrifugation experiments show that the recombinant protein has a tendency for self-aggregation depending on the buffer system (Fig. 7). However, it should be noted that the recombinant protein domain described in this paper is most probably not strictly identical with the chloroplast form of the enzyme, because the cleavage site for processing has not yet been determined.
Acknowledgments
This work is dedicated to Professor Horst Kessler on the occasion of his 60th birthday. We thank A. Werner and F. Wendling for help with the preparation of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 369), the Fonds der Chemischen Industrie, and the Hans-Fischer-Gesellschaft.
Footnotes
References
- 1.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broers S T J. Ph.D. thesis. Zürich, Switzerland: Eidgenössiche Technische Hochschule; 1994. [Google Scholar]
- 3.Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk M, Bacher A. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- 4.Rohmer M. In: Comprehensive Natural Products Chemistry. Barton D, Nakanishi K, editors. Vol. 2. Oxford: Pergamon; 1999. pp. 45–68. [Google Scholar]
- 5.Schwarz M K. Ph.D. thesis. Zürich, Switzerland: Eidgenössiche Technische Hochschule; 1994. [Google Scholar]
- 6.Sprenger G A, Schörken U, Wiegert T, Grolle S, deGraaf A A, Taylor S V, Begley T P, Bringer-Meyer S, Sahm H. Proc Natl Acad Sci USA. 1997;94:12857–12862. doi: 10.1073/pnas.94.24.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lois L M, Campos N, Putra S R, Danielsen K, Rohmer M, Boronat A. Proc Natl Acad Sci USA. 1998;95:2105–2110. doi: 10.1073/pnas.95.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange B M, Wildung M R, McCaskill D, Croteau R. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouvier F, d'Harlingue A, Suire C, Backhaus R A, Camara B. Plant Physiol. 1998;117:1423–1431. doi: 10.1104/pp.117.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi S, Kuzuyama T, Watanabe H, Seto H. Proc Natl Acad Sci USA. 1998;95:9879–9884. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange B M, Croteau R. Arch Biochem Biophys. 1999;365:170–174. doi: 10.1006/abbi.1999.1168. [DOI] [PubMed] [Google Scholar]
- 12.Schwender J, Müller C, Zeidler J, Lichtenthaler H K. FEBS Lett. 1999;455:140–144. doi: 10.1016/s0014-5793(99)00849-2. [DOI] [PubMed] [Google Scholar]
- 13.Jomaa H, Wiesner J, Sanderbrand S, Altinicicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenthaler H K, et al. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 14.Rohdich F, Wungsintaweekul J, Fellermeier M, Sagner S, Herz S, Kis K, Eisenreich W, Bacher A, Zenk M H. Proc Natl Acad Sci USA. 1999;96:11758–11763. doi: 10.1073/pnas.96.21.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzuyama T, Takagi M, Kaneda K, Dairi T, Seto H. Tetrahedron Lett. 2000;41:703–706. [Google Scholar]
- 16.Lüttgen H, Rohdich F, Herz S, Wungsintaweekul J, Hecht S, Schuhr C A, Fellermeier M, Sagner S, Zenk M H, Bacher A, et al. Proc Natl Acad Sci USA. 2000;97:1062–1067. doi: 10.1073/pnas.97.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herz S, Wungsintaweekul J, Schuhr C A, Hecht S, Lüttgen H, Sagner S, Fellermeier M, Eisenreich W, Zenk M H, Bacher A, et al. Proc Natl Acad Sci USA. 2000;97:2486–2490. doi: 10.1073/pnas.040554697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kis K, Wungsintaweekul J, Eisenreich W, Zenk M H, Bacher A. J Org Chem. 2000;65:587–592. doi: 10.1021/jo9905118. [DOI] [PubMed] [Google Scholar]
- 19.Logemann J, Schell J, Willmitzer L. Anal Biochem. 1987;163:303–304. [Google Scholar]
- 20.Bullock W O, Fernandez J M, Short J M. BioTechniques. 1987;5:376–379. [Google Scholar]
- 21.Zamenhof P J, Villarejo M. J Bacteriol. 1972;110:171–178. doi: 10.1128/jb.110.1.171-178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanger F, Nicklen S, Coulson A R. Proc Acad Natl Sci USA. 1977;74:5463–5468. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane T M, Shah B D, Ridgeway T M, Pelletier S L. In: Analytical Ultracentrifugation in Biochemistry and Polymer Science. Harding S E, Rowe A J, Horton J C, editors. Cambridge: Royal Society of Chemistry; 1992. pp. 90–125. [Google Scholar]
- 24.Simpson G, Filipowicz W. Plant Mol Biol. 1996;32:1–41. doi: 10.1007/BF00039375. [DOI] [PubMed] [Google Scholar]
- 25.Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk M H. Proc Natl Acad Sci USA. 1997;94:10600–10605. doi: 10.1073/pnas.94.20.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtenthaler H K, Schwender J, Disch A, Rohmer M. FEBS Lett. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- 27.Emanuelsson O, Nielsen H, von Heijne G. Protein Sci. 1999;8:978–984. doi: 10.1110/ps.8.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]