Abstract
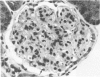
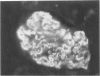
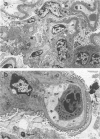
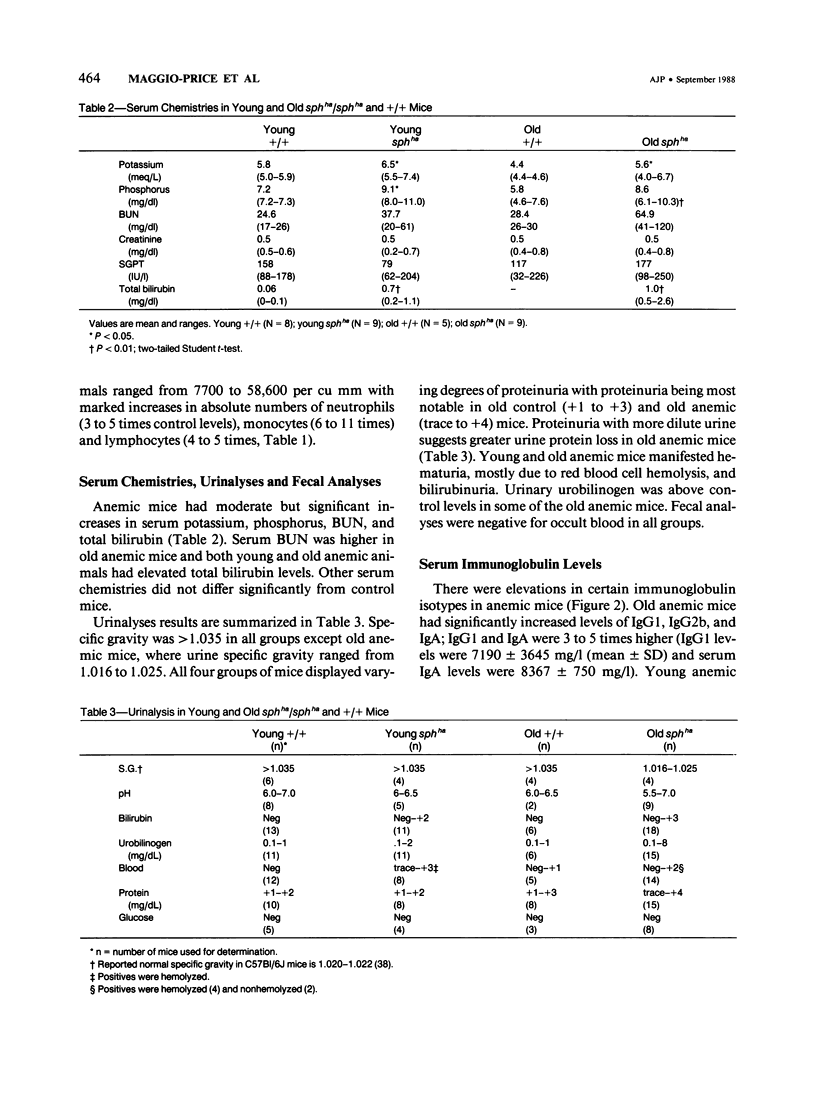
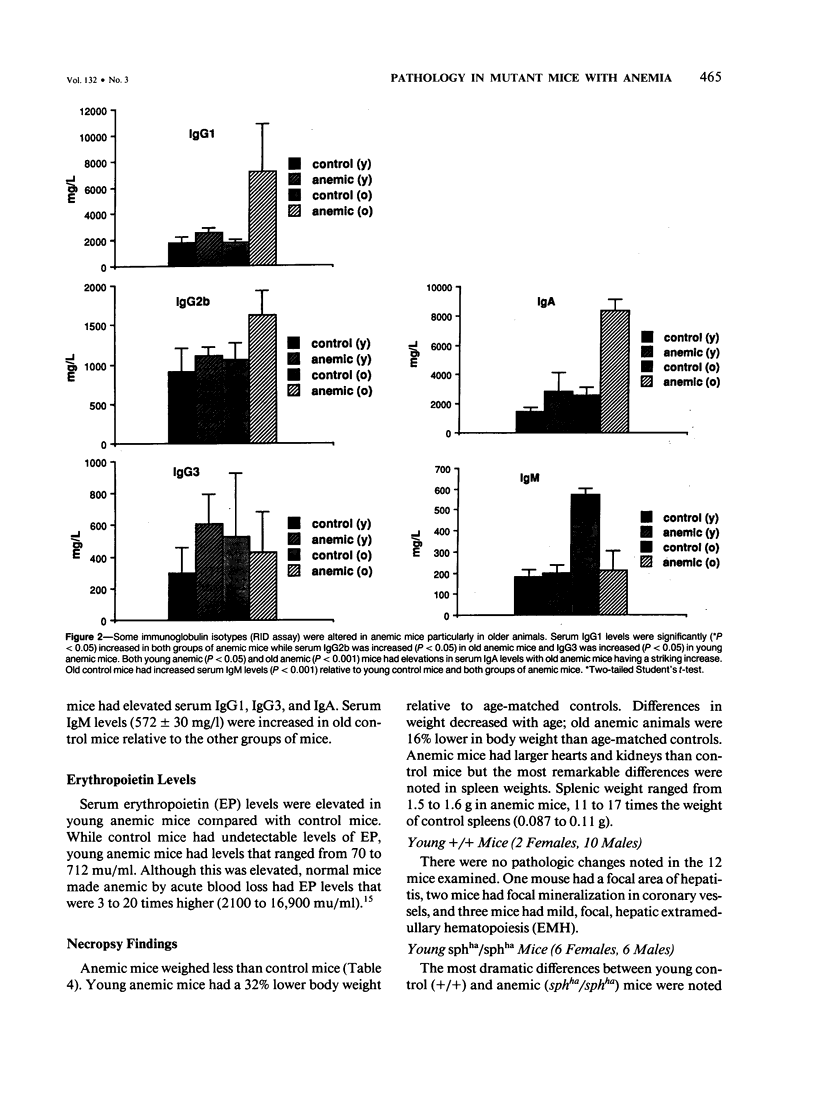
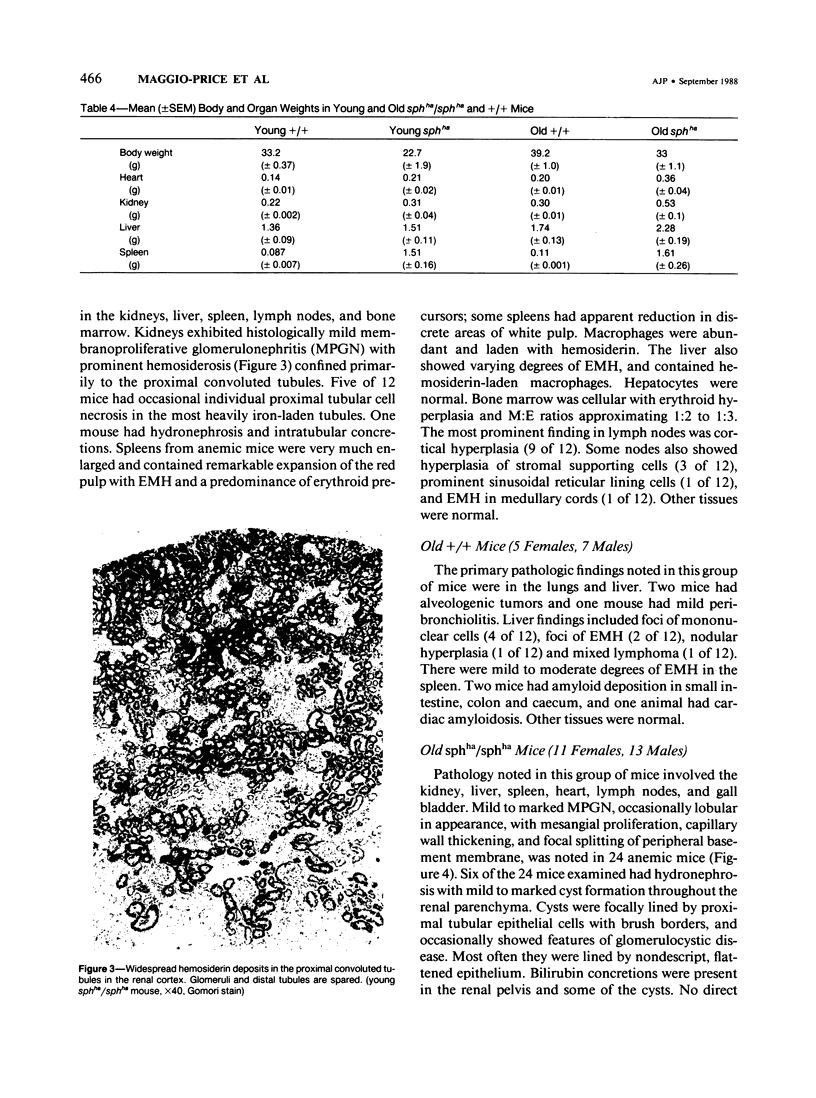
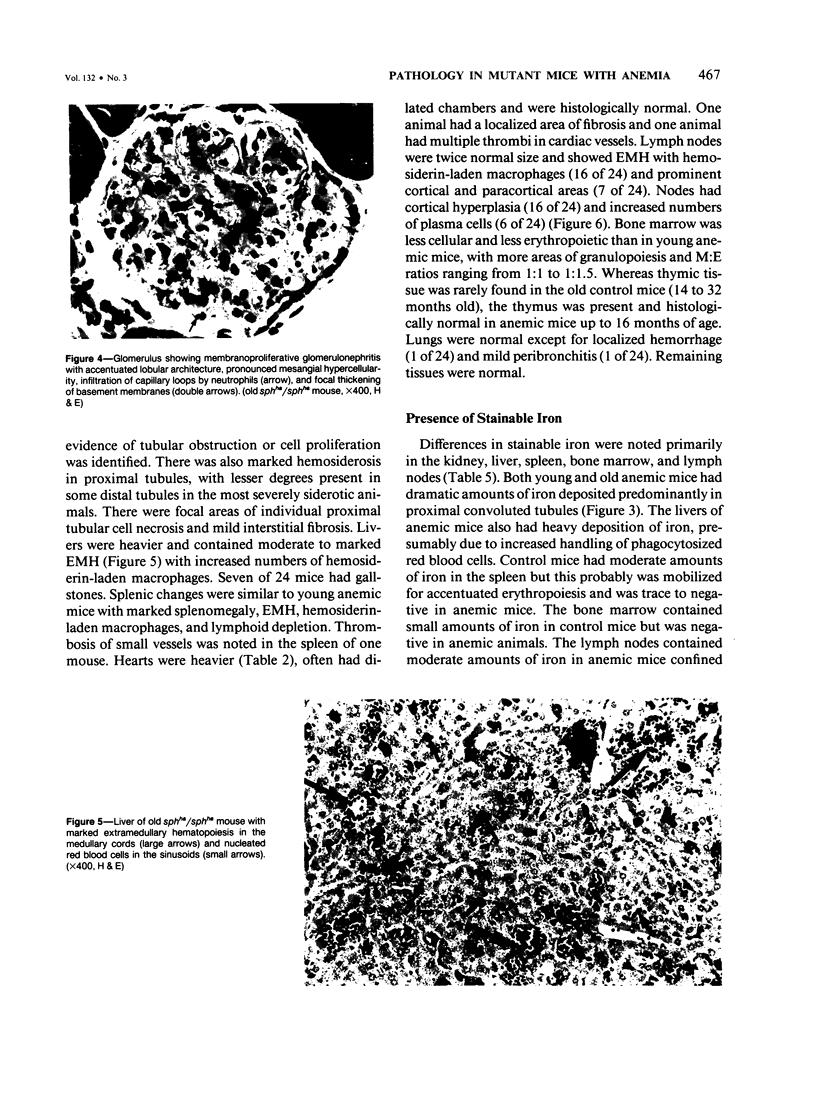
A colony of mice with congenital hemolytic anemia, sphha/sphha, were evaluated over a 3-year period. Prominent findings included decreased survivability, reticulocytosis, increased peripheral blood leukocytes, extramedullary hematopoiesis in liver and spleen, lymphoid hyperplasia and membranoproliferative glomerulonephritis. Older (12 to 21 months) anemic animals had elevated serum levels of IgG1 and IgA. There was deposition of C3, IgG, IgM, and IgA in renal glomeruli of both control and anemic mice, but deposition of IgM and IgA was more prominent and widely distributed in anemic animals and correlated with mesangial expansion and the presence of electron dense deposits in the mesangium and in glomerular capillary walls. Prominent renal tubular hemosiderosis was noted in young and old anemic mice. The relation between the hemolytic anemia and glomerular disease is unclear but these mice may be an animal model useful for exploration of changes attendant with chronic hemolysis and evaluation of renal disease that accompanies hemolytic anemia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein S. E. Inherited hemolytic disease in mice: a review and update. Lab Anim Sci. 1980 Apr;30(2 Pt 1):197–205. [PubMed] [Google Scholar]
- Bodine D. M., 4th, Birkenmeier C. S., Barker J. E. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984 Jul;37(3):721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Brookoff D., Maggio-Price L., Bernstein S., Weiss L. Erythropoiesis in ha/ha and sph/sph mice, mutants which produce spectrin-deficient erythrocytes. Blood. 1982 Mar;59(3):646–651. [PubMed] [Google Scholar]
- Chalifoux L. V., Bronson R. T., Sehgal P., Blake B. J., King N. W. Nephritis and hemolytic anemia in owl monkeys (Aotus trivirgatus). Vet Pathol. 1981 Apr;18(Suppl 6):23–37. doi: 10.1177/0300985881018s0603. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Heck J. A., Strober W. T-cell regulation of murine IgA synthesis. J Exp Med. 1979 Mar 1;149(3):632–643. doi: 10.1084/jem.149.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. E., Reindorf C. Serum immunoglobulin levels in sickle cell disease and thalassemia major. Am J Dis Child. 1968 Dec;116(6):586–590. doi: 10.1001/archpedi.1968.02100020590003. [DOI] [PubMed] [Google Scholar]
- George C. R., Parbtani A., Cameron J. S. Mouse malaria nephropathy. J Pathol. 1976 Dec;120(4):235–249. doi: 10.1002/path.1711200407. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Gordon-Smith E. C. Lymphocytes and haemopoiesis. Br J Haematol. 1981 Feb;47(2):163–169. doi: 10.1111/j.1365-2141.1981.tb02776.x. [DOI] [PubMed] [Google Scholar]
- Greenquist A. C., Shohet S. B., Bernstein S. E. Marked reduction of spectrinin hereditary spherocytosis in the common house mouse. Blood. 1978 Jun;51(6):1149–1155. [PubMed] [Google Scholar]
- Hirsch M. S., Allison A. C., Harvey J. J. Immune complexes in mice infected neonatally with Moloney leukaemogenic and murine sarcoma viruses. Nature. 1969 Aug 16;223(5207):739–740. doi: 10.1038/223739a0. [DOI] [PubMed] [Google Scholar]
- JOE M., TEASDALE J. M., MILLER J. R. A new mutation (sph) causing neonatal jaundice in the house mouse. Can J Genet Cytol. 1962 Jun;4:219–225. doi: 10.1139/g62-026. [DOI] [PubMed] [Google Scholar]
- Jones D. B. Membranoproliferative glomerulonephritis. One of many diesases? Arch Pathol Lab Med. 1977 Sep;101(9):457–461. [PubMed] [Google Scholar]
- Kinashi T., Harada N., Severinson E., Tanabe T., Sideras P., Konishi M., Azuma C., Tominaga A., Bergstedt-Lindqvist S., Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986 Nov 6;324(6092):70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- Kreimer-Birnbaum M., Bannerman R. M., Russell E. S., Bernstein S. E. Pyrrole pigments in normal and congenitally anaemic mice (+:+, W-W v , ha-ha, nb-nb, mk-mk, f-f and sla-Y). Comp Biochem Physiol A Comp Physiol. 1972 Sep 1;43(1):21–30. doi: 10.1016/0300-9629(72)90464-1. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Meyers P. A., Moore M. A. Synthesis and release of erythroid colony- and burst-potentiating activities by purified populations of murine peritoneal macrophages. J Exp Med. 1980 Apr 1;151(4):839–852. doi: 10.1084/jem.151.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARDI P., RUOL A. Renal hemosiderosis in the hemolytic anemias: diagnosis by means of needle biopsy. Blood. 1960 Jul;16:1029–1038. [PubMed] [Google Scholar]
- Landaw S. A. Kinetic aspects of endogenous carbon monoxide production in experimental animals. Ann N Y Acad Sci. 1970 Oct 5;174(1):32–48. doi: 10.1111/j.1749-6632.1970.tb49770.x. [DOI] [PubMed] [Google Scholar]
- Landaw S. A., Russell E. S., Bernstein S. E. Splenic destruction of newly-formed red blood cells and shortened erythrocyte survival in mice with congenital microcytosis. Scand J Haematol. 1970;7(6):516–524. doi: 10.1111/j.1600-0609.1970.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Maggio-Price L., Brookoff D., Weiss L. Changes in hematopoietic stem cells in bone marrow of mice with Plasmodium berghei malaria. Blood. 1985 Nov;66(5):1080–1085. [PubMed] [Google Scholar]
- Maggio-Price L., Wolf N. S., Priestley G. V., Pietrzyk M. E., Bernstein S. E. Evaluation of stem cell reserve using serial bone marrow transplantation and competitive repopulation in a murine model of chronic hemolytic anemia. Exp Hematol. 1988 Sep;16(8):653–659. [PubMed] [Google Scholar]
- Markham R. V., Jr, Sutherland J. C., Mardiney M. R., Jr The ubiquitous occurrence of immune complex localization in the renal glomeruli of normal mice. Lab Invest. 1973 Jul;29(1):111–120. [PubMed] [Google Scholar]
- Markham R. V., Sutherland J. C., Cimino E. F., Drake W. P., Mardiney M. R. Immune complexes localized in the renal glomeruli of AKR mice: the presence of MuLV gs-I and C-type RNA tumor virus gs-3 determinants. Rev Eur Etud Clin Biol. 1972 Aug-Sep;17(7):690–694. [PubMed] [Google Scholar]
- Natsuume-Sakai S., Motonishi K., Migita S. Quantitative estimations of five classes of immunoglobulin in inbred mouse strains. Immunology. 1977 Jun;32(6):861–866. [PMC free article] [PubMed] [Google Scholar]
- Okudaira H., Terada E., Ito T., Yamamoto K., Mizoguchi Y., Ogita T., Nomura T. Autoimmune glomerulonephritis and hemolytic anemia in a new laboratory animal, the Afghan pika. Clin Immunol Immunopathol. 1981 Dec;21(3):375–386. doi: 10.1016/0090-1229(81)90226-9. [DOI] [PubMed] [Google Scholar]
- Pardo V., Strauss J., Kramer H., Ozawa T., McIntosh R. M. Nephropathy associated with sickle cell anemia: an autologous immune complex nephritis. II. Clinicopathologic study of seven patients. Am J Med. 1975 Nov;59(5):650–659. doi: 10.1016/0002-9343(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Sassa S., Drummond G. S., Bernstein S. E., Kappas A. Long-term administration of massive doses of Sn-protoporphyrin in anemic mutant mice (sphha/sphha). J Exp Med. 1985 Sep 1;162(3):864–876. doi: 10.1084/jem.162.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Drummond G. S., Bernstein S. E., Kappas A. Tin-protoporphyrin suppression of hyperbilirubinemia in mutant mice with severe hemolytic anemia. Blood. 1983 May;61(5):1011–1013. [PubMed] [Google Scholar]
- Schilling R. F. Hereditary spherocytosis: a study of splenectomized persons. Semin Hematol. 1976 Jul;13(3):169–176. [PubMed] [Google Scholar]
- Shadduck R., Howard D., Stohlman F., Jr A difference in erythropoietin production between anemic and hypoxic mice. Proc Soc Exp Biol Med. 1968 May;128(1):132–136. doi: 10.3181/00379727-128-32961. [DOI] [PubMed] [Google Scholar]
- Strauss J., Pardo V., Koss M. N., Griswold W., McIntosh R. M. Nephropathy associated with sickle cell anemia: an autologous immune complex nephritis. I. Studies on nature of glomerular-bound antibody and antigen identification in a patient with sickle cell disease and immune deposit glomerulonephritis. Am J Med. 1975 Mar;58(3):382–387. doi: 10.1016/0002-9343(75)90604-x. [DOI] [PubMed] [Google Scholar]
- Unger A. E., Harris M. J., Bernstein S. E., Falcone J. C., Lux S. E. Hemolytic anemia in the mouse. Report of a new mutation and clarification of its genetics. J Hered. 1983 Mar-Apr;74(2):88–92. doi: 10.1093/oxfordjournals.jhered.a109747. [DOI] [PubMed] [Google Scholar]
- Wehner R. W., Smith R. G. Progressive cytomegalovirus glomerulonephritis - An experimental model. Am J Pathol. 1983 Sep;112(3):313–325. [PMC free article] [PubMed] [Google Scholar]
- White R. H. Quartan malarial nephrotic syndrome. Nephron. 1973;11(2):147–162. doi: 10.1159/000180227. [DOI] [PubMed] [Google Scholar]