Abstract
A site-specific recombination system that probes the relative probabilities that pairs of chromosomal loci collide with one another in living cells of budding yeast was used to explore the relative contributions of pairing, recombination, synaptonemal complex formation, and telomere clustering to the close juxtaposition of homologous chromosome pairs during meiosis. The level of Cre-mediated recombination between a pair of loxP sites located at an allelic position on homologous chromosomes was 13-fold greater than that between a pair of loxP sites located at ectopic positions on nonhomologous chromosomes. Mutations affecting meiotic recombination initiation and the processing of DNA double-strand breaks (DSBs) into single-end invasions (SEIs) reduced the levels of allelic Cre-mediated recombination levels by three- to sixfold. The severity of Cre/loxP phenotypes is presented in contrast to relatively weak DSB-independent pairing defects as assayed using fluorescence in situ hybridization for these mutants. Mutations affecting synaptonemal complex (SC) formation or crossover control gave wild-type levels of allelic Cre-mediated recombination. A delay in attaining maximum levels of allelic Cre-mediated recombination was observed for a mutant defective in telomere clustering. None of the mutants affected ectopic levels of recombination. These data suggest that stable, close homolog juxtaposition in yeast is distinct from pre-DSB pairing interactions, requires both DSB and SEI formation, but does not depend on crossovers or SC.
Keywords: Meiosis, homolog pairing, recombination, synaptonemal complex, budding yeast
During meiosis, chromosomes are segregated sequentially in two different ways. In the first meiotic division, homologous chromosomes segregate from one another. In the second meiotic division, as in mitosis, sister chromatids segregate from one another. Interactions between homologous chromosomes develop and are stabilized throughout meiosis I prophase at both the DNA level and at the homolog-axis level. Events contributing to these interactions include pairing, recombination, synapsis, and telomere clustering (for review, see Roeder 1997; Zickler and Kleckner 1998, 1999; Paques and Haber 1999; Walker and Hawley 2000; Villeneuve and Hillers 2001; Burgess 2002). Ultimately, the formation of crossover DNA products ensures the accurate reductional segregation of chromosomes in the first meiotic division.
In budding yeast, transient, unstable homolog pairing interactions can be detected in premeiotic cells and during meiosis I prophase when analyzed by fluorescent in situ hybridization (FISH). For this assay, physical connections between homologs are inferred from their ability to limit the distance two loci separate from one another in chromosome spreads prepared from osmotically lysed cells (Weiner and Kleckner 1994). Pairing contacts formed during meiotic prophase occur even in the absence of recombination and synapsis (see below; Loidl et al. 1994; Weiner and Kleckner 1994; Nag et al. 1995; Rockmill et al. 1995; Cha et al. 2000). Premeiotic and meiotic pairing are separated by a period of unpairing during DNA replication (Weiner and Kleckner 1994).
Meiotic recombination is initiated by the formation of double-strand breaks (DSBs) after DNA replication and at about the same time that meiotic pairing is detected (for review, see Keeney 2001). The 5′ ends of the broken DNA are resected to reveal 3′ single-stranded DNA tails that subsequently interact with the homolog via single-end invasions (SEIs) (Sun et al. 1991; Hunter and Kleckner 2001). SEIs differentiate into crossover and noncrossover recombination events, with the former likely involving a double-Holliday junction (dHJ) intermediate (Schwacha and Kleckner 1995; Allers and Lichten 2001; Hunter and Kleckner 2001).
In parallel to the DNA events of recombination there is a progression of structural/axial events that have been defined by electron microscopy (EM) and by fluorescent immunocytology (for review, see Roeder 1997; Zickler and Kleckner 1999). Meiotic chromosomes are arranged in linear arrays of chromatin loops whose bases form a structural axis that is elaborated by proteins during meiotic prophase (Moens and Pearlman 1988). The axes of two homologs are ultimately connected along their lengths by the synaptonemal complex (SC). Axial elements, which will become the lateral elements of the SC, are formed concomitant with DSB formation (Padmore et al. 1991). The transverse elements of the SC, which hold the lateral elements in alignment, arise at the time of SEIs (Hunter and Kleckner 2001). DHJs arise during pachytene when SC formation is complete (Schwacha and Kleckner 1995), and crossover DNA products generally appear at the end of this stage (Padmore et al. 1991). It is thought that synapsis does not drive homolog alignment in yeast, but instead reinforces the DNA contacts formed over the course of meiotic prophase (e.g., through pairing and recombination; Roeder 1997; Zickler and Kleckner 1999).
Associations between homolog axes in yeast have been visualized by EM in spread chromosomes isolated from mutants where the SC is absent (Sym et al. 1993). Such axial associations may correspond to those observed in plants prior to SC formation (Albini and Jones 1987; Anderson and Stack 1988; Franklin et al. 1999), and in mouse spermatocytes at zygotene, when synapsis is initiated (Tarsounas et al. 1999). Associations observed in yeast likely involve DNA contacts mediated by the homologous recombination machinery, because their formation is dependent on the RecA homologs, Rad51 and Dmc1 (Rockmill et al. 1995).
The overall organization of chromosomes in the nucleus also influences the association of homologs. During meiosis, telomeres cluster at the nuclear envelope in the bouquet orientation (for review, see Zickler and Kleckner 1998). The bouquet stage in yeast precedes synapsis and likely coincides with, although is not dependent on, early meiotic recombination events (Trelles-Sticken et al. 1999). Finally, a mutation in NDJ1 reduces the level of bouquet formation and also confers a delay in pairing interactions and in SC formation (Chua and Roeder 1997; Conrad et al. 1997; Trelles-Sticken et al. 2000).
The analysis of mutant phenotypes in yeast has suggested a functional interdependence among the above pathways. For example, to date, all mutants defective in DSB formation are also defective for axial element and/or SC formation (for review, see Roeder 1997; Zickler and Kleckner 1999). In contrast, some but not all mutants defective for DSB formation are also defective for pairing (for review, see Burgess 2002). Pairing interactions detected using FISH have been shown to be dependent on the SPO11 gene product; however, a mutation in the putative catalytic residue required for DSB formation, spo11-y135F, allows for nearly full levels of meiotic pairing (Cha et al. 2000). Other mutants including hop1, mek1, and dmc1 are also defective for the formation or processing of DSBs yet allow for high pairing levels relative to wild-type cells (Loidl et al. 1994; Weiner and Kleckner 1994; Nag et al. 1995; Rockmill et al. 1995).
Here we determined the relative contributions made to close homolog juxtaposition by DSB-independent pairing, recombination, SC formation, and the bouquet arrangement. We have developed and applied a noninvasive, quantitative assay that probes meiosis-specific associations between allelic loci in living cells using site-specific recombination (Cre/loxP). Phenotypic analysis of 12 mutants affecting meiotic chromosome associations suggests that the homolog-specific associations detected using the Cre/loxP assay are distinct from DSB-independent pairing interactions and genetically separable from synapsis. Instead meiotic recombination was found to be an important determinant of close meiotic homolog juxtaposition. Differences in phenotypes between different classes of recombination mutants suggest that close, stable juxtaposition is mediated through either pre-SEIs or SEIs and not specifically through a crossover-only pathway. A delay in close homolog juxtaposition was observed for the bouquet-defective mutant.
Results
Application of an exogenous site-specific recombination system to study chromosome colocalization in meiotic cells of yeast
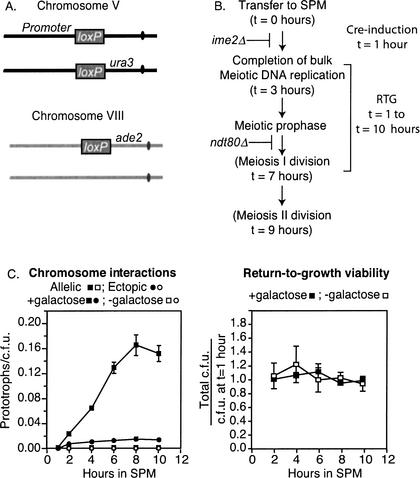
We modified a previously described exogenous site-specific recombination system (Cre/loxP; Burgess and Kleckner 1999) to assess the relative probabilities with which chromosomal loci collide with one another during meiosis I prophase in budding yeast. For this assay, pairs of loxP sites located at either an allelic position on homologous chromosomes or at ectopic positions on nonhomologous chromosomes undergo Cre-mediated recombination to give a genetically detectable product. Specifically, a promoter located on chromosome V (GPD1p-loxP) is recombined with a promoterless reporter gene on either chromosome V (loxP-ura3) or chromosome VIII (loxP-ade2) to activate expression of that gene and generate a prototrophic strain (Fig. 1A). Prior analysis of Cre/loxP-mediated prototroph formation in mitotically dividing cells showed that the rate of recombination per cell division reflects the frequency of collisions between chromosomal loci and thus their relative spatial proximity to one another (Burgess and Kleckner 1999). Here, the relative spatial position of chromosomal loci during meiosis was inferred by measuring commitment to prototroph formation after cells were returned to mitotic growth following entry into meiotic prophase (Fig. 1B,C, left). The return-to-growth (RTG) procedure is widely used and allows for the analysis of mutants that would otherwise arrest during meiosis or give inviable spores (Sherman and Roman 1963; Esposito and Esposito 1974; Zenvirth et al. 1997).
Figure 1.
(A) Orientation and chromosome location of promoter (pGPD1-loxP) and two reporter (loxP-ura3 and loxP-ade2) constructs used in Cre/loxP assay relative to their adjacent centromeres (not drawn to scale; see text for details). (B) Temporal order of meiotic events relative to Cre-induction and return-to-growth (RTG) procedures. Timing of meiotic events is based on Padmore et al. (1991). All strains used in this study contain the ndt80Δ mutation, which blocks the exit from meiotic prophase I. The ime2Δ mutation blocks entry into meiotic DNA replication. Other mutations used in this study affect chromosomal events that occur during meiotic prophase (See text). (C, left) Commitment to prototroph formation following RTG in the presence (black symbols) or absence (open symbols) of induction of Cre-expression by the addition of galactose at t = 1 h after transfer into SPM. Data from the ndt80Δ strain designated here as “WT” are shown. Allelic interactions are reported by Ura+ prototrophs/cfu (squares), and ectopic interactions are reported by Ade+ prototrophs/cfu (circles). Right: Survivability (total cfu / cfu at t = 1 h) of ndt80Δ cells after RTG throughout the meiotic time course in the presence or absence of galactose.
Two additional modifications were made to the strains to study Cre/loxP recombination during meiosis. First, since this study involved the analysis of mutations that could differentially affect the timing or ability of cells to proceed through the first meiotic division or result in chromosome missegregation at the MI division, the NDT80 gene was deleted. The ndt80Δ mutation arrests cells in prophase prior to the first meiotic division, allowing return to mitotic growth by plating onto nutritional media (Fig. 1C, right; Xu et al. 1997). Second, the direct influence of telomere clustering was minimized for this study by selecting positions for loxP sites equidistant from both the adjacent centromeres and the telomeres of similarly sized chromosomes, V and VIII. The loxP sites are oriented so that recombination results in the reciprocal exchange of chromosome arms, thereby giving rise to viable products upon RTG (see below).
The use of two different reporter constructs in the same cell could potentially confound the analysis if they are in competition with one another. To address this issue specifically, we measured allelic and ectopic Cre-mediated recombination levels in strains containing the allelic loxP-ura3 and the ectopic loxP-ade2 reporter constructs either together or alone. Similar levels of Ura+ prototrophs were generated in the presence or absence of the ectopic loxP-ade2 reporter (Table 1, cf. Ura+ t = 8 h, line 1 with line 2). Moreover, similar levels of Ade+ prototrophs were generated in either the presence or absence of the allelic loxP-ura3 reporter (Table 1, cf. Ade+ t = 8, lines 1 and 3). Thus additional loxP sites on separate chromosomes do not compete with one another. By this same reasoning, loxP sites on sister chromatids likely do not compete with allelic or ectopic interactions.
Table 1.
Dependence of Ura+ and Ade+ prototroph formation on loxP-ura3 and loxP-ade2 chromosome location
| Chromosome and Genotype
|
Ura+ prototrophs/c.f.u.
|
Ade+ prototrophs/c.f.u.
|
|||||
|---|---|---|---|---|---|---|---|
|
V1
|
V2
|
VIII1
|
VIII2
|
t = 1a
|
t = 8
|
t = 1
|
t = 8
|
| GPDlp-loxP | loxP-ura3 | loxP-ade2 | — | 0.0009 ± 0.0004 | 0.142 ± 0.008 | 0.00004 ± 0.00006 | 0.017 ± 0.002 |
| GDPlp-loxP | loxP-ura3 | — | — | 0.0007 ± 0.0003 | 0.141 ± 0.019 | 0.00003 ± 0.00005 | <0.00003 |
| GPDlp-loxP | — | loxP-ade2 | — | <0.00007 | <0.00005 | 0.00013 ± 0.00022 | 0.012 ± 0.001 |
| GPDlp-loxP | loxP-ade2 | — | — | <0.00003 | <0.00003 | 0.0003 ± 0.00012 | 0.113 ± 0.004 |
Hours after transfer to SPM.
Any differences observed in Cre-mediated recombination levels should be attributable to the chromosome location of each reporter and not to the identity of reporter gene itself. This pattern was confirmed by measuring the level of Ade+ prototrophs generated when the loxP-ade2 reporter construct was located on chromosome V as the “allelic” interaction reporter instead of loxP-ura3. Similar levels of prototrophs were obtained in the two cases (Table 1, cf. Ura+ t = 8, line 2 with Ade+ t = 8, line 4; see also Fig. 3B).
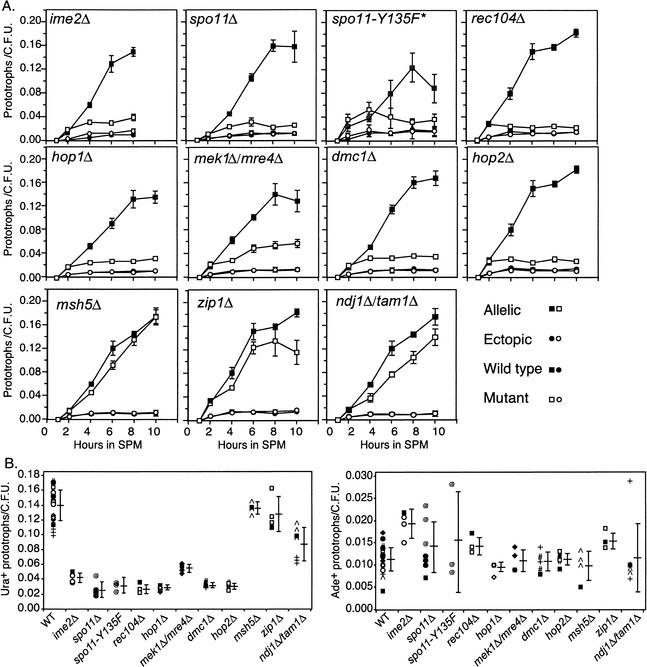
Figure 3.
Commitment to prototroph formation following Cre-induction at t = 1 h after transfer into SPM. Allelic and ectopic events are reported as in Figure 1. (A) Data from ndt80Δ strains are shown using black symbols while data from double mutants are shown using open symbols. All measurements are the average (± S.D.) of three independent cultures of ndt80Δ and double mutant strains processed in parallel. The asterisk in the spo11-Y135F panel denotes that both the wild-type and mutant alleles contain a triple-hemagglutinin and hexahistidine tags that may affect their function (see text). (B) Summary of all experiments carried out on WT (ndt80Δ) and double mutant strains. Each symbol represents a different day that an experiment was performed, and each point represents the number of prototrophs/cfu given for an individual culture. The average and standard deviation are shown by the horizontal and vertical bars, respectively. Note the difference in scales for Ura+ and Ade+ prototrophs/cfu.
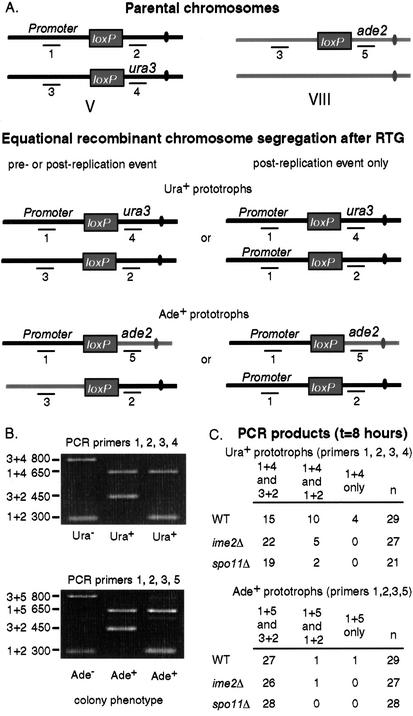
For the assay to be effective at reporting on collisions between meiotic chromosomes, commitment to Cre-mediated recombination must occur during meiotic prophase. If the recombination events were to occur prior to meiotic DNA replication, balanced reciprocal recombinants would be detected in DNA samples isolated from every prototroph (e.g., products 1 + 4 and 2 + 3; Fig. 2A, left). In contrast, if the recombination events occurred after DNA replication of the loxP sites and were then followed by an equational division after RTG, the selected recombinant product (e.g., GPD1p-loxP-ura3) would be recovered in every prototroph, whereas its reciprocal product (−loxP−) would be obtained in 50% of prototrophs. An unbalanced nonrecombinant chromosome (GPD1p-loxP) would be obtained in the other 50% of prototrophs (e.g., products 1 + 4 and 1 + 2; Fig. 2A, right). Other patterns can and do arise; for example, if a Cre/loxP recombination event promoted the reductional segregation of chromosomes upon return to growth (e.g., product 1 + 4 only; data not shown). By this reasoning, the fraction of recombination events occurring during meiotic prophase would equal twice the number of prototrophs containing both a recombinant and a nonrecombinant chromosome divided by the total number of prototrophs with two PCR products.
Figure 2.
Configuration of markers flanking loxP sites before and after Cre-mediated recombination as determined by PCR. (A) Oligonucleotide primers (1, 2, 3, 4, and 5) give unique sized products. Oligos 1, 2, 3, and 4 are described elsewhere (Burgess and Kleckner 1999). Oligo 5 is 5′-CATCGTATGCCAAAGTCC-3′ and anneals to the ADE2 gene. Parental markers and two expected types of recombinant marker configurations following equational chromosome segregation after RTG are diagramed (see text for details). (B) Expected size of PCR products for nonprototrophs (parental class) and prototrophic strains (recombinant class). (C) Number of observed PCR product classes among Ura+ prototrophs (top) and Ade+ prototrophs (bottom) analyzed by RTG 8 h after transfer into SPM (7 h after Cre-induction) in wild-type, ime2Δ, and spo11Δ strains.
PCR analysis to detect both recombinant and nonrecombinant chromosomes was carried out on Ura+ and Ade+ prototrophs arising after t = 8 h on SPM for one of the time course experiments (Fig. 2B). Based on the results reported in Figure 2C, we estimate that ∼80% of allelic (2 × 10/25) and ∼7% of ectopic (2 × 1/28) Cre/loxP recombination events occurred after replication of the loxP sites, either during meiotic prophase or RTG (see below).
During meiosis, levels of Cre-mediated recombination between an allelic site are greater than between ectopic sites
Because homolog colocalization is a prominent feature of meiosis, it was anticipated that the level of allelic Cre-mediated recombination events (i.e., Ura+ prototrophs/cfu) generated during meiosis would be greater than the level of ectopic recombination events (i.e., Ade+ prototrophs/cfu). Analysis of 28 experiments shows this to be the case. The level of Ura+ prototrophs formed in samples removed at t = 8 h after transfer to SPM was on average 12.7-fold greater than the level of Ade+ prototrophs. The average level of Ura+ prototrophs/cfu was 0.14 ± 0.02, and the average level of Ade+ prototrophs was 0.011 ± 0.003. A negligible number of Ura+ and Ade+ prototrophs were present prior to galactose induction at t = 1 h (e.g., Table 1) and at t = 8 h in the absence of induction (e.g., Fig. 1C), thereby ruling out the possibility that prototrophs arose by meiotic gene conversion.
The rate of recovery of prototrophs formed by allelic Cre-mediated recombination remained constant (0.019 ± 0.006 Ura+ prototrophs/cfu/h) through the first seven h following Cre-induction (i.e., t = 1 through t = 8 h in SPM). In contrast, the rate for ectopic interactions was biphasic; during the first h, the rate of Ade+ prototroph formation (0.005 ± 0.002 Ade+ prototrophs/cfu/h) was about fourfold lower than the rate of Ura+ prototroph formation. For the period of time between 2 and 8 h after transfer to SPM, the rate dropped to 0.000 ± 0.001 Ade+ prototrophs/cfu/h. That is, Cre-mediated recombination events are no longer detected two h after transfer of the cells to SPM and one h after Cre induction. This timing corresponds approximately to the time of DNA replication (Padmore et al. 1991).
The sharp decline in the rate of commitment to prototroph formation for ectopic Cre-mediated recombination events after t = 2 h in SPM suggested that either ectopic sites could no longer participate in a recombination event following replication of the loxP sites or that recombinants could form, but were nonviable following RTG. PCR analysis revealed that one wild-type Ade+ prototroph gave both a reciprocal plus a parental PCR product, suggesting that ectopic recombination following replication of the loxP sites can give rise to viable cell products (Fig. 2C). More were recovered in other experiments (data not shown). Thus, it appears that ectopic recombination between loxP sites during meiotic prophase is severely limited. These results, taken together with those reported above, suggest that the Ura+/Ade+ ratio for meiotic recombination events may be 10-fold, or more (see below), greater than the 13-fold difference detected at the t = 8 h timepoint.
Allelic Cre-mediated recombination levels are greater in meiotic cells than in cells blocked for the entry into meiosis
The level of Cre-mediated recombination in premeiotic cells was compared directly to levels observed in meiotic cells by analyzing an ime2Δ strain in parallel with wild type (i.e., ime2Δ ntd80Δ and ntd80Δ). IME2 is required for the entry into meiosis; ime2Δ gives a prolonged premeiotic G1 arrest phenotype when incubated in SPM (Foiani et al. 1996). Allelic Cre-mediated recombination levels in the ime2Δ strain were reduced compared to those observed in wild-type cells for all timepoints following t = 2 h (Fig. 3A). At t = 8 h, the average level of Ura+ prototrophs in the ime2Δ mutant was reduced threefold relative to the wild-type level (Fig. 3B). Ectopic Cre-mediated recombination was slightly elevated compared with the wild-type control (Fig. 3B). No decrease in survivability during RTG was observed (data not shown). In the ime2Δ strain at t = 8 h in SPM, 5/27 Ura+ prototrophs gave PCR products indicative of a postreplication event (1 + 2 and 1 + 4; Fig. 2C), suggesting that some events may occur after RTG and not during meiotic prophase. One of 27 Ade+ prototrophs gave a postreplication segregation pattern (Fig. 2C).
Interestingly, the homologous Cre-mediated recombination events were not decreased to ectopic levels but instead were about twofold greater. The difference between allelic and ectopic levels likely represents homologous associations between chromosomes in premeiotic cells. Previous studies using FISH have shown that pairs of loci on homologous chromosomes are paired ∼50% of the time in premeiotic cells, mitotically dividing cells, and in cells arrested in G1 with alpha factor (Weiner and Kleckner 1994; Burgess et al. 1999). We tested whether ime2Δ cells undergoing prolonged arrest in G1 would show wild-type premeiotic pairing levels by FISH. The fraction of nuclei in which the distance between allelic foci in spread nuclear preparations was less than 0.7 μm was on average 0.48 (i.e., ∼50%, Table 2). These results suggest that two levels of interaction between homologs are detected using the Cre/loxP assay. The first is the more prominent meiosis-specific interactions between allelic sequences, and the second is the less robust premeiotic associations.
Table 2.
Pairing levels by FISH
| Genotype
|
Experiment
|
Pairing levela
|
No. of nuclei scored
|
% of ndt80Δ levels
|
|
|---|---|---|---|---|---|
| VIIIobs; XIobs; bkgd
|
VIIIobs-bkgd; XIobs-bkgd
|
||||
| ndt80Δ | 1 | 0.70; 0.78; 0.03 | 0.67; 0.75 | 93 | |
| ndt80Δ | 2 | 0.83; 0.88; 0.03 | 0.80; 0.85 | 105 | |
| ndt80Δ | 3 | 0.92; 0.89; 0.03 | 0.89; 0.86 | 65 | |
| ndt80Δ | 4 | 0.89; 0.87; <0.01 | 0.89; 0.87 | 98 | |
| ndt80Δ | 5 | 0.89; 1.0; 0.03 | 0.86; 0.97 | 19 | |
| ndt80Δ | 6 | 0.95; 0.90; 0.08 | 0.87; 0.82 | 102 | |
| Average (VIII; XI) | 0.83 ± 0.09; 0.85 ± 0.07 | 100 | |||
| ime2Δ ndt80Δ | 6 | 0.61; 0.95; 0.11 | 0.50; 0.48 | 95 | |
| ime2Δ ndt80Δ | 7 | 0.54; 0.58; 0.09 | 0.43; 0.47 | 135 | |
| Average (VIII; XI) | 0.47 ± 0.04; 0.48 ± 0.00 | NA | |||
| spo11Δ ndt80Δ | 2 | 0.31; 0.38; 0.08 | 0.23; 0.30 | 103 | |
| spo11Δ ndt80Δ | 3 | 0.74; 0.50; 0.12 | 0.62; 0.38 | 38 | |
| spo11Δ ndt80Δ | 5 | 0.57; 0.59; 0.05 | 0.52; 0.54 | 117 | |
| Average (VIII; XI) | 0.46 ± 0.20; 0.41 ± 0.12 | 52 | |||
| spo11-Y135F-HA3HIS6 ndt80Δ | 8 | 0.45; 0.43; 0.07 | 0.38; 0.36 | 150 | 44 |
| spo11-HA3HIS6 ndt80Δ | 2 | 0.94; 0.97; 0.06 | 0.88; 0.91 | 98 | |
| spo11-HA3HIS6 ndt80Δ | 8 | 0.82; 0.74; 0.03 | 0.79; 0.71 | 96 | |
| Average (VIII; XI) | 0.83 ± 0.06; 0.81 ± 0.14 | 98 | |||
| hoplΔ ndt80Δ | 4 | 0.60; 0.64; 0.03 | 0.57; 0.61 | 125 | |
| hoplΔ ndt80Δ | 5 | 0.52; 0.57; 0.06 | 0.46; 0.51 | 54 | |
| Average (VIII; XI) | 0.52 ± 0.08; 0.56 ± 0.07 | 64 | |||
| meklΔ ndt80Δ | 4 | 0.56; 0.63; 0.01 | 0.55; 0.62 | 108 | |
| meklΔ ndt80Δ | 6 | 0.67; 0.72; 0.05 | 0.62; 0.67 | 209 | |
| Average (VIII; XI) | 0.59 ± 0.05; 0.64 ± 0.03 | 74 | |||
Pairing assessed at t = 6 hours in SPM with fluorescently labeled cosmid probes hybridized to spread nuclei preparations. Cosmid probe q, nick translation labeled with Alexa-488, hybridizes chromosome VIII while probe g, aminoallyl labeled with Cy3, identifies chromosome XI (see Materials and Methods). Observed pairing levels are fraction of nuclei scored with a distance between foci of 0.7 μm or less. Background pairing levels for each hybridization determined as fraction of nuclei scored with nonhomologous pairs of loci that were ≤ 0.7 μm apart.
Allelic Cre/loxP recombination is significantly decreased by mutations that block initiation of meiotic recombination yet allow for high levels of homolog pairing
To better understand the genetic requirements for establishing the close juxtaposition of homologous chromosomes during meiosis, we next determined the Cre/loxP phenotypes of mutants known to affect pairing and/or meiotic recombination. SPO11 is required for homolog pairing as assayed by FISH (Loidl et al. 1994; Weiner and Kleckner 1994) and is also required for DSB formation (Cao et al. 1990; Bergerat et al. 1997; Keeney et al. 1997). Allelic Cre-mediated recombination levels were reduced in a spo11Δ strain sixfold relative to wild type, while ectopic Cre-mediated recombination levels were indistinguishable from the wild-type strain (Fig. 3). Interestingly, the level of allelic events was not decreased to ectopic levels in a spo11Δ mutant. Instead, the profile of allelic Cre-mediated events paralleled the ime2Δ mutant and were about twofold greater than ectopic interactions, again suggestive of premeiotic associations. Accordingly, the majority of Ura+ prototrophs arising in the spo11Δ strain by t = 8 h in SPM contained both reciprocal recombination products, suggesting that most events occurred prior to meiotic DNA replication (Fig. 2C). Survivability during RTG was not compromised by the spo11Δ mutation (data not shown). A deletion in the REC104 gene, which is required for DSB formation and likely acts at a similar step in the DSB formation pathway as SPO11, gave a similar phenotype (Fig. 3; Bullard et al. 1996; Jiao et al. 1999).
Another class of mutants that abolish or greatly decrease DSB formation was shown to exhibit nearly wild-type levels of meiotic pairing (∼90% of wild-type) when assayed using FISH. spo11-Y135F abolishes DSBs, whereas hop1Δ and mek1Δ/mre4Δ were reported to give DSB levels up to 10% and 10%–20% of wild-type cells, respectively (Leem and Ogawa 1992; Schwacha and Kleckner 1994; Mao-Draayer et al. 1996; Bergerat et al. 1997; Cha et al. 2000). Allelic Cre-mediated recombination levels were reduced fivefold for spo11-Y135F and hop1Δ mutants and threefold for the mek1Δ/mre4Δ mutant (Fig. 3). None of the mutants exhibited a change in ectopic levels or survivability by RTG (Fig. 3; data not shown). Because the spo11-Y135F allele was tagged at the C-terminus with both a triple-hemagglutinin epitope and a hexahistidine sequence, a wild-type SPO11 allele containing the same tagged sequences was tested in parallel, and gave slightly reduced levels compared to the untagged version (Fig. 3A). Other phenotypes have been observed for the tagged wild-type SPO11 gene, including reduced DSB levels at low temperatures (Kee and Keeney 2002).
One possible reason to account for the difference in severity of Cre/loxP phenotypes compared to previously published pairing phenotypes exhibited by the spo11-y135F, hop1Δ, and mek1Δ/mre4Δ mutants is that initial pairing interactions may be destabilized after prolonged arrest in the ndt80Δ mutant background. Interpretation of the Cre/loxP results thus depends on knowing the homolog pairing phenotypes of these mutants in the ndt80Δ genetic background at these later timepoints. Meiotic homolog pairing interactions were measured by FISH in the wild-type, spo11Δ, spo11-Y135F, hop1Δ, and mek1Δ/mre4Δ strains used for Cre/loxP analysis (i.e., containing the ndt80Δ mutation) 6 h after transfer to SPM. Probes to chromosomes VIII and XI were selected for the analysis based on their distal position relative to their adjacent centromeres and telomeres.
In the ndt80Δ mutant (i.e., wild-type strains in this study), the average fraction of nuclei that gave distances of less than 0.7 μm between the FISH signals in spread nuclear preparations for two different loci was 0.84, which is similar to previously reported pairing levels for this mutant (Weiner and Kleckner 1994). The spo11Δ ndt80Δ mutant gave average pairing levels of 0.44 (Table 2), which is about fourfold higher than the values reported previously for spo11Δ NDT80 (Weiner and Kleckner 1994). Interestingly, relatively low levels (0.38) were given by the spo11Y135F strain, which in an NDT80 background gave wild-type levels of pairing (Table 2; Cha et al. 2000). Similar results were reported by Neale et al. (2002). Thus for this mutant, prolonged arrest may result in decreased pairing levels.
In contrast, the hop1Δ ndt80Δ and mek1Δ/mre4Δ ndt80Δ strains gave pairing levels of 0.54 and 0.62, which are 64% and 74% of ndt80Δ levels at t = 6 h after transfer to SPM, respectively (Table 2; Fig. 4). These levels, relative to the control, are slightly lower than previously reported in an NDT80 strain background, but not so low as to account for the observed low levels of allelic Cre-mediated recombination in these strains. Taken together, these results in conjunction with the Cre/loxP results for these mutants presented above suggest that close juxtaposition of homologs depends on DSB formation and is distinct from DSB-independent homolog pairing interactions.
Figure 4.
Percentile ranked distances between allelic and ectopic foci from meiotic cultures 6 h after transfer into SPM. Six distances among pairs of hybridization signals are individually plotted from the smallest distance to the largest distance for the nuclei analyzed. Data on the x-axis are plotted as the relative nucleus rank number for each strain. (Open squares) Allelic foci distances for chromosome XI; (closed squares) allelic foci distances for chromosome VIII; (diamonds) ectopic foci distances for chromosomes XI and VIII; (dashed line) the cutoff limit for pairing (d < 0.7μm; see Weiner and Kleckner 1994). The three strains were processed in parallel and correspond to experiment 4 shown in Table 2.
Allelic Cre-mediated recombination levels are decreased significantly in mutants that fail to process meiotic DSBs beyond resection but are not reduced in mutants that influence crossover control
DSBs formed in dmc1Δ and hop2Δ mutants accumulate and are hyperresected (Bishop et al. 1992; Leu et al. 1998). SEIs and dHJ intermediates formed between homologous chromosomes are absent in a dmc1Δ mutant (Schwacha and Kleckner 1997; Hunter and Kleckner 2001). Interestingly, a dmc1Δ mutant gives relatively high levels of pairing compared to a spo11Δ mutant when measured by FISH (0.61 vs. 0.16 respectively; Weiner and Kleckner 1994; Rockmill et al. 1995), whereas a hop2Δ mutant gives lower levels (0.15–0.45) depending on the chromosome assayed (Leu et al. 1998). Both mutants exhibit nearly normal amounts of SC, albeit with delayed kinetics. One notable difference between these mutants is that synapsis occurs between homologs in a dmc1Δ mutant and between nonhomologous chromosomes in a hop2Δ mutant (Rockmill et al. 1995; Leu et al. 1998).
Allelic Cre/loxP recombination levels in both the dmc1Δ and hop2Δ mutants were indistinguishable from the spo11Δ mutant and were reduced fivefold compared to wild-type cells (Fig. 3). Similar to the DSB-defective mutants (above), ectopic Cre-mediated recombination was not affected in either mutant (Fig. 3). Additionally, no decrease in survivability during RTG was observed (data not shown).
msh5Δ represents a class of mutants that exhibit decreased levels of meiotic crossing over but wild-type levels of total recombination events (Hollingsworth et al. 1995). The MSH5 gene product is thought to act at an intermediate-to-late step in the recombination pathway to modulate crossover control (Roeder 1997; Zickler and Kleckner 1999). In contrast to other mutants affecting meiotic recombination, allelic Cre-mediated recombination levels in the msh5Δ mutant at t = 8 h were very similar to wild-type levels, if not very slightly delayed (Fig. 3). Ectopic recombination levels were indistinguishable from wild-type levels (Fig. 3). No decrease in survivability during RTG was observed (data not shown).
Allelic Cre-mediated recombination events are not significantly reduced in a zip1Δ mutant, which abolishes SC formation
The zip1Δ mutant exhibits a complete absence of synapsis, yet homologs pair at nearly wild-type levels as detected by FISH (Sym et al. 1993; Nag et al. 1995). Like the msh5Δ mutant, the zip1Δ mutant also exhibits decreased levels of meiotic crossing over without a corresponding decrease in the levels of total recombination interactions (Sym and Roeder 1994). The zip1Δ mutant gave nearly wild-type levels of allelic Cre-mediated recombination events (Fig. 3). The ectopic events were slightly elevated (1.4-fold) in the zip1Δ mutant compared to wild type (Fig. 3). No reduction in survivability during RTG was observed (data not shown).
Allelic Cre-mediated recombination events are delayed in an ndj1Δ/tam1Δ mutant, which disrupts bouquet formation
The ndj1Δ/tam1Δ mutant was analyzed to investigate whether allelic interhomolog interactions detected using the Cre/loxP assay are delayed, as are axial elements and SC formation. At t = 8 h, the ndj1Δ/tam1Δ mutant gave a modest but significant decrease of allelic Cre-mediated recombination events compared to wild type (Fig. 3). Interestingly, the level of prototrophs did not plateau as in other mutants defective for Cre/loxP interactions, but instead continued to rise up to the t = 10 h timepoint. That is, the ndj1Δ/tam1Δ mutant gave a curve that appears to be offset about 2 h from the curve given by the wild-type strain. Ectopic recombination events were indistinguishable from wild-type levels (Fig. 3). No decrease in survivability during RTG was observed (data not shown).
Discussion
Site-specific recombination can be used to report on the stabilization of associations between chromosomal loci during meiosis
This study found significant differences in the levels of commitment to Cre-mediated recombination between a pair of loxP sites located at an allelic position on homologous chromosomes compared to a pair of loxP sites located at ectopic positions on nonhomologous chromosomes during meiotic prophase. Allelic Cre-mediated recombination levels were at least 13-fold greater than ectopic levels. Significant differences were also found for allelic Cre-mediated recombination in a subset of strains containing mutations known to affect meiotic associations between homologous chromosomes. Mutations affecting meiotic recombination initiation and processing of DSBs beyond the resection stage reduced the levels of allelic Cre-mediated recombination three- to sixfold without affecting ectopic levels. No significant effect was observed for mutations defective specifically in SC formation or crossover control. A mutation causing a delay in both pairing interactions and SC formation also resulted in a delay in the maximum level of allelic Cre-mediated recombination events. These results, taken together, suggest that the frequency of Cre-mediated recombination reports on the probability with which chromosomal loci collide with one another during meiotic prophase, and furthermore, that such collisions are governed by constraints imposed by factors that promote homologous chromosome associations during meiosis. We refer to these associations as close, stable homolog juxtaposition.
Meiotic homolog associations do not restrict interactions between ectopic regions of the genome
No increase in ectopic Cre/loxP recombination was observed for any mutant that exhibited a decrease in allelic Cre-mediated interactions. In contrast, heteroallelic gene conversion frequencies measured between ectopic sites, in either spore clones or by RTG, have been shown to increase relative to wild type in several situations where interactions between homologous chromosomes are delayed or compromised, for example, in ndj1Δ and hop2Δ mutants (Leu et al. 1998; Goldman and Lichten 2000). These data have previously been interpreted as indicating that associations between homologous chromosomes restrict the ability of ectopically located short homologous sequences to interact and recombine (Goldman and Lichten 2000). Such restriction may be imposed spatially (e.g., sterically) with homolog associations precluding interactions between ectopically located sequences, or alternatively, through the use of preferred recombination pathways that predominate when homolog pairing interactions are intact. Given that the Cre/loxP assay reports on the relative spatial proximity of loci, and that ectopic Cre/loxP interactions are not increased in pairing defective mutants, the second interpretation is favored.
DSB-independent pairing interactions are not sufficient to promote close, stable homolog juxtaposition in premeiotic cells
Comparison of Cre/loxP recombination levels in meiotic cells compared to those in the premeiotically arrested ime2Δ mutant suggest that allelic chromosomal loci are spatially closer together during meiosis than in premeiotic cells. Chromosome conformation capture (3C), reporting on the overall spatial organization of chromosomes, was recently shown to produce similar results (Dekker et al. 2002). In addition, these findings are consistent with the behavior of allelic GFP-tagged chromosomal loci, which were shown to interact more frequently in meiotic nuclei compared to premeiotic nuclei of yeast (Aragon-Alcaide and Strunnikov 2000).
Nonetheless, DSB-independent homolog pairing interactions which are distinct from close, stable homolog juxtaposition can be detected by both FISH and Cre/loxP in premeiotic cells, meiotic cells where recombination fails to occur, and in mitotically dividing cells (this study; Loidl et al. 1994; Weiner and Kleckner 1994; Nag et al. 1995; Burgess and Kleckner 1999; Burgess et al. 1999). In addition, preferential homolog associations in premeiotic cells are detected by 3C analysis (Dekker et al. 2002). GFP-tagged chromosome analysis, however, does not show preferential premeiotic homolog associations, possibly because the self-association of the lacO-repeat arrays obscures their detection (Aragon-Alcaide and Strunnikov 2000).
Although loxP sites on sister chromatids likely do not compete with interhomolog or ectopic Cre/loxP events, it is possible that only events that juxtapose loxP sites to an extent that approaches that of sister chromatid cohesion will yield a significant signal. Thus, homologs could be closely paired by FISH criteria in premeiotic cells yet not be close enough to give meiotic levels of Cre/loxP recombination.
Both DSB-independent pairing and recombination interactions contribute to close, stable homolog juxtaposition during meiosis
We show here that the meiosis-specific interactions detected using the Cre/loxP assay are dependent on the function of genes required for DSB formation and processing. Several of these genes when mutated exhibit high levels of homolog pairing interactions as detected by FISH and low levels of Cre/loxP interactions. These results suggest that the high levels of Cre-mediated events observed in wild-type cells are reporting on a meiosis-specific stage of close spatial juxtaposition of homologous chromosomes that is dependent on meiotic recombination and is distinct from DSB-independent pairing interactions.
Of the five mutants analyzed that are defective for forming wild-type levels of DSBs, mek1Δ/mre4Δ gave the most modest reduction in Cre/loxP interactions compared to wild-type strains. The greater levels of allelic Cre-mediated recombination in the mek1Δ mutant compared to spo11Δ may reflect the low levels of DSBs formed in mek1Δ. Interestingly, the observed levels of DSBs formed in the mek1Δ and hop1Δ mutants are very similar; however, the hop1Δ mutant gives the same levels of Cre-mediated recombination as spo11Δ. Schwacha and Kleckner (1994) have reported aberrant joint molecule (i.e., double-Holliday junction) formation in this mutant. In their study, DSBs formed in the hop1Δ mutant were channeled into a sister chromatid repair pathway later in meiosis instead of an interhomolog recombination pathway. The lack of interhomolog recombination agrees with and perhaps accounts for the reduced Cre/loxP interactions observed in the hop1Δ, despite some DSB formation.
Although close juxtaposition of homologous chromosomes during meiosis is distinct from DSB-independent pairing interactions, it may nonetheless depend on both recombination interactions and DSB-independent pairing contacts. Between 175 and 260 meiotic recombination events per cell were estimated to occur in yeast, based on both the total genetic map length and a nearly 2:1 ratio of meiotic noncrossover:crossover formation (Bishop 1994). A similar number of DSB hotspots were detected with an average spacing of 48 kb in noncentromeric regions (Gerton et al. 2000). A similar spacing of DSB-independent pairing contacts was calculated for premeiotic and meiotic cells by FISH (Weiner and Kleckner 1994; Burgess et al. 1999). If the sites of initial pairing interactions are the same as the sites of DSB formation, then it is difficult to imagine that the sum of recombination events and pairing contacts would serve to bring homologs any closer together than they already are in premeiotic cells. One way to reconcile this issue is to consider that the sites of DSB formation are distinct from the sites of pairing contacts and that close homolog juxtaposition is dependent on both initial pairing contacts and recombination interactions. A model where functional differentiation of two types of regions occurs along meiotic chromosomes was proposed in which GC-rich regions are primarily involved in recombination interactions (J-regions, involving “joint molecules”) whereas AT-rich regions are primarily involved in DSB-independent pairing interactions (P-regions, Zickler and Kleckner 1999). Close homolog juxtaposition as detected by the Cre/loxP assay during meiosis may represent interactions between homologs involving both J-regions and P-regions. In contrast, premeiotic homolog associations detected using both FISH and the Cre/loxP assay (above) may represent P-type interactions only.
Close, stable homolog juxtaposition does not require crossover resolution or genes involved in modulation of crossover control
In ndt80Δ arrested cells, Holliday junction intermediates are unresolved and very few crossovers form, whereas recombination events not associated with crossing over are unaffected (Allers and Lichten 2001). Since the strains used in the present study all contained the ndt80Δ mutation, close homolog juxtaposition detected using the Cre/loxP assay does not depend on the formation of crossover DNA products. Moreover, two mutations that conferred a twofold decrease in the levels of crossover formed without affecting total recombination events, that is, zip1Δ and msh5Δ, gave nearly the same levels of allelic Cre-mediated recombination levels as the ndt80Δ wild type. Taken together, these results suggest that close homolog juxtaposition is likely dependent either on an intermediate from which both the noncrossover and crossover pathways diverge or the noncrossover pathway specifically, but not solely on the crossover pathway.
Relationship of close, stable homolog juxtaposition to SEIs and axial associations
Because close homolog juxtaposition does not occur in mutants able to form resected DSB products but does occur in mutants which fail to resolve crossovers, it follows that juxtaposition might be dependent on some stage of the meiotic recombination pathway that falls between resection and resolution. Double-Holliday junctions leading to crossovers are preceded temporally, and coupled mechanistically, to single-end invasion (SEI) events during meiosis (Hunter and Kleckner 2001). Since the close homolog juxtaposition observed using the Cre/loxP assay shares many of the same genetic requirements as SEIs (e.g., dependence on DSB formation and DMC1 function), it is attractive to speculate that they are mechanistically related, or even equivalent.
RecA-dependent axial associations have been detected at the cytological level in yeast and in other species, as noted earlier. Analysis of the kinetic and genetic requirements of SEI formation has led to the proposal that axial associations represent nascent DSB-homolog interactions that precede SEI formation (Hunter and Kleckner 2001). We have shown here that close homolog juxtaposition, as assayed by the Cre/loxP assay, depends on the same genetic requirements as both axial associations and SEIs. Together, these data suggest that such close juxtaposition is mediated, at least in part, through axial associations of the type described in plants and yeast. Another important contribution may come from DSB-independent pairing interactions (see above).
Implications
EM images of a zip1Δ mutant in yeast show that the axes of two chromosomes can be held in alignment without the intimate association provided by the SC (Sym et al. 1993). The distance between unsynapsed axes, however, is up to 0.4 μm, whereas the distance between synapsed axes is about 0.1 μm. Despite this difference in interaxis distances, allelic Cre-mediated recombination levels are similar in wild type and zip1Δ mutants. One interpretation of this result is that at least some DNA segments of two synapsed homologs (e.g., the locus characterized in this study) are not any closer together functionally than two DNA segments juxtaposed by recombinational interactions. It is possible that interactions between the pair of allelic loxP sites used in this study may be constrained from fully interacting with one another in fully synapsed chromosomes despite their closer proximity. Alternatively, the sites used in this study may be located far away from the chromosomal axis so that the more intimate association of axes afforded by the SC does not contribute to the closer spatial position of loxP sites. Future analysis of interactions between loxP sites located at different positions relative to their own axis will address this question. At this time, however, the relationship of chromosomal sequences and axis attachment sites is unknown.
Materials and methods
Yeast strains
All yeast strains are isogenic derivatives of SK1 (Kane and Roth 1974). Two parental haploid strains were constructed from which all strains described here are derived: SBY1338 (MATa ho∷hisG lys2 ura3Δ∷hisG leu2∷hisG ade2Δ∷hisG trp1∷hisG GAL3 flo8∷LEU2-loxP-ura3 ndt80Δ∷LEU2-loxP-ade2) and SBY1448 (MATα ho∷hisG lys2∷GAL1-Cre-LYS2 ura3Δ∷hisG leu2∷hisG ade2Δ∷hisG trp1∷hisG GAL3 flo8∷LEU2-pGPD1-loxP-lacZ ndt80Δ∷LEU2). Alleles designated “hisG” have been described (Alani et al. 1987, 1990; Cao et al. 1990). An SK1 strain containing the GAL3 allele was kindly provided by Neil Hunter (Harvard University, Cambridge, MA). flo8∷LEU2-pGPD1-loxP-lacZ, flo8∷LEU2-loxP-ura3, and flo8∷LEU2-loxP-ade2 markers were introduced by transformation of pSB285 cut with NotI, pSB288 cut with NotI and Bsp120I, and pSB286 cut with NotI and Bsp120I, respectively. ndt80Δ∷LEU2-loxP-ade2 was constructed by PCR amplification of pSB186 using the primer pairs homologous to the upstream and downstream regions of the NDT80 gene fused to the Bluescript polylinker sequences. The resultant PCR product was used to replace genomic sequences −485 to +1224 bp relative to the start codon of the NDT80 gene. GAL1-cre was targeted to the LYS2 locus by cutting pSB290 with BglII prior to transformation. Genomic integrations were confirmed by PCR and by 2:2 segregation of markers. The ndt80Δ∷LEU2 allele was described by Xu et al. (1995).
Knockout mutations of meiosis-specific genes were generated independently in SBY1338 and SBY1448 by transformation using PCR-based disruption by replacing the entire open reading frame with the marker kanMX4 (Wach et al. 1994) to make hop2Δ∷kanMX4, zip1Δ∷kanMX4, ndj1Δ∷kanMX4, rec104Δ∷kanMX4, and dmc1Δ∷ kanMX4 . For ime2Δ∷kanMX4, hop1Δ∷kanMX4, and mek1Δ/mre4Δ∷kanMX4 strains, knockouts were made similarly except that previously constructed knockout strains were purchased from Research Genetics, and PCR primers were designed to amplify regions ∼200 bp upstream and downstream of the disrupted open reading frames for use in transformation. All allele replacements were confirmed by PCR using criteria described by Winzeler et al. (1999) or by Southern blotting. SK1 strains containing spo11Δ∷kanMX4, spo11-Y135F-HA3HIS6∷kanMX4, or spo11-HA3HIS6∷kanMX4 alleles were kindly provided by Scott Keeney and described in Kee and Keeney (2002). Diploids used in this study were constructed by crossing the SBY1338- and SBY1438-derived knockout strains.
Plasmid construction
Construction of pSB133 and pSB124 containing LEU2-pGPD1-loxP-lacZ and LEU2-loxP-ura, respectively, were described by Burgess and Kleckner (1999). PSB186 is a Bluescript KS+-derived (Stratagene) plasmid containing the LEU2-loxP-ade2 construct which comprises the 1.2-kb XhoI–SalI fragment containing the LEU2 gene from YEp13 (Berben et al. 1991), the 35-bp SalI–PstI region from the Bluescript polylinker, the 74-bp PstI–BamHI region including the loxP site from pBS64 (Sauer et al. 1987) and a 1.8-kb region of ADE2 gene flanked by BamHI and SacI restriction sites including sequences 11 bp upstream of the ATG through 123 bp downstream of the stop codon. The LEU2-pGPD1-loxP-lacZ (Bsp120I–NotI fragment of pSB133), LEU2-loxP-ura3 (XhoI–SmaI fragment of pSB124) and the LEU2-loxP-ade2(XhoI–Ecl136II fragment from pSB186) constructs were inserted at the BglII site of the FLO8 gene in pSB284 to make pSB285, pSB286, and pSB288, respectively. PSB284 was made by inserting the PCR-amplified FLO8 gene from SK1 into the HindIII and EcoRI site of Bluescript KS+. The pGAL1-cre transcriptional fusion described in Burgess and Kleckner (1999) (pSB92) was modified by removing the ARS1 and CEN4 sequences contained on a PstI–SmaI fragment to make pSB290.
Media
YP (1% yeast extract, 2% Bacto-peptone) was supplemented to make the following media types: YPD (2% dextrose, 0.004% tryptophan, and 0.01% adenine sulfate); YPD-ADE (2% dextrose, 0.004% tryptophan); YPA (1% potassium acetate, 0.004% tryptophan ,and 0.01% adenine sulfate); and YPG (3% glycerol, 0.004% tryptophan, and 0.01% adenine sulfate). Solid media was made by adding 2% Bacto-agar before autoclaving. SPM (1% potassium acetate, 0.02% raffinose, 0.1X amino acid mix); SC-URA media and amino acid mix were prepared as described by Burke et al. (2000).
Synchronous meiotic time courses
Synchronization of meiotic cultures is described in Padmore et al. (1991). Transfer of cells to SPM culture marks t = 0 of the meiotic time course. Expression of the gene encoding Cre recombinase was induced with the addition of 0.03% galactose at t = 1 h. This level of galactose is sufficient to give high levels of induction of prototroph formation without affecting the kinetics or efficiency of the meiosis I divisions (McCarroll and Esposito 1994; data not shown). Sample aliquots were pulled from the culture at t = 1 (before induction), 2, 4, 6, 8, and 10 h. Cell aliquots of 1 mL were pelleted, resuspended in 2% glucose, sonicated 5 sec at 15% maximum power using the microtip of a 550 Sonic ZD-dismembrator (Fisher Scientific), and diluted appropriately for plating on selective and nonselective media. Growth on SC-URA plates after five d was used to score Ura+ prototrophs, and the formation of white colonies on YPD-ADE plates grown 2 d at 30°C was used to score Ade+ prototrophs among the pink Ade− auxotrophs.
FISH analysis
Approximately 8 × 108 cells were collected after incubation in SPM for 6 h, without the addition of galactose, and treated with 0.1% sodium azide. Nuclear spreads were carried out as described in Weiner and Kleckner (1994) except that PMSF was omitted and all washes were carried out at room temperature. Cosmid probes “q” and “g” are described in Burgess et al. (1999). The cosmid source for probe q is ATCC70891 (B. Dujon, Institut Pasteur, Paris, France) while g is pUKG141. Probe g was 3′-end labeled with aminoallyl-dUTP (Sigma) according to the method of Dernberg and Sedat (1998) except that the probe was first digested in 5 mM HEPES (pH 7.7), 25 mM NaCl, 5 mM MgCl2, 50μg/mL BSA, 1mM DTT with AluI, HaeIII, MspI, RsaI, MseI, and Sau3A, and labeling was carried out in the presence of 40 μM aminoallyl-dUTP and 30 μM dTTP in the initial mix with an additional 30 μM dTTP spike 30 min into the 2 h incubation at 37°C. Probe g was then reacted with Cy3-NHS ester dye (Amersham, catalog no. PA23001) per the manufacturer's instructions except that ∼5 μg of DNA was added to the dye reaction and incubated at 37°C for 2 h before quenching with 37.5 mM glycine (pH 8), followed by a further incubation at 37°C for 10 min. Probe q was labeled in the presence of 40 μM Alexa 488-dUTP (Molecular Probes) by nick translation using DNA Polymerase I/DNaseI (GIBCO BRL) in a reaction containing 50 mM Tris-HCl (pH 7.8), 5 mM MgCl2, 10 mM 2-mercaptoethanol, 10 μg/mL BSA, 40 μM dATP, dCTP, and dGTP, 8 μM dTTP with an additional 4 U/μL DNase (GIBCO) at 15°C for 1 h. Reactions were stopped by the addition of EDTA (pH 8.0) to a final concentration of 50 mM and heated to 65°C for 15 min. Each probe was purified through a G-50 Sephadex spin column prior to slide hybridization.
Hybridization and washing of probes was carried out essentially as described in Weiner and Kleckner (1994) except that 0.4× SSC, 1% BSA, and 4% dextran sulfate were used for the hybridization and no detection antibodies were used. Nuclei were visualized at 1000× using a Zeiss Axioscope epifluorescence microscope equipped with HyQ TRITC, FITC, and Cascade blue filter sets (Chroma). Digital images were collected using a Hamamatsu Orca CCD camera and analyzed using Openlab (Improvision) software.
Acknowledgments
We thank Nancy Kleckner, Neil Hunter, and Scott Keeney for plasmids and yeast strains. We are indebted to Nancy Kleckner for advice and support in the early stages of this work. We thank Alastair Goldman, Owen Hughes, Neil Hunter, and Joshua Chang Mell for comments on the manuscript. This work was supported by grants to S.M.B. from the Arnold and Mabel Beckman Foundation and from the American Cancer Society (no. RSG CCG-101133).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL smburgess@ucdavis.edu; FAX (530) 752-3085.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.983802.
References
- Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- Albini SM, Jones GH. Synaptonemal complex spreading in Allium cepa and A. fistulosum I. The intiation and sequence of pairing. Chromosoma. 1987;95:324–338. [Google Scholar]
- Allers T, Lichten M. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell. 2001;106:47–57. doi: 10.1016/s0092-8674(01)00416-0. [DOI] [PubMed] [Google Scholar]
- Anderson LK, Stack SM. Nodules associated with axial cores and synaptonemal complexes during zygotene in Psilotum nudum. Chromosoma. 1988;97:96–100. [Google Scholar]
- Aragon-Alcaide L, Strunnikov AV. Functional dissection of in vivo interchromosome association in Saccharomyces cerevisiae. Nat Cell Biol. 2000;2:812–818. doi: 10.1038/35041055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berben G, Dumont J, Gilliquet V, Bolle PA, Hilger F. The YDp plasmids: A uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast. 1991;7:475–477. doi: 10.1002/yea.320070506. [DOI] [PubMed] [Google Scholar]
- Bergerat A, de Massy B, Gadelle D, Varoutas PC, Nicolas A, Forterre P. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- Bishop DK. RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell. 1994;79:1081–1092. doi: 10.1016/0092-8674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Bishop DK, Park D, Xu L, Kleckner N. DMC1: A meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- Bullard SA, Kim S, Galbraith AM, Malone RE. Double strand breaks at the HIS2 recombination hot spot in Saccharomyces cerevisiae. Proc Natl Acad Sci. 1996;93:13054–13059. doi: 10.1073/pnas.93.23.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SM. Homologous chromosome associations and nuclear order in meiotic and mitotically dividing cells of budding yeast. Adv Genet. 2002;46:49–90. doi: 10.1016/s0065-2660(02)46004-x. [DOI] [PubMed] [Google Scholar]
- Burgess SM, Kleckner N. Collisions between yeast chromosomal loci in vivo are governed by three layers of organization. Genes & Dev. 1999;13:1871–1883. doi: 10.1101/gad.13.14.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SM, Kleckner N, Weiner BM. Somatic pairing of homologs in budding yeast: Existence and modulation. Genes & Dev. 1999;13:1627–1641. doi: 10.1101/gad.13.12.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Dawson D, Stearns T. Methods in yeast genetics: A Cold Spring Harbor Laboratory course manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Cao L, Alani E, Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990;61:1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- Cha RS, Weiner BM, Keeney S, Dekker J, Kleckner N. Progression of meiotic DNA replication is modulated by interchromosomal interaction proteins, negatively by Spo11p and positively by Rec8p. Genes & Dev. 2000;14:493–503. [PMC free article] [PubMed] [Google Scholar]
- Chua PR, Roeder GS. Tam1, a telomere-associated meiotic protein, functions in chromosome synapsis and crossover interference. Genes & Dev. 1997;11:1786–1800. doi: 10.1101/gad.11.14.1786. [DOI] [PubMed] [Google Scholar]
- Conrad MN, Dominguez AM, Dresser ME. Ndj1p, a meiotic telomere protein required for normal chromosome synapsis and segregation in yeast. Science. 1997;276:1252–1255. doi: 10.1126/science.276.5316.1252. [DOI] [PubMed] [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner K. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- Dernburg AF, Sedat JW. Mapping three-dimensional chromosome architecture in situ. Methods Cell Biol. 1998;53:187–233. doi: 10.1016/s0091-679x(08)60880-8. [DOI] [PubMed] [Google Scholar]
- Esposito RE, Esposito MS. Genetic recombination and commitment to meiosis in Saccharomyces. Proc Natl Acad Sci. 1974;71:3172–3176. doi: 10.1073/pnas.71.8.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foiani M, Nadjar-Boger E, Capone R, Sagee S, Hashimshoni T, Kassir Y. A meiosis-specific protein kinase, Ime2, is required for the correct timing of DNA replication and for spore formation in yeast meiosis. Mol Gen Genet. 1996;253:278–288. doi: 10.1007/s004380050323. [DOI] [PubMed] [Google Scholar]
- Franklin AE, McElver J, Sunjevaric I, Rothstein R, Bowen B, Cande WZ. Three-dimensional microscopy of the Rad51 recombination protein during meiotic prophase. Plant Cell. 1999;11:809–824. doi: 10.1105/tpc.11.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerton JL, DeRisi J, Shroff R, Lichten M, Brown PO, Petes TD. Global mapping of meiotic recombination hotspots and coldspots in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci. 2000;97:11383–11390. doi: 10.1073/pnas.97.21.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman ASH, Lichten M. Restriction of ectopic recombination by interhomolog interactions during Saccharomyces cerevisiae meiosis. Proc Natl Acad Sci. 2000;97:9537–9542. doi: 10.1073/pnas.97.17.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth NM, Ponte L, Halsey C. MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes & Dev. 1995;9:1728–1739. doi: 10.1101/gad.9.14.1728. [DOI] [PubMed] [Google Scholar]
- Hunter N, Kleckner N. The single-end invasion: An asymmetric intermediate at the double- strand break to double-holliday junction transition of meiotic recombination. Cell. 2001;106:59–70. doi: 10.1016/s0092-8674(01)00430-5. [DOI] [PubMed] [Google Scholar]
- Jiao K, Bullard SA, Salem L, Malone RE. Coordination of the initiation of recombination and the reductional division in meiosis in Saccharomyces cerevisiae. Genetics. 1999;152:117–128. doi: 10.1093/genetics/152.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane SM, Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974;118:8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee K, Keeney S. Functional interactions between SPO11 and REC102 during initiation of meiotic recombination in Saccharomyces cerevisiae. Genetics. 2002;160:111–122. doi: 10.1093/genetics/160.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S. The mechanism and control of meiotic recombination initiation. Curr Top Dev Biol. 2001;52:1–53. doi: 10.1016/s0070-2153(01)52008-6. [DOI] [PubMed] [Google Scholar]
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- Leem SH, Ogawa H. The MRE4 gene encodes a novel protein kinase homologue required for meiotic recombination in Saccharomyces cerevisiae. Nucleic Acids Res. 1992;20:449–457. doi: 10.1093/nar/20.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu JY, Chua PR, Roeder GS. The meiosis-specific Hop2 protein of S. cerevisiae ensures synapsis between homologous chromosomes. Cell. 1998;94:375–386. doi: 10.1016/s0092-8674(00)81480-4. [DOI] [PubMed] [Google Scholar]
- Loidl J, Klein F, Scherthan H. Homologous pairing is reduced but not abolished in asynaptic mutants of yeast. J Cell Biol. 1994;125:1191–1200. doi: 10.1083/jcb.125.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao-Draayer Y, Galbraith AM, Pittman DL, Cool M, Malone RE. Analysis of meiotic recombination pathways in the yeast Saccharomyces cerevisiae. Genetics. 1996;144:71–86. doi: 10.1093/genetics/144.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll RM, Esposito RE. SPO13 negatively regulates the progression of mitotic and meiotic nuclear division in Saccharomyces cerevisiae. Genetics. 1994;138:47–60. doi: 10.1093/genetics/138.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Pearlman RE. Chromatin organization at meiosis. BioEssays. 1988;9:151–153. doi: 10.1002/bies.950090503. [DOI] [PubMed] [Google Scholar]
- Nag DK, Scherthan H, Rockmill B, Bhargava J, Roeder GS. Heteroduplex DNA formation and homolog pairing in yeast meiotic mutants. Genetics. 1995;141:75–86. doi: 10.1093/genetics/141.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MJ, Ramachandran M, Trelles-Sticken E, Scherthan H, Goldman ASH. Wild-type levels of Spo11-induced double strand breaks are required for normal regulation of single strand resection during meiosis. Mol Cell. 2002;9:835–846. doi: 10.1016/s1097-2765(02)00498-7. [DOI] [PubMed] [Google Scholar]
- Padmore R, Cao L, Kleckner N. Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell. 1991;66:1239–1256. doi: 10.1016/0092-8674(91)90046-2. [DOI] [PubMed] [Google Scholar]
- Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockmill B, Sym M, Scherthan H, Roeder GS. Roles for two RecA homologs in promoting meiotic chromosome synapsis. Genes & Dev. 1995;9:2684–2695. doi: 10.1101/gad.9.21.2684. [DOI] [PubMed] [Google Scholar]
- Roeder GS. Meiotic chromosomes: It takes two to tango. Genes & Dev. 1997;11:2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- Sauer B, Whealy M, Robbins A, Enquist L. Site-specific insertion of DNA into a pseudorabies virus vector. Proc Natl Acad Sci. 1987;84:9108–9112. doi: 10.1073/pnas.84.24.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell. 1994;76:51–63. doi: 10.1016/0092-8674(94)90172-4. [DOI] [PubMed] [Google Scholar]
- ————— Identification of double Holliday junctions as intermediates in meiotic recombination. Cell. 1995;83:783–791. doi: 10.1016/0092-8674(95)90191-4. [DOI] [PubMed] [Google Scholar]
- ————— Interhomolog bias during meiotic recombination: Meiotic functions promote a highly differentiated interhomolog-only pathway. Cell. 1997;90:1123–1135. doi: 10.1016/s0092-8674(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Sherman F, Roman H. Evidence for two types of allelic recombination in yeast. Genetics. 1963;48:255–261. doi: 10.1093/genetics/48.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Treco D, Szostak JW. Extensive 3′-overhanging, single-stranded DNA associated with the meiosis-specific double-strand breaks at the ARG4 recombination initiation site. Cell. 1991;64:1155–1161. doi: 10.1016/0092-8674(91)90270-9. [DOI] [PubMed] [Google Scholar]
- Sym M, Roeder GS. Crossover interference is abolished in the absence of a synaptonemal complex protein. Cell. 1994;79:283–292. doi: 10.1016/0092-8674(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Sym M, Engebrecht JA, Roeder GS. ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell. 1993;72:365–378. doi: 10.1016/0092-8674(93)90114-6. [DOI] [PubMed] [Google Scholar]
- Tarsounas M, Morita T, Pearlman RE, Moens PB. RAD51 and DMC1 form mixed complexes associated with mouse meiotic chromosome cores and synaptonemal complexes. J Cell Biol. 1999;147:207–220. doi: 10.1083/jcb.147.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelles-Sticken E, Loidl J, Scherthan H. Bouquet formation in budding yeast: Initiation of recombination is not required for meiotic telomere clustering. J Cell Sci. 1999;112:651–658. doi: 10.1242/jcs.112.5.651. [DOI] [PubMed] [Google Scholar]
- Trelles-Sticken E, Dresser ME, Scherthan H. Meiotic telomere protein Ndj1p is required for meiosis-specific telomere distribution, bouquet formation and efficient homologue pairing. J Cell Biol. 2000;151:95–106. doi: 10.1083/jcb.151.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve AM, Hillers KJ. Whence meiosis? Cell. 2001;106:647–650. doi: 10.1016/s0092-8674(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Walker MY, Hawley RS. Hanging on to your homolog: The roles of pairing, synapsis and recombination in the maintenance of homolog adhesion. Chromosoma. 2000;109:3–9. doi: 10.1007/s004120050407. [DOI] [PubMed] [Google Scholar]
- Weiner BM, Kleckner N. Chromosome pairing via multiple interstitial interactions before and during meiosis in yeast. Cell. 1994;77:977–991. doi: 10.1016/0092-8674(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- Xu L, Ajimura M, Padmore R, Klein C, Kleckner N. NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:6572–6581. doi: 10.1128/mcb.15.12.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Weiner BM, Kleckner N. Meiotic cells monitor the status of the interhomolog recombination complex. Genes & Dev. 1997;11:106–118. doi: 10.1101/gad.11.1.106. [DOI] [PubMed] [Google Scholar]
- Zenvirth D, Loidl J, Klein S, Arbel A, Shemesh R, Simchen G. Switching yeast from meiosis to mitosis: Double-strand break repair, recombination and synaptonemal complex. Genes Cells. 1997;2:487–498. doi: 10.1046/j.1365-2443.1997.1370335.x. [DOI] [PubMed] [Google Scholar]
- Zickler D, Kleckner N. The leptotene-zygotene transition of meiosis. Annu Rev Genet. 1998;32:619–697. doi: 10.1146/annurev.genet.32.1.619. [DOI] [PubMed] [Google Scholar]
- ————— Meiotic chromosomes: Integrating structure and function. Annu Rev Genet. 1999;33:603–754. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]