Abstract
The DRB region of the MHC in primate species is known to display abundant region configuration polymorphism with regard to the number and content of genes present per haplotype. Furthermore, depending on the species studied, the different DRB genes themselves may display varying degrees of allelic polymorphism. Because of this combination of diversity (differential gene number) and polymorphism (allelic variation), molecular typing methods for the primate DRB region are cumbersome. All intact DRB genes present in humans and rhesus macaques appear to possess, however, a complex and highly divergent microsatellite. Microsatellite analysis of a sizeable panel of outbred rhesus macaques, covering most of the known Mamu-DRB haplotypes, resulted in the definition of unique genotyping patterns that appear to be specific for a given haplotype. Subsequent examination of a representative panel of human cells illustrated that this approach also facilitates high-resolution HLA-DRB typing in an easy, quick, and reproducible fashion. The genetic composition of this complex microsatellite is shown to be in concordance with the phylogenetic relationships of various HLA-DRB and Mamu-DRB exon 2 gene/lineage sequences. Moreover, its length variability segregates with allelic variation of the respective gene. This simple protocol may find application in a variety of research avenues such as transplantation biology, disease association studies, molecular ecology, paternity testing, and forensic medicine.
Keywords: biological science, MHC
Gene products of the MHC play a key role in generating adaptive immune responses. Most vertebrate species express two distinct classes of MHC cell surface markers that execute differential biological functions. MHC class I glycoproteins are involved in the presentation of peptides of intracellular origin to cytotoxic T cells, possibly resulting in the lysis of infected or malignant structures. MHC class II molecules bind peptides from extracellular sources, and subsequent interaction with T helper cells may result, for instance, in antibody production. During vertebrate evolution, the MHC class I and II region genes have been subject to many expansion and contraction processes, and as a consequence a given (sub)region may harbor multiple, highly related genes. A next layer of complexity is the phenomenon that some of these MHC genes display unprecedented levels of polymorphism. The compound evolutionary history of MHC genes, in combination with their abundant levels of polymorphism, represents a challenge for the research community, because one has to come up with a simple and understandable nomenclature system. For example, in the human population five HLA-DRB region configurations have been defined. Each configuration contains a DRA gene in conjunction with a unique combination of polymorphic genes, designated DRB1 to DRB9 (1), which arose from a intricate series of duplication and crossing-over events during primate evolution (2, 3). Some of these genes are functional, whereas others have various types of deleterious mutations and are considered to represent pseudogenes (4, 5). The HLA-DRB1 gene is, at least in part, responsible for the fact that each of the human region configurations displays plentiful allelic heterogeneity (6).
The Mamu-DRB region in rhesus macaques (Macaca mulatta) is also highly plastic (7, 8), and at least 30 region configurations have been documented that, as found in humans, display marked differences with regard to number and content of genes present per haplotype (7, 9, 10). Most of the Mamu-DRB alleles belong to loci/lineages that are shared with humans, and these alleles have been named accordingly (6, 11, 12). For example, HLA-DRB1*0301 and Mamu-DRB1*0306 define highly similar alleles encoded by apparently orthologous genes in different primate species. This high degree of genetic resemblance is also reflected by similarities at the functional level, such as peptide selection and T cell activation (13). Different primate species may also share similar pseudogenes. HLA-DRB6 orthologues have been found, for instance, in chimpanzees (14–16), gorillas (17), and different macaque species (17–19). Additionally, particular lineage members that may be regarded as functional in one species remain untranscribed in another species (20). Some pseudogenes located in the primate MHC region have remained stable entities over long evolutionary time spans. Recently it was shown that intact gene segments of pseudogenes can be recruited again for immune functions by exon shuffling (21). Although some Mamu-DRB loci/lineages display moderate levels of polymorphism, within the region configurations themselves only marginally low levels of allelic polymorphism are observed (7, 22).
Allelic polymorphism ensures that different allotypes select distinct peptides for T cell activation, preventing one particular pathogen from sweeping through an entire population. Particular HLA class II alleles are indeed associated with either susceptibility or resistance to contract particular diseases (23–26). Apart from its genuine biological function, in transplantation protocols HLA-DR matching may also influence graft survival positively (27–29).
Because of the complexity of the HLA-DRB region, routine typing is performed by using different techniques (or a combination thereof) such as sequence-specific oligonucleotide typing (PCR-SSOP) for lineage-specific low resolution analysis (30) or sequence-specific priming (PCR-SSP) with unique primer sets for characterizing various HLA-DRB genes and their alleles. Because rhesus macaques possess complex DRB region configurations, laborious methods such as exon 2 or full-length DRB cloning and subsequent sequencing are inevitable for accurate typing.
In close proximity to exon 2, responsible for most sequence variation, maps a complex dinucleotide repeat, D6S2878, that is present in the majority of the HLA-DRB genes (31–33). This microsatellite, located in the beginning of intron 2, displays a complex profile concerning repeat length variability as well as sequence variation (34). This repeat is also attractive from an evolutionary point of view, because it is present in apes and Old and New World monkeys as well (33–36). Therefore, D6S2878 is considered to represent a promising marker for the development of a DR haplotyping protocol in different primate species, provided that a single set of informative primers can be developed. Because the DR regions in humans (high allelic variability in combination with a low number of region configurations) and rhesus macaques (low allelic variability and a high number of region configurations) represent each other's extremes, they were selected as candidate systems for setting up a fast and accurate high-resolution haplotyping protocol.
Results
Mamu-DRB Region Configuration Definition by Microsatellite Markers.
The rhesus macaques studied are part of a large self-sustaining colony that has been thoroughly pedigreed based on segregation of serologically and molecularly defined markers (9, 20). The current panel covers 22 different region configurations characterized by unique combinations from two to six distinct Mamu-DRB genes (Table 1). Limited allelic polymorphism is detected within region configurations 1, 11, 15, 18, 19, and 21. To denote allelic variation observed, for instance, in region configuration 1, the relevant haplotypes have been designated 1a and 1b (Table 1). The gene frequencies of the different region configurations as encountered in our population of animals are provided as well, in concert with the number of animals tested. Some haplotypes are frequently observed whereas others appear to be rare [supporting information (SI) Table 3].
Table 1.
Mamu-DRB haplotypes defined by exon 2 sequencing and D6S2878 genotyping
| Hap | First DRB locus | STR | Second DRB locus | STR | Third DRB locus | STR | Fourth DRB locus | STR | Fifth DRB locus | STR | Sixth DRB locus | STR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | DRB1*0406 | 205, 207, 211 | DRB5*0301 | 169 | ||||||||
| 1b | DRB1*0411 | 223 | DRB5*0304 | 153 | ||||||||
| 2 | DRB3*0403 | 192 | DRB*W305 | 227 | ||||||||
| 3† | DRB1*0412 | 199 | DRB*W3701 | 235 | ||||||||
| 4† | DRB1*1010 | 205 | DRB6*0121 | 216 | ||||||||
| 5† | DRB3*0408 | 203 | DRB*W404 | 293 | ||||||||
| 6† | DRB3*0411 | 190 | DRB*W314 | 219 | ||||||||
| 7‡ | DRB4*0102 | 291 | DRB5*0306 | 173 | ||||||||
| 8‡ | DRB1*0321 | 181 | DRB1*0322/3 | 209 | ||||||||
| 9‡ | DRB1*0321 | 181 | DRB1*0322/3 | 209 | DRB1*1003§ | 175? | ||||||
| 10‡ | DRB1*0309 | 229 | DRB*W2507 | 181 | DRB6*0109 | ? | ||||||
| 11a | DRB1*0303 | 195 | DRB1*1007 | 197 | DRB6*0103 | (202–208) | ||||||
| 11b | DRB1*0312 | 195 | DRB1*1007 | 201 | DRB6*0103 | ? | ||||||
| 11c | DRB1*0306 | 226 | DRB1*1007 | 197 | DRB6*0103 | ? | ||||||
| 11d | DRB1*0306 | 226–230 | DRB1*1003 | 199–213 | DRB6*0107 | 182 | ||||||
| 12 | DRB1*0309 | 233–247 | DRB6*0101 | ? | DRB*W201 | 263 (261, 267) | ||||||
| 13 | DRB6*0112 | 214 | DRB*W2501 | 214, 218? | DRB*W2002 | 218 | DRB6*0106? | 205? | ||||
| 14 | DRB6*0114 | 218 | DRB*W303 | 193 | DRB*W401 | 187 | ||||||
| 15a | DRB1*0403 | 229 | DRB6*0107 | 184 | DRB*W501 | ? | DRB6*? | 208 | ||||
| 15b‡ | DRB1*0403 | 229 | DRB6*0107 | 183 | DRB*W502 | ? | DRB6*0120 | 208 | ||||
| 16 | DRB1*0404 | 225 | DRB6*0102 | 164 | DRB*W307 | 195 | DRB*W702 | 188 | ||||
| 17 | DRB1*0701 | 197 | DRB3*0405 | 237 | DRB5*0303 | 169 | DRB6*0123 | 184 | ||||
| 18a† | DRB1*0306 | 214 | DRB1*07032 | 211 | DRB6*0124 | 170 | DRB*W2603 | 177 | DRB6*0120 | ? | ||
| 18b‡ | DRB1*0306 | 214 | DRB1*07033 | 207 | DRB6*0124 | 168 | DRB*W2603 | 177 | DRB6*0120 | 208 | ||
| 19a | DRB1*0318 | 181 | DRB6*0105 | 190, 192 | DRB*W604 | 201, 205 | DRB*W603 | 275, 277 | DRB6*0104 (+ALU) | 222 (220) | ||
| 19b | DRB1*0318 | 181 | DRB6*0105 | 194 | DRB*W604 | 197 | DRB*W611 | 275 | DRB6*01? | 222 | ||
| 20† | DRB3*0403 | 182 | DRB*W402 | 185 | DRB*W2701 | 227 | DRB6*0116 | 206 | DRB6*0107 | 182 | ||
| 21a | DRB6*0111 | 178 | DRB*W606 | 211, 213, 197 | DRB*W2104 | 211, 213 | DRB*W2603 | 177, 187 | DRB6*0122 | 236 | ||
| 21b‡ | DRB6*0108 | 176 | DRB*W606 | 213 | DRB*W2104 | 213 | DRB*W2604 | 177 | DRB6*0122 | 232 | ||
| 22 | DRB1*0310 | 241 | DRB6*0118 | 210 | DRB*W101 | 219, 223 | DRB*W602 | 279 (285) | DRB*W609 | 219 (223) | DRB6*0106 | 192 |
Data in parentheses are STR length observed in some (one to three) animals. Question marks indicate STRs not detected or rarely detected but presence of gene ascertained by sequencing. “205?” and “175?” indicate STRs detected but not confirmed by sequencing. Hap, haplotype.
†Animals with Chinese origin.
‡Animals with Burmese origin.
§DRB1*1003 belong to haplotypes 8 or 9.
Nearly all Mamu-DRB loci/lineages possess the D6S2878 microsatellite, and the relevant DNA segment could be amplified by means of the unique primer set developed for this protocol (Table 1). Amplification failures have only been observed for a few DRB6/DRBW pseudogenes for which the corresponding amplicons could be scarcely detected, most probably because of primer inconsistencies. The overall results illustrate, however, that the lengths of the amplified short tandem repeat (STR) products are highly variable and range from 153 to 293 bp (Table 1). Additionally, various STR markers seem to be predictive for the presence of a particular Mamu-DRB allele, and, moreover, family studies demonstrated that they segregate in a Mendelian manner. Subsequent sequencing of the DNA segment ranging from exon 2 to intron 2 was conducted to unequivocally link each D6S2878 allele to an individual DRB gene/allele. This approach proved that diverse STR lengths could distinguish even highly similar alleles, differing for only one or two nucleotides. An instance is provided by the Mamu-DRB1*07032 and Mamu-DRB1*07033 alleles, which are part of the two haplotypes belonging to configuration 18 (Table 1).
On average, most of the STRs linked to an individual allele appear to be rather conservative in composition and length. In the case of the frequently observed haplotype 11a (SI Table 3), for example, no length variability for both DRB1 gene-associated D6S2878 alleles is observed, which also holds true for the STR that is linked to DRB5*0301 as seen in haplotype 1a (Table 1). The DRB1*0406-linked STR of the latter configuration, however, may slightly vary in length. Such differences, as can be seen for example in haplotype 12, are reproducible and do segregate in families with a particular haplotype as defined by serological methods (37). Because each region configuration is composed of an exclusive combination of different Mamu-DRB genes, the most essential conclusion that can be drawn is that the combination of STR markers appears to be unique for a given region configuration/haplotype.
HLA-DRB Haplotype Definition by Microsatellite Analysis.
Genotyping for the highly divergent (GT)x(GA)y microsatellite allows speedy and accurate DR haplotyping in rhesus macaques, a species known to possess a high number of region configurations; these display, however, low levels of allelic variation. Next, it was investigated whether the same approach would work in a species that possesses a relatively low number of region configurations that parade abundant levels of allelic polymorphism. Therefore, in the first instance, a thoroughly characterized panel of 160 unrelated human samples of Caucasoid origin was chosen to conduct such an analysis. This selection also comprised 64 homozygous typing cells, facilitating the simple definition of haplotypes. Furthermore, the panel included the five known HLA-DRB region configurations, designated DR8, DR1, DR51, DR52, and DR53 (38), but also covered the 13 most common DR serotypes present in the Caucasoid population, differing for their DRB1 alleles (Table 2). To denote allelic variation as observed within some serotypes, the different DRB haplotypes have been designated a, b, c, and so on (Table 2). The number of samples tested per haplotype, as well as the gene frequencies of the serotypes (39), have also been summarized (SI Table 3).
Table 2.
HLA-DRB haplotypes defined by sequencing and D6S2878 genotyping
| Haplotype | First DRB locus | STR | Second DRB locus | STR | Third DRB locus | STR |
|---|---|---|---|---|---|---|
| DR8 | ||||||
| DR8a | DRB1*0801(03) | 170 (172) | ||||
| DR8b | DRB1*080302 | 176 | ||||
| DR1 | ||||||
| DR1a/DR103 | DRB1*010101/0103 | 154 (156) | DRB6*010101 | 136 | ||
| DR1b | DRB1*010201 | 160 | DRB6*0101 | 136 | ||
| DR10 | DRB1*100101 | 161 | DRB6*0101 | 136 | ||
| DR52 | ||||||
| DR17a | DRB1*030101 | 172 | DRB3*010101(2) | 180 | ||
| DR17b | DRB1*0301 | 178–184 | DRB3*0202(01 | 208 (206, 210) | ||
| DR11a | DRB1*110101 | 182–192 | DRB3*020201 | 206–218 | ||
| DR11b | DRB1*110201 | 186 | DRB3*020201 | 214 | ||
| DR11c | DRB1*110401 | 188 | DRB3*020201 | 208 (210) | ||
| DR12a | DRB1*120101 | 200 | DRB3*010102 | 180 | ||
| DR12b | DRB1*1201 | 206 (208) | DRB3*02(0201) | 210, 212 | ||
| DR12c | DRB1*120201 | 216 | DRB3*030101 | 186 | ||
| DR13a | DRB1*1301(01) | 194 | DRB3*0101(02) | 180 | ||
| DR13b | DRB1*1301(01) | 194, 196 | DRB3*02(0201) | 204–214 | ||
| DR13c | DRB1*1302(01) | 188, 206–218 | DRB3*0301/0101 | 186 | ||
| DR14a | DRB1*140101 | 192 | DRB3*0211 | 212 | ||
| DR14b | DRB1*140101 | 186, 188, 196 | DRB3*020201 | 210 | ||
| DR14c | DRB1*140101 | 198 | DRB3*0201 | 214 | ||
| DR53 | ||||||
| DR4a | DRB1*0401(01) | 180, 182, 184 | DRB4*(010101) | 178, 180 (176, 182/4) | DRB7*010101 | 135 |
| DR4b | DRB1*0402 | 194 | DRB4*010101 | 176 | DRB7*010101 | 135 |
| DR4c | DRB1*0404 | 192 (196) | DRB4*010101 | 178 | DRB7*010101 | 135 |
| DR4d | DRB1*040701 | 184 | DRB4*010101 | 178 | DRB7*010101 | 135 |
| DR4e | DRB1*0408 | 180 | DRB4*010101 | 178 | DRB7*010101 | 135 |
| DR7 | DRB1*070101 | 149 | DRB4*010101 | 170, 176, 180 | DRB7*010101/2 | 135 |
| DR9 | DRB1*09(0102) | 170 (196) | DRB4*010101 | 180 | DRB7*010101 | 135 |
| DR51 | ||||||
| DR15a | DRB1*150101 | 186 | DRB5*0101(01) | 180 | DRB6*(0201) | 154 |
| DR15b | DRB1*150101 | 186, 190 (184, 188) | DRB5*0101(01) | 184 (190) | DRB6*(0201) | 152 |
| DR15c | DRB1*150201 | 202–208 | DRB5*0102 | 220 | DRB6*(0201) | 174 |
| DR16 | DRB1*160101 | 178 | DRB5*? | 184 | DRB6*0202 | 144 |
Data in parentheses indicate STR lengths detected only once or twice.
Again, a specially designed generic primer pair allowed amplification of the relevant intron 2 segment for virtually all HLA-DRB genes/alleles. Amplification failed only for one particular HLA-DRB5 allele present on DR16 haplotypes. This may be due to a mutated primer site. Subsequent extensive sequencing of exon 2–intron 2 DNA segments verified for each of the HLA-DRB alleles the unique linkage to its adjacent D6S2878 allele. The lengths of the respective repeats are highly polymorphic in the human population as well and range from 135 to 220 bp (Table 2). Moreover, STR lengths are highly predictive for the presence of individual HLA-DRB alleles, as was also shown earlier for rhesus monkeys. Again, differential STR lengths can make the distinction between highly similar -DRB alleles, differing for a few nucleotides: for example in the case of DRB1*0801(03) and DRB1*080302 (Table 2).
The DR53 region configuration contains a pseudogene, named HLA-DRB7, which is absent in macaques. This pseudogene displays no allelic variation, and indeed the associated D6S2878 is invariant in length independent of the adjacent -DRB1 allele. Thus, the DRB7-associated STR typifies all of the haplotypes belonging to the DR53 region configuration (Table 2). The same holds true for the DRB6 allele and its adjacent STR, which are characteristic for the DR1 region configuration, covering the DR1 and DR10 serotypes. As observed in rhesus macaques, some HLA-DRB alleles appear to be associated with multiple D6S2878 variants. A case is provided by the HLA-DRB1*140101 allele observed within the DR14 haplotypes, but some of the repeats grouping in the DR11a and DR13c family of haplotypes also show length differences (Table 2). Family analyses are needed to demonstrate their segregation. Most of the human repeats, however, appear to be conservative in composition and length and are linked to an individual allele. Within this panel of 30 different HLA-DRB haplotypes, all but two can be readily dissected based on by their D6S2878 profiles. Thus, complex DR region configuration/haplotype information, as present in at least two populations of primate species, is readily obtained and defined based on D6S2878 genotyping after a simple amplification protocol conducted with one primer set.
Genetic Stability and Evolutionary History of the STR-DRB Complex.
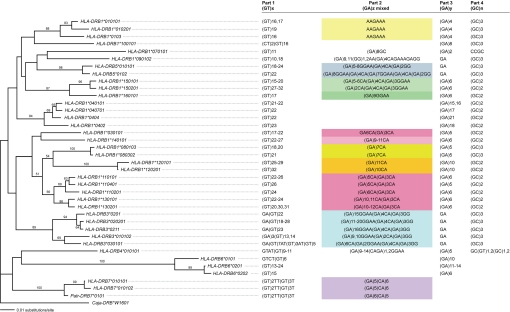
The D6S2878 microsatellite has a composite character in primates (33–36), and phylogenetic comparisons of different DRB1 sequences obtained from humans and chimpanzees indicated that the ancestral structure most likely must have been a (GT)x(GA)y dinucleotide repeat. The HLA-DRB1-associated microsatellite comprises three sections exhibiting different evolutionary stabilities. The 5′(GT)x repeat represents the longest segment and evolves most rapidly, which is a known feature for long, uninterrupted dinucleotide repeats. The middle section or the (GA)z part is shorter and interrupted; its constellation appears to correlate well with different lineages/loci, and its length seems to segregate with specific DRB1 alleles. The length of the 3′(GA)y part appears to be specific for a certain DRB lineage/locus (34). All HLA-DRB exon 2 sequences described in this study have been subjected to phylogenetic analyses, on which the D6S2878 sequences have been superimposed (Fig. 1). As can be seen, this article extends the knowledge on this particular microsatellite but also underscores its compound character (32, 34). Additionally, a short dinucleotide (GC)1–3 part could be observed at the 3′ end of the microsatellite of all HLA-DRB genes except for those belonging to the DRB6 and DRB7 pseudogenes. The variation seen in this newly recognized section of the microsatellite seems to be prognostic for certain HLA-DRB lineages or loci, respectively. The 5′(GT)x part is indeed the most polymorphic, which may, especially in the case of fairly long (GT)x repeats, evolve faster than the mutation rate operative on exon 2 itself (Fig. 1). This phenomenon, already described for DQCAR (40), could explain why, for instance, the HLA-DRB1*1302 allele is associated with a STR displaying length variation (Table 2).
Fig. 1.
Phylogenetic analysis of HLA-DRB exon 2 with D6S2878 sequences superimposed. The tree was rooted by including the Caja-DRB*W1601 allele, and bootstrap values are shown. Identical or similar colors indicate identity or similarities of the (GA)z mixed part of D6S2878.
The ancient HLA-DRB6 and HLA-DRB7 pseudogenes appear to miss the middle, interrupted (GA)z or the (GA)y part, respectively. The HLA-DRB6*0101 gene harbors a genetically stable D6S2878 showing no length differences at all. This is most probably because of its composition represented by a short and interrupted 5′(GT) and 3′(GA) part (41, 42). For HLA-DRB6*0201 the opposite is true, and the long and uninterrupted (GT)x and (GA)y parts are indicative for unstable STR lengths (Table 2 and Fig. 1) (43).
The Mamu-DRB exon 2 sequences have also been subjected to phylogenetic analyses, and the genetic composition of the D6S2878 sequences has been superimposed (SI Fig. 2). Like in humans, D6S2878 has a compound character. The newly described fourth 3′(GC) dinucleotide part is also present in rhesus macaques with repeat lengths ranging from 1 to 5. Comparable to the human situation, Mamu-DRB6 alleles form a distinct clade in the phylogenetic tree. Because the DRB6 locus is thought to predate the divergence of Old World monkeys, great apes, and homonoids, it is not surprising that the same ancestral (GT)x(GA)y structure was detected in rhesus monkeys just as in humans. In contrast to the human microsatellite, some Mamu-DRB lineages/loci are characterized by a multipart (GT)x and/or a (GA)z middle segment. In general, the rhesus macaque STR appears to be even more complex than its human equivalent. This multifaceted composition seems to correlate with the high number of DRB region configuration/haplotype diversity observed in rhesus macaques. Despite the complex microsatellite patterns observed in the rhesus macaque, the composition and length of repeats associated with known Mamu-DRB alleles are, as expected, highly similar. The rhesus macaque DRB region has been subject to several rounds of duplication and contraction processes. For that reason, it is difficult to understand which highly related genes located on different region configurations/haplotypes represent separate loci or whether these sequences have an allelic affiliation. This microsatellite will be helpful in sorting out such genetic relationships.
Discussion
Microsatellite D6S2878 is considered to represent a promising marker for the development of a quick and accurate DRB haplotyping protocol in primate species, on the condition that one single set of informative primers can be developed. Indeed, a specifically designed primer pair allowed amplification of the relevant intron 2 segment for virtually all HLA-DRB and Mamu-DRB genes/alleles. For both species, amplification artifacts have rarely been observed and such types of problems are easily overcome by designation of specific primers that can be added to the same reaction mixture. The D6S2878 STR was proven to be highly variable in length, not only in humans but also in the rhesus macaque, thus verifying this microsatellite as a useful marker for DRB typing of both species. Phylogenetic analyses of human as well as rhesus macaque exon 2 sequences have been performed and compared with microsatellite composition. For humans, the evolutionary relationships of exon 2 and the adjacent microsatellite seem to segregate closely together (Fig. 1). In rhesus macaques, the microsatellite composition was far more variable than in humans, and a comparison of microsatellite and exon 2 phylogeny does not always seem to match the microsatellite composition (SI Fig. 2). One explanation for these results may be that rhesus macaque DRB loci/lineages are much older than their human equivalents, and this can be the reason for the higher diversity of the adjacent microsatellite as well. Furthermore, preliminary results of intron sequences illustrate that some of the Mamu-DRB sequences that are considered alleles of a given locus probably represent monomorphic loci themselves. However, truly allelic variants manifest the reliability of the comparison of exon 2 and the D6S2878 marker not only in humans but also in rhesus macaques.
Microsatellite typing was in the first instance performed on large human and rhesus macaque panels for which the typing information was known. The most essential conclusion to be drawn (Tables 1 and 2) is that in both species the combination of STR markers appears to be unique for a given haplotype. Within the rhesus macaque panel of 31 haplotypes all could be defined unambiguously; within the human panel of 30 haplotypes all but two could be thus defined. As stated earlier, ambiguities can easily be solved by development of additional primer pairs. As a control, a blind test was performed with 47 human and 26 rhesus monkey samples. More than 90% of the samples were scored correctly for their Mhc-DRB haplotypes. Some of the samples were not scored properly because they contained allotypes that were not present in the original test panel. Thus, this D6S2878 typing protocol provides a highly reliable method for Mamu-DRB and HLA-DRB haplotyping, with its main advantage being simplicity and speed. Because differently labeled primers can be used for microsatellite typing, multiplexing is possible, and 96 samples or even more can be analyzed within one test panel. The simplicity of the test is especially useful for Mamu-DRB haplotyping, which is otherwise extremely time-consuming because of the unprecedented high number of different -DRB region configurations. For the human situation, this approach is very helpful in the analysis of large amounts of samples, as they are needed, for example, in population and/or disease association studies. Furthermore, this method may also be of use in forensic medicine as well as in paternity-testing protocols. Additionally, high-resolution -DRB haplotyping will simplify donor–recipient matching in organ as well as bone marrow transplantation. Because the D6S2878 STR is an old entity, it may also be used to study other populations of primate species.
The evolutionary stability of this microsatellite has been a matter of debate (32–34, 36, 44). The HLA-DRB7-associated D6S2878 allele is especially remarkable, because this repeat has the shortest (GT)x as well as the (GA)y part and is highly stable in length, showing no polymerase slippage at all. This is in accordance with the fact that short and/or interrupted repeats are more stable than long, uninterrupted dinucleotides (41–43). To what extent the repeat composition may have a direct or indirect influence on the low mutation rate of the adjacent exon 2 segment of the DRB7 pseudogene is not well understood at present. It has been proposed, for example, that microsatellites near genes may increase and probably also decrease local mutation rates (45). Interestingly, exon 2 of the DRB7 pseudogene, present on the only shared DR region configuration of humans and chimpanzees, is highly conserved between both species. Moreover, the D6S2878 sequence is nearly identical (Fig. 1). It has been suggested that the intron 2 segment containing the (GT)x(GA)y repeat may bind a zinc-dependent protein and forms non B-DNA structures; thus, functionality of these so-called “junk” DNA sequences cannot be ruled out and should be subjected to further analysis (44).
Materials and Methods
Samples.
The 167 rhesus monkeys analyzed, housed at the Biomedical Primate Research Centre's breeding colony, originated mostly from India, but some are also of Burmese or Chinese origin. Seven of these animals are completely homozygous for their MHC region and derived from consanguineous matings; two additional animals are homozygous for their Mamu-A, Mamu-B, and Mamu-DR serotypes. Genomic DNA of human individuals or rhesus macaques was extracted from EDTA blood samples or from immortalized B cell lines using a standard salting-out procedure. Of the 160 human samples tested, 64 were HLA-DRB homozygous, 17 of which belong to a thoroughly characterized homozygous typing cell panel of the 14th International Histocompatibility Workshop (2005).
STR-DRB Genotyping.
The relevant DNA segment in rhesus macaques was amplified with a forward primer located at the end of exon 2 (5′-Mamu-DRB-STR, TTC ACA GTG CAG CGG CGA GGT) and two labeled reverse primers in intron 2 (3′-Mamu-DRB-STR_VIC, ACA CCT GTG CCC TCA GAA CT; and 3′-Mamu-DRB-STR_FAM_1007, ACA TCT GTG TCC TCA GAC CT). For human samples a labeled forward primer located at the end of exon 2 (5′-HLA-DRB-STR_VIC, GAG AGC TTC ACA GTG CAG C) and one reverse primer in intron 2 (3′-HLA-DRB-STR, GAG AGG ATT CTA AAT GCT CAC) was used. The labeled primers were synthesized by Applied Biosystems (Foster City, CA), and the unlabeled primers were synthesized by Invitrogen (Paisley, Scotland). The PCR for rhesus macaques was performed in a 25-μl reaction volume containing 1 unit of Taq polymerase (Invitrogen) with 1.0 μM of the unlabeled forward primer, 0.6 μM of the VIC-labeled reversed primer, 0.2 μM of the FAM-labeled reversed primer, 2.5 mM MgCl2, 0.2 mM of each dNTP, 1× PCR buffer II (Invitrogen), and 100 ng of DNA.
The PCR mixture for the human STR amplification was the same as that used for rhesus macaques with 0.1 μM of the VIC-labeled forward primer and 0.1 μM of the unlabeled reverse primer. The cycling parameters for both amplifications were a 5-min 95°C initial denaturation step followed by five cycles of 1 min at 94°C, 45 s at 58°C, and 45 s at 72°C. Then the program was followed by 25 cycles of 45 s at 94°C, 30 s at 58°C, and 45 s at 72°C. A final extension step was performed at 72°C for 30 min. The amplified DNA was prepared for genotyping according to the manufacturer's guidelines and analyzed on an ABI 3130 genetic analyzer (Applied Biosystems). STR analysis was performed with the Genemapper program (Applied Biosystems), and all samples were analyzed at least twice.
PCR, Cloning, and Sequencing.
Seventy-five different Mamu-DRB alleles and 38 HLA-DRB alleles were sequenced from exon 2 to intron 2 including the microsatellite with a generic 5′ DRB–exon 2 primer (CGT GTC CCC ACA GCA CGT TTC) together with the same 3′ primers as used for DRB-STR genotyping but without label. The PCRs for rhesus monkey and human DRB were performed in a 100-μl volume containing 4 units of Taq polymerase (Invitrogen) with 0.2 μM of each primer, 2.5 mM MgCl2, 0.2 mM of each dNTP, 1× PCR buffer II (Invitrogen), and 200 ng of DNA. The cycling parameters were the same as described for STR-DRB genotyping. The resulting amplicons were cloned and sequenced as described recently (19, 22). The resulting sequences were analyzed by using the Sequence Navigator program (Applied Biosystems).
Phylogenetic Analyses.
Multiple sequence alignments of exon 2 of human and rhesus macaque -DRB sequences were created by using MacVector version 8.1.1 (Oxford Molecular Group), and phylogenetic analyses were then performed by using PAUP version 4.0b.10 (46). Pairwise distances were calculated by using Kimura-2 parameter and the neighbor-joining method for creating a phylogram. Confidence estimates of grouping were calculated according to the bootstrap method generated from 1,000 replicates.
Supplementary Material
Acknowledgments
We thank W. Verduin and J. Anholts for human DNA preparation, D. Devine for editing the manuscript, and H. v. Westbroek for artwork. This study was supported in part by National Institutes of Health Grant 1 1-R24-RR16038-01 (Catalog of Federal Assistance 93.306) and by National Institutes of Health/National Center for Research Resources Project U24-RR1814401.
Abbreviation
- STR
short tandem repeat.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the EMBL Nucleotide Sequence Database (accession nos. AM490200–AM490205).
This article contains supporting information online at www.pnas.org/cgi/content/full/0702964104/DC1.
References
- 1.Marsh SG. Tissue Antigens. 1998;51:467–507. doi: 10.1111/j.1399-0039.1998.tb02984.x. [DOI] [PubMed] [Google Scholar]
- 2.Bontrop RE, Otting N, de Groot NG, Doxiadis GG. Immunol Rev. 1999;167:339–350. doi: 10.1111/j.1600-065x.1999.tb01403.x. [DOI] [PubMed] [Google Scholar]
- 3.Klein J, Satta Y, O'Huigin C, Takahata N. Annu Rev Immunol. 1993;11:269–295. doi: 10.1146/annurev.iy.11.040193.001413. [DOI] [PubMed] [Google Scholar]
- 4.Raymond CK, Kas A, Paddock M, Qiu R, Zhou Y, Subramanian S, Chang J, Palmieri A, Haugen E, Kaul R, Olson MV. Genome Res. 2005;15:1250–1257. doi: 10.1101/gr.3554305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traherne JA, Horton R, Roberts AN, Miretti MM, Hurles ME, Stewart CA, Ashurst JL, Atrazhev AM, Coggill P, Palmer S, et al. PLoS Genet. 2006;2:e9. doi: 10.1371/journal.pgen.0020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson J, Waller MJ, Parham P, de Groot N, Bontrop R, Kennedy LJ, Stoehr P, Marsh SG. Nucleic Acids Res. 2003;31:311–314. doi: 10.1093/nar/gkg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doxiadis GG, Otting N, de Groot NG, Noort R, Bontrop RE. J Immunol. 2000;164:3193–3199. doi: 10.4049/jimmunol.164.6.3193. [DOI] [PubMed] [Google Scholar]
- 8.Slierendregt BL, Otting N, van Besouw N, Jonker M, Bontrop RE. J Immunol. 1994;152:2298–2307. [PubMed] [Google Scholar]
- 9.Doxiadis GG, Otting N, de Groot NG, Bontrop RE. Immunol Rev. 2001;183:76–85. doi: 10.1034/j.1600-065x.2001.1830106.x. [DOI] [PubMed] [Google Scholar]
- 10.Khazand M, Peiberg C, Nagy M, Sauermann U. Tissue Antigens. 1999;54:615–624. doi: 10.1034/j.1399-0039.1999.540612.x. [DOI] [PubMed] [Google Scholar]
- 11.Ellis SA, Bontrop RE, Antczak DF, Ballingall K, Davies CJ, Kaufman J, Kennedy LJ, Robinson J, Smith DM, Stear MJ, et al. Immunogenetics. 2006;57:953–958. doi: 10.1007/s00251-005-0071-4. [DOI] [PubMed] [Google Scholar]
- 12.Klein J, Bontrop RE, Dawkins RL, Erlich HA, Gyllensten UB, Heise ER, Jones PP, Parham P, Wakeland EK, Watkins DI. Immunogenetics. 1990;31:217–219. doi: 10.1007/BF00204890. [DOI] [PubMed] [Google Scholar]
- 13.Geluk A, Elferink DG, Slierendregt BL, van Meijgaarden KE, de Vries RR, Ottenhoff TH, Bontrop RE. J Exp Med. 1993;177:979–987. doi: 10.1084/jem.177.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergstrom TF, Erlandsson R, Engkvist H, Josefsson A, Erlich HA, Gyllensten U. Immunol Rev. 1999;167:351–365. doi: 10.1111/j.1600-065x.1999.tb01404.x. [DOI] [PubMed] [Google Scholar]
- 15.Mayer WE, O'Huigin C, Klein J. Proc Natl Acad Sci USA. 1993;90:10720–10724. doi: 10.1073/pnas.90.22.10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno-Pelayo MA, Fernandez-Soria VM, Paz-Artal E, Ferre-Lopez S, Rosal M, Morales P, Varela P, Arnaiz-Villena A. Immunogenetics. 1999;49:843–850. doi: 10.1007/s002510050563. [DOI] [PubMed] [Google Scholar]
- 17.Kenter M, Otting N, de Weers M, Anholts J, Reiter C, Jonker M, Bontrop RE. Hum Immunol. 1993;36:205–218. doi: 10.1016/0198-8859(93)90127-m. [DOI] [PubMed] [Google Scholar]
- 18.Doxiadis GG, Otting N, de Groot NG, de Groot N, Rouweler AJ, Noort R, Verschoor EJ, Bontjer I, Bontrop RE. Immunogenetics. 2003;55:540–551. doi: 10.1007/s00251-003-0590-9. [DOI] [PubMed] [Google Scholar]
- 19.Doxiadis GG, Rouweler AJ, de Groot NG, Louwerse A, Otting N, Verschoor EJ, Bontrop RE. Immunogenetics. 2006;58:259–268. doi: 10.1007/s00251-006-0083-8. [DOI] [PubMed] [Google Scholar]
- 20.de Groot N, Doxiadis GG, De Groot NG, Otting N, Heijmans C, Rouweler AJ, Bontrop RE. J Immunol. 2004;172:6152–6157. doi: 10.4049/jimmunol.172.10.6152. [DOI] [PubMed] [Google Scholar]
- 21.Doxiadis GG, van der Wiel MK, Brok HP, de Groot NG, Otting N, 't Hart BA, van Rood JJ, Bontrop RE. Proc Natl Acad Sci USA. 2006;103:5864–5868. doi: 10.1073/pnas.0600643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penedo MC, Bontrop RE, Heijmans CM, Otting N, Noort R, Rouweler AJ, de Groot N, de Groot NG, Ward T, Doxiadis GG. Immunogenetics. 2005;57:198–209. doi: 10.1007/s00251-005-0787-1. [DOI] [PubMed] [Google Scholar]
- 23.Jones EY, Fugger L, Strominger JL, Siebold C. Nat Rev Immunol. 2006;6:271–282. doi: 10.1038/nri1805. [DOI] [PubMed] [Google Scholar]
- 24.Nepom GT, Erlich H. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- 25.Tisch R, McDevitt H. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 26.Undlien DE, Lie BA, Thorsby E. Trends Genet. 2001;17:93–100. doi: 10.1016/s0168-9525(00)02180-6. [DOI] [PubMed] [Google Scholar]
- 27.Dankers MK, Roelen DL, Nagelkerke NJ, de Lange P, Persijn GG, Doxiadis II, Claas FH. Hum Immunol. 2004;65:13–19. doi: 10.1016/j.humimm.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Heemskerk MH, de Paus RA, Lurvink EG, Koning F, Mulder A, Willemze R, van Rood JJ, Falkenburg JH. Proc Natl Acad Sci USA. 2001;98:6806–6811. doi: 10.1073/pnas.111162298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opelz G, Wujciak T, Dohler B, Scherer S, Mytilineos J. Rev Immunogenet. 1999;1:334–342. [PubMed] [Google Scholar]
- 30.Verduyn W, Doxiadis II, Anholts J, Drabbels JJ, Naipal A, D'Amaro J, Persijn GG, Giphart MJ, Schreuder GM. Hum Immunol. 1993;37:59–67. doi: 10.1016/0198-8859(93)90143-o. [DOI] [PubMed] [Google Scholar]
- 31.Andersson G, Larhammar D, Widmark E, Servenius B, Peterson PA, Rask L. J Biol Chem. 1987;262:8748–8758. [PubMed] [Google Scholar]
- 32.Epplen C, Santos EJ, Guerreiro JF, van Helden P, Epplen JT. Hum Genet. 1997;99:399–406. doi: 10.1007/s004390050379. [DOI] [PubMed] [Google Scholar]
- 33.Riess O, Kammerbauer C, Roewer L, Steimle V, Andreas A, Albert E, Nagai T, Epplen JT. Immunogenetics. 1990;32:110–116. doi: 10.1007/BF00210448. [DOI] [PubMed] [Google Scholar]
- 34.Bergstrom TF, Engkvist H, Erlandsson R, Josefsson A, Mack SJ, Erlich HA, Gyllensten U. Am J Hum Genet. 1999;64:1709–1718. doi: 10.1086/302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriener K, O'Huigin C, Tichy H, Klein J. Immunogenetics. 2000;51:169–178. doi: 10.1007/s002510050028. [DOI] [PubMed] [Google Scholar]
- 36.Trtkova K, Mayer WE, O'Huigin C, Klein J. Mol Phylogenet Evol. 1995;4:408–419. doi: 10.1006/mpev.1995.1038. [DOI] [PubMed] [Google Scholar]
- 37.Bontrop RE, Otting N, Slierendregt BL, Lanchbury JS. Immunol Rev. 1995;143:33–62. doi: 10.1111/j.1600-065x.1995.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 38.Schreuder GM, Hurley CK, Marsh SG, Lau M, Fernandez-Vina M, Noreen HJ, Setterholm M, Maiers M. Tissue Antigens. 2005;65:1–55. doi: 10.1111/j.1399-0039.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 39.Marsh SG, Parham P, Barber LD. The HLA FactsBook. London: Academic; 2000. [Google Scholar]
- 40.Lin L, Jin L, Lin X, Voros A, Underhill P, Mignot E. Tissue Antigens. 1998;52:9–18. doi: 10.1111/j.1399-0039.1998.tb03018.x. [DOI] [PubMed] [Google Scholar]
- 41.Petes TD, Greenwell PW, Dominska M. Genetics. 1997;146:491–498. doi: 10.1093/genetics/146.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wierdl M, Dominska M, Petes TD. Genetics. 1997;146:769–779. doi: 10.1093/genetics/146.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin L, Macaubas C, Hallmayer J, Kimura A, Mignot E. Proc Natl Acad Sci USA. 1996;93:15285–15288. doi: 10.1073/pnas.93.26.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maueler W, Bassili G, Arnold R, Renkawitz R, Epplen JT. Gene. 1999;226:9–23. doi: 10.1016/s0378-1119(98)00573-3. [DOI] [PubMed] [Google Scholar]
- 45.Vowles EJ, Amos W. PLoS Biol. 2004;2:E199. doi: 10.1371/journal.pbio.0020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swafford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, MA: Sinauer; 2002. Version 4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.