Abstract
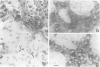
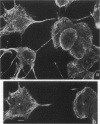
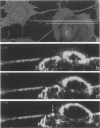
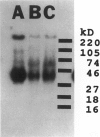
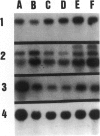
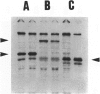
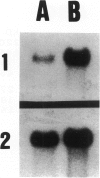
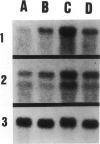
We developed and characterized a coculture system composed of a fat-storing cell clone (CFSC-2G) and freshly isolated hepatocytes that can reproduce in vitro some of the physical and functional relationships observed in vivo. Hepatocytes in the coculture are polarized, are smaller in size than hepatocytes plated on plastic, maintain a cuboidal shape, and have a tendency to form cords. Fat-storing cells, which are initially extended, retract and leave spaces that resemble liver sinusoids. Both cell types in the coculture system are functional for at least two weeks as determined by the expression of high levels of liver-specific protein mRNAs as well as by the production and secretion of liver-specific proteins into the culture medium. The hepatocytes maintain relatively high levels of asialoglycoprotein receptor on their cell surface and form functional gap junctional complexes with fat-storing cells. Hence, this coculture system retains a number of differentiated functions of hepatocytes, making it a useful model to study cell-cell interactions in culture and to analyze regulation of hepatocyte functions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedossa P., Houglum K., Trautwein C., Holstege A., Chojkier M. Stimulation of collagen alpha 1(I) gene expression is associated with lipid peroxidation in hepatocellular injury: a link to tissue fibrosis? Hepatology. 1994 May;19(5):1262–1271. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Arenson D. M., Maher J. J., Roll F. J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987 Mar;79(3):801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher N. L., Robinson G. S., Farmer S. R. Effects of extracellular matrix on hepatocyte growth and gene expression: implications for hepatic regeneration and the repair of liver injury. Semin Liver Dis. 1990 Feb;10(1):11–19. doi: 10.1055/s-2008-1040453. [DOI] [PubMed] [Google Scholar]
- Chojkier M. Hepatocyte collagen production in vivo in normal rats. J Clin Invest. 1986 Aug;78(2):333–339. doi: 10.1172/JCI112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement B., Guguen-Guillouzo C., Campion J. P., Glaise D., Bourel M., Guillouzo A. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: modulation of albumin secretion and accumulation of extracellular material. Hepatology. 1984 May-Jun;4(3):373–380. doi: 10.1002/hep.1840040305. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Kant J. A. Molecular cloning of cDNA for the alpha, beta, and gamma chains of rat fibrinogen. A family of coordinately regulated genes. J Biol Chem. 1981 Sep 25;256(18):9718–9723. [PubMed] [Google Scholar]
- Fishman G. I., Hertzberg E. L., Spray D. C., Leinwand L. A. Expression of connexin43 in the developing rat heart. Circ Res. 1991 Mar;68(3):782–787. doi: 10.1161/01.res.68.3.782. [DOI] [PubMed] [Google Scholar]
- Friedman S. L. Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis. 1990 Feb;10(1):20–29. doi: 10.1055/s-2008-1040454. [DOI] [PubMed] [Google Scholar]
- Geerts A., Greenwel P., Cunningham M., De Bleser P., Rogiers V., Wisse E., Rojkind M. Identification of connective tissue gene transcripts in freshly isolated parenchymal, endothelial, Kupffer and fat-storing cells by northern hybridization analysis. J Hepatol. 1993 Aug;19(1):148–158. doi: 10.1016/s0168-8278(05)80188-6. [DOI] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Goltz J. S., Wolkoff A. W., Novikoff P. M., Stockert R. J., Satir P. A role for microtubules in sorting endocytic vesicles in rat hepatocytes. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7026–7030. doi: 10.1073/pnas.89.15.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet F., Normand C., Morin O. Cellular interactions promote tissue-specific function, biomatrix deposition and junctional communication of primary cultured hepatocytes. Hepatology. 1988 Sep-Oct;8(5):1010–1018. doi: 10.1002/hep.1840080506. [DOI] [PubMed] [Google Scholar]
- Greenwel P., Iraburu M. J., Reyes-Romero M., Meraz-Cruz N., Casado E., Solis-Herruzo J. A., Rojkind M. Induction of an acute phase response in rats stimulates the expression of alpha 1(I) procollagen messenger ribonucleic acid in their livers. Possible role of interleukin-6. Lab Invest. 1995 Jan;72(1):83–91. [PubMed] [Google Scholar]
- Greenwel P., Rubin J., Schwartz M., Hertzberg E. L., Rojkind M. Liver fat-storing cell clones obtained from a CCl4-cirrhotic rat are heterogeneous with regard to proliferation, expression of extracellular matrix components, interleukin-6, and connexin 43. Lab Invest. 1993 Aug;69(2):210–216. [PubMed] [Google Scholar]
- Greenwel P., Schwartz M., Rosas M., Peyrol S., Grimaud J. A., Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991 Dec;65(6):644–653. [PubMed] [Google Scholar]
- Gressner A. M., Bachem M. G. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990 Feb;10(1):30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Lotfi S., Gressner G., Lahme B. Identification and partial characterization of a hepatocyte-derived factor promoting proliferation of cultured fat-storing cells (parasinusoidal lipocytes). Hepatology. 1992 Nov;16(5):1250–1266. [PubMed] [Google Scholar]
- Grimaud J. A., Druguet M., Peyrol S., Chevalier O., Herbage D., El Badrawy N. Collagen immunotyping in human liver: light and electron microscope study. J Histochem Cytochem. 1980 Nov;28(11):1145–1156. doi: 10.1177/28.11.7000887. [DOI] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson D. M., Clayton D. F., Darnell J. E., Jr, Reid L. M. Posttranscriptional modulation of gene expression in cultured rat hepatocytes. Mol Cell Biol. 1984 Sep;4(9):1929–1934. doi: 10.1128/mcb.4.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Mendoza-Figueroa T. Cultivation of adult rat hepatocytes on 3T3 cells: expression of various liver differentiated functions. Differentiation. 1989 Aug;41(2):148–157. doi: 10.1111/j.1432-0436.1989.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loréal O., Levavasseur F., Fromaget C., Gros D., Guillouzo A., Clément B. Cooperation of Ito cells and hepatocytes in the deposition of an extracellular matrix in vitro. Am J Pathol. 1993 Aug;143(2):538–544. [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak K. M., Lieber C. S. Lipocytes and transitional cells in alcoholic liver disease: a morphometric study. Hepatology. 1988 Sep-Oct;8(5):1027–1033. doi: 10.1002/hep.1840080508. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984 Jul;51(1):57–74. [PubMed] [Google Scholar]
- Mead J. E., Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B., HAUSMAN D. H., PODBER E. The localization of adenosine triphosphatase in liver: in situ staining and cell fractionation studies. J Histochem Cytochem. 1958 Jan;6(1):61–71. doi: 10.1177/6.1.61. [DOI] [PubMed] [Google Scholar]
- Nakano M., Worner T. M., Lieber C. S. Perivenular fibrosis in alcoholic liver injury: ultrastructure and histologic progression. Gastroenterology. 1982 Oct;83(4):777–785. [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. II. A cytochemical method for light and electron microscopy. J Histochem Cytochem. 1972 Dec;20(12):1006–1023. doi: 10.1177/20.12.1006. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Rosen O. M., Rubin C. S. Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol. 1980 Oct;87(1):180–196. doi: 10.1083/jcb.87.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenthal M., Neubauer K., Baralle F. E., Peters H., Meyer zum Büschenfelde K. H., Ramadori G. Rat hepatocytes in primary culture synthesize and secrete cellular fibronectin. Exp Cell Res. 1992 Dec;203(2):289–296. doi: 10.1016/0014-4827(92)90001-o. [DOI] [PubMed] [Google Scholar]
- Otto J. M., Grenett H. E., Fuller G. M. The coordinated regulation of fibrinogen gene transcription by hepatocyte-stimulating factor and dexamethasone. J Cell Biol. 1987 Sep;105(3):1067–1072. doi: 10.1083/jcb.105.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. L. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986 Jul;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Knittel T., Odenthal M., Schwögler S., Neubauer K., Meyer zum Büschenfelde K. H. Synthesis of cellular fibronectin by rat liver fat-storing (Ito) cells: regulation by cytokines. Gastroenterology. 1992 Oct;103(4):1313–1321. doi: 10.1016/0016-5085(92)91522-6. [DOI] [PubMed] [Google Scholar]
- Reid L. M., Jefferson D. M. Culturing hepatocytes and other differentiated cells. Hepatology. 1984 May-Jun;4(3):548–559. doi: 10.1002/hep.1840040332. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Gatmaitan Z., Mackensen S., Giambrone M. A., Ponce P., Reid L. M. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980 Oct;87(1):255–263. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber M. A., Zern M. A., Shafritz D. A. Use of in situ hybridization to identify collagen and albumin mRNAs in isolated mouse hepatocytes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4017–4020. doi: 10.1073/pnas.80.13.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz E. G., Li D., Omiecinski C. J., Muller-Eberhard U., Kleinman H. K., Elswick B., Guzelian P. S. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988 Mar;134(3):309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa T., Yamazaki M., Mori Y., Yonaha T., Yoshizato K. Morphological and immuno-cytochemical characterization of a hetero-spheroid composed of fibroblasts and hepatocytes. J Cell Sci. 1992 Mar;101(Pt 3):495–501. doi: 10.1242/jcs.101.3.495. [DOI] [PubMed] [Google Scholar]
- Tong J. Z., De Lagausie P., Furlan V., Cresteil T., Bernard O., Alvarez F. Long-term culture of adult rat hepatocyte spheroids. Exp Cell Res. 1992 Jun;200(2):326–332. doi: 10.1016/0014-4827(92)90179-c. [DOI] [PubMed] [Google Scholar]
- Traub O., Look J., Dermietzel R., Brümmer F., Hülser D., Willecke K. Comparative characterization of the 21-kD and 26-kD gap junction proteins in murine liver and cultured hepatocytes. J Cell Biol. 1989 Mar;108(3):1039–1051. doi: 10.1083/jcb.108.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- Webber E. M., FitzGerald M. J., Brown P. I., Bartlett M. H., Fausto N. Transforming growth factor-alpha expression during liver regeneration after partial hepatectomy and toxic injury, and potential interactions between transforming growth factor-alpha and hepatocyte growth factor. Hepatology. 1993 Dec;18(6):1422–1431. [PubMed] [Google Scholar]
- Weiner F. R., Czaja M. J., Jefferson D. M., Giambrone M. A., Tur-Kaspa R., Reid L. M., Zern M. A. The effects of dexamethasone on in vitro collagen gene expression. J Biol Chem. 1987 May 25;262(15):6955–6958. [PubMed] [Google Scholar]
- Weiner F. R., Giambrone M. A., Czaja M. J., Shah A., Annoni G., Takahashi S., Eghbali M., Zern M. A. Ito-cell gene expression and collagen regulation. Hepatology. 1990 Jan;11(1):111–117. doi: 10.1002/hep.1840110119. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Ochalski A., Hertzberg E. L., Nagy J. I. LM and EM immunolocalization of the gap junctional protein connexin 43 in rat brain. Brain Res. 1990 Feb 5;508(2):313–319. doi: 10.1016/0006-8993(90)90415-8. [DOI] [PubMed] [Google Scholar]
- Zern M. A., Chakraborty P. R., Ruiz-Opazo N., Yap S. H., Shafritz D. A. Development and use of a rat albumin cDNA clone to evaluate the effect of chronic ethanol administration on hepatic protein synthesis. Hepatology. 1983 May-Jun;3(3):317–322. doi: 10.1002/hep.1840030307. [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989 Dec;109(6 Pt 2):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]