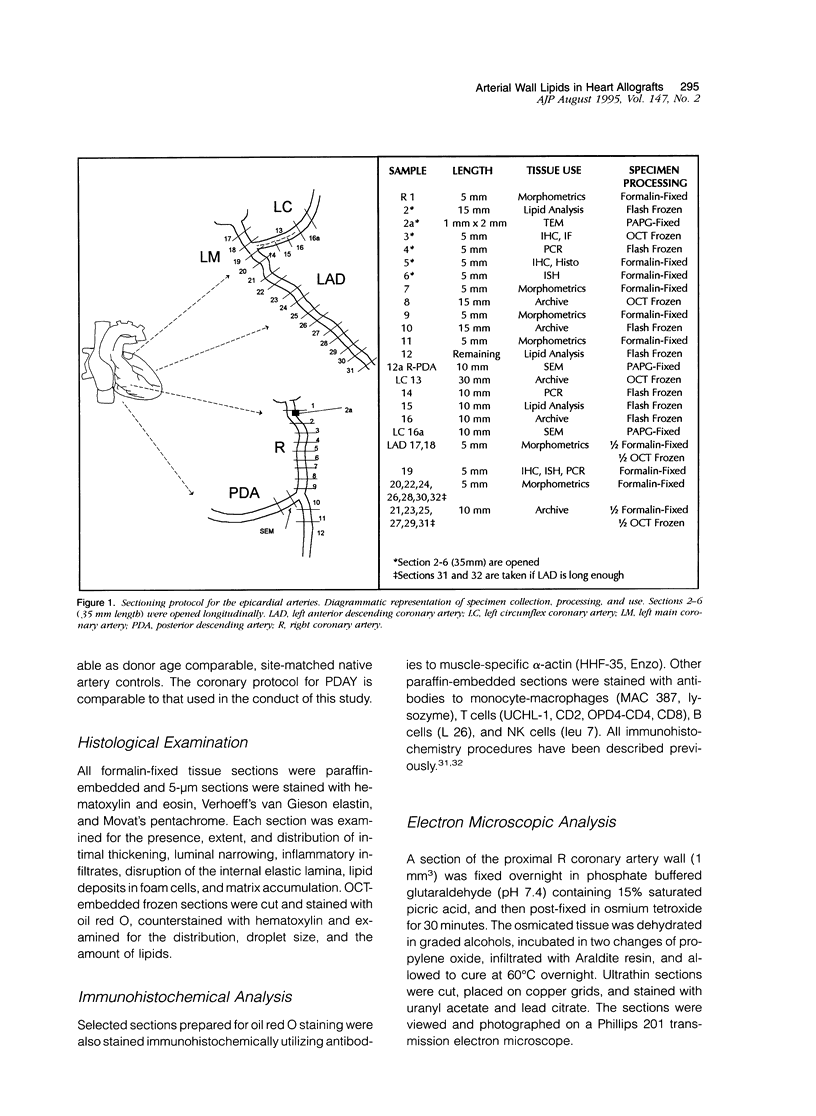
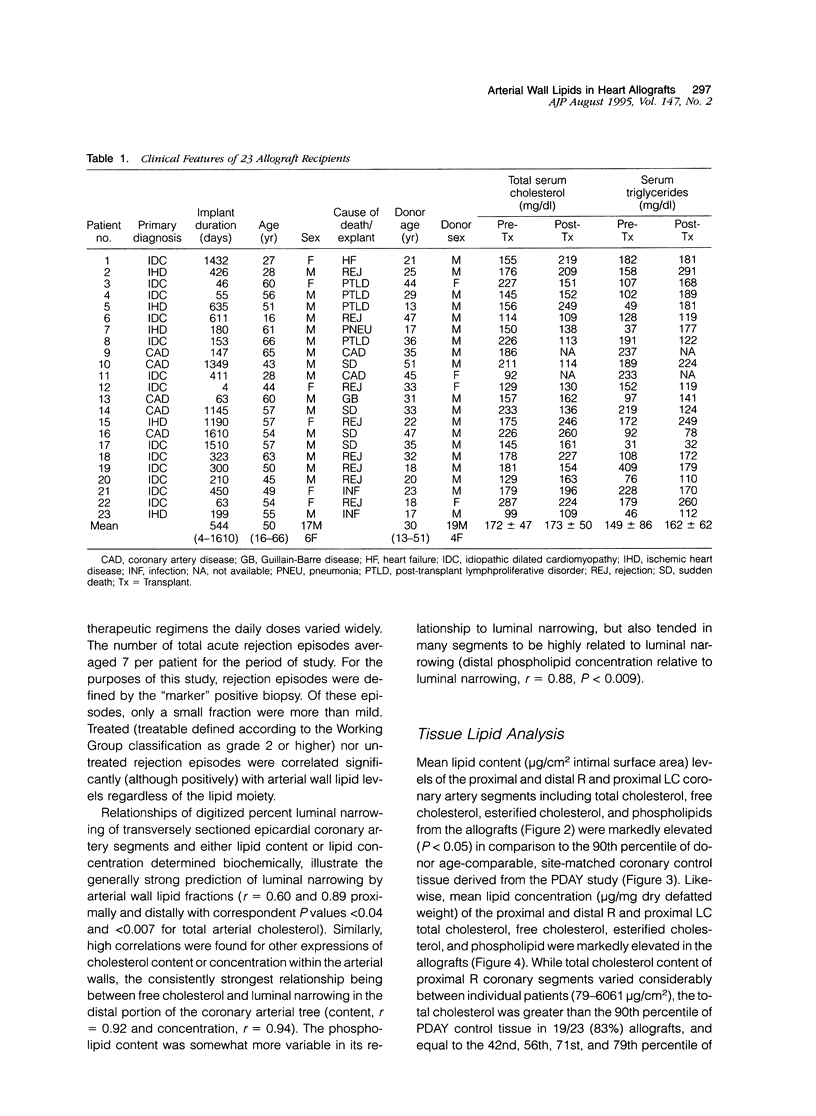
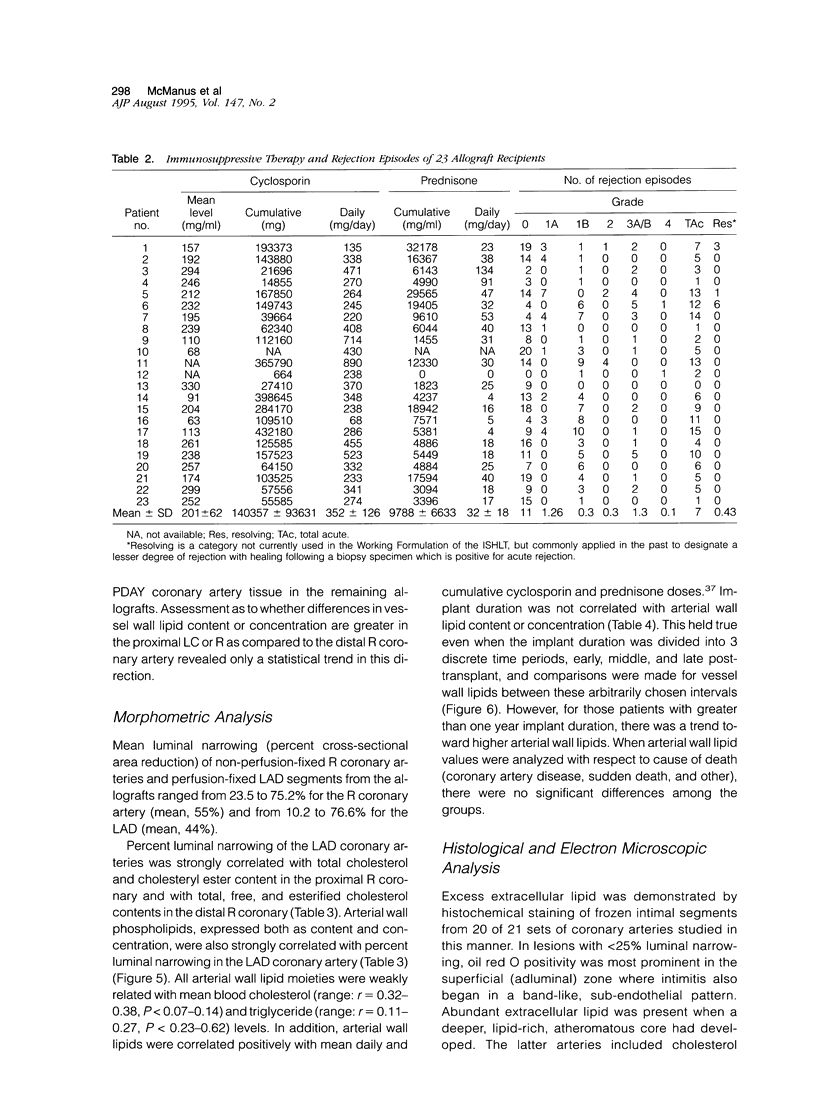
Abstract
Transplant arteriopathy is a major late complication in human heart allograft recipients and the pathogenesis of such arteriopathy remains uncertain. The degree to which lipids and atheromata are involved in the arteriopathic lesions remains unsettled, and there is uncertainty regarding the significance of insudation or retention of lipids within the coronary artery walls of transplanted hearts. On current immunosuppressive regimens, most patients experience an increased serum total cholesterol and low-density lipoprotein cholesterol after transplant. Elevation of these blood lipids has an undetermined relationship to arteriopathy. We carried out morphological, morphometric, immunohistochemical, ultrastructural, and biochemical studies of particular coronary artery segments from 23 unselected explant or autopsy allografts and donor age-matched native coronary controls. Patients died of cardiac and non-cardiac reasons over a period of 4 to 1610 days after transplant. Atheromata were frequent, and diffuse intra- and extra-cellular accumulation of lipids in both intimal and medial walls was documented by oil red O positivity, immunohistochemical staining (muscle-specific alpha-actin), transmission and scanning electron microscopy, and biochemical analysis. Mean total cholesterol, esterified cholesterol, free cholesterol, and phospholipid content (microgram/cm2 intimal surface area) and concentration (microgram/mg dry defatted weight) in arteriopathic coronaries were > 10-fold higher than in comparable native coronary segments. Extent of lipids in the arterial walls was highly correlated with digitized percent luminal narrowing, mean daily and cumulative cyclosporin dose, and mean cumulative prednisone dose. Our data suggests strongly that lipid accumulation is an important early and persistent phenomenon in the development of transplant arteriopathy.
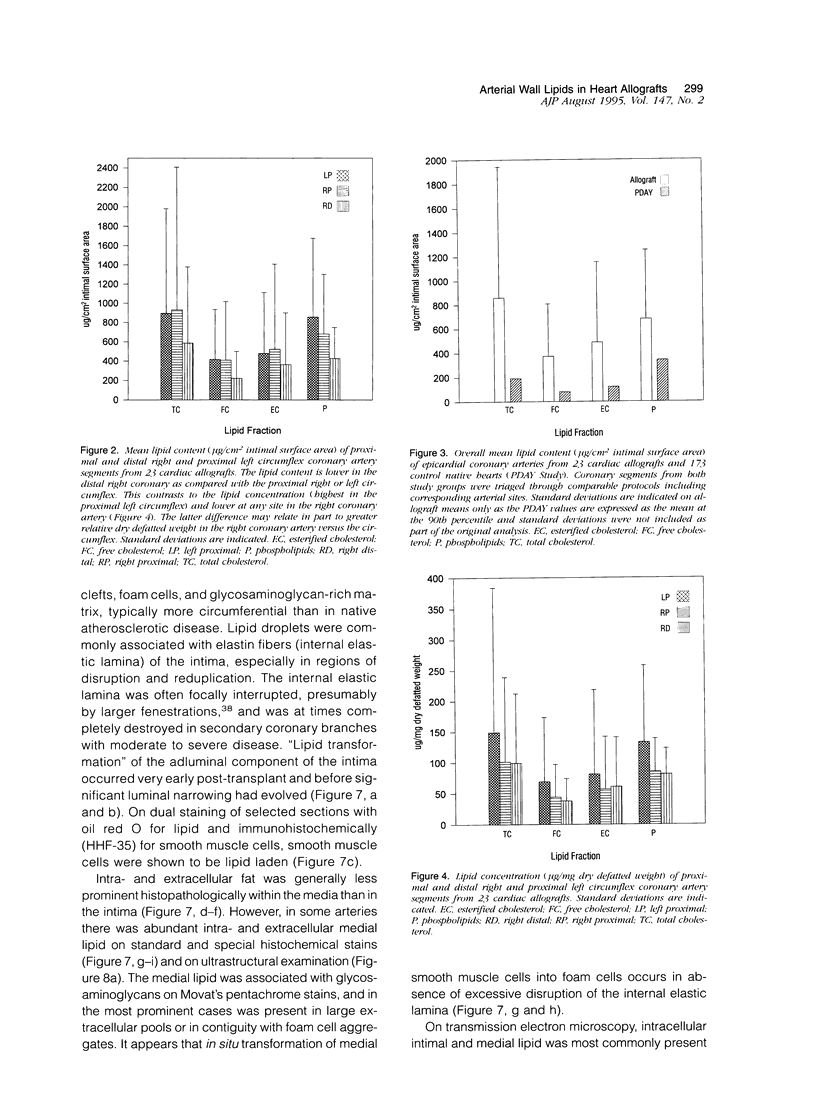
Full text
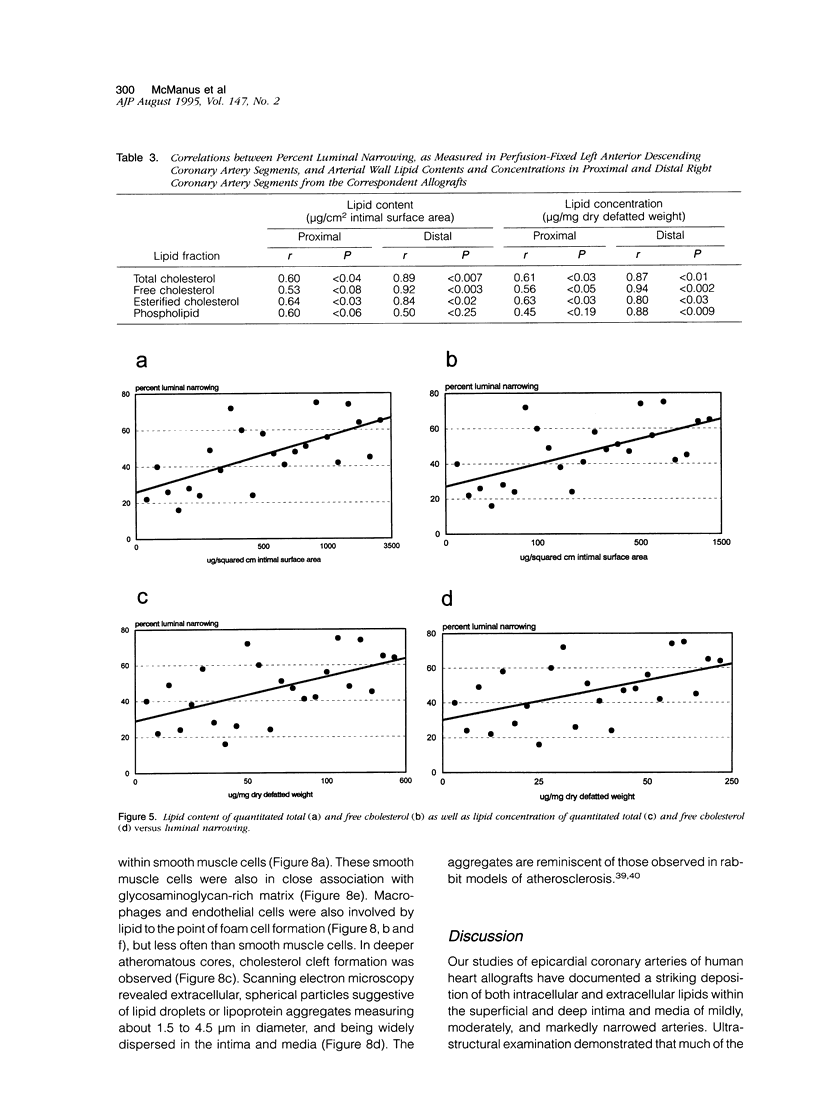
PDF


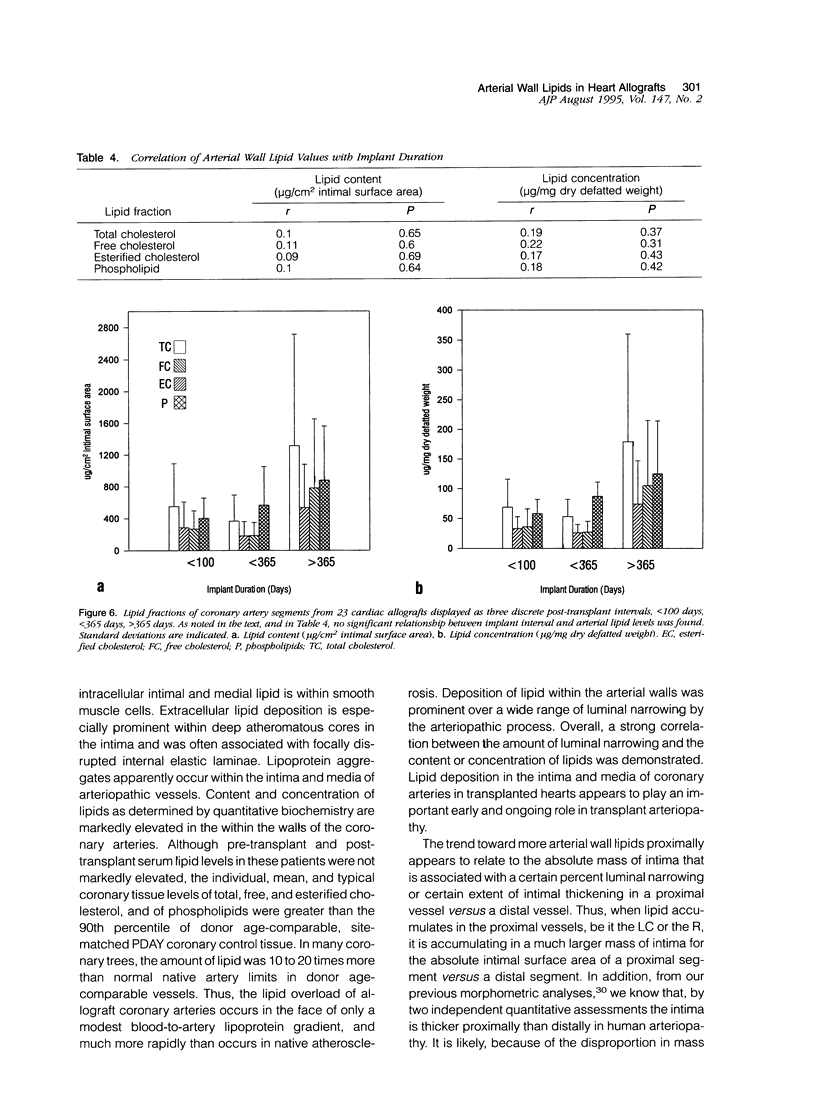
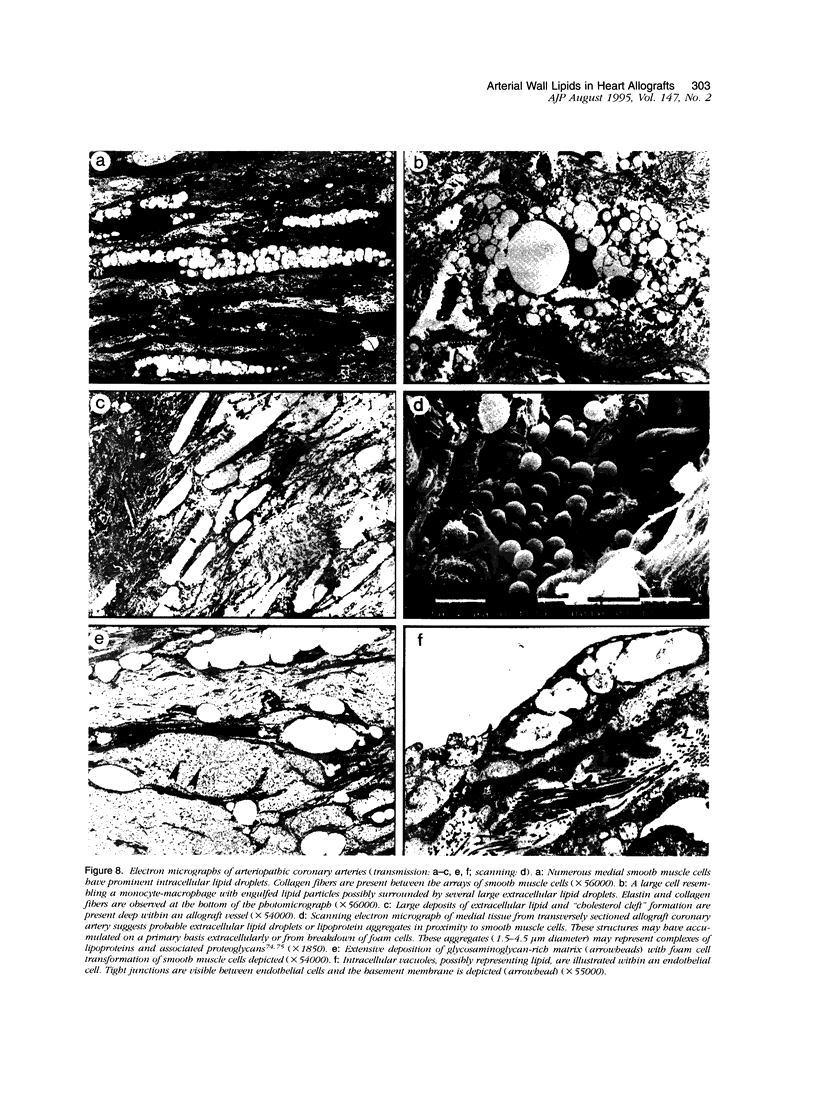





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballantyne C. M., Radovancevic B., Farmer J. A., Frazier O. H., Chandler L., Payton-Ross C., Cocanougher B., Jones P. H., Young J. B., Gotto A. M., Jr Hyperlipidemia after heart transplantation: report of a 6-year experience, with treatment recommendations. J Am Coll Cardiol. 1992 May;19(6):1315–1321. doi: 10.1016/0735-1097(92)90340-s. [DOI] [PubMed] [Google Scholar]
- Beisiegel U., Niendorf A., Wolf K., Reblin T., Rath M. Lipoprotein(a) in the arterial wall. Eur Heart J. 1990 Aug;11 (Suppl E):174–183. doi: 10.1093/eurheartj/11.suppl_e.174. [DOI] [PubMed] [Google Scholar]
- Bieber C. P., Stinson E. B., Shumway N. E., Payne R., Kosek J. Cardiac transplantation in man. VII. Cardiac allograft pathology. Circulation. 1970 May;41(5):753–772. doi: 10.1161/01.cir.41.5.753. [DOI] [PubMed] [Google Scholar]
- Billingham M. E. Cardiac transplant atherosclerosis. Transplant Proc. 1987 Aug;19(4 Suppl 5):19–25. [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Brasile L., Zerbe T., Rabin B., Clarke J., Abrams A., Cerilli J. Identification of the antibody to vascular endothelial cells in patients undergoing cardiac transplantation. Transplantation. 1985 Dec;40(6):672–675. doi: 10.1097/00007890-198512000-00020. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Busch G. J., Galvanek E. G., Reynolds E. S., Jr Human renal allografts. Analysis of lesions in long-term survivors. Hum Pathol. 1971 Jun;2(2):253–298. doi: 10.1016/s0046-8177(71)80037-0. [DOI] [PubMed] [Google Scholar]
- Camejo G., Olofsson S. O., Lopez F., Carlsson P., Bondjers G. Identification of Apo B-100 segments mediating the interaction of low density lipoproteins with arterial proteoglycans. Arteriosclerosis. 1988 Jul-Aug;8(4):368–377. doi: 10.1161/01.atv.8.4.368. [DOI] [PubMed] [Google Scholar]
- Camejo G. The interaction of lipids and lipoproteins with the intercellular matrix of arterial tissue: its possible role in atherogenesis. Adv Lipid Res. 1982;19:1–53. doi: 10.1016/b978-0-12-024919-0.50007-2. [DOI] [PubMed] [Google Scholar]
- DUGUID J. B. Pathogenesis of atherosclerosis. Lancet. 1949 Nov 19;2(6586):925–927. doi: 10.1016/s0140-6736(49)91503-2. [DOI] [PubMed] [Google Scholar]
- Davies H., al-Tikriti S. Coronary arterial pathology in the transplanted human heart. Int J Cardiol. 1989 Oct;25(1):99–117. doi: 10.1016/0167-5273(89)90169-1. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Selden S. C., 3rd, Schwartz S. M. Enhanced rates of fluid pinocytosis during exponential growth and monolayer regeneration by cultured arterial endothelial cells. J Cell Physiol. 1980 Feb;102(2):119–127. doi: 10.1002/jcp.1041020204. [DOI] [PubMed] [Google Scholar]
- Dunmore P. J., Song S. H., Roach M. R. A comparison of the size of fenestrations in the internal elastic lamina of young and old porcine aortas as seen with the scanning electron microscope. Can J Physiol Pharmacol. 1990 Feb;68(2):139–143. doi: 10.1139/y90-022. [DOI] [PubMed] [Google Scholar]
- Dunn M. J., Crisp S. J., Rose M. L., Taylor P. M., Yacoub M. H. Anti-endothelial antibodies and coronary artery disease after cardiac transplantation. Lancet. 1992 Jun 27;339(8809):1566–1570. doi: 10.1016/0140-6736(92)91832-s. [DOI] [PubMed] [Google Scholar]
- Emeson E. E., Shen M. L. Accelerated atherosclerosis in hyperlipidemic C57BL/6 mice treated with cyclosporin A. Am J Pathol. 1993 Jun;142(6):1906–1915. [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. J., Hajjar D. P., Minick C. R. Enhancement of cholesterol and cholesteryl ester accumulation in re-endothelialized aorta. Am J Pathol. 1980 Apr;99(1):81–104. [PMC free article] [PubMed] [Google Scholar]
- Ferns G., Reidy M., Ross R. Vascular effects of cyclosporine A in vivo and in vitro. Am J Pathol. 1990 Aug;137(2):403–413. [PMC free article] [PubMed] [Google Scholar]
- Fielding P. E., Vlodavsky I., Gospodarowicz D., Fielding C. J. Effect of contact inhibition on the regulation of cholesterol metabolism in cultured vascular endothelial cells. J Biol Chem. 1979 Feb 10;254(3):749–755. [PubMed] [Google Scholar]
- Foerster A. Vascular rejection in cardiac transplantation. A morphological study of 25 human cardiac allografts. APMIS. 1992 Apr;100(4):367–376. [PubMed] [Google Scholar]
- French J. E. Atherosclerosis in relation to the structure and function of the arterial intima, with special reference to th endothelium. Int Rev Exp Pathol. 1966;5:253–353. [PubMed] [Google Scholar]
- Gao S. Z., Schroeder J. S., Hunt S., Stinson E. B. Retransplantation for severe accelerated coronary artery disease in heart transplant recipients. Am J Cardiol. 1988 Nov 1;62(13):876–881. doi: 10.1016/0002-9149(88)90885-5. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Grattan M. T., Moreno-Cabral C. E., Starnes V. A., Oyer P. E., Stinson E. B., Shumway N. E. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989 Jun 23;261(24):3561–3566. [PubMed] [Google Scholar]
- Gurecki J., Warty V., Sanghvi A. The transport of cyclosporine in association with plasma lipoproteins in heart and liver transplant patients. Transplant Proc. 1985 Aug;17(4):1997–2002. [PubMed] [Google Scholar]
- Guyton J. R., Klemp K. F. Early extracellular and cellular lipid deposits in aorta of cholesterol-fed rabbits. Am J Pathol. 1992 Oct;141(4):925–936. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Fabricant C. G., Minick C. R., Fabricant J. Virus-induced atherosclerosis. Herpesvirus infection alters aortic cholesterol metabolism and accumulation. Am J Pathol. 1986 Jan;122(1):62–70. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Falcone D. J., Fowler S., Minick C. R. Endothelium modifies the altered metabolism of the injured aortic wall. Am J Pathol. 1981 Jan;102(1):28–39. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Nicholson A. C., Hajjar K. A., Sando G. N., Summers B. D. Decreased messenger RNA translation in herpesvirus-infected arterial cells: effects on cholesteryl ester hydrolase. Proc Natl Acad Sci U S A. 1989 May;86(9):3366–3370. doi: 10.1073/pnas.86.9.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. P., Russell G. I., Parvin S. D., Veitch P. S., Walls J. Alterations in lipid and carbohydrate metabolism attributable to cyclosporin A in renal transplant recipients. Br Med J (Clin Res Ed) 1986 Jan 4;292(6512):16–16. doi: 10.1136/bmj.292.6512.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. P., Russell G. I., Parvin S. P., Veitch P. S., Walls J. Metabolic effects of conversion from cyclosporine to azathioprine in renal transplant recipients. Proc Eur Dial Transplant Assoc Eur Ren Assoc. 1985;21:1010–1014. [PubMed] [Google Scholar]
- Hilbrands L. B., Demacker P. N., Hoitsma A. J. Cyclosporin and serum lipids in renal transplant recipients. Lancet. 1993 Mar 20;341(8847):765–767. [PubMed] [Google Scholar]
- Hruban R. H., Beschorner W. E., Baumgartner W. A., Augustine S. M., Ren H., Reitz B. A., Hutchins G. M. Accelerated arteriosclerosis in heart transplant recipients is associated with a T-lymphocyte-mediated endothelialitis. Am J Pathol. 1990 Oct;137(4):871–882. [PMC free article] [PubMed] [Google Scholar]
- Kosek J. C., Bieber C., Lower R. R. Heart graft arteriosclerosis. Transplant Proc. 1971 Mar;3(1):512–514. [PubMed] [Google Scholar]
- Leszczynski D., Zhao Y., Yeagley T. J., Foegh M. L. Direct and endothelial cell-mediated effect of cyclosporin A on the proliferation of rat smooth muscle cells in vitro. Am J Pathol. 1993 Jan;142(1):149–155. [PMC free article] [PubMed] [Google Scholar]
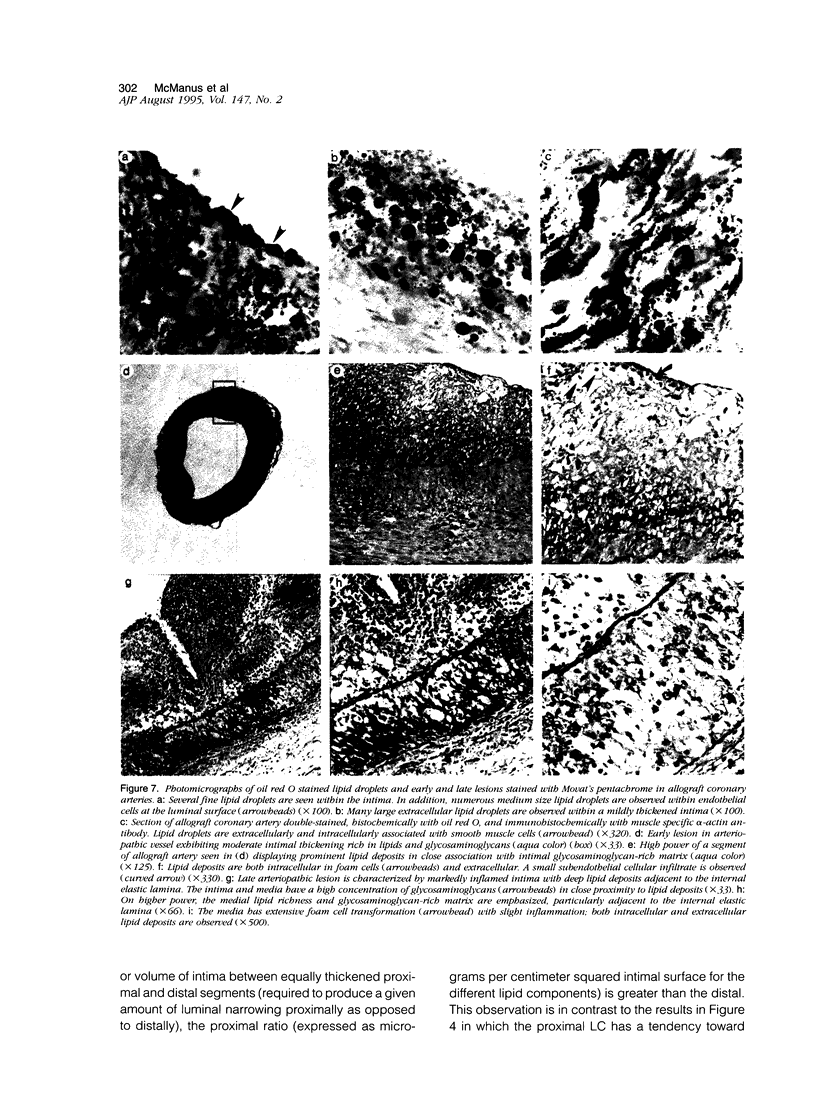
- Lin H., Wilson J. E., Kendall T. J., Radio S. J., Cornhill F. J., Herderick E., Winters G. L., Costanzo M. R., Porter T., Thieszen S. L. Comparable proximal and distal severity of intimal thickening and size of epicardial coronary arteries in transplant arteriopathy of human cardiac allografts. J Heart Lung Transplant. 1994 Sep-Oct;13(5):824–833. [PubMed] [Google Scholar]
- Linder J., Cassling R. S., Rogler W. C., Wilson J. E., Markin R. S., Sears T. D., McManus B. M. Immunohistochemical characterization of lymphocytes in uninflamed ventricular myocardium. Implications for myocarditis. Arch Pathol Lab Med. 1985 Oct;109(10):917–920. [PubMed] [Google Scholar]
- Liu G., Butany J. Morphology of graft arteriosclerosis in cardiac transplant recipients. Hum Pathol. 1992 Jul;23(7):768–773. doi: 10.1016/0046-8177(92)90346-5. [DOI] [PubMed] [Google Scholar]
- Malcom G. T., Strong J. P., Restrepo C. Atherosclerosis and lipid composition of the abdominal aorta. Comparison of autopsied New Orleans and Guatemalan men. Lab Invest. 1984 Jan;50(1):79–86. [PubMed] [Google Scholar]
- Malcom G. T., Strong J. P. The expression of results of lipid determinations in arterial tissues: mass per unit weight vs mass per unit area. Atherosclerosis. 1981 Nov-Dec;40(3-4):273–277. doi: 10.1016/0021-9150(81)90137-4. [DOI] [PubMed] [Google Scholar]
- McManus B. M., Malcom G., Kendall T. J., Gulizia J. M., Wilson J. E., Winters G., Costanzo M. R., Thieszen S., Radio S. J. Lipid overload and proteoglycan expression in chronic rejection of the human transplanted heart. Clin Transplant. 1994 Jun;8(3 Pt 2):336–340. [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. G., Insull W., Jr Effect of regenerated endothelium on lipid accumulation in the arterial wall. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1724–1728. doi: 10.1073/pnas.74.4.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll A., Duffield R., Lewis B. Flux of plasma lipoproteins into human arterial intima. Comparison between grossly normal and atheromatous intima. Atherosclerosis. 1981 May;39(2):229–242. doi: 10.1016/0021-9150(81)90073-3. [DOI] [PubMed] [Google Scholar]
- Orekhov A. N., Tertov V. V., Smirnov V. N. Lipids in cells of atherosclerotic and uninvolved human aorta. II. Lipid metabolism in primary culture. Exp Mol Pathol. 1985 Oct;43(2):187–195. doi: 10.1016/0014-4800(85)90039-5. [DOI] [PubMed] [Google Scholar]
- Raine A. E., Carter R., Mann J. I., Chapman J. R., Morris P. J. Increased plasma LDL cholesterol after renal transplantation associated with cyclosporine immunosuppression. Transplant Proc. 1987 Feb;19(1 Pt 2):1820–1821. [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Rudas L., Pflugfelder P. W., McKenzie F. N., Menkis A. H., Novick R. J., Kostuk W. J. Serial evaluation of lipid profiles and risk factors for development of hyperlipidemia after cardiac transplantation. Am J Cardiol. 1990 Nov 1;66(15):1135–1138. doi: 10.1016/0002-9149(90)90518-6. [DOI] [PubMed] [Google Scholar]
- Rudas L., Pflugfelder P. W., McKenzie F. N., Menkis A. H., Novick R. J., Kostuk W. J. Serial evaluation of lipid profiles and risk factors for development of hyperlipidemia after cardiac transplantation. Am J Cardiol. 1990 Nov 1;66(15):1135–1138. doi: 10.1016/0002-9149(90)90518-6. [DOI] [PubMed] [Google Scholar]
- Salisbury B. G., Falcone D. J., Minick C. R. Insoluble low-density lipoprotein-proteoglycan complexes enhance cholesteryl ester accumulation in macrophages. Am J Pathol. 1985 Jul;120(1):6–11. [PMC free article] [PubMed] [Google Scholar]
- Salomon R. N., Hughes C. C., Schoen F. J., Payne D. D., Pober J. S., Libby P. Human coronary transplantation-associated arteriosclerosis. Evidence for a chronic immune reaction to activated graft endothelial cells. Am J Pathol. 1991 Apr;138(4):791–798. [PMC free article] [PubMed] [Google Scholar]
- Sandkamp M., Funke H., Schulte H., Köhler E., Assmann G. Lipoprotein(a) is an independent risk factor for myocardial infarction at a young age. Clin Chem. 1990 Jan;36(1):20–23. [PubMed] [Google Scholar]
- Schonfeld G. Lipoproteins in atherogenesis. Artery. 1979 Apr;5(4):305–329. [PubMed] [Google Scholar]
- Schwenke D. C., Carew T. E. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis. 1989 Nov-Dec;9(6):908–918. doi: 10.1161/01.atv.9.6.908. [DOI] [PubMed] [Google Scholar]
- Scott P. J., Hurley P. J. The distribution of radio-iodinated serum albumin and low-density lipoprotein in tissues and the arterial wall. Atherosclerosis. 1970 Jan-Feb;11(1):77–103. doi: 10.1016/0021-9150(70)90008-0. [DOI] [PubMed] [Google Scholar]
- Segarra A., Chacón P., Vilardell M., Piera L. L. Cyclosporin and serum lipids in renal transplant recipients. Lancet. 1993 Mar 20;341(8847):766–767. [PubMed] [Google Scholar]
- Sinapius D. Lipid deposition in the media of human coronary arteries. Atherosclerosis. 1980 Sep;37(1):87–96. doi: 10.1016/0021-9150(80)90096-9. [DOI] [PubMed] [Google Scholar]
- Smith E. B., Staples E. M. Distribution of plasma proteins across the human aortic wall--barrier functions of endothelium and internal elastic lamina. Atherosclerosis. 1980 Dec;37(4):579–590. doi: 10.1016/0021-9150(80)90065-9. [DOI] [PubMed] [Google Scholar]
- Speir E., Modali R., Huang E. S., Leon M. B., Shawl F., Finkel T., Epstein S. E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994 Jul 15;265(5170):391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Sukhova G. K., Libby P. Interaction of the allogeneic state and hypercholesterolemia in arterial lesion formation in experimental cardiac allografts. Arterioscler Thromb. 1994 May;14(5):734–745. doi: 10.1161/01.atv.14.5.734. [DOI] [PubMed] [Google Scholar]
- Thomson J. G. Production of severe atheroma in a transplanted human heart. Lancet. 1969 Nov 22;2(7630):1088–1092. doi: 10.1016/s0140-6736(69)90700-4. [DOI] [PubMed] [Google Scholar]
- Uretsky B. F., Murali S., Reddy P. S., Rabin B., Lee A., Griffith B. P., Hardesty R. L., Trento A., Bahnson H. T. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987 Oct;76(4):827–834. doi: 10.1161/01.cir.76.4.827. [DOI] [PubMed] [Google Scholar]
- Uys C. J., Rose A. G. Pathologic findings in long-term cardiac transplants. Arch Pathol Lab Med. 1984 Feb;108(2):112–116. [PubMed] [Google Scholar]
- Vasile E., Simionescu M., Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983 Jun;96(6):1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters G. L., Kendall T. J., Radio S. J., Wilson J. E., Costanzo-Nordin M. R., Switzer B. L., Remmenga J. A., McManus B. M. Posttransplant obesity and hyperlipidemia: major predictors of severity of coronary arteriopathy in failed human heart allografts. J Heart Transplant. 1990 Jul-Aug;9(4):364–371. [PubMed] [Google Scholar]