Abstract
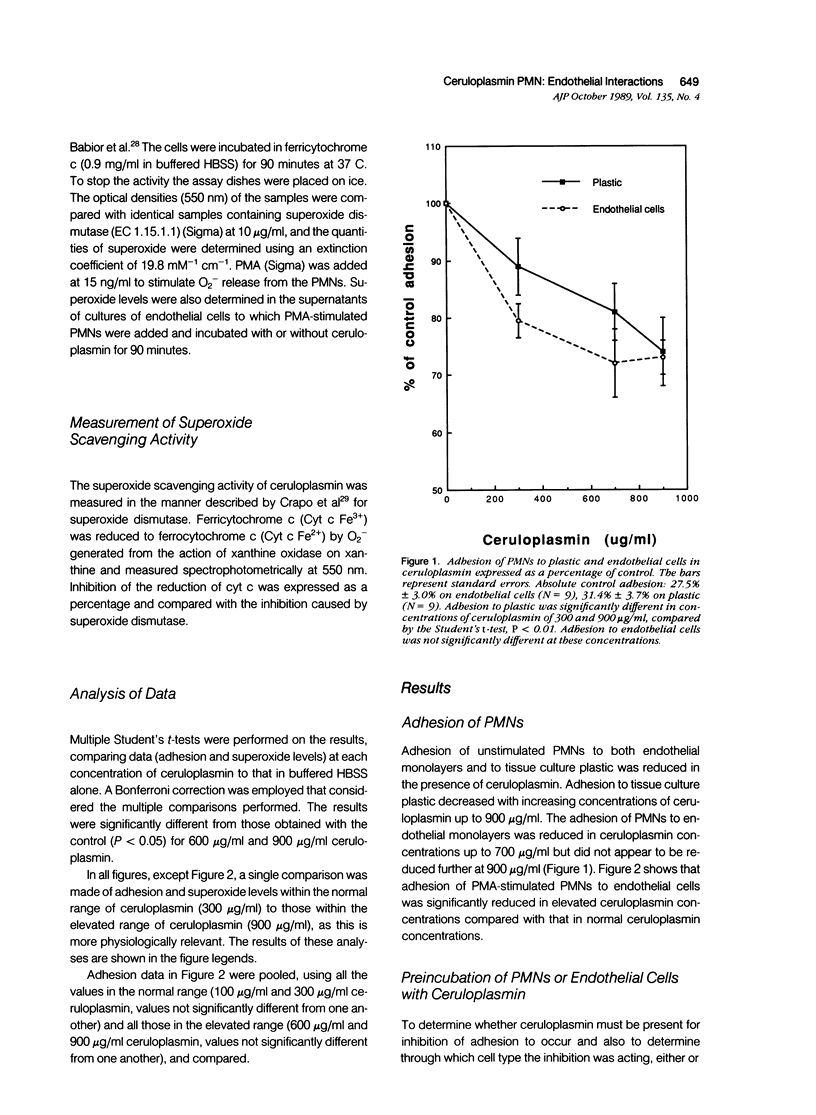
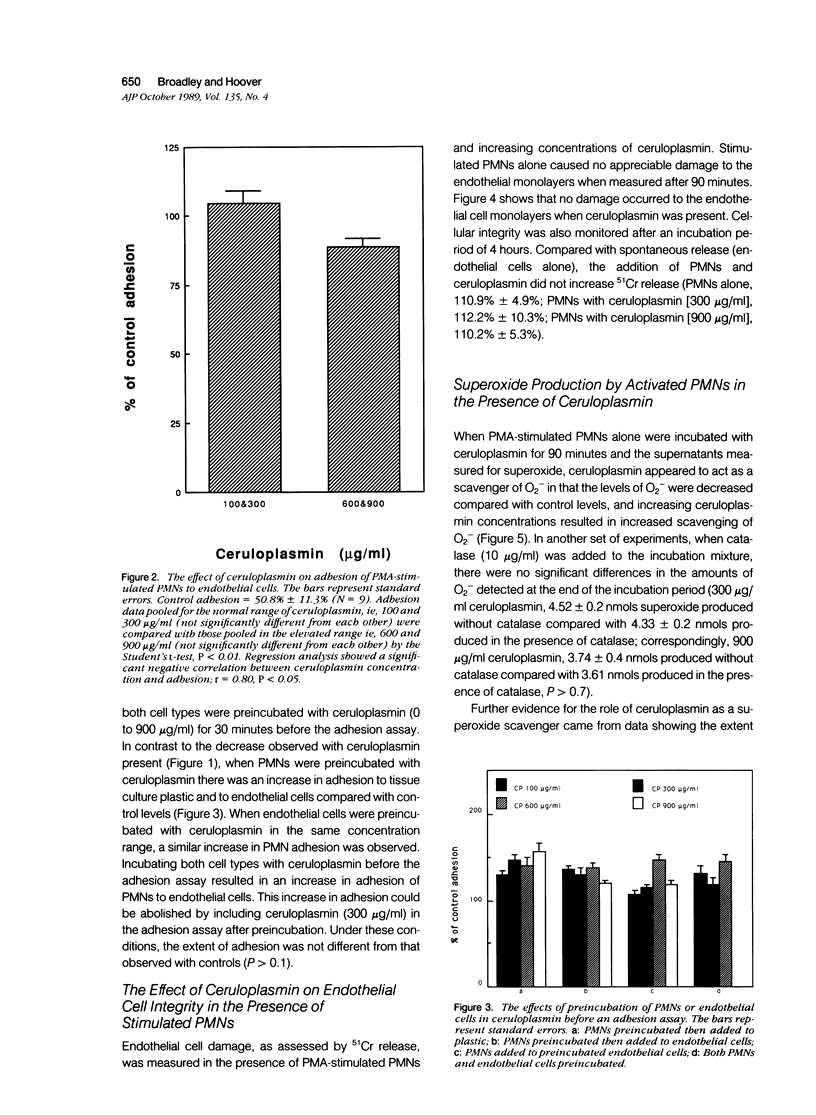
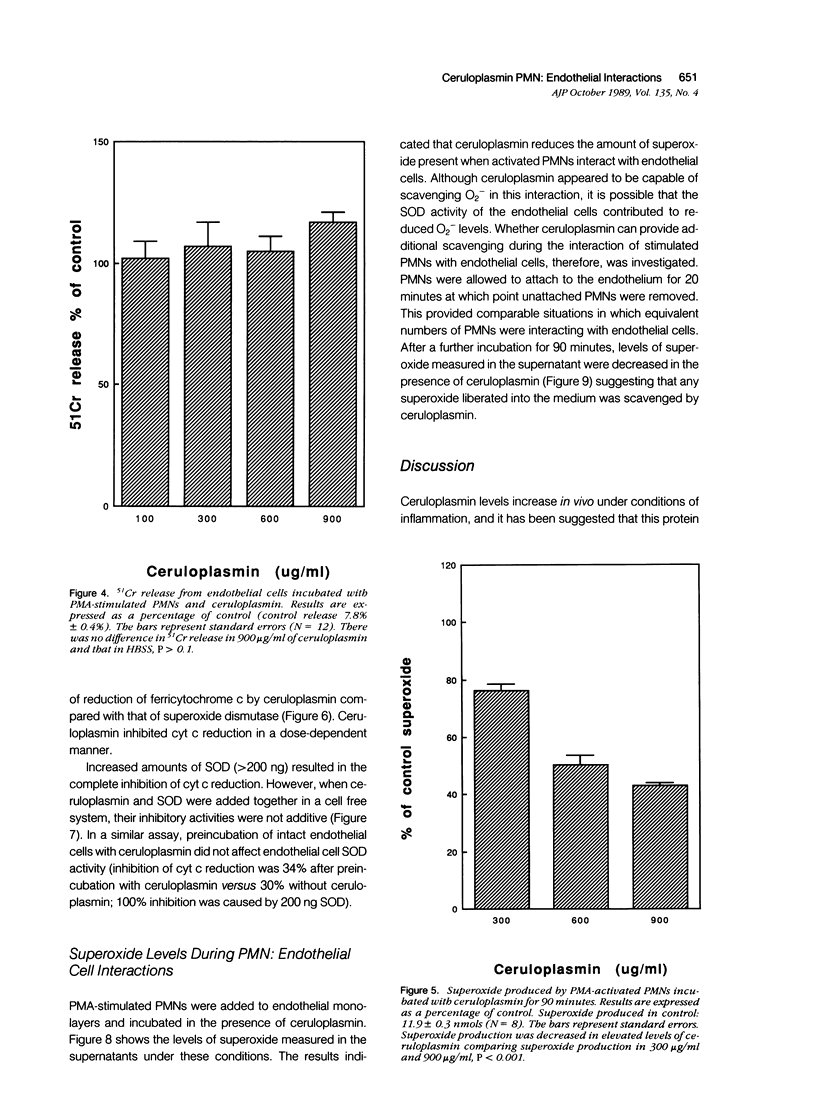
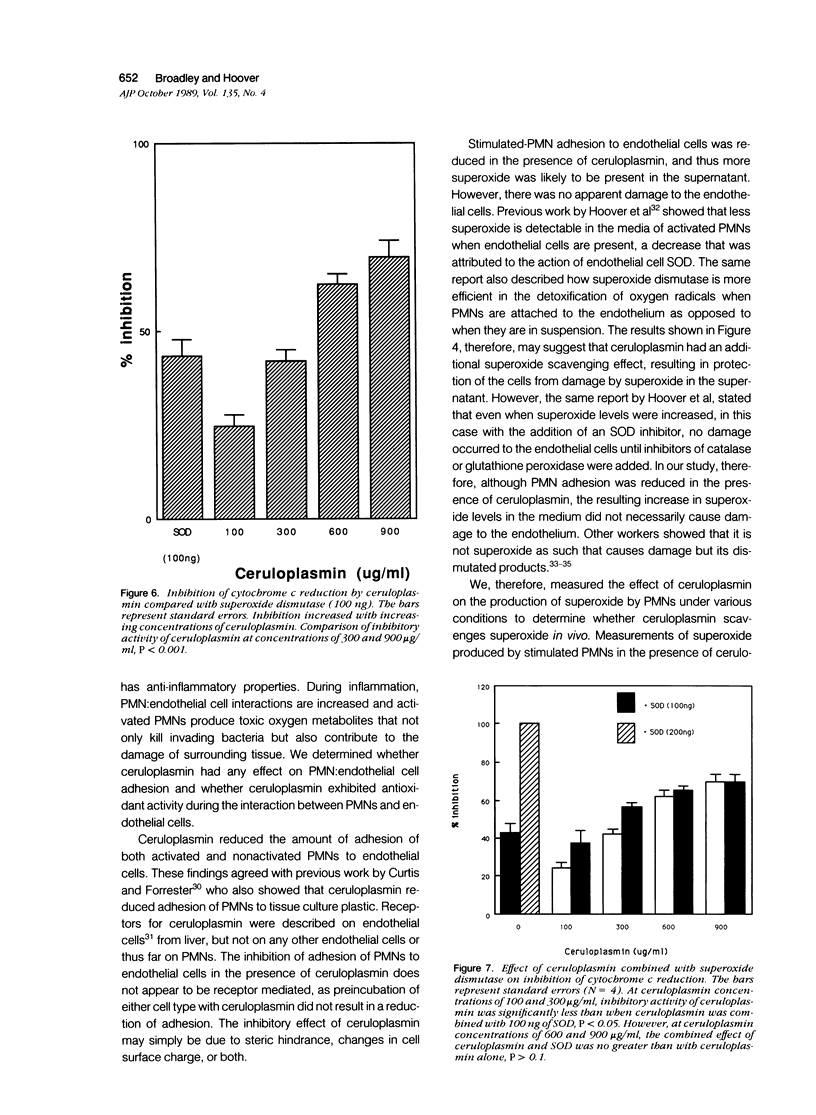
The plasma protein, ceruloplasmin, has been implicated as an anti-inflammatory agent, although this property has not been demonstrated unequivocally in vivo. The role of this protein in an in vitro system of cultured endothelial cells and polymorphonuclear leukocytes (PMNs) was investigated. One of the initial steps in an inflammatory response is increased adhesion between PMNs and the endothelial lining of the blood vessels. The results showed that ceruloplasmin interferes with this process and reduces the number of phorbol myristate acetate-activated leukocytes that adhere to endothelium. Preincubation of either the activated PMNs or the endothelium with ceruloplasmin did not produce the same results, suggesting that the continuous presence of ceruloplasmin is required. During attachment PMNs become activated and release a variety of substances, including toxic oxygen species such as superoxide and hydrogen peroxide. In the in vitro system used in this study no injury occurred to the endothelial cells, as measured by 51Cr release, when activated PMNs were added with ceruloplasmin. The data show that ceruloplasmin reduced, in a dose dependent manner, the levels of superoxide produced by the activated PMNs, further supporting ceruloplasmin's previously reported role as a scavenger of superoxide. Ceruloplasmin also reduced the levels of superoxide when activated PMNs were in contact with endothelial cells. Although ceruloplasmin interfered with the copper-dependent scavenger enzyme, superoxide dismutase (SOD), in a cell-free system, ceruloplasmin had no effect on SOD in intact endothelial cells. These results suggest that ceruloplasmin may act as an anti-inflammatory agent by reducing the number of PMNs attaching to endothelium and by acting as an extracellular scavenger of superoxide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Hill H. A., Mahood J. F., Willson R. L., Wolfenden B. S. Does caeruloplasmin dismute superoxide? No. FEBS Lett. 1980 Aug 25;118(1):127–129. doi: 10.1016/0014-5793(80)81233-6. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985 Dec;121(3):394–403. [PMC free article] [PubMed] [Google Scholar]
- Blake D. R., Blann A., Bacon P. A., Farr M., Gutteridge J. M., Halliwell B. Ferroxidase and ascorbate oxidase activities of caeruloplasmin in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1983 May;64(5):551–553. doi: 10.1042/cs0640551. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Butler E. N., Repine J. E. Hyperoxia damages cultured endothelial cells causing increased neutrophil adherence. Am Rev Respir Dis. 1983 Sep;128(3):469–472. doi: 10.1164/arrd.1983.128.3.469. [DOI] [PubMed] [Google Scholar]
- Conforti A., Franco L., Milanino R., Velo G. P. Copper and ceruloplasmin (Cp) concentrations during the acute inflammatory process in the rat. Agents Actions. 1982 Jul;12(3):303–307. doi: 10.1007/BF01965394. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Curtis A. S., Forrester J. V. The competitive effects of serum proteins on cell adhesion. J Cell Sci. 1984 Oct;71:17–35. doi: 10.1242/jcs.71.1.17. [DOI] [PubMed] [Google Scholar]
- Deshmukh V. K., Raman P. H., Dhuley J. N., Naik S. R. Role of ceruloplasmin in inflammation: increased serum ceruloplasmin levels during inflammatory conditions and its possible relationship with anti-inflammatory agents. Pharmacol Res Commun. 1985 Jul;17(7):633–642. doi: 10.1016/0031-6989(85)90070-0. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. A new function for ceruloplasmin as an acute-phase reactant in inflammation: a scavenger of superoxide anion radicals. Trans Assoc Am Physicians. 1979;92:360–369. [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin: an acute phase reactant that scavenges oxygen-derived free radicals. Ann N Y Acad Sci. 1982;389:368–379. doi: 10.1111/j.1749-6632.1982.tb22150.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Antioxidant properties of caeruloplasmin towards iron- and copper-dependent oxygen radical formation. FEBS Lett. 1983 Jun 27;157(1):37–40. doi: 10.1016/0014-5793(83)81111-9. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Antioxidant properties of the proteins caeruloplasmin, albumin and transferrin. A study of their activity in serum and synovial fluid from patients with rheumatoid arthritis. Biochim Biophys Acta. 1986 Jan 30;869(2):119–127. doi: 10.1016/0167-4838(86)90286-4. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Hill C., Blake D. R. Copper stimulated phospholipid membrane peroxidation: antioxidant activity of serum and synovial fluid from patients with rheumatoid arthritis. Clin Chim Acta. 1984 May 16;139(1):85–90. doi: 10.1016/0009-8981(84)90195-5. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Stocks J. Caeruloplasmin: physiological and pathological perspectives. Crit Rev Clin Lab Sci. 1981;14(4):257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. L., Karnovsky M. J., Austen K. F., Corey E. J., Lewis R. A. Leukotriene B4 action on endothelium mediates augmented neutrophil/endothelial adhesion. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2191–2193. doi: 10.1073/pnas.81.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. L., Robinson J. M., Karnovsky M. J. Adhesion of polymorphonuclear leukocytes to endothelium enhances the efficiency of detoxification of oxygen-free radicals. Am J Pathol. 1987 Feb;126(2):258–268. [PMC free article] [PubMed] [Google Scholar]
- Jayson M. I., Davis P., Whicher J. T., Walters G. Serum copper and caeruloplasmin in ankylosing spondylitis, systemic sclerosis, and morphea. Ann Rheum Dis. 1975 Oct;35(5):443–445. doi: 10.1136/ard.35.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka M., Tavassoli M. The role of liver endothelium in the binding and uptake of ceruloplasmin: studies with colloidal gold probe. J Ultrastruct Res. 1985 Feb;90(2):194–202. doi: 10.1016/0889-1605(85)90109-0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cohen L. Receptor-mediated regulation of superoxide production in human neutrophils stimulated by phorbol myristate acetate. J Clin Invest. 1981 Nov;68(5):1314–1320. doi: 10.1172/JCI110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox I. S. The role of copper in prostaglandin synthesis. Biochim Biophys Acta. 1973 Apr 13;306(1):74–81. doi: 10.1016/0005-2760(73)90210-5. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Gole G. A. Cellular and molecular mechanisms in angiogenesis. Trans Ophthalmol Soc U K. 1980 Sep;100(3):354–358. [PubMed] [Google Scholar]
- McAuslan B. R., Reilly W. G., Hannan G. N., Gole G. A. Angiogenic factors and their assay: activity of formyl methionyl leucyl phenylalanine, adenosine diphosphate, heparin, copper, and bovine endothelium stimulating factor. Microvasc Res. 1983 Nov;26(3):323–338. doi: 10.1016/0026-2862(83)90080-8. [DOI] [PubMed] [Google Scholar]
- Milanino R., Mazzoli S., Passarella E., Tarter G., Velo G. P. Carrageenan oedema in copper-deficient rats. Agents Actions. 1978 Dec;8(6):618–622. doi: 10.1007/BF01998891. [DOI] [PubMed] [Google Scholar]
- Raju K. S., Alessandri G., Ziche M., Gullino P. M. Ceruloplasmin, copper ions, and angiogenesis. J Natl Cancer Inst. 1982 Nov;69(5):1183–1188. [PubMed] [Google Scholar]
- SCHEINBERG I. H., GITLIN D. Deficiency of ceruloplasmin in patients with hepatolenticular degeneration (Wilson's disease). Science. 1952 Oct 31;116(3018):484–485. doi: 10.1126/science.116.3018.484. [DOI] [PubMed] [Google Scholar]
- SCHEINBERG I. H., MORELL A. G. Exchange of ceruloplasmin copper with ionic Cu64 with reference to Wilson's disease. J Clin Invest. 1957 Aug;36(8):1193–1201. doi: 10.1172/JCI103515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEINBERG I. H., MORELL A. G. Exchange of ceruloplasmin copper with ionic Cu64 with reference to Wilson's disease. J Clin Invest. 1957 Aug;36(8):1193–1201. doi: 10.1172/JCI103515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder P. R., Al-Timimi D., McMurray W., White A. G., Zoob B. C., Dormandy T. L. Serum copper and related variables in rheumatoid arthritis. Ann Rheum Dis. 1978 Feb;37(1):67–70. doi: 10.1136/ard.37.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder P. R., McMurray W., White A. G., Dormandy T. L. Synovial fluid copper and related variables in rheumatoid and degenerative arthritis. Ann Rheum Dis. 1978 Feb;37(1):71–72. doi: 10.1136/ard.37.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinar E., Navok T., Chevion M. The analogous mechanisms of enzymatic inactivation induced by ascorbate and superoxide in the presence of copper. J Biol Chem. 1983 Dec 25;258(24):14778–14783. [PubMed] [Google Scholar]
- Sorenson J. R. Copper complexes - a unique class of anti-arthritic drugs. Prog Med Chem. 1978;15:211–260. doi: 10.1016/s0079-6468(08)70257-1. [DOI] [PubMed] [Google Scholar]
- Sorenson J. R. Evaluation of copper complexes as potential anti-arthritic drugs. J Pharm Pharmacol. 1977 Jul;29(7):450–452. doi: 10.1111/j.2042-7158.1977.tb11367.x. [DOI] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashoji S., Kajimoto G. Antioxidant effect of caeruloplasmin on microsomal lipid peroxidation. FEBS Lett. 1983 Feb 21;152(2):168–170. doi: 10.1016/0014-5793(83)80371-8. [DOI] [PubMed] [Google Scholar]
- van Steveninck J., van der Zee J., Dubbelman T. M. Site-specific and bulk-phase generation of hydroxyl radicals in the presence of cupric ions and thiol compounds. Biochem J. 1985 Nov 15;232(1):309–311. doi: 10.1042/bj2320309. [DOI] [PMC free article] [PubMed] [Google Scholar]


