Abstract
Bluetongue virus (BTV) is an insect-vectored emerging pathogen of ruminants with the potential for devastating economic impact on European agriculture. BTV and many other members of the Reoviridae have remained stubbornly refractory to the development of methods for the rescue of infectious virus from cloned nucleic acid (reverse genetics). Partially disassembled virus particles are transcriptionally active, synthesizing viral transcripts in the cytoplasm of infected cells, in essence delivering viral nucleic acids in situ. With the goal of generating a reverse-genetics system for BTV, we examined the possibility of recovering infectious BTV by the transfection of BSR cells with BTV transcripts (single-stranded RNA [ssRNA]) synthesized in vitro using BTV core particles. Following transfection, viral-protein synthesis was detected by immunoblotting, and confocal examination of the cells showed a punctate cytoplasmic distribution of inclusion bodies similar to that seen in infected cells. Viral double-stranded RNA (dsRNA) was isolated from ssRNA-transfected cells, demonstrating that replication of the ssRNA had occurred. Additionally, infectious virus was present in the medium of transfected cells, as demonstrated by the passage of infectivity in BSR cells. Infectivity was sensitive to single-strand-specific RNase A, and cotransfection of genomic BTV dsRNA with transcribed ssRNA demonstrated that the ssRNA species, rather than dsRNA, were the active components. We conclude that it is possible to recover infectious BTV wholly from ssRNA, which suggests a means for establishing helper virus-independent reverse-genetics systems for members of the Reoviridae.
The introduction of specific mutations into the cloned genomes of many viruses and the study of the phenotype of the recovered infectious virus (reverse genetics) has allowed more directed approaches to the study of their gene products and genomes. Initially, viruses amenable to study by reverse genetics were those that have a single-stranded RNA (ssRNA) genome that also functions as mRNA, such as bacteriophage Qβ and poliovirus (11, 17). In recent years, RNA viruses with genomes of opposite sense to their mRNA, such as measles virus, influenza virus, and bunyaviruses, have been recovered entirely from cDNA clones (1, 8, 9, 12). Despite these successes, however, a number of viruses have remained refractory to similar techniques.
Bluetongue virus (BTV) is transmitted between ruminant hosts by several species of biting midges of the genus Culicoides, which determine its geographic range. BTV, endemic in many tropical countries, is an emerging pathogen of ruminants in Europe, with the potential to have a severe economic impact on European agriculture (10). BTV belongs to the genus Orbivirus of the family Reoviridae and has a segmented genome consisting of 10 linear double-stranded RNA (dsRNA) molecules (19, 22). The segments are classified into three size classes: large (L1 to L3), medium (M4 to M6), and small (S7 to S10). The virus particle has a layered structure, the outer layer of which is lost before the remaining core particle enters the cytoplasm of the host cell (5, 20, 21). The core particle is transcriptionally active, synthesizing and extruding multiple capped message sense copies of each viral genome segment into the host cell cytoplasm (5, 20, 21). These transcripts have the dual roles of encoding the viral proteins and serving as templates for the synthesis of the new viral dsRNA genome segments present in progeny virus particles. BTV replication occurs concomitantly with the establishment of virus inclusion bodies in the cytoplasm, and the dsRNA genome of the virus is never freely distributed in the cytosol (15). While such a strategy minimizes the host interferon response, uniquely among RNA virus replication cycles, it obfuscates a simple genome → replication intermediate → genome flow diagram and makes uncertain the assumption that intervention is possible at any one point, as would be required for the recovery of infectious BTV from cloned copies of the genome. Indeed, the development of reverse genetics has been slow for viruses of the family Reoviridae; Roner and Joklik reported the first helper virus-based system for the genus Orthoreovirus in 2001 (13). It was demonstrated that transfection with viral ssRNA produced by virus cores, in combination with an in vitro-synthesized transcript of a cloned virus genome segment, led to the recovery of virus derived from the input RNA. In this system, infection with the helper virus is necessary to recover progeny virus derived from the viral ssRNA (14). More recently, a helper virus-based system for the genus Rotavirus was created when Komoto et al. showed that a viral genome segment could be recovered in infectious virus when it was expressed using the recombinant vaccinia virus T7 RNA polymerase system in cells that were also infected with rotavirus (3). To date, there are no reports of other genera in the Reoviridae being manipulated by reverse genetics or of a demonstration that infectious virus can be recovered from RNA alone.
As in other members of the family Reoviridae, the core particles of BTV synthesize and release capped ssRNA transcripts into the cytoplasm of infected cells, using the genomic dsRNA segments as templates. Apart from the production of viral ssRNA transcripts, the core particle itself has no known role in infection. The extruded transcripts act both as mRNAs for the synthesis of the viral proteins and as templates for the synthesis of new dsRNA genome segments. In theory, both of these roles would be satisfied if the viral ssRNA was delivered to the host cell cytoplasm by a route other than transcription from an infectious viral core. Here, we investigate the possibility that bluetongue virus ssRNA could be infectious without the use of a helper virus. Purified bluetongue virus type 1 (BTV-1) cores were used to synthesize viral ssRNA in vitro and then completely removed from the viral ssRNA. We report that bluetongue virus ssRNA directs high levels of viral-protein synthesis in at least 50% of transfected cells and, furthermore, is infectious in 1 in 10,000 transfected cells, leading to the recovery of infectious virus.
MATERIALS AND METHODS
Cell lines and virus.
BSR cells (a clone of BHK-21 cells) were cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 5% (vol/vol) fetal bovine serum (Invitrogen).
BTV-1 stocks were generated by infecting BSR cells at a multiplicity of infection (MOI) of 0.1 and harvesting the medium 3 to 4 days postinfection.
Purification of bluetongue virus cores.
BSR cultures were infected with BTV-1 at an MOI of 0.02. Transcriptionally active BTV-1 cores were purified using a modification of the methods of Mertens et al. (6) and Van Dijk and Huismans (18). Briefly, BTV-1-infected BSR cells were lysed at 64 h postinfection in chilled 100 mM Tris-HCl, pH 8.8, 50 mM NaCl, 10 mM EDTA, 0.1% NP-40. Nuclei were removed by centrifugation at 2,500 × g at 4°C for 5 min. α-Chymotrypsin was added to 60 μg/ml, and the sample was incubated at 35°C for 1 h. The sample was made 0.2% (wt/vol) for N-lauroyl sarcosine (sodium salt) and incubated at 25°C for 1 h. Ultracentrifugation on a sucrose cushion consisting of 40% (wt/vol) sucrose, 600 mM MgCl2, 20 mM Tris-HCl, pH 8.0, was carried out at 141,000 × g and 20°C for 2 h. The pellet was resuspended in 20 mM Tris-HCl, pH 8.0, 0.5% NP-40 and incubated at 4°C for 16 h. A second ultracentrifuge spin through a sucrose cushion consisting of 40% (wt/vol) sucrose, 20 mM Tris-HCl, pH 8.0, at 141,000 × g and 20°C for 2 h was performed. The pellet was resuspended in 200 μl 20 mM Tris-HCl, pH 8.0.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of purified BTV-1 cores.
Proteins in purified cores were separated using 10% polyacrylamide gels with the Laemmli buffer system (4). The gels were stained with Coomassie blue R250.
Synthesis of bluetongue virus ssRNA in vitro.
Viral ssRNA was synthesized in vitro using reaction conditions similar to those described by Mertens and Payne (7). Briefly, BTV-1 cores were incubated at 40 μg/ml and 30°C for 5 to 6 h in BTV core transcription buffer (100 mM Tris-HCl, pH 8.0, 4 mM ATP, 2 mM GTP, 2 mM CTP, 2 mM UTP, 500 μM S-adenosylmethionine, 6 mM dithiothreitol, 9 mM MgCl2, 0.5 U/μl RNasin Plus [Promega]).
Purification of bluetongue virus ssRNA.
The initial step in removal of the infectious cores from the in vitro transcription reaction was ultracentrifugation in a TLS-55 rotor at 214,000 × g and 4°C for 25 min. The supernatants were spun a second time, as described above, to further remove residual cores. The supernatants were made 2 M for LiCl, using 8 M LiCl (Sigma), and incubated at 4°C for 16 h to precipitate ssRNA. Samples were microcentrifuged at 17,000 × g at 4°C for 15 min. The pellets were dissolved in 200 μl proteinase K solution (10 μg/ml in OptiMEM I [Invitrogen]) and incubated at 35°C for 30 min. The samples were deproteinized by extracting them twice with phenol-chloroform, 0.1% (wt/vol) SDS and twice with chloroform. The aqueous phase was used directly in transfections (see below).
Denaturing agarose gel electrophoresis.
Purified BTV ssRNA was analyzed by electrophoresis on 1% agarose in MOPS (morpholinepropanesulfonic acid) electrophoresis buffer in the presence of formaldehyde, using standard techniques (16).
Transfection of BSR cells with purified bluetongue virus RNA or bluetongue virus cores.
BSR monolayers in six-well plates were transfected with BTV-1 RNA or BTV-1 cores using Lipofectamine 2000 Reagent (Invitrogen). RNA or cores and Lipofectamine 2000 were individually diluted in OptiMEM I and mixed according to the manufacturer's instructions. Complexes were added to the monolayers and not removed.
Infectivity assay of BTV ssRNA or BTV cores.
Ninety to 100% confluent BSR monolayers in six-well plates (2 × 106 cells) were transfected as described above. At 8 h posttransfection, the culture medium was replaced with a 6-ml overlay consisting of minimal essential medium (Invitrogen), 2% fetal calf serum, 1.5% (wt/vol) agarose type VII (Sigma). The assay mixtures were incubated at 35°C, 5% CO2 for 72 h to allow plaques to appear when BTV-1 ssRNA was used or 48 h when BTV-1 cores were used. For plaque picking, the monolayers were stained with Neutral Red at 80 μg/ml for 8 h. For counting purposes, the assay mixtures were fixed with 10% formaldehyde in phosphate-buffered saline (PBS) (Oxoid) for 3 h and stained with crystal violet.
Indirect immunofluorescence.
BSR cells were seeded on glass coverslips in six-well plates and transfected with BTV-1 ssRNA or infected with BTV-1 at an MOI of 1. Transfected or infected cells were washed once in PBS (Oxoid) and fixed in 4% (wt/vol) paraformaldehyde in PBS. The cells were permeabilized in 1% (vol/vol) Tween 20 in PBS. Staining was performed using a rabbit polyclonal antiserum raised to BTV10 NS2 (R658), followed by tetramethylrhodamine isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma). Nuclear staining was achieved by using Hoescht 33342 at 3.3 μg/ml in the secondary-antibody solution. Digital images were recorded with an LSM510 microscope (Carl Zeiss Ltd., United Kingdom) using a 63× oil objective. Images were processed with the LSM image browser software (Carl Zeiss Ltd., United Kingdom).
Immunoblot analysis of BSR cells transfected with BTV-1 ssRNA.
Standard immunoblotting techniques were used with rabbit polyclonal antiserum R658 raised against NS2 or mouse monoclonal antibody 2C3 raised against VP6. Proteins were visualized using alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) or alkaline phosphatase-conjugated sheep anti-mouse IgG (Sigma), respectively.
Extraction of dsRNA from transfected BSR cells.
Total RNA was extracted from cells using Tri-Reagent (Sigma) according to the manufacturer's instructions. The RNA was precipitated with an equal volume of isopropanol and dissolved in 30 μl distilled water. Samples were made 2 M for LiCl, using 8 M LiCl (Sigma), and incubated at 4°C for 16 h to precipitate the ssRNA. The samples were microcentrifuged at 17,000 × g at 4°C for 15 min, and the supernatant containing dsRNA was precipitated with an equal volume of isopropanol. Samples were microcentrifuged at 17,000 × g at 4°C for 10 min, and the pellets were washed twice in 70% (vol/vol) ethanol before being dissolved in distilled water.
Nondenaturing polyacrylamide gel electrophoresis.
RNA samples were run on 9% polyacrylamide gels at 130 V in Tris-glycine buffer (pH 8.4). The gels were stained with ethidium bromide solution (0.5 μg/ml) for 30 min.
Preparation of BSR cells for phase-contrast microscopy.
Cells were washed once in PBS and fixed using 4% paraformaldehyde in PBS for 30 min. The cells were washed once in PBS, and phase-contrast images were recorded with a Nikon Eclipse TS100 microscope using an attached digital camera.
RNase A digestion of bluetongue virus RNA or bluetongue virus cores.
RNase A digests were carried out in 5-μl reaction mixtures in OptiMEM I at 25°C for 30 min. The digested material was immediately used to transfect BSR cells, as described.
RESULTS
Purification of bluetongue virus cores and in vitro synthesis of BTV-1 ssRNA.
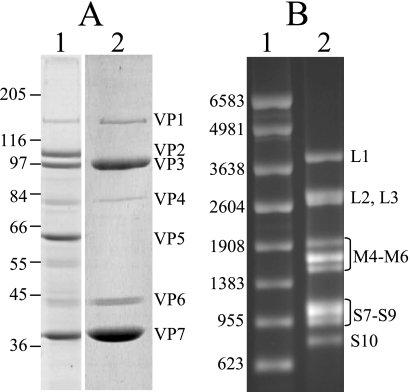
Since BTV cores are transcriptionally active, yielding full-length capped ssRNAs, we used a high-yielding and robust serotype of BTV (BTV-1) to prepare BTV cores by purification from infected BSR cells, as described in Materials and Methods. SDS-PAGE analysis of the purified cores revealed the presence of all five of the core proteins (VP1, VP3, VP4, VP6, and VP7) in the expected ratios and the absence of both of the outer capsid proteins, VP2 and VP5 (Fig. 1A).
FIG. 1.
Purification of BTV-1 cores and synthesis of BTV-1 ssRNA in vitro. (A) Ten percent SDS-PAGE of purified BTV-1 cores. Lane 1, purified BTV-1 virions; lane 2, purified BTV1 cores. The numbers on the left indicate the protein molecular mass markers in kDa, and the viral proteins are indicated on the right. (B) Denaturing 1% agarose gel electrophoresis of purified BTV-1 ssRNA. Lane 1, 1-μg ssRNA markers (Promega). The numbers on the left indicate the lengths of the markers in nucleotides. Lane 2, 1 μg BTV-1 ssRNA. The transcripts of the viral genome segments are indicated on the right.
BTV ssRNA was synthesized in vitro using the transcriptional activities of BTV cores as described in Materials and Methods. To ensure the removal of the cores, the reaction mixture was ultracentrifuged twice and treated with proteinase K, followed by two extractions with phenol-chloroform, as described in Materials and Methods. The selective precipitation of ssRNA with LiCl was included to separate the ssRNA from dsRNA. Denaturing agarose gel electrophoresis analysis of the purified BTV ssRNA showed that all 10 viral transcripts were synthesized and purified, with no evidence of premature termination or gross degradation (Fig. 1B). This suggests that in vitro-synthesized BTV ssRNA has the same coding potential as BTV ssRNA produced within an infected cell.
Validation of BTV ssRNA purification.
The removal of all core particles was essential to allow study of the infectivity of BTV ssRNA. To determine whether the ssRNA purification method removed the infectivity from cores, a test of the proteinase K digestion step, followed by the two phenol-chloroform extractions, was performed as described in Materials and Methods. This was done using three independent preparations of 50 μg of purified BTV-1 cores, and the infectivity of each preparation was tested by attempting virus recovery by the transfection of 2 × 106 BSR cells. Ten percent of each preparation (equivalent to 5 μg of cores) was used to transfect BSR cells and overlaid with agarose, as described in Materials and Methods. After 72 h of incubation, no plaques were detected in the treated samples, in contrast to the appearance of plaques derived from 5 ng untreated cores in 48 h (Fig. 2). This result demonstrated that no residual infectivity derived from BTV cores remained following the procedures used to purify ssRNA from transcribing core preparations.
FIG. 2.
Validation of the removal of infectious cores. BSR cell monolayers transfected with treated or untreated BTV-1 cores and overlaid with agarose as described in Materials and Methods. (A) BSR monolayer transfected with the purified nucleic acid derived from proteinase K digestion and two phenol-chloroform extractions of 5 μg BTV-1 cores. (B) BSR monolayer transfected with 5 ng untreated BTV-1 cores.
Expression of bluetongue virus proteins in BSR cells transfected with purified BTV-1 ssRNA.
To determine whether the purified ssRNAs could synthesize viral proteins in vivo, 2 × 106 BSR cells seeded on glass coverslips were transfected with 4 μg (615 fmol of the ssRNA form of the genome) BTV-1 ssRNA. Fixation and staining of cells was carried out at 16 h posttransfection. The viral inclusion body-forming protein, NS2, was used as a marker to assess the expression of BTV proteins. In ∼50% of the cells, NS2 staining was found in a cytoplasmic punctate distribution (Fig. 3A and B). The staining pattern of NS2 in transfected cells was similar to that produced in BTV-1-infected cells (Fig. 3C and D), although the sizes of the inclusion bodies were usually smaller in the transfected cells. The production of numerous discrete inclusions containing the viral NS2 protein demonstrated that transfection could effectively deliver BTV ssRNA to the cytoplasm of BSR cells. Analysis of BSR cells transfected with 6 ng to 2 μg (0.92 fmol to 308 fmol) BTV-1 ssRNA by immunofluorescence showed that cells expressing NS2 in the typical punctate pattern were present with as little as 6 ng of RNA, but at a frequency of <1% of the cells (Fig. 3E and data not shown).
FIG. 3.
Expression of NS2 in BSR cells transfected with BTV-1 ssRNA. BSR cells were grown on glass coverslips in six-well plates and either transfected with BTV-1 ssRNA (A, B, and E) or infected with BTV-1 (C and D). NS2 (red) was stained by indirect immunofluorescence, and nuclei (blue) were stained with Hoescht 33342, as described in Materials and Methods. (A) BSR cells transfected with 4 μg (615 fmol) BTV-1 ssRNA and fixed after 16 h. (B) Enlargement of the area indicated in panel A. (C) BSR cells infected with BTV-1 at an MOI of 1 and fixed after 8 h. (D) Enlargement of the area indicated in panel C. (E) BSR cells transfected with 6 ng (0.92 fmol) BTV-1 ssRNA and fixed after 16 h.
To assess the expression of viral proteins over time in BSR cells transfected with BTV ssRNA, the inclusion body protein NS2 and the minor structural protein VP6 were used as markers. Replicates of 1.5 × 106 BSR cells were transfected with 2 μg (308 fmol) BTV ssRNA and harvested at intervals, with the presence/absence of cytopathic effect (CPE) recorded concurrently. The expression of NS2 was detected from 16 h onward, with an increase in expression occurring between 48 and 64 h, which correlated with the appearance in the cultures of CPE indistinguishable from that caused by viral infection (Fig. 4). The expression of VP6 was detectable from 64 h onward, again correlating with the appearance of CPE (Fig. 4).
FIG. 4.
Time course of the expression of NS2 and VP6 in BSR cells transfected with BTV-1 ssRNA. BSR cells (1.5 × 106) were transfected with 2 μg (308 fmol) BTV-1 ssRNA. Lysates were harvested at intervals after transfection/infection and analyzed by SDS-PAGE, followed by immunoblotting using anti-NS2 rabbit polyclonal antibody R658 (A) or anti-VP6 mouse monoclonal antibody 2C3 (B). The numbers above the lanes indicate the times of harvest after transfection, in hours. Lane V is BSR infected with BTV-1 at an MOI of 1 and harvested at 72 h. The arrows indicate the positions of the NS2 and VP6 bands, and the numbers on the right represent the protein molecular mass markers in kDa.
Recovery of infectious BTV-1 from BSR cells transfected with purified BTV-1 ssRNA.
To determine whether infectious virus could be generated from BTV ssRNA, large quantities of ssRNA were initially tested, as it was expected that the recovery of virus would occur at a lower frequency than viral-protein expression in transfected cells. BSR cells (2 × 106) were transfected with 4 to 8 μg (0.62 to 1.23 pmol) BTV-1 ssRNA as described above. At 3 days posttransfection, a cytopathic effect indistinguishable from that caused by BTV-1 infection was seen in 100% of the cells (data not shown). In order to determine whether the viral ssRNA had been replicated to produce new genomic dsRNA, the dsRNA was extracted from the transfected cells at 3 days posttransfection, as described in Materials and Methods. Analysis of the dsRNA by electrophoresis on nondenaturing 9% polyacrylamide gels revealed that all 10 dsRNA segments had been synthesized in the cells transfected with BTV-1 ssRNA (Fig. 5). To determine whether infectious virus was present, 2.5 × 105 BSR cells were inoculated with 50 μl of culture medium taken from the transfected cells 3 days posttransfection. The inoculated BSR cells displayed cytopathic effect throughout the cultures at 3 days postinfection (Fig. 6 and data not shown), demonstrating that infectious virus was present in the medium of the transfected BSR cells. The recovery of infectious virus from transfected 293T cells was also possible (data not shown), but the CPE took longer to become apparent and investigations were continued using only BSR cells.
FIG. 5.
Synthesis of bluetongue virus dsRNA in BSR cells transfected with BTV-1 ssRNA. BSR cells in six-well plates were transfected with BTV-1 ssRNA, and after 3 days, dsRNA was extracted and run on nondenaturing 9% polyacrylamide gels, as described in Materials and Methods. Five hundred nanograms of BTV-1 genomic dsRNA was extracted from purified cores (lane 1). The BSR cells were transfected with 4 μg (615 fmol) BTV-1 ssRNA (lane 2), 6 μg (923 fmol) BTV-1 ssRNA (lane 3), or 8 μg (1.23 pmol) BTV-1 ssRNA (lane 4) or not transfected (lane 5).
FIG. 6.
Passage of virus derived from BSR cells transfected with BTV-1 ssRNA. BSR cells (2.5 × 105) were infected with 50 μl medium from BSR cells transfected with 8 μg (1.23 pmol) BTV-1 ssRNA (A), 2,500 PFU BTV-1 (B), or 50 μl medium from untreated BSR cells (C). The images were captured at 72 h postinfection.
Infectivity assay for purified BTV-1 ssRNA.
In order to quantify the virus rescue events and allow the recovery of virus from individual rescue events, BSR monolayers were transfected with BTV-1 ssRNA and overlaid with agarose (see Materials and Methods). Plaques were produced that were similar in appearance to those produced by infection with BTV-1 and were recovered using as little as 60 ng BTV-1 ssRNA (Fig. 7A). Virus from individual plaques was amplified by picking the plaques and infecting 106 BSR cells. Four days postinfection, dsRNA was extracted and analyzed by electrophoresis on nondenaturing 9% polyacrylamide gels. The dsRNA profiles of BTV-1 plaques derived from transfection were indistinguishable from that of BTV-1 derived from infection (Fig. 7B). These data demonstrated that infectious BTV-1 was present in each plaque.
FIG. 7.
Quantitation of virus rescue events in BSR cells transfected with BTV-1 ssRNA. (A) BSR monolayers were transfected with a dilution series of BTV-1 ssRNA and overlaid with agarose, as described in Materials and Methods. The wells received 0 ng (well 1), 60 ng [9.23 fmol] (well 2), 200 ng [30.8 fmol] (well 3), 600 ng [92.3 fmol] (well 4), 2 μg [308 fmol] (well 5), or 6 μg [923 fmol] (well 6) of BTV-1 ssRNA. The monolayers were fixed and stained with crystal violet 3 days after transfection. The numbers to the left of the wells indicate the numbers of plaques. (B) Nondenaturing 9% polyacrylamide gel analysis of dsRNA extracted from virus amplified from isolated plaques. BSR cells (106) in six-well plates were infected with virus picked from individual plaques, and dsRNA was extracted after 4 days. Lane 1, 500 ng BTV-1 dsRNA extracted from BSR infected with BTV-1 stock virus; lanes 2 and 3, 500 ng BTV-1 dsRNA extracted from BSR infected with individual BTV-1 plaques derived from transfection.
The infectivity of purified BTV-1 ssRNA is sensitive to RNase A.
The infectivity assay provided an independent means of assessing the nature of the infectious material in the BTV-1 ssRNA. To characterize the infectious material in the BTV-1 ssRNA, its sensitivity to RNase A was compared with that of BTV-1 cores, in which the genome is protected by the VP3 and VP7 layers of the capsid. Two micrograms (308 fmol) BTV-1 ssRNA or 20 ng BTV-1 cores was digested with RNase A prior to transfection of the BSR cells and agarose overlay, as described in Materials and Methods. The infectivity of the BTV-1 ssRNA was reduced to zero by 10 pg of RNase A, whereas the infectivity of the BTV-1 cores was unaffected by as much as 1 μg of RNase A (Fig. 8). The RNase A sensitivity of the infectious material in BTV-1 ssRNA was 100,000 times greater than that of cores and independently eliminated the possibility that some cores had survived the purification of the ssRNA, demonstrating that unprotected RNA was the source of the infectivity.
FIG. 8.
The infectivity of BTV-1 ssRNA is sensitive to RNase A. BSR monolayers were transfected with RNase A digests of either 2 μg (307 fmol) BTV-1 ssRNA (A) or 20 ng BTV-1 cores (B) and overlaid with agarose, as described in Materials and Methods. The quantities of RNase A used were as follows: wells 1, no RNase A; wells 2, 100 ag; wells 3, 1 fg; wells 4, 10 fg; wells 5, 100 fg; wells 6, 1 pg; wells 7, 10 pg; wells 8, 100 pg; wells 9, 1 ng; wells 10, 10 ng; wells 11, 100 ng; wells 12, 1 μg. The monolayers were fixed and stained with crystal violet 3 days after transfection (A) or 2 days after transfection (B).
The addition of BTV-1 dsRNA to BTV-1 ssRNA does not increase the rescue of infectious virus.
The possibility remained that trace quantities of genomic dsRNA were being rescued by the viral proteins encoded by the ssRNA. The packaging of the BTV-1 dsRNA into assembling particles might occur when the BTV-1 ssRNA had directed sufficient viral-protein synthesis to assemble core particles. To address this point, BTV-1 dsRNA was purified from cores, as described for the purification of BTV-1 ssRNA, starting at the proteinase K digestion step (see Materials and Methods). Twenty-five to 800 ng (1.92 to 61.5 fmol of genomes) purified BTV-1 dsRNA was mixed with 2 μg (308 fmol) BTV-1 ssRNA, and the effect on the number of virus rescue events was assessed in transfected BSR cells using the infectivity assay (Fig. 9). The addition of BTV-1 dsRNA did not increase the infectivity of the BTV-1 ssRNA, demonstrating that it is the BTV-1 ssRNA that is rescued and not trace amounts of contaminating dsRNA that may be present.
FIG. 9.
The infectivity of BTV-1 ssRNA is not increased by the cotransfection of BTV-1 dsRNA. BSR monolayers were cotransfected with a constant amount of BTV-1 ssRNA plus various amounts of BTV-1 dsRNA or were transfected with BTV-1 dsRNA alone. Wells 1 to 5 were transfected with 2 μg (307 fmol) BTV-1 ssRNA plus the following quantities of BTV-1 dsRNA: well 1, 0 ng; well 2, 25 ng (1.92 fmol); well 3, 50 ng (3.85 fmol); well 4, 200 ng (15.4 fmol); well 5, 800 ng (61.5 fmol). Well 6 was transfected with 800 ng (61.5 fmol) BTV-1 dsRNA only. The monolayers were fixed and stained with crystal violet 3 days after transfection. The numbers to the left of wells indicate the numbers of plaques.
DISCUSSION
In the Reoviridae family, the role of the core particles is to synthesize viral ssRNA in the cytoplasm of the infected cell, where they serve as mRNAs for viral-protein synthesis and as templates for the synthesis of new genomic dsRNAs. In this model, the delivery of BTV ssRNA to the cytoplasm of a cell by a route other than extrusion from an infecting core particle should lead to the production of infectious virus. In this study, we investigated the possibility that BTV-1 transcripts synthesized in vitro are infectious when introduced into BSR cells by transfection.
Initially, the expression of viral proteins in transfected cells was investigated. The transfection of BSR cells with viral ssRNA directed sufficient viral-protein expression to produce a punctate pattern of NS2 staining, similar to that seen in the viral inclusion bodies of infected cells (Fig. 3). Furthermore an increase in the expression of both NS2 and the minor structural protein VP6 coincided with the appearance of CPE in the transfected BSR cells.
The possibility of recovering infectious virus was assessed by the transfection of BSR cells with microgram quantities of BTV-1 ssRNA. When BSR cells were transfected with 4 to 8 μg viral ssRNA, a CPE was visible in 100% of the cells by 3 days posttransfection. Viral genomic dsRNA was synthesized in the transfected cells (Fig. 5), demonstrating that replication of the viral genome had occurred, a process as yet inseparable from the assembly of progeny particles in members of the Reoviridae. Furthermore, the CPE was reproduced in BSR cells incubated with culture medium from the transfected cells, demonstrating the presence of infectious virus (Fig. 6). We concluded from these experiments that infectious BTV-1 was produced in BSR cells transfected with purified BTV-1 ssRNA.
The number of virus rescue events was quantified by overlaying the transfected BSR cells with agarose. Using this infectivity assay, the recovery of viral plaques was robust over a range of amounts of input ssRNA (Fig. 7). The relationship between the amount of input RNA and the number of rescue events was approximately linear between 0 and 2 μg of ssRNA, giving a value of 140 plaques/μg ssRNA. The fact that sufficient viral-protein expression for virus rescue was produced by as little as 60 ng of ssRNA may be explained by the observation that transfection with nanogram quantities of ssRNA leads to high levels of protein expression in a low proportion of cells and no detectible protein expression in the remaining cells (Fig. 3E).
The infectivity assay allowed the RNase A sensitivity of infectivity derived from ssRNA to be compared with that of core particles. Infectivity derived from cores was shown to be resistant to at least 100,000 times more RNase A than infectivity derived from viral ssRNA (Fig. 8). We conclude from this result that the infectivity present in purified BTV-1 ssRNA is not derived from core particles surviving the purification methods used; rather, it is dependent on the integrity of unprotected viral RNA present.
The possibility that the infectivity of the viral ssRNA may rely on trace amounts of viral dsRNA was investigated. The cotransfection of viral ssRNA and viral genomic dsRNA derived from cores did not affect the number of virus rescue events compared to transfection with viral ssRNA alone (Fig. 9). From this result, we conclude that the recovery of virus does not depend on the packaging of trace amounts of viral dsRNA into assembling particles. In addition, the possibility that virus recovery is due to partly disrupted core particles being “reassembled” with the newly synthesized viral proteins is ruled out by this result. In summary, we conclude that in vitro-synthesized BTV transcripts are infectious when introduced into BSR cells by transfection. This is the first report of viral ssRNA being infectious without the use of a helper virus in the Reoviridae family.
A calculation from the number of plaques recovered demonstrates that rescue of infectious virus occurs in approximately 1 in 10,000 transfected cells when microgram quantities of viral ssRNA are used, whereas high levels of viral-protein expression are detectible in ∼50% of the cells. One explanation for this difference is that there may be a critical amount of viral ssRNA that must be introduced into the cell for a productive infection to be initiated. Below this threshold, there would be no rescue, but viral proteins would be synthesized. In addition, if rescue is to occur, a transfected cell must receive copies of each of the 10 transcripts. Furthermore, enough copies of each transcript must be present so that both viral proteins are synthesized, and enough of each transcript must remain available for packaging and replication. Once progeny cores are assembled, the transcripts produced by these progeny cores will lead to an amplification of gene expression and so to further core particle and virion production. A second possibility involves the poorly understood processes of genome packaging and genome replication, which in the Reoviridae occur within the viral inclusion bodies. The introduction of viral ssRNA into cells by transfection omits the presence of a core particle, which has been shown to be targeted to the viral inclusion bodies in the case of mammalian orthoreoviruses (2). It has been suggested that the core produces ssRNA within the inclusion body, thus supplying viral ssRNA for packaging and replication at the site of assembly of progeny cores. Delivery of viral RNA by transfection would not reproduce this situation, and a reduction in the recovery of virus would be expected. A third possibility is that the infectivity of the ssRNA is reduced by low levels of RNase contamination remaining in the purified cores. Degradation of the RNA may not be sufficient to prevent the synthesis of viral proteins, but it could be sufficient to reduce the probability of packaging a full complement of 10 intact RNA segments.
Roner et al. established that orthoreovirus ssRNA is infectious in L929 cells when a helper virus is used, but viral ssRNA alone was not found to be infectious (14). The lack of infectivity of orthoreovirus ssRNA may indicate that there is a genuine difference between the assembly of mammalian orthoreovirus and bluetongue virus. Alternatively, details specific to the system may account for the lack of virus rescue observed, such as the detection of viral-protein expression in only 4% of transfected cells. Roner et al. demonstrated that in the presence of a helper virus, a mixture of viral ssRNA and dsRNA was more infectious than ssRNA alone and that dsRNA was infectious without ssRNA (14). These observations were not reproduced by the cotransfection of BSR cells with BTV-1 ssRNA and dsRNA. In the former case, it is unclear whether this points to differences between the requirements of the two genera for packaging the viral genome or whether the additional presence of a helper virus in the orthoreovirus system is the cause. In the latter case, the lack of infectivity of BTV dsRNA should be expected, as there is no known mechanism for BTV dsRNA to be used as a template for the synthesis of viral proteins.
The recovery of infectious BTV from ssRNA demonstrates that, beyond the generation of transcripts in the cytoplasm of the infected cell, there is no essential role for the core particle. The discovery that BTV ssRNA is infectious brings the realization of a reverse-genetics system for orbiviruses a step closer. To produce a reverse-genetics system from this finding will require the replacement of a segment of viral ssRNA with a transcript produced from a cDNA clone. Such a method for the targeted replacement of a single ssRNA has been developed for use in the helper virus-based system used for orthoreovirus (13). Experiments are under way to determine whether heterologous genetic information can be introduced into the BTV genome by this approach, thus making possible the directed study of orbivirus proteins and RNAs.
Acknowledgments
We thank P. P. C. Mertens (IAH, United Kingdom) for the kind gift of the BTV-1 South African isolate and I. M. Jones (Reading University, United Kingdom) for critically reading the manuscript.
This work was partly supported by the BBSRC and the Wellcome Trust, United Kingdom.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Bridgen, A., and R. M. Elliott. 1996. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc. Natl. Acad. Sci. USA 93:15400-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broering, T. J., J. Kim, C. L. Miller, C. D. Piggott, J. B. Dinoso, M. L. Nibert, and J. S. Parker. 2004. Reovirus nonstructural protein mu NS recruits viral core surface proteins and entering core particles to factory-like inclusions. J. Virol. 78:1882-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komoto, S., J. Sasaki, and K. Taniguchi. 2006. Reverse genetics system for introduction of site-specific mutations into the double-stranded RNA genome of infectious rotavirus. Proc. Natl. Acad. Sci. USA 103:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 5.Martin, S. A., and H. J. Zweerink. 1972. Isolation and characterization of two types of bluetongue virus particles. Virology 50:495-506. [DOI] [PubMed] [Google Scholar]
- 6.Mertens, P. P., J. N. Burroughs, and J. Anderson. 1987. Purification and properties of virus particles, infectious subviral particles, and cores of bluetongue virus serotypes 1 and 4. Virology 157:375-386. [DOI] [PubMed] [Google Scholar]
- 7.Mertens, P. P., and C. C. Payne. 1978. S-Adenosyl-l-homocysteine as a stimulator of viral RNA synthesis by two distinct cytoplasmic polyhedrosis viruses. J. Virol. 26:832-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann, G., and Y. Kawaoka. 2004. Reverse genetics systems for the generation of segmented negative-sense RNA viruses entirely from cloned cDNA. Curr. Top Microbiol. Immunol. 283:43-60. [DOI] [PubMed] [Google Scholar]
- 9.Neumann, G., M. A. Whitt, and Y. Kawaoka. 2002. A decade after the generation of a negative-sense RNA virus from cloned cDNA—what have we learned? J. Gen. Virol. 83:2635-2662. [DOI] [PubMed] [Google Scholar]
- 10.Purse, B. V., P. S. Mellor, D. J. Rogers, A. R. Samuel, P. P. C. Mertens, and M. Baylis. 2005. Climate change and the recent emergence of bluetongue in Europe. Nat. Rev. Microbiology 3:171-181. [DOI] [PubMed] [Google Scholar]
- 11.Racaniello, V. R., and D. Baltimore. 1981. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science 214:916-919. [DOI] [PubMed] [Google Scholar]
- 12.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roner, M. R., and W. K. Joklik. 2001. Reovirus reverse genetics: incorporation of the CAT gene into the reovirus genome. Proc. Natl. Acad. Sci. USA 98:8036-8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roner, M. R., L. A. Sutphin, and W. K. Joklik. 1990. Reovirus RNA is infectious. Virology 179:845-852. [DOI] [PubMed] [Google Scholar]
- 15.Roy, P. 2005. Bluetongue virus proteins and particles and their role in virus entry, assembly, and release. Adv. Virus Res. 64:69-123. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 17.Taniguchi, T., M. Palmieri, and C. Weissmann. 1978. QB DNA-containing hybrid plasmids giving rise to QB phage formation in the bacterial host. Nature 274:223-228. [DOI] [PubMed] [Google Scholar]
- 18.Van Dijk, A. A., and H. Huismans. 1980. The in vitro activation and further characterization of the bluetongue virus-associated transcriptase. Virology 104:347-356. [DOI] [PubMed] [Google Scholar]
- 19.Verwoerd, D. W. 1969. Purification and characterization of bluetongue virus. Virology 38:203-212. [DOI] [PubMed] [Google Scholar]
- 20.Verwoerd, D. W., H. J. Els, E. M. De Villiers, and H. Huismans. 1972. Structure of the bluetongue virus capsid. J. Virol. 10:783-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verwoerd, D. W., and H. Huismans. 1972. Studies on the in vitro and the in vivo transcription of the bluetongue virus genome. Onderstepoort J. Vet. Res. 39:185-191. [PubMed] [Google Scholar]
- 22.Verwoerd, D. W., H. Louw, and R. A. Oellermann. 1970. Characterization of bluetongue virus ribonucleic acid. J. Virol. 5:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]