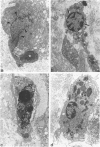
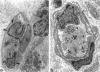
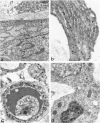
Abstract
Granulation tissue formation and contraction is an important step of second intention wound healing. Granulation tissue develops from the connective tissue surrounding the damaged or missing area and its cellular components are mainly small vessel and inflammatory cells as well as fibroblasts and myofibroblasts. As the wound closes and evolves into a scar, there is an important decrease in cellularity; in particular myofibroblasts disappear. The question arises as to which process is responsible for this cellular loss. During a previous investigation on the expression of alpha-smooth muscle actin in myofibroblasts (Darby I, Skalli O, Gabbiani G, Lab Invest, 1990, 63:21-29), we have observed that in late phases of wound healing, many myofibroblasts show changes compatible with apoptosis and suggested that this type of cell death could be responsible for the disappearance of myofibroblasts. We have now tested this hypothesis by means of morphometry at the electron microscopic level and by in situ end labeling of fragmented DNA. Our results indicate that the number of myofibroblastic and vascular cells undergoing apoptosis increases as the wound closes and support the assumption that this is the mechanism of granulation tissue evolution into a scar. The regulation of apoptotic phenomena during wound healing may be important in scar establishment and development of pathological scarring.
Full text
PDF


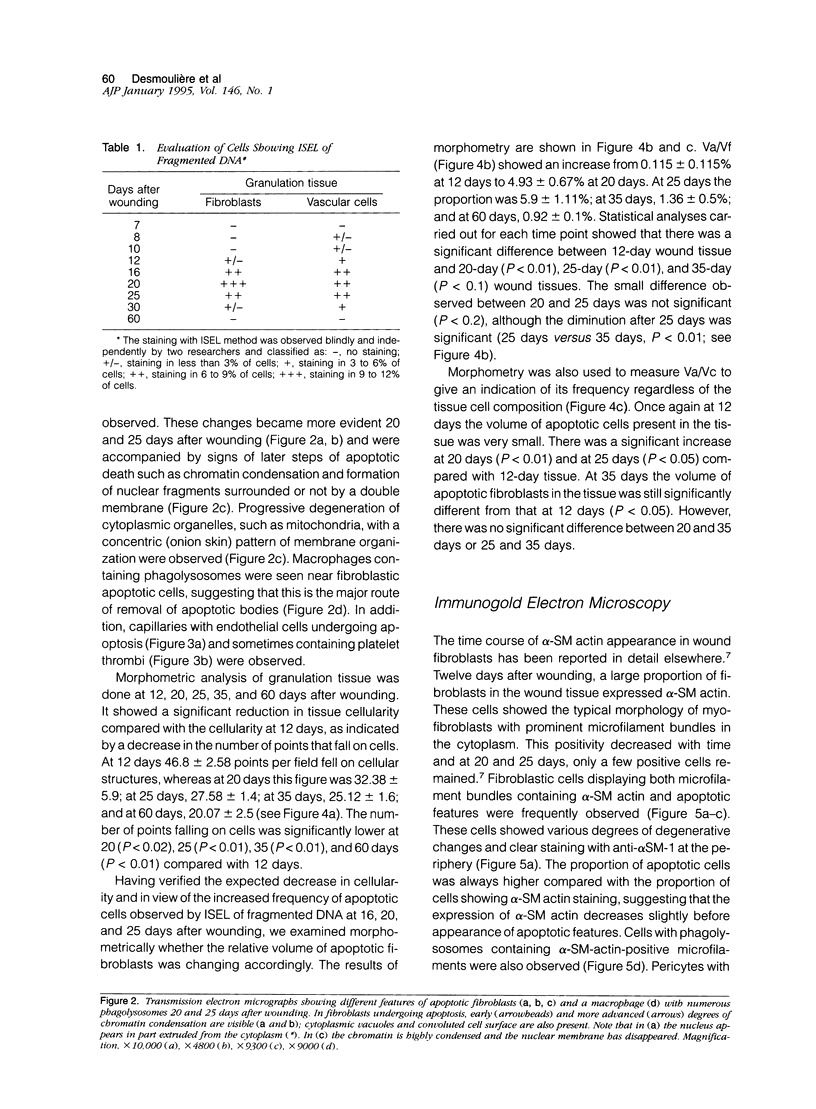
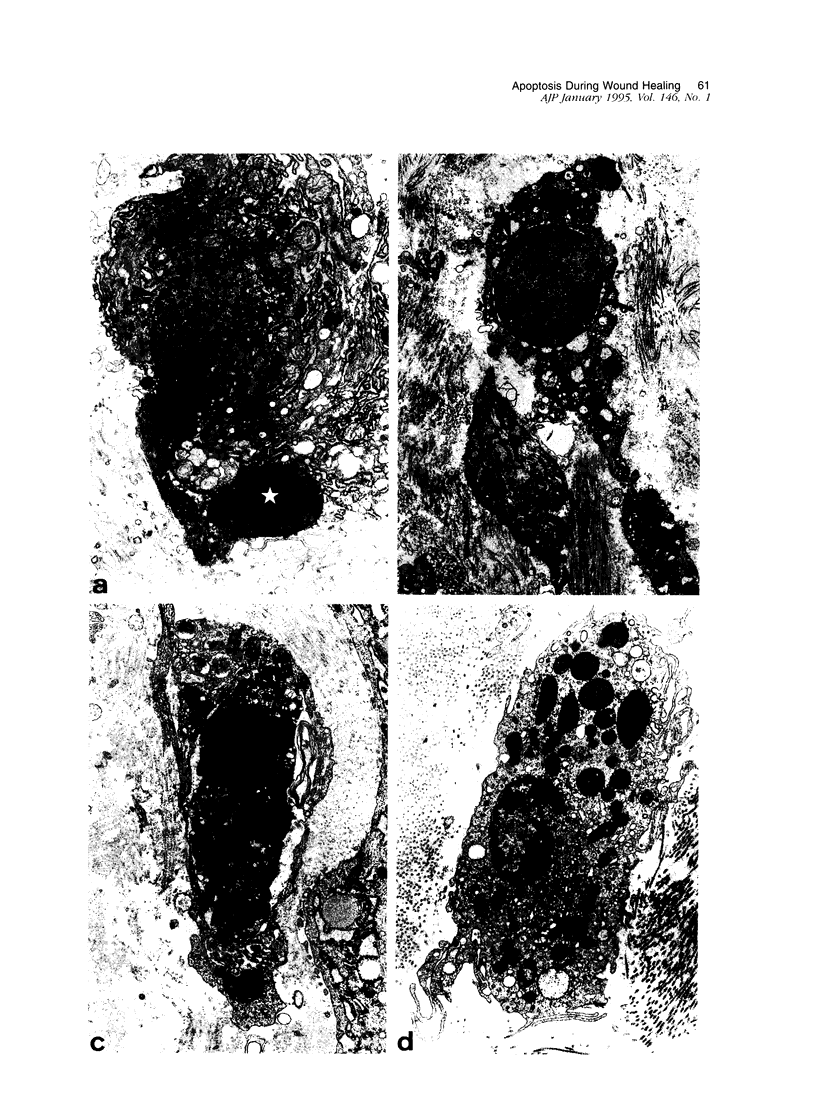
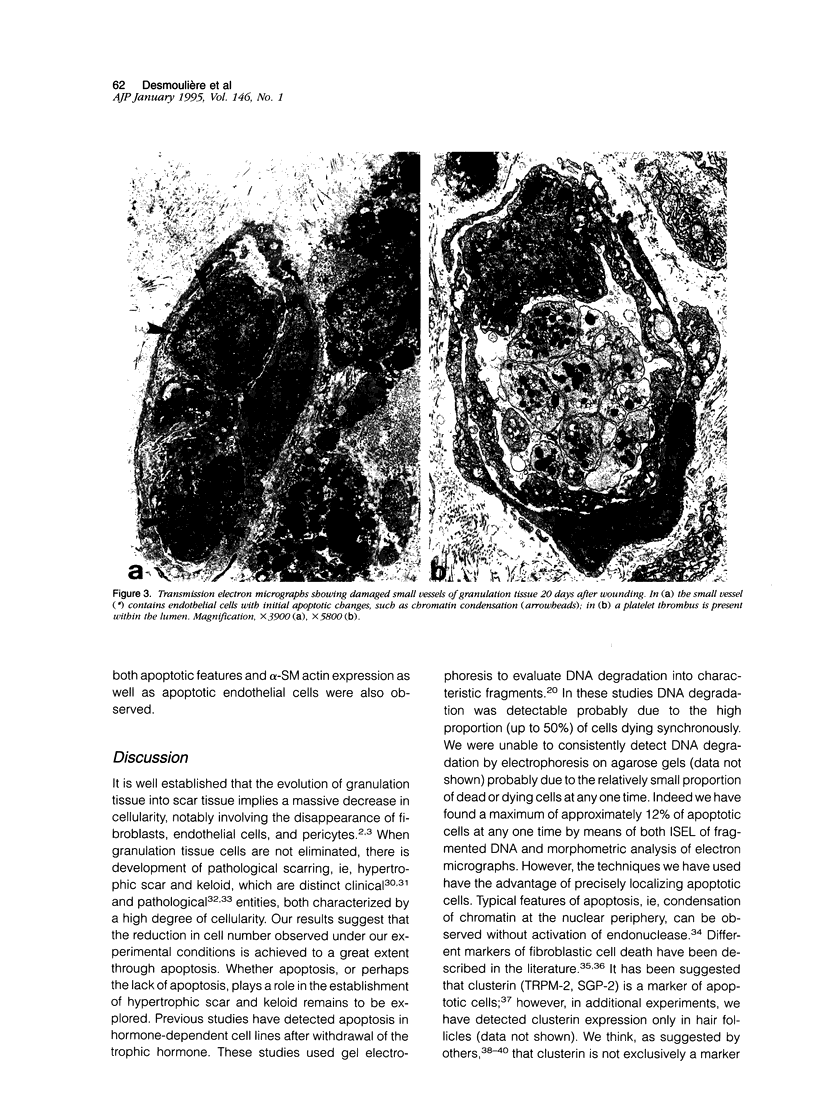
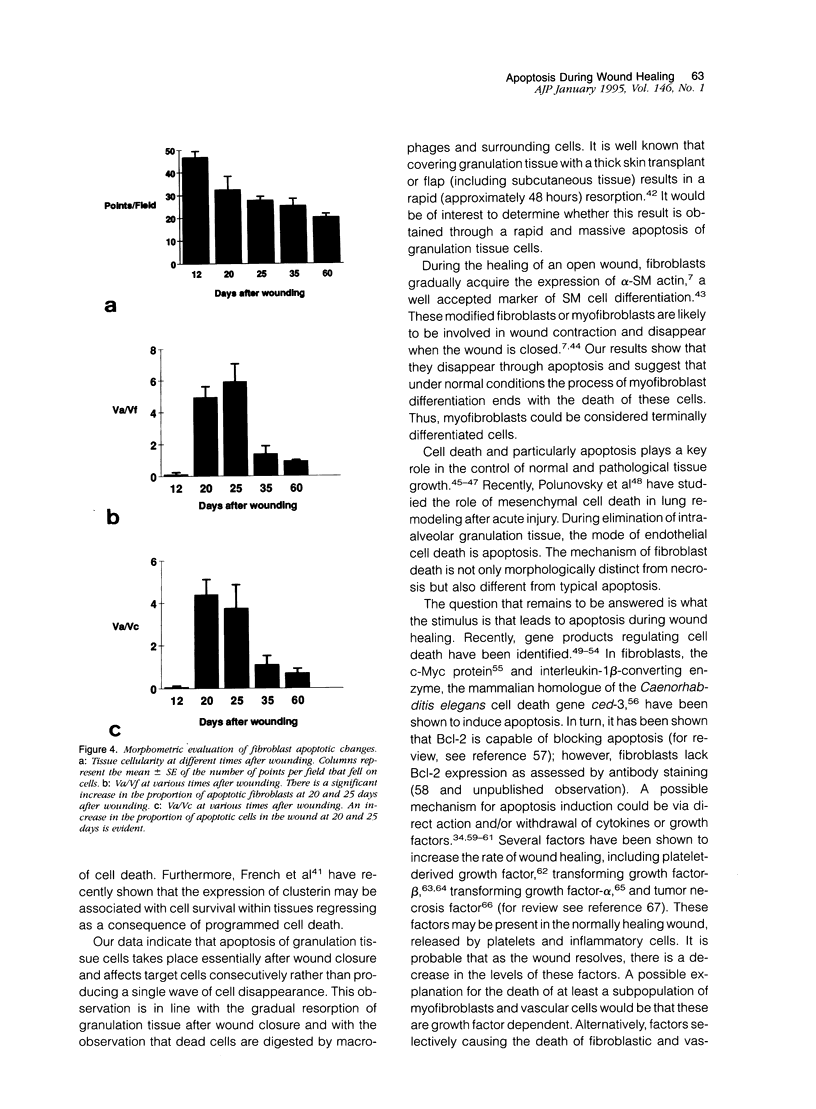


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolph S., Hameister H. In situ nick translation of metaphase chromosomes with biotin-labeled d-UTP. Hum Genet. 1985;69(2):117–121. doi: 10.1007/BF00293280. [DOI] [PubMed] [Google Scholar]
- Adzick N. S., Longaker M. T. Scarless fetal healing. Therapeutic implications. Ann Surg. 1992 Jan;215(1):3–7. doi: 10.1097/00000658-199201000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari B., Coates P. J., Greenstein B. D., Hall P. A. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993 May;170(1):1–8. doi: 10.1002/path.1711700102. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Beck L. S., DeGuzman L., Lee W. P., Xu Y., Siegel M. W., Amento E. P. One systemic administration of transforming growth factor-beta 1 reverses age- or glucocorticoid-impaired wound healing. J Clin Invest. 1993 Dec;92(6):2841–2849. doi: 10.1172/JCI116904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn W. R., Cosman B. Histologic basis of keloid and hypertrophic scar differentiation. Clinicopathologic correlation. Arch Pathol. 1966 Jul;82(1):65–71. [PubMed] [Google Scholar]
- Bursch W., Oberhammer F., Schulte-Hermann R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol Sci. 1992 Jun;13(6):245–251. doi: 10.1016/0165-6147(92)90077-j. [DOI] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993 Jul;306(1):42–48. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis: the physiologic pathway of cell death. Hosp Pract (Off Ed) 1993 Dec 15;28(12):35–43. doi: 10.1080/21548331.1993.11442887. [DOI] [PubMed] [Google Scholar]
- Danscher G. Localization of gold in biological tissue. A photochemical method for light and electronmicroscopy. Histochemistry. 1981;71(1):81–88. doi: 10.1007/BF00592572. [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Don M. M., Ablett G., Bishop C. J., Bundesen P. G., Donald K. J., Searle J., Kerr J. F. Death of cells by apoptosis following attachment of specifically allergized lymphocytes in vitro. Aust J Exp Biol Med Sci. 1977 Aug;55(4):407–417. doi: 10.1038/icb.1977.38. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Desmoulière A., Diegelmann R. F., Cohen I. K., Compton C. C., Garner W. L., Kapanci Y., Gabbiani G. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994 Jul;145(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Evans V. G. Multiple pathways to apoptosis. Cell Biol Int. 1993 May;17(5):461–476. doi: 10.1006/cbir.1993.1087. [DOI] [PubMed] [Google Scholar]
- French L. E., Wohlwend A., Sappino A. P., Tschopp J., Schifferli J. A. Human clusterin gene expression is confined to surviving cells during in vitro programmed cell death. J Clin Invest. 1994 Feb;93(2):877–884. doi: 10.1172/JCI117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Schmid E., Winter S., Chaponnier C., de Ckhastonay C., Vandekerckhove J., Weber K., Franke W. W. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994 Feb;124(4):401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert L., Pandey S., Wang E. Commitment to cell death is signaled by the appearance of a terminin protein of 30 kDa. Exp Cell Res. 1994 Jan;210(1):10–18. doi: 10.1006/excr.1994.1002. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Clusterin: the intriguing guises of a widely expressed glycoprotein. Trends Biochem Sci. 1992 Apr;17(4):154–159. doi: 10.1016/0968-0004(92)90325-4. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Winterford C. M., Harmon B. V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994 Apr 15;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischer C. W., Shetlar M. R., Chvapil M. Hypertrophic scars and keloids: a review and new concept concerning their origin. Scan Electron Microsc. 1982;(Pt 4):1699–1713. [PubMed] [Google Scholar]
- Laster S. M., Wood J. G., Gooding L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988 Oct 15;141(8):2629–2634. [PubMed] [Google Scholar]
- Lee S., Christakos S., Small M. B. Apoptosis and signal transduction: clues to a molecular mechanism. Curr Opin Cell Biol. 1993 Apr;5(2):286–291. doi: 10.1016/0955-0674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- Martin P., Hopkinson-Woolley J., McCluskey J. Growth factors and cutaneous wound repair. Prog Growth Factor Res. 1992;4(1):25–44. doi: 10.1016/0955-2235(92)90003-z. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Green D. R., Cotter T. G. Dicing with death: dissecting the components of the apoptosis machinery. Trends Biochem Sci. 1994 Jan;19(1):26–30. doi: 10.1016/0968-0004(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Mooney D. P., O'Reilly M., Gamelli R. L. Tumor necrosis factor and wound healing. Ann Surg. 1990 Feb;211(2):124–129. doi: 10.1097/00000658-199002000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton B. C. Transforming growth factor-beta stimulates endometrial stromal apoptosis in vitro. Endocrinology. 1994 Mar;134(3):1055–1060. doi: 10.1210/endo.134.3.8119142. [DOI] [PubMed] [Google Scholar]
- Murer-Orlando M. L., Peterson A. C. In situ nick translation of human and mouse chromosomes detected with a biotinylated nucleotide. Exp Cell Res. 1985 Apr;157(2):322–334. doi: 10.1016/0014-4827(85)90117-x. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Oberhammer F., Bursch W., Tiefenbacher R., Fröschl G., Pavelka M., Purchio T., Schulte-Hermann R. Apoptosis is induced by transforming growth factor-beta 1 within 5 hours in regressing liver without significant fragmentation of the DNA. Hepatology. 1993 Nov;18(5):1238–1246. [PubMed] [Google Scholar]
- Peitsch M. C., Mannherz H. G., Tschopp J. The apoptosis endonucleases: cleaning up after cell death? Trends Cell Biol. 1994 Feb;4(2):37–41. doi: 10.1016/0962-8924(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polunovsky V. A., Chen B., Henke C., Snover D., Wendt C., Ingbar D. H., Bitterman P. B. Role of mesenchymal cell death in lung remodeling after injury. J Clin Invest. 1993 Jul;92(1):388–397. doi: 10.1172/JCI116578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaye B., Mosselmans R., Fiers W., Dumont J. E., Galand P. Tumor necrosis factor induces apoptosis (programmed cell death) in normal endothelial cells in vitro. Am J Pathol. 1991 Feb;138(2):447–453. [PMC free article] [PubMed] [Google Scholar]
- Rockwell W. B., Cohen I. K., Ehrlich H. P. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989 Nov;84(5):827–837. doi: 10.1097/00006534-198911000-00021. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. E., Dvergsten J., Correa-Rotter R. Clusterin: an enigmatic protein recruited by diverse stimuli. J Lab Clin Med. 1993 Feb;121(2):205–214. [PubMed] [Google Scholar]
- Rotello R. J., Hocker M. B., Gerschenson L. E. Biochemical evidence for programmed cell death in rabbit uterine epithelium. Am J Pathol. 1989 Mar;134(3):491–495. [PMC free article] [PubMed] [Google Scholar]
- Rudolph R. Inhibition of myofibroblasts by skin grafts. Plast Reconstr Surg. 1979 Apr;63(4):473–480. doi: 10.1097/00006534-197904000-00005. [DOI] [PubMed] [Google Scholar]
- Russell J. H., Masakowski V., Rucinsky T., Phillips G. Mechanisms of immune lysis. III. Characterization of the nature and kinetics of the cytotoxic T lymphocyte-induced nuclear lesion in the target. J Immunol. 1982 May;128(5):2087–2094. [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Schwartzman R. A., Cidlowski J. A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993 Apr;14(2):133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. Death-defying acts: a meeting review on apoptosis. Genes Dev. 1993 Dec;7(12A):2277–2284. doi: 10.1101/gad.7.12a.2277. [DOI] [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yamada T., Ohyama H. Radiation-induced interphase death of rat thymocytes is internally programmed (apoptosis). Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Jan;53(1):65–75. doi: 10.1080/09553008814550431. [DOI] [PubMed] [Google Scholar]